Dynamic Imaging of Lipid Order and Heterogeneous Microviscosity in Mitochondrial Membranes of Potato Tubers Under Abiotic Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Stress Treatment
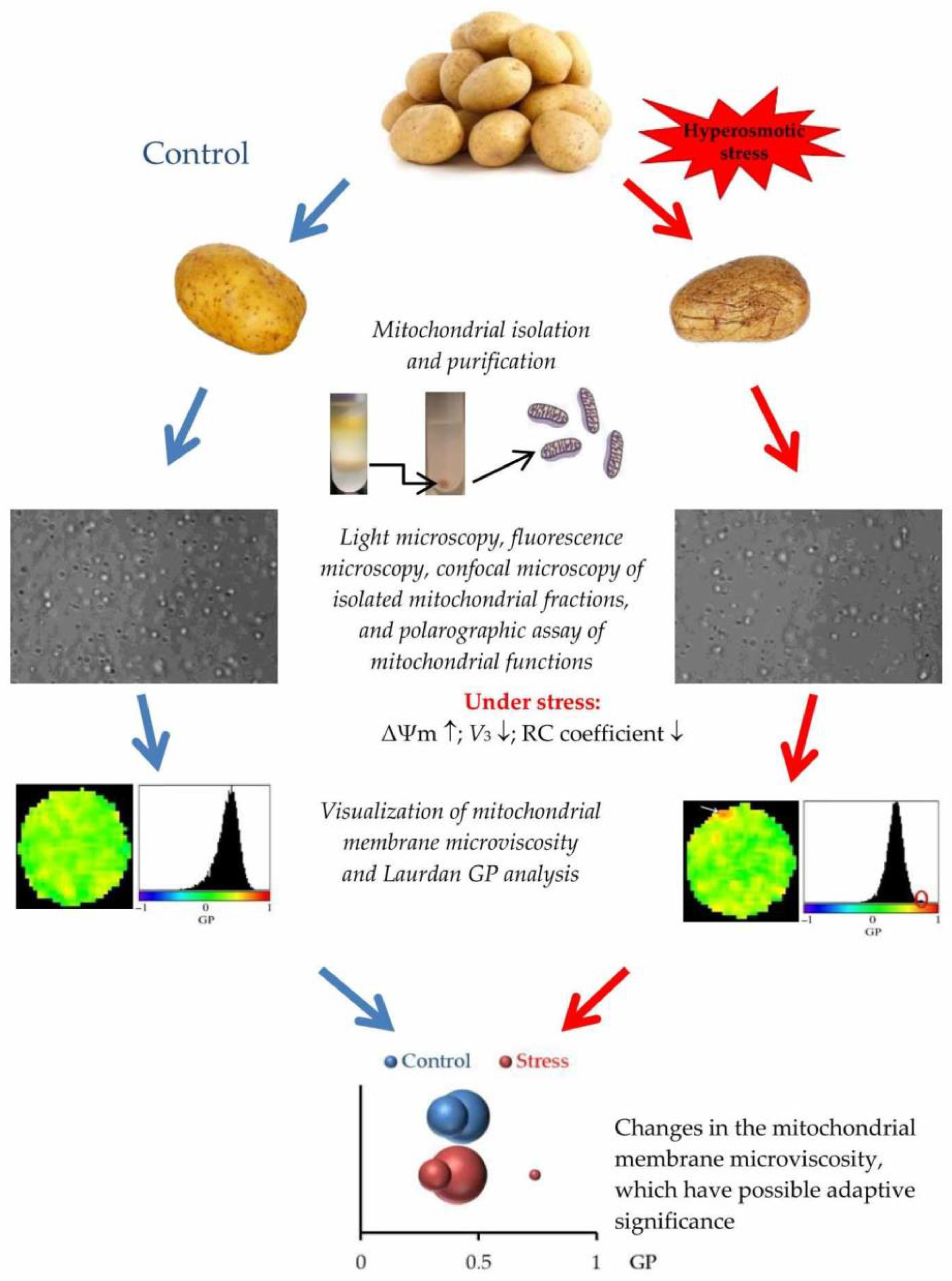
2.2. Isolation of Mitochondria, Respiratory Measurements, and Integrity of the Outer Mitochondrial Membrane
2.3. Fluorescence Microscopy and Imaging of Mitochondrial Activity
2.4. Confocal Microscopy and the Assessment of the Physical State of Mitochondrial Membranes
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIC | Akaike information criterion |
| BIC | Bayesian information criterion |
| CCCP | Carbonyl cyanide m-chlorophenyl hydrazone |
| ETC | Electron transport chain |
| GP | Generalized polarization |
| Laurdan | 2-dimethylamino-6-lauroylnaphthalene |
| MO | MitoTracker Orange CMTMRos |
| OXPHOS | Oxidative phosphorylation system |
| RC | Respiratory control |
| ROS | Reactive oxygen species |
| V3 | Phosphorylating respiration |
| V4 | Non-phosphorylating respiration |
| ΔμH+ | Chemiosmotic potential |
| ΔΨm | Membrane potential |
References
- Peng, S.B.; Huang, J.L.; Sheehy, J.E.; Laza, R.C.; Visperas, R.M.; Zhong, X.H.; Centeno, G.S.; Khush, G.S.; Cassman, K.G. Rice yields decline with higher night temperature from global warming. Proc. Natl. Acad. Sci. USA 2004, 101, 9971–9975. [Google Scholar] [CrossRef]
- Koshkin, E.I.; Andreeva, I.V.; Guseinov, G.G. Impact of global climate change on productivity and stress tolerance of field crops. Agrochem 2019, 12, 83–96. [Google Scholar] [CrossRef]
- Alotaibi, M. Climate change, its impact on crop production, challenges, and possible solutions. Not. Bot. Horti Agrobot. Cluj-Napoca 2023, 51, 13020. [Google Scholar] [CrossRef]
- Atkin, O.; Macherel, D. The crucial role of plant mitochondria in orchestrating drought tolerance. Ann. Bot. 2009, 103, 581–597. [Google Scholar] [CrossRef]
- Lateef, M.; Naawe, E.K.; Hasan, Z.; Çalışkan, M.E. An overview of the impact of drought stress on potatoes in the era of climate change. In Drought Stress; Chaudhry, U.K., Öztürk, Z.N., Gökçe, A.F., Eds.; Springer: Cham, Switzerland, 2025; pp. 239–263. [Google Scholar] [CrossRef]
- Logan, D.C. The mitochondrial compartment. J. Exp. Bot. 2006, 57, 1225–1243. [Google Scholar] [CrossRef]
- Ukolova, I.V.; Kondakova, M.A.; Kondratov, I.G.; Sidorov, A.V.; Borovskii, G.B.; Voinikov, V.K. New insights into the organisation of the oxidative phosphorylation system in the example of pea shoot mitochondria. Biochim. Biophys. Acta—Bioenerg. 2020, 1861, 148264. [Google Scholar] [CrossRef]
- Skulachev, V.P. Bioenergetic aspects of apoptosis, necrosis and mitoptosis. Apoptosis 2006, 11, 473–485. [Google Scholar] [CrossRef]
- Grabelnych, O.I.; Borovik, O.A.; Tauson, E.L.; Pobezhimova, T.P.; Katyshev, A.I.; Pavlovskaya, N.S.; Koroleva, N.A.; Lyubushkina, I.V.; Bashmakov, V.Y.; Popov, V.N.; et al. Mitochondrial energy-dissipating systems (alternative oxidase, uncoupling proteins, and external NADH dehydrogenase) are involved in development of frost-resistance of winter wheat seedlings. Biochemistry (Mosc) 2014, 79, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Pastore, D.; Trono, D.; Laus, M.N.; Di Fonzo, N.; Flagella, Z. Possible plant mitochondria involvement in cell adaptation to drought stress. A case study: Durum wheat mitochondria. J. Exp. Bot. 2007, 58, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Beckman, K.B.; Ames, B.N. The free radical theory of aging matures. Physiol. Rev. 1998, 78, 547–581. [Google Scholar] [CrossRef]
- James, A.M.; Murphy, M.P. How mitochondrial damage affects cell function. J. Biomed. Sci. 2002, 9, 475–487. [Google Scholar] [CrossRef]
- Yun, J.; Finkel, T. Mitohormesis. Cell Metab. 2014, 19, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Lázaro, J.J.; Jiménez, A.; Camejo, D.; Iglesias-Baena, I.; del Martí, M.C.; Lázaro-Payo, A.; Barranco-Medina, S.; Sevilla, F. Dissecting the integrative antioxidant and redox systems in plant mitochondria. Effect of stress and S-nitrosylation. Front. Plant Sci. 2013, 4, 460. [Google Scholar] [CrossRef] [PubMed]
- Liberatore, K.L.; Dukowic-Schulze, S.; Miller, M.E.; Chen, C.; Kianian, S.F. The role of mitochondria in plant development and stress tolerance. Free Radic. Biol. Med. 2016, 100, 238–256. [Google Scholar] [CrossRef] [PubMed]
- Møller, I.M.; Rasmusson, A.G.; van Aken, O. Plant mitochondria—Past, present and future. Plant J. 2021, 108, 912–959. [Google Scholar] [CrossRef]
- Porcher, A.; Kangasjärvi, S. Plant biology: Unlocking mitochondrial stress signals. Curr. Biol. 2024, 34, R59–R61. [Google Scholar] [CrossRef]
- Mouritsen, O.G. The liquid-ordered state comes of age. Biochim. Biophys. Acta (BBA)-Biomembr. 2010, 1798, 1286–1288. [Google Scholar] [CrossRef]
- Morigaki, K.; Tanimoto, Y. Evolution and development of model membranes for physicochemical and functional studies of the membrane lateral heterogeneity. Biochim. Biophys. Acta (BBA)-Biomembr. 2018, 1860, 2012–2017. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Bharti, D.; Levental, I. Membrane heterogeneity beyond the plasma membrane. Front. Cell Dev. Biol. 2020, 8, 580814. [Google Scholar] [CrossRef]
- Kuimova, M.K. Mapping viscosity in cells using molecular rotors. Phys. Chem. Chem. Phys. 2012, 14, 12671–12686. [Google Scholar] [CrossRef]
- Simons, K.; van Meer, G. Lipid sorting in epithelial cells. Biochemistry 1988, 27, 6197–6202. [Google Scholar] [CrossRef]
- Lagerholm, B.C.; Weinreb, G.E.; Jacobson, K.; Thompson, N.L. Detecting microdomains in intact cell membranes. Annu. Rev. Phys. Chem. 2005, 56, 309–336. [Google Scholar] [CrossRef]
- van Meer, G.; Voelker, D.R.; Feigenson, G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell Biol. 2008, 9, 112–124. [Google Scholar] [CrossRef]
- Sezgin, E.; Levental, I.; Mayor, S.; Eggeling, C. The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell Biol. 2017, 18, 361–374. [Google Scholar] [CrossRef]
- Zheng, Y.Z.; Berg, K.B.; Foster, L.J. Mitochondria do not contain lipid rafts, and lipid rafts do not contain mitochondrial proteins. J. Lipid Res. 2009, 50, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, T.; Manganelli, V.; Grasso, M.; Mattei, V.; Ferri, A.; Misasi, R.; Sorice, M. Role of mitochondrial raft-like microdomains in the regulation of cell apoptosis. Apoptosis 2015, 20, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Rozentsvet, O.; Nesterkina, I.; Ozolina, N.; Nesterov, V. Detergent-resistant microdomains (lipid rafts) in endomembranes of the wild halophytes. Funct. Plant Biol. 2019, 46, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Nurminsky, V.N.; Nesterov, V.N.; Rosentsvet, O.A.; Rakevich, A.L.; Bukin, Y.S.; Kapustina, I.S.; Ozolina, N.V. Analysis of lipid order in raft structures of mitochondrial membranes of halophytes with the aid of fluorescence microscopy. Biochem. (Mosc.) Suppl. Ser. A Membr. Cell Biol. 2021, 15, 249–256. [Google Scholar] [CrossRef]
- Neuburger, M.; Journet, E.P.; Bligny, R.; Carde, J.-P.; Douce, R. Purification of plant mitochondria by isopycnic centrifugation in density gradients of Percoll. Arch. Biochem. Biophys. 1982, 217, 312–323. [Google Scholar] [CrossRef]
- Grabelnykh, O.I.; Yakovenko, K.V.; Polyakova, E.A.; Korsukova, A.V.; Stepanov, A.V.; Fedotova, O.A.; Zabanova, N.S.; Lyubushkina, I.V.; Pobezhimova, T.P.; Borovskii, G.B. Functioning of mitochondria in transgenic potato tubers with the gox gene for glucose oxidase from Penicillium funiculosum during different storage periods. Russ. J. Plant Physiol. 2022, 69, 112. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–274. [Google Scholar] [CrossRef]
- Nikiforov, N.G.; Ryabova, A.; Kubekina, M.V.; Romanishkin, I.D.; Trofimov, K.A.; Chegodaev, Y.S.; Ivanova, E.; Orekhov, A.N. Two subpopulations of human monocytes that differ in mitochondrial membrane potential. Biomedicines 2021, 9, 153. [Google Scholar] [CrossRef]
- Lyubushkina, I.V.; Fedyaeva, A.V.; Stepanov, A.V.; Grabelnych, O.I. High temperatures induce ROS generation and damage to respiratory activity in Saccharum officinarum suspension cells. J. Sib. Fed. Univ. Biol. 2021, 14, 354–369. [Google Scholar] [CrossRef]
- Bagatolli, L.A. To see or not to see: Lateral organization of biological membranes and fluorescence microscopy. Biochim. Biophys. Acta 2006, 1758, 1541–1556. [Google Scholar] [CrossRef]
- Watanabe, N.; Goto, Y.; Suga, K.; Nyholm, T.K.M.; Slotte, J.P.; Umakoshi, H. Solvatochromic modeling of Laurdan for multiple polarity analysis of dihydrosphingomyelin bilayer. Biophys. J. 2019, 116, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Harris, F.M.; Best, K.B.; Bell, J.D. Use of laurdan fluorescence intensity and polarization to distinguish between changes in membrane fluidity and phospholipid order. Biochim. Biophys. Acta 2002, 1565, 123–128. [Google Scholar] [CrossRef]
- Wheeler, G.; Tyler, K.M. Widefield microscopy for live imaging of lipid domains and membrane dynamics. Biochim. Biophys. Acta 2011, 1808, 634–641. [Google Scholar] [CrossRef]
- Owen, D.M.; Rentero, C.; Magenau, A.; Abu-Siniyeh, A.; Gaus, K. Quantitative imaging of membrane lipid order in cells and organisms. Nat. Protoc. 2011, 7, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Guaragnella, N.; Di Noia, M.A.; Primavera, A. Mitochondrial (dys) function: A double edge sword in cell stress response. Front. Cell Death 2024, 3, 1467272. [Google Scholar] [CrossRef]
- Hill, S.; Sataranatarajan, K.; van Remmen, H. Role of signaling molecules in mitochondrial stress response. Front. Genet. 2018, 9, 225. [Google Scholar] [CrossRef] [PubMed]
- Ukolova, I.V.; Borovskii, G.B. OXPHOS organization and activity in mitochondria of plants with different life strategies. Int. J. Mol. Sci. 2023, 24, 15229. [Google Scholar] [CrossRef]
- Gaus, K.; Gratton, E.; Kable, E.P.; Jones, A.S.; Gelissen, I.; Kritharides, L.; Jessup, W. Visualizing lipid structure and raft domains in living cells with two-photon microscopy. Proc. Natl. Acad. Sci. USA 2003, 100, 15554–15559. [Google Scholar] [CrossRef]
- Gaus, K.; Zech, T.; Harder, T. Visualizing membrane microdomains by Laurdan 2-photon microscopy. Mol. Membr. Biol. 2006, 23, 41–48. [Google Scholar] [CrossRef]
- Weber, P.; Wagner, M.; Schneckenburger, H. Fluorescence imaging of membrane dynamics in living cells. J. Biomed. Opt. 2010, 15, 046017. [Google Scholar] [CrossRef]
- Wenzel, M.; Vischer, N.O.E.; Strahl, H.; Hamoen, L.W. Assessing membrane fluidity and visualizing fluid membrane domains in bacteria using fluorescent membrane dyes. Bio-protoc. 2018, 8, e3063. [Google Scholar] [CrossRef]
- Levitan, I. Evaluating membrane structure by Laurdan imaging: Disruption of lipid packing by oxidized lipids. Curr. Top. Membr. 2021, 88, 235–256. [Google Scholar] [CrossRef]
- Gunther, G.; Malacrida, L.; Jameson, D.M.; Gratton, E.; Sánchez, S.A. LAURDAN since Weber: The quest for visualizing membrane heterogeneity. Acc. Chem. Res. 2021, 54, 976–987. [Google Scholar] [CrossRef]
- Pokorna, S.; Ventura, A.E.; Santos, T.C.B.; Hof, M.; Prieto, M.; Futerman, A.H.; Silva, L.C. Laurdan in live cell imaging: Effect of acquisition settings, cell culture conditions and data analysis on generalized polarization measurements. J. Photochem. Photobiol. B Biol. 2022, 228, 112404. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Sharma, P.; Chaturvedi, V.; Pati, A.K. Organic fluorophores for studying lipid membrane structures and dynamics. Chem. Asian J. 2025, e00328. [Google Scholar] [CrossRef] [PubMed]
- Orlikowska-Rzeznik, H.; Krok, E.; Chattopadhyay, M.; Lester, A.; Piatkowski, L. Laurdan discerns lipid membrane hydration and cholesterol content. J. Phys. Chem. B 2023, 127, 3382–3391. [Google Scholar] [CrossRef]
- Boyd, M.A.; Kamat, N.P. Visualizing tension and growth in model membranes using optical dyes. Biophys. J. 2018, 115, 1307–1315. [Google Scholar] [CrossRef]
- Zapata-Mercado, E.; Azarova, E.V.; Hristova, K. Effect of osmotic stress on live cell plasma membranes, probed via Laurdan general polarization measurements. Biophys. J. 2022, 121, 2411–2418. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; George, G.; Raja, S.O.; Kandaswamy, P.; Kumar, M.; Thutupalli, S.; Laxman, S.; Gulyani, A. A molecular rotor FLIM probe reveals dynamic coupling between mitochondrial inner membrane fluidity and cellular respiration. Proc. Natl. Acad. Sci. USA 2023, 120, e2213241120. [Google Scholar] [CrossRef] [PubMed]
- Knippenberg, S.; De, K.; Aisenbrey, C.; Bechinger, B.; Osella, S. Hydration- and temperature-dependent fluorescence spectra of Laurdan conformers in a DPPC membrane. Cells 2024, 13, 1232. [Google Scholar] [CrossRef]
- Watanabe, N.; Suga, K.; Slotte, J.P.; Nyholm, T.K.M.; Umakoshi, H. Lipid-surrounding water molecules probed by time-resolved emission spectra of Laurdan. Langmuir 2019, 35, 6762–6770. [Google Scholar] [CrossRef]
- Dinic, J.; Biverståhl, H.; Mäler, L.; Parmryd, I. Laurdan and di-4-ANEPPDHQ do not respond to membrane-inserted peptides and are good probes for lipid packing. Biochim. Biophys. Acta 2011, 1808, 298–306. [Google Scholar] [CrossRef] [PubMed]
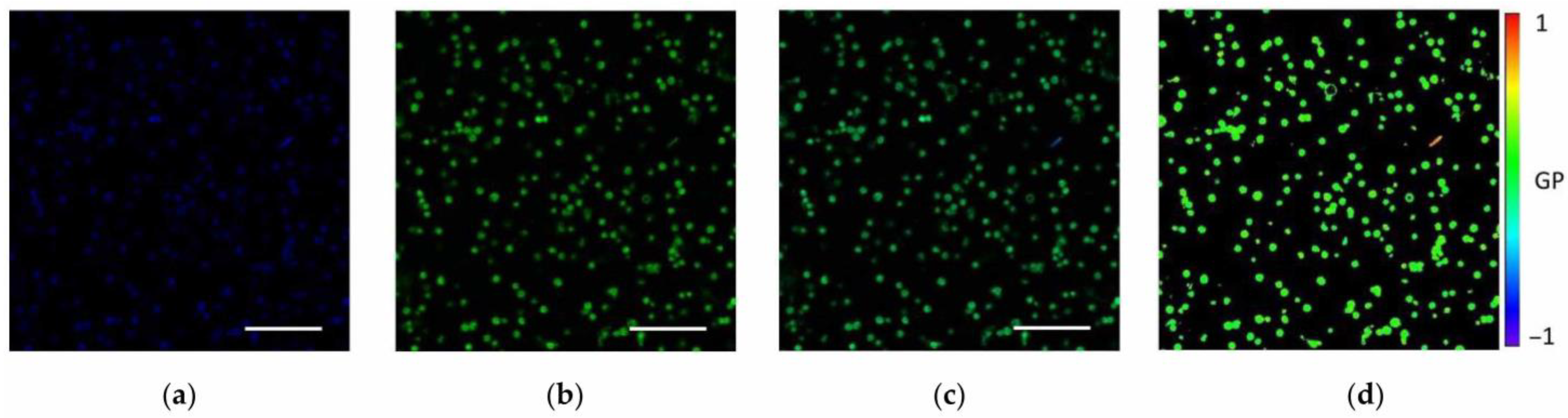
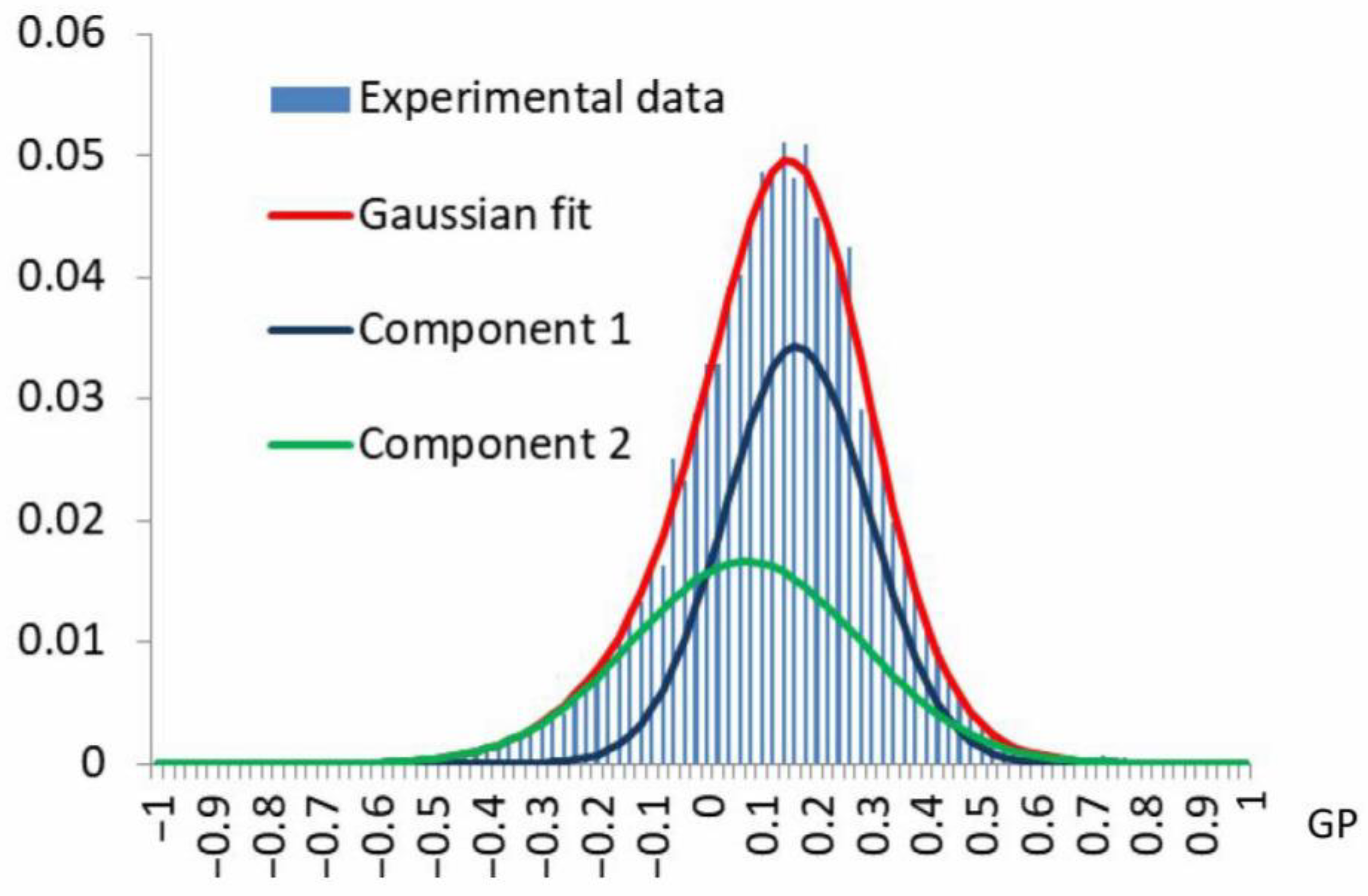
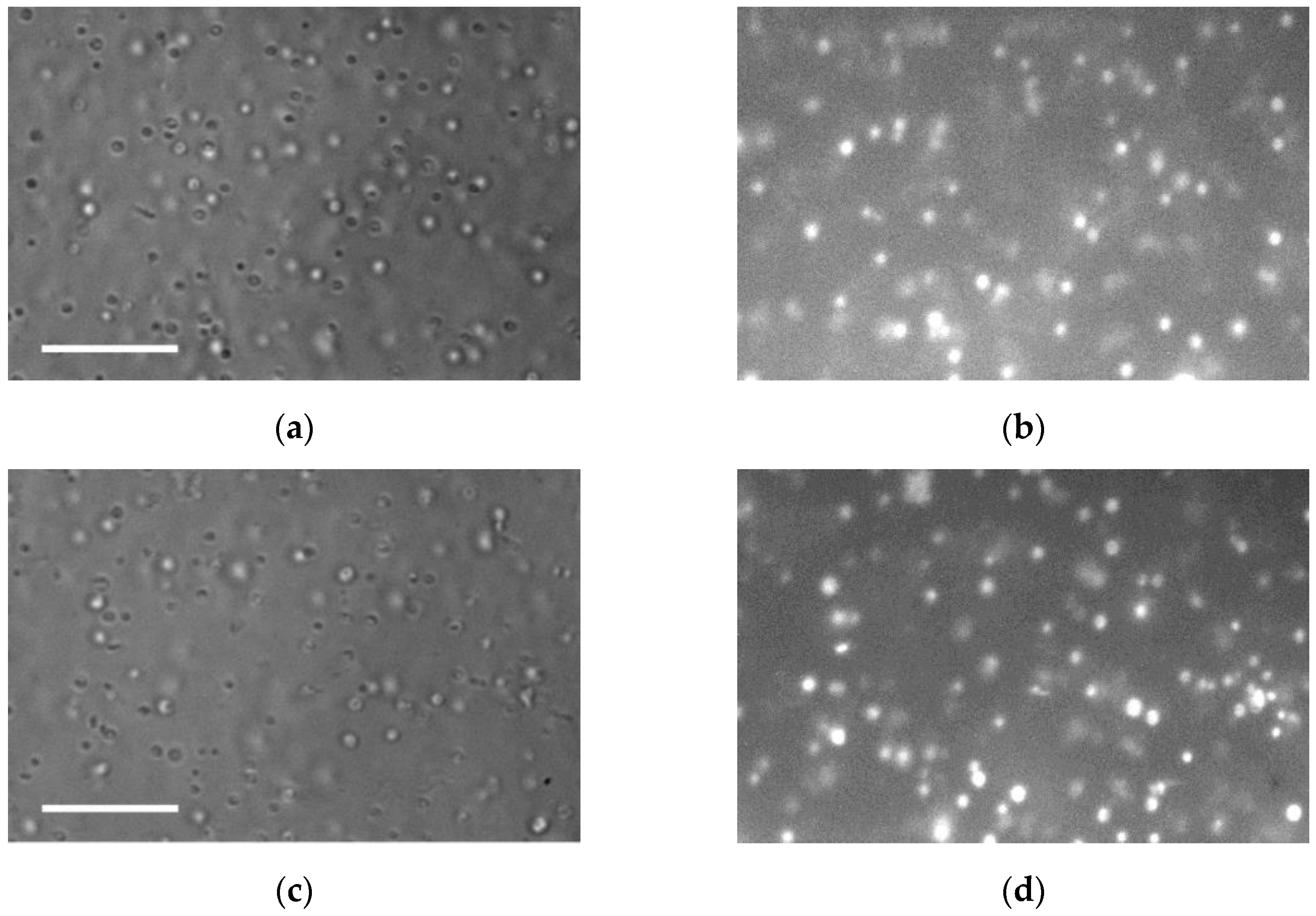
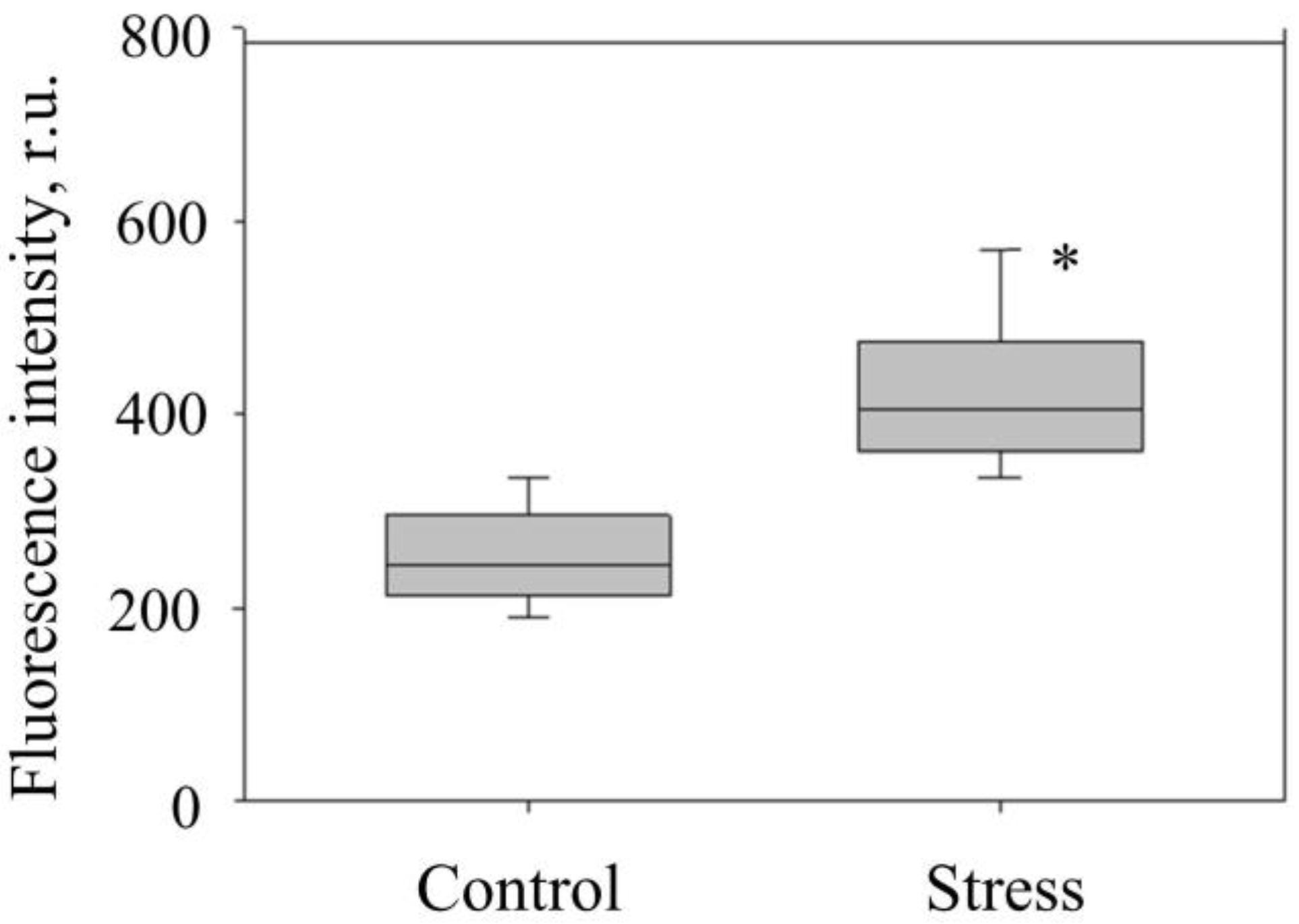
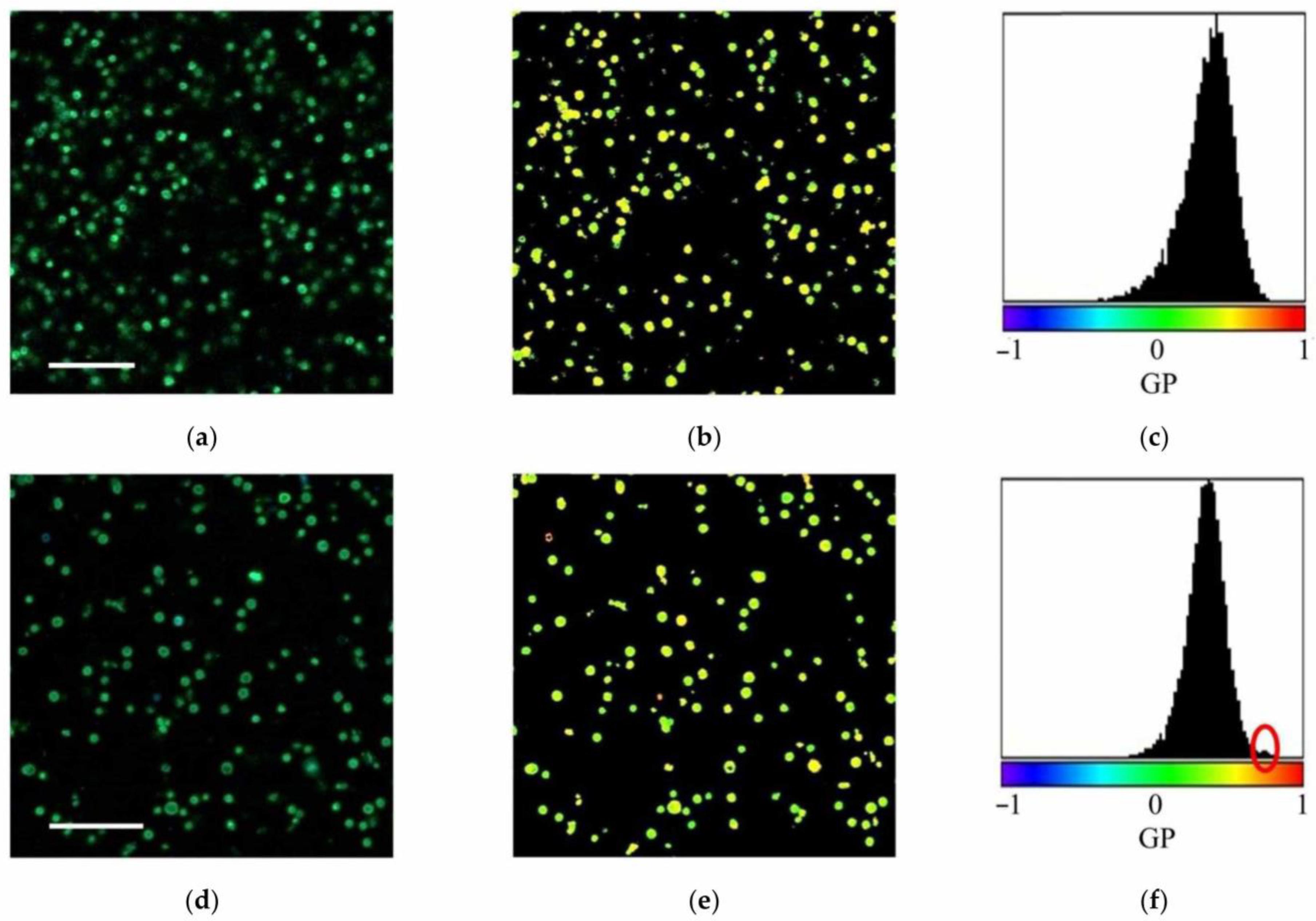
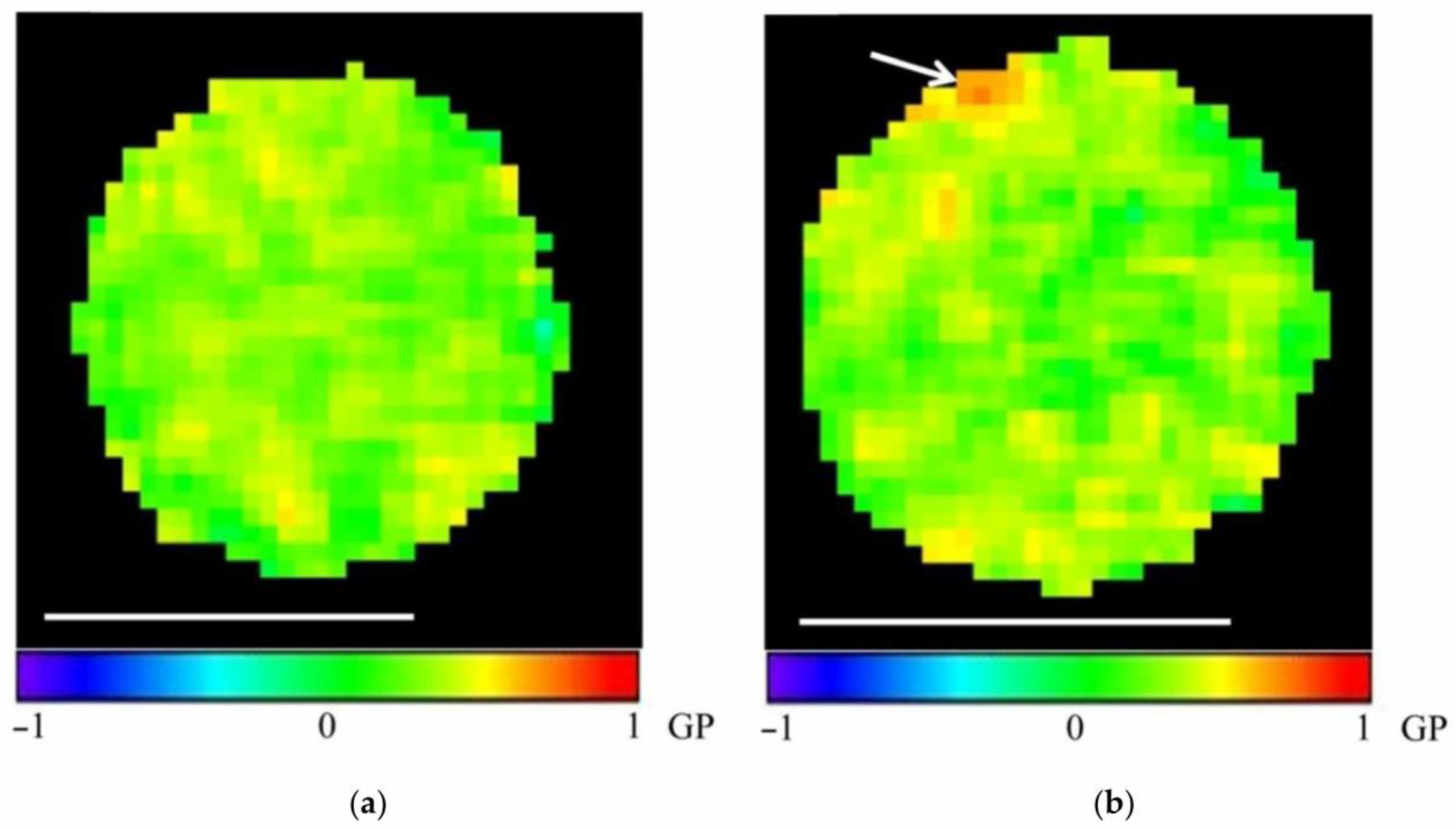
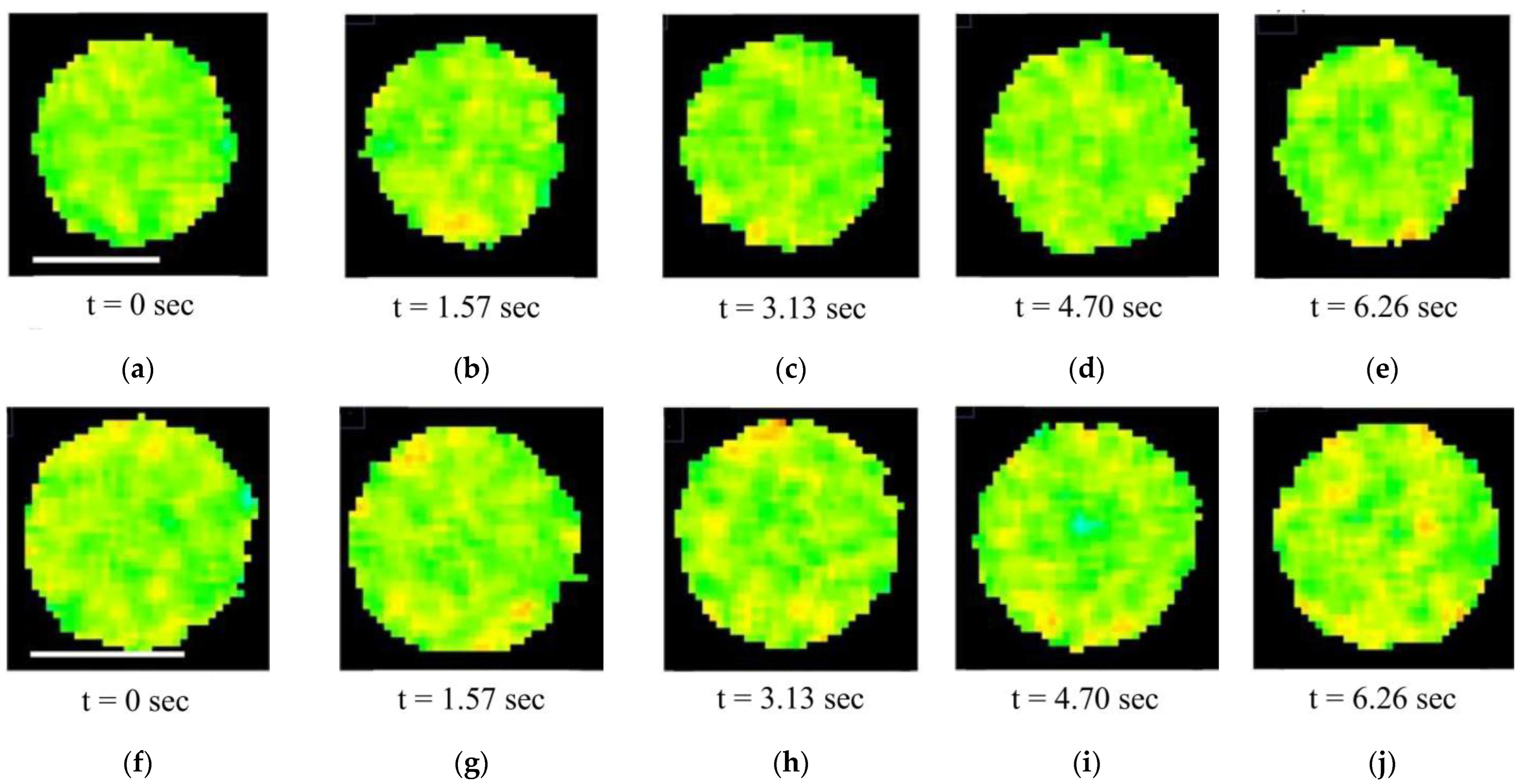
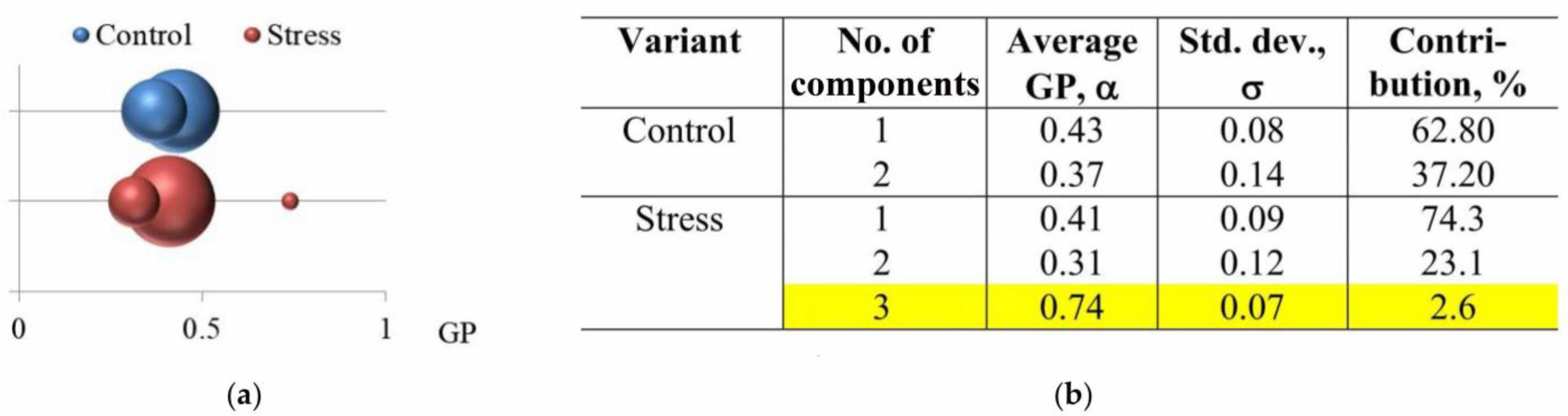
| Measurable Parameter | Control | Stress |
|---|---|---|
| V3, nmol O2 × (min × mg protein)−1 | 141.0 ± 25.1 | 61.6 ± 12.9 * |
| V4, nmol O2 × (min × mg protein)−1 | 55.7 ± 11.3 | 49.5 ± 12.7 |
| RC coefficient | 2.55 ± 0.07 | 1.26 ± 0.06 * |
| Integrity, % | 90 ± 2 | 88 ± 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nurminsky, V.N.; Shamanova, S.I.; Grabelnych, O.I.; Ozolina, N.V.; Wang, Y.; Perfileva, A.I. Dynamic Imaging of Lipid Order and Heterogeneous Microviscosity in Mitochondrial Membranes of Potato Tubers Under Abiotic Stress. Membranes 2025, 15, 302. https://doi.org/10.3390/membranes15100302
Nurminsky VN, Shamanova SI, Grabelnych OI, Ozolina NV, Wang Y, Perfileva AI. Dynamic Imaging of Lipid Order and Heterogeneous Microviscosity in Mitochondrial Membranes of Potato Tubers Under Abiotic Stress. Membranes. 2025; 15(10):302. https://doi.org/10.3390/membranes15100302
Chicago/Turabian StyleNurminsky, Vadim N., Svetlana I. Shamanova, Olga I. Grabelnych, Natalia V. Ozolina, Yuguang Wang, and Alla I. Perfileva. 2025. "Dynamic Imaging of Lipid Order and Heterogeneous Microviscosity in Mitochondrial Membranes of Potato Tubers Under Abiotic Stress" Membranes 15, no. 10: 302. https://doi.org/10.3390/membranes15100302
APA StyleNurminsky, V. N., Shamanova, S. I., Grabelnych, O. I., Ozolina, N. V., Wang, Y., & Perfileva, A. I. (2025). Dynamic Imaging of Lipid Order and Heterogeneous Microviscosity in Mitochondrial Membranes of Potato Tubers Under Abiotic Stress. Membranes, 15(10), 302. https://doi.org/10.3390/membranes15100302






