Development and Characterization of Sawdust-Based Ceramic Membranes for Textile Effluent Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Ceramic Membrane
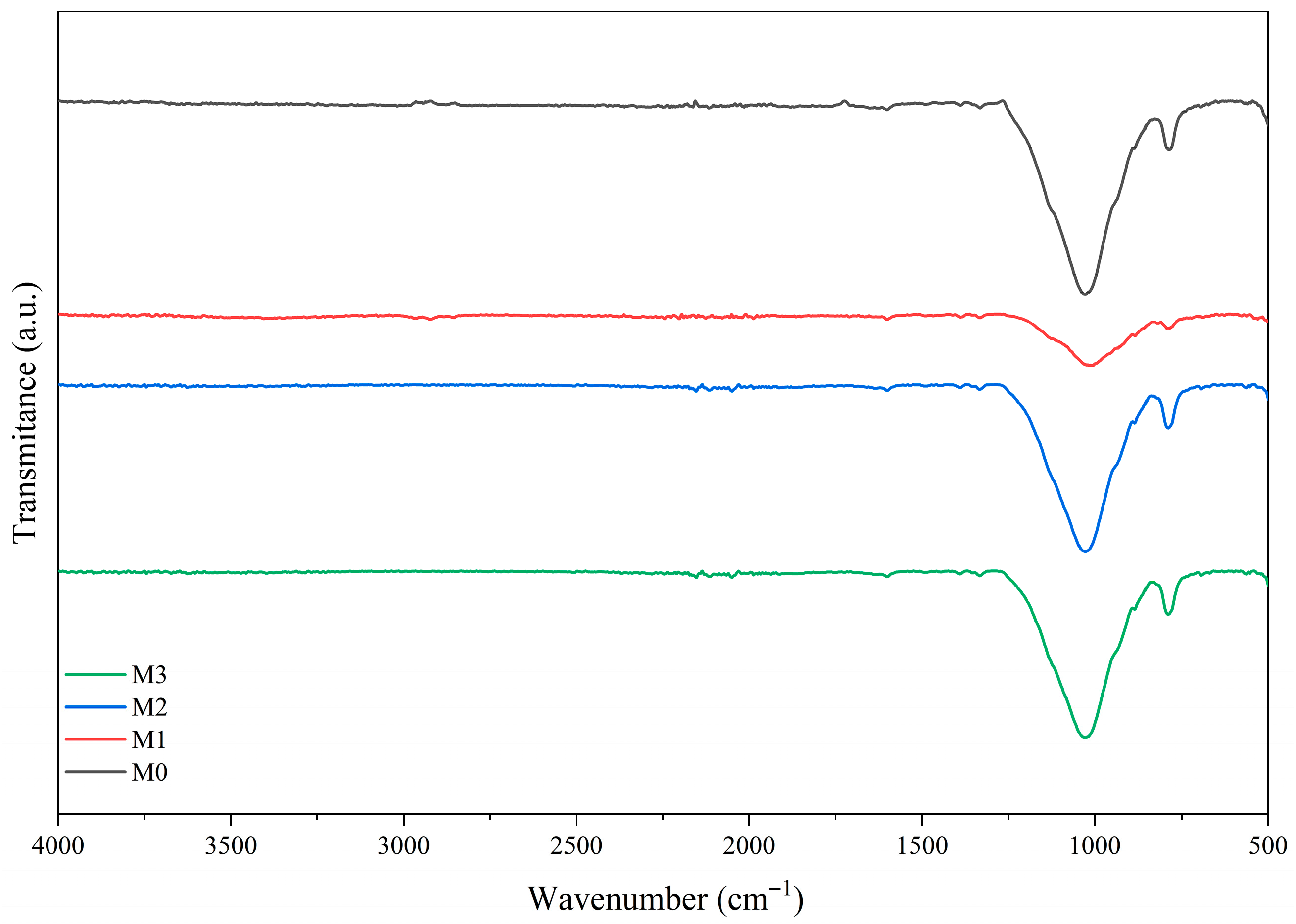
2.2. Characterization
2.3. Permeation Performance
Data Analysis
2.4. Adsorption Isotherm Studies
3. Results and Discussion
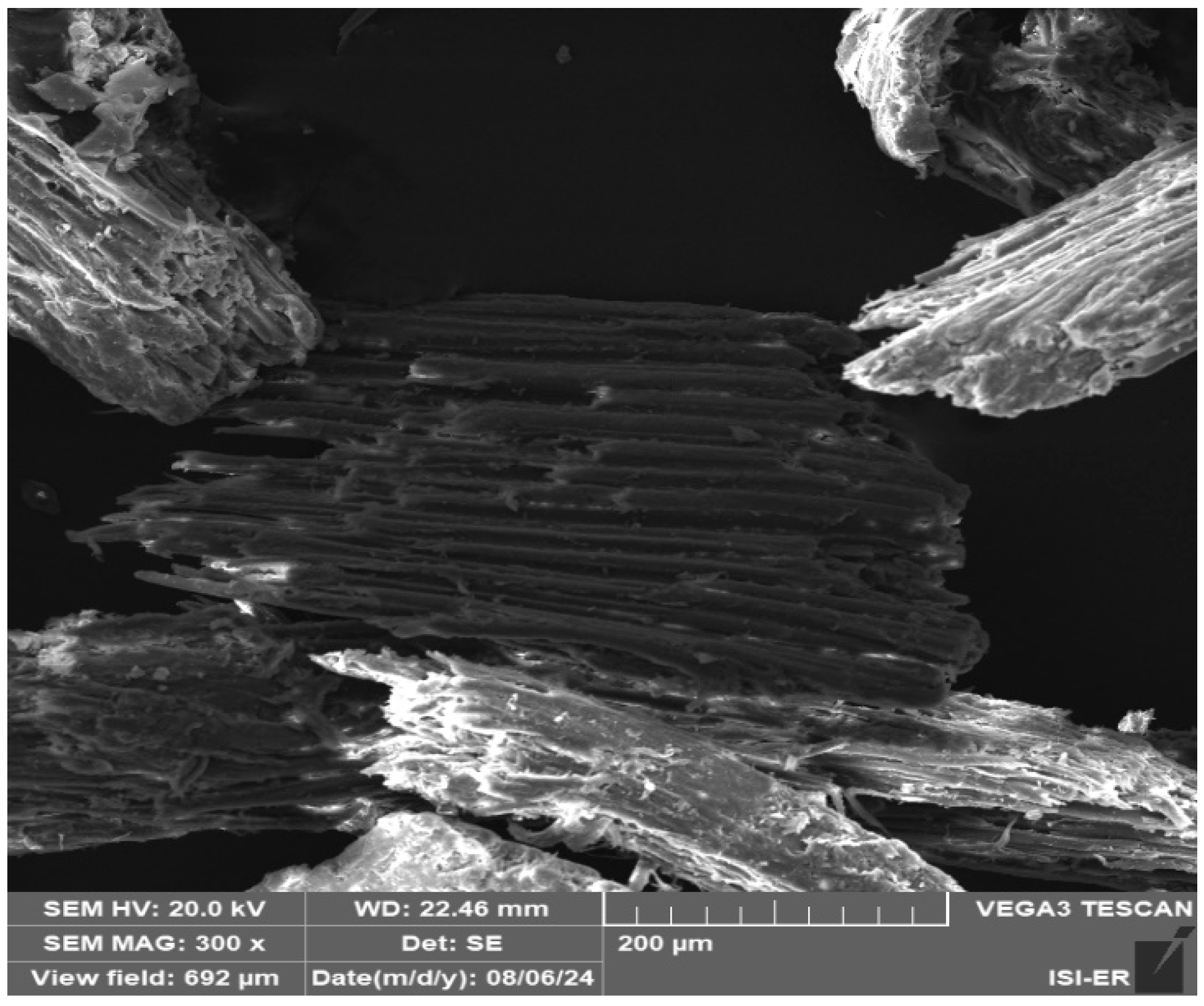
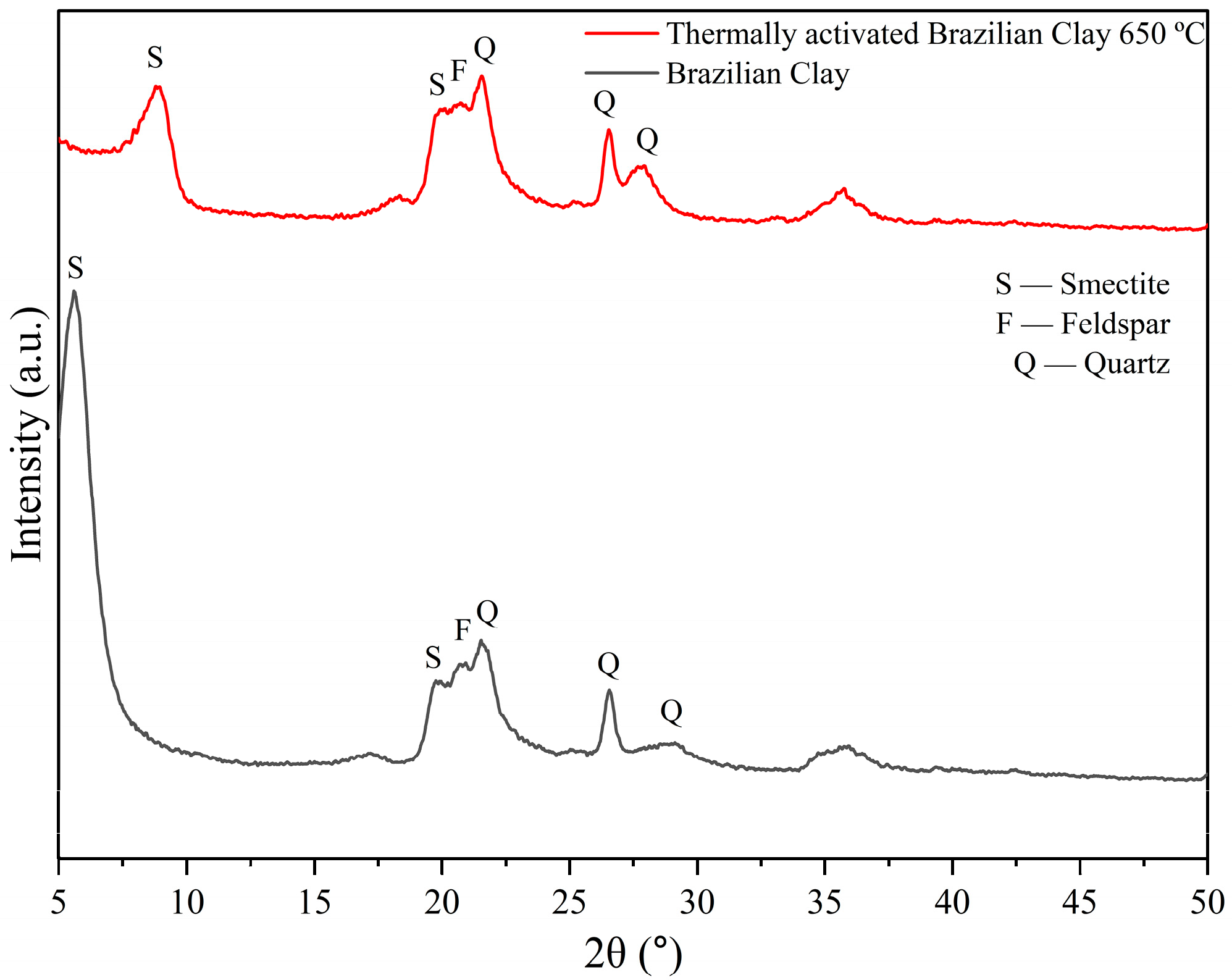
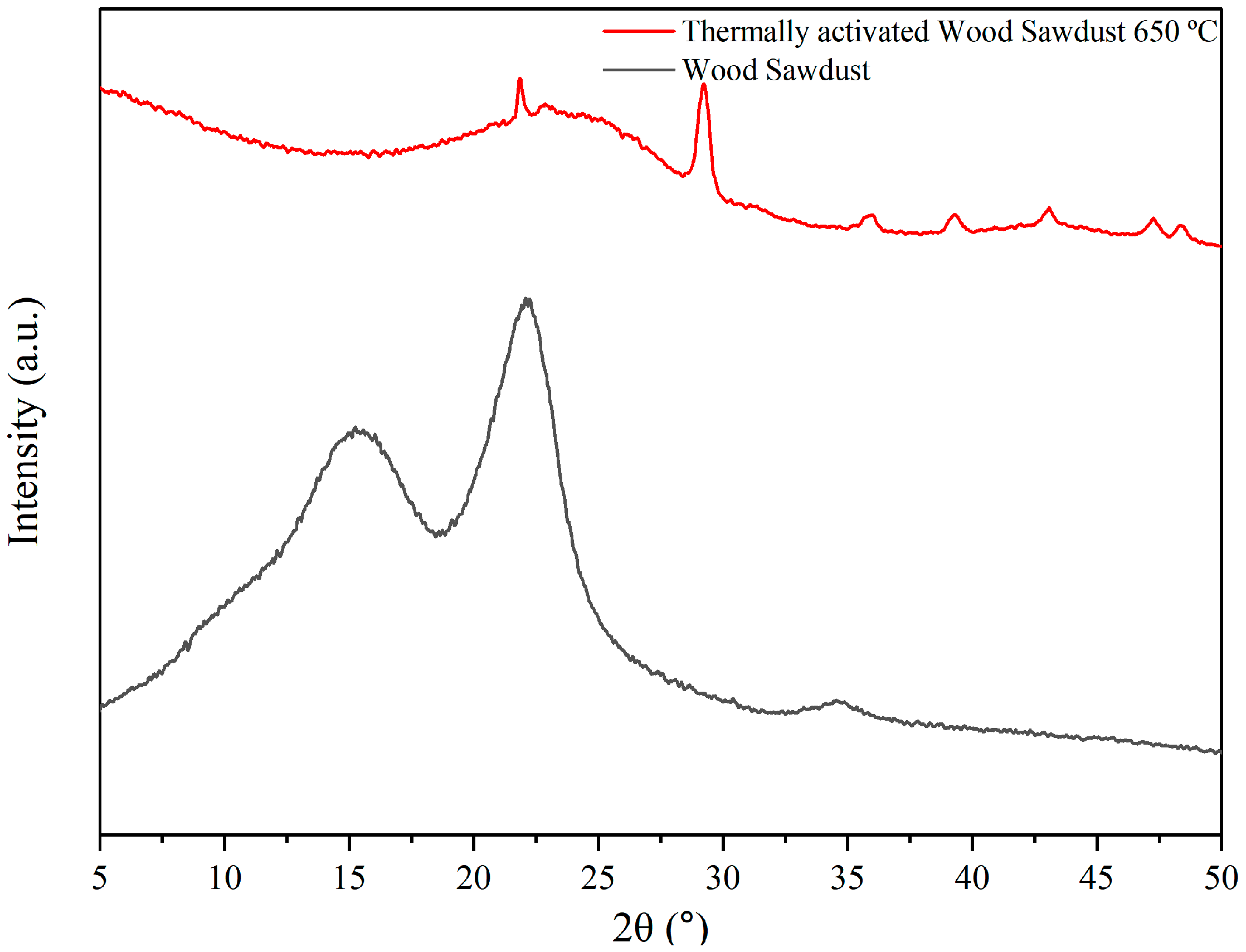
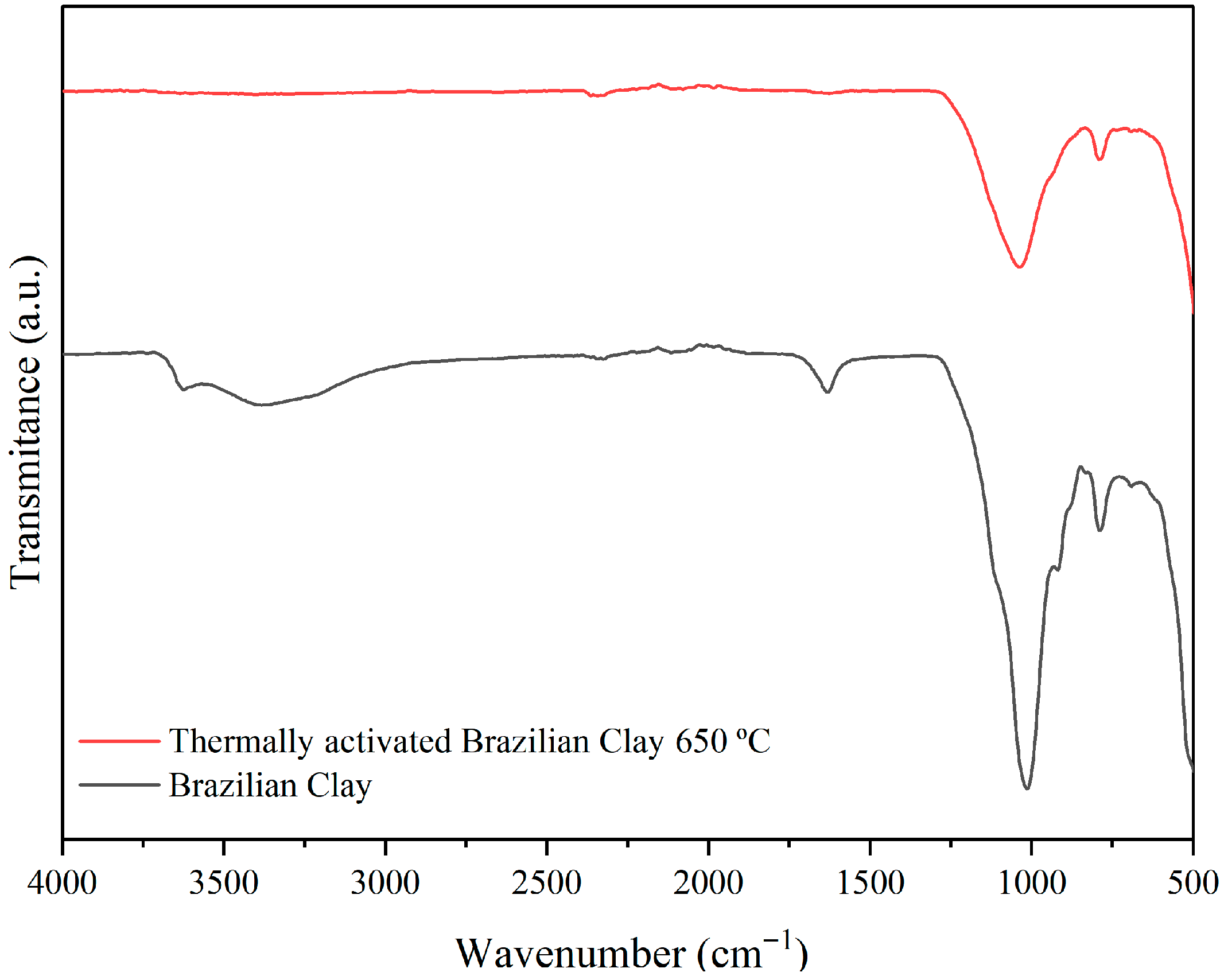
3.1. Raw Materials Analysis
3.2. Low-Cost Ceramic Membranes Analysis
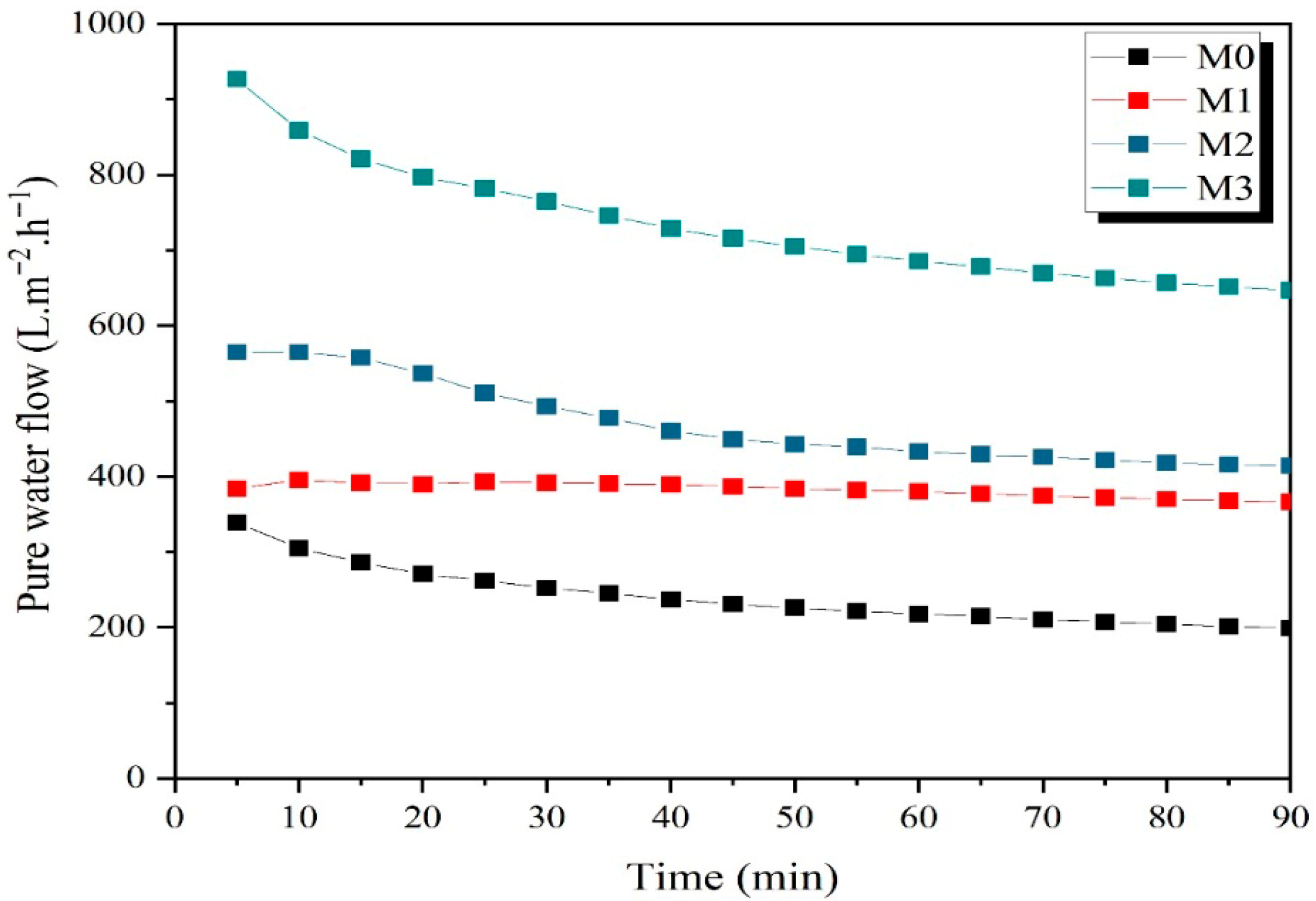
3.3. Pure Water Permeate Flow
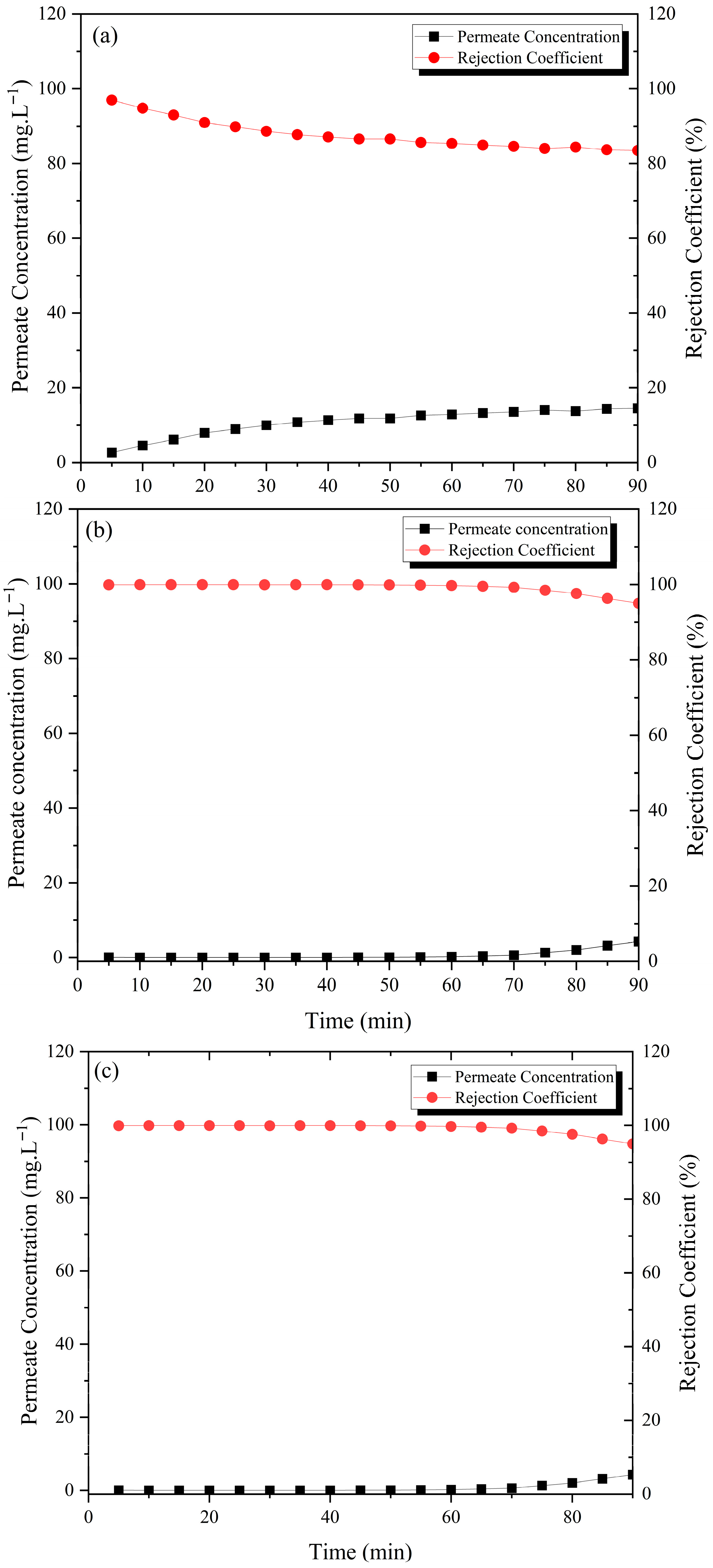
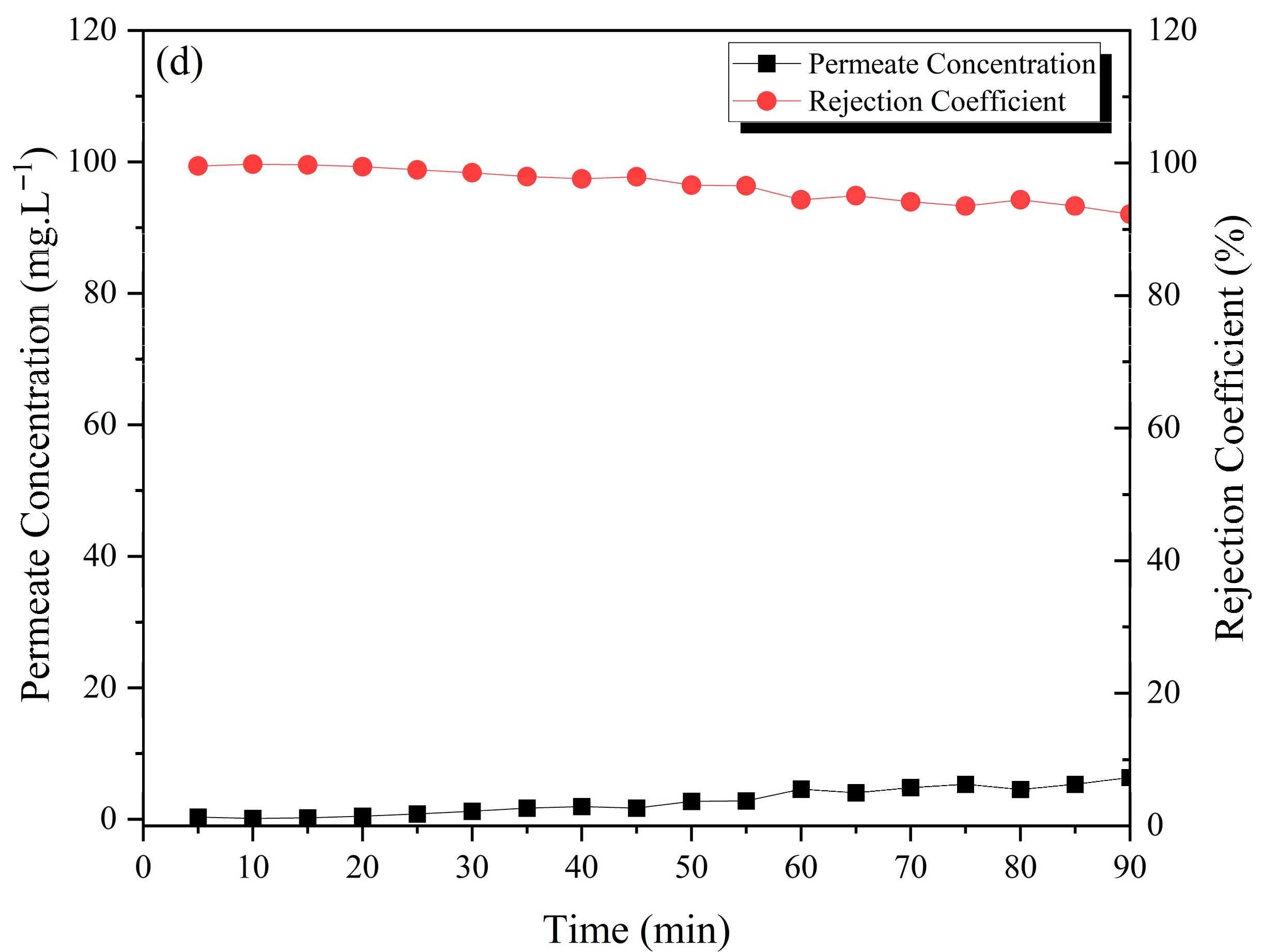
3.4. Treatment of Methylene Blue Wastewater by Ceramic Membranes
3.5. Data Analysis
3.6. Adsorption
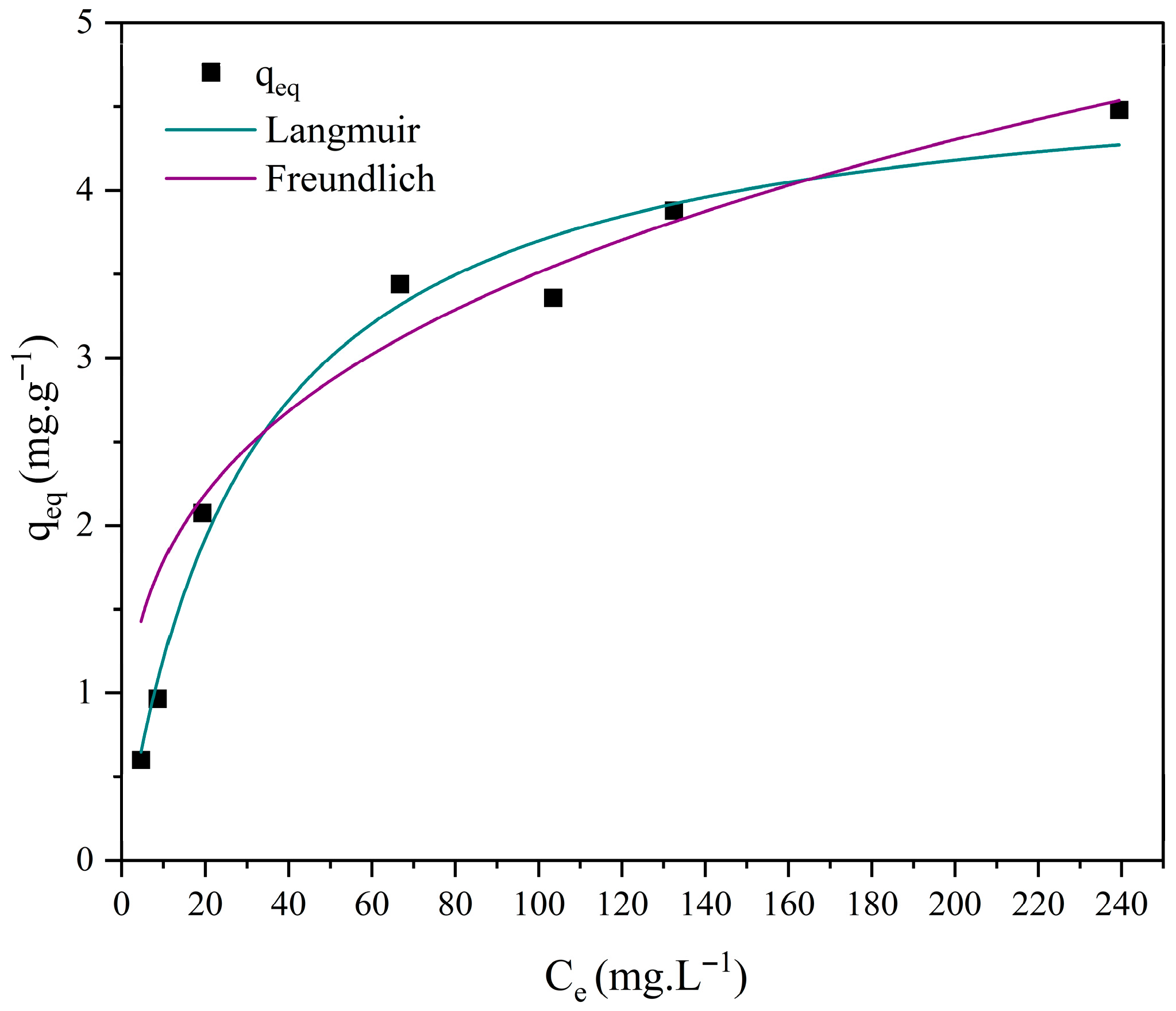
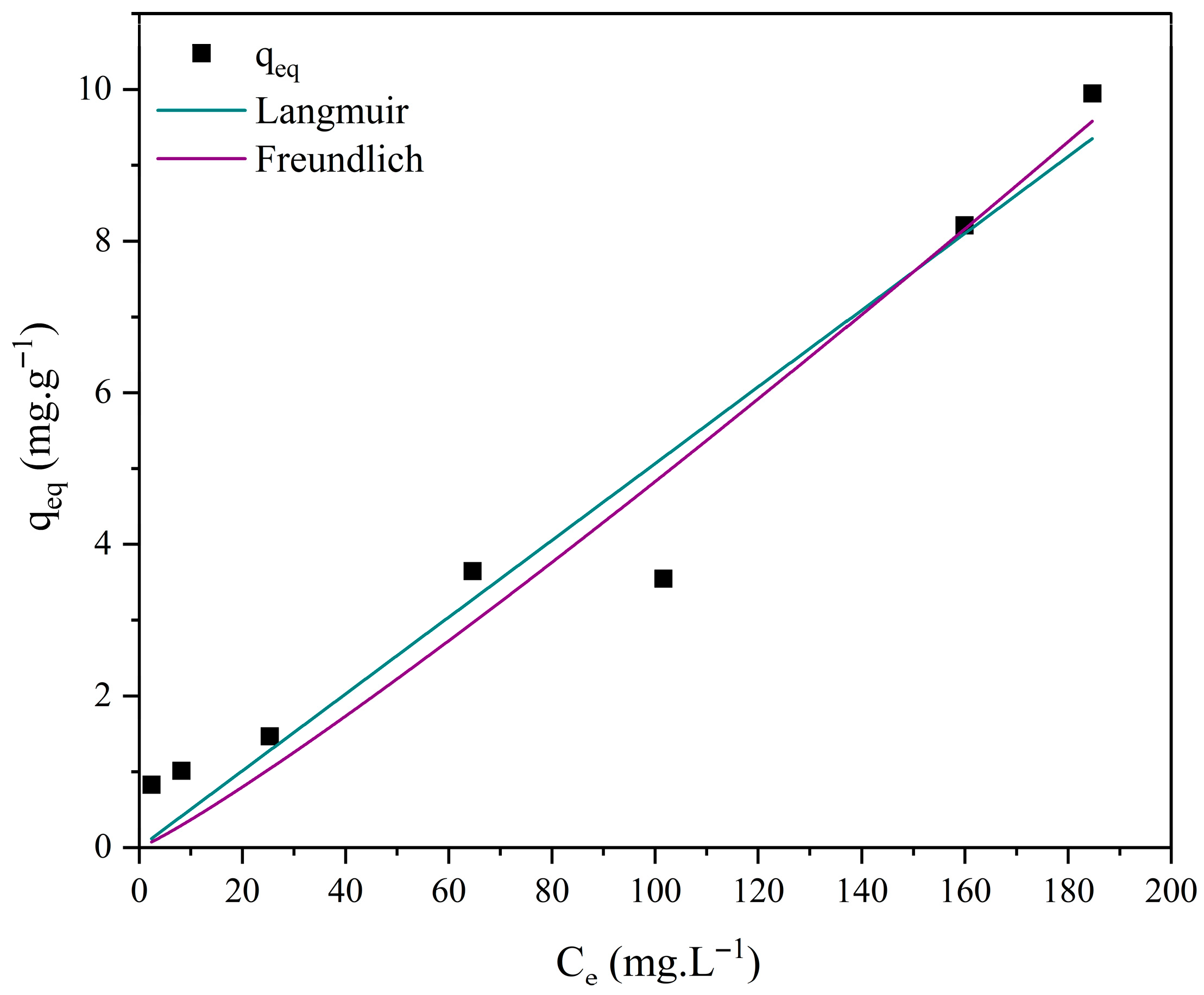
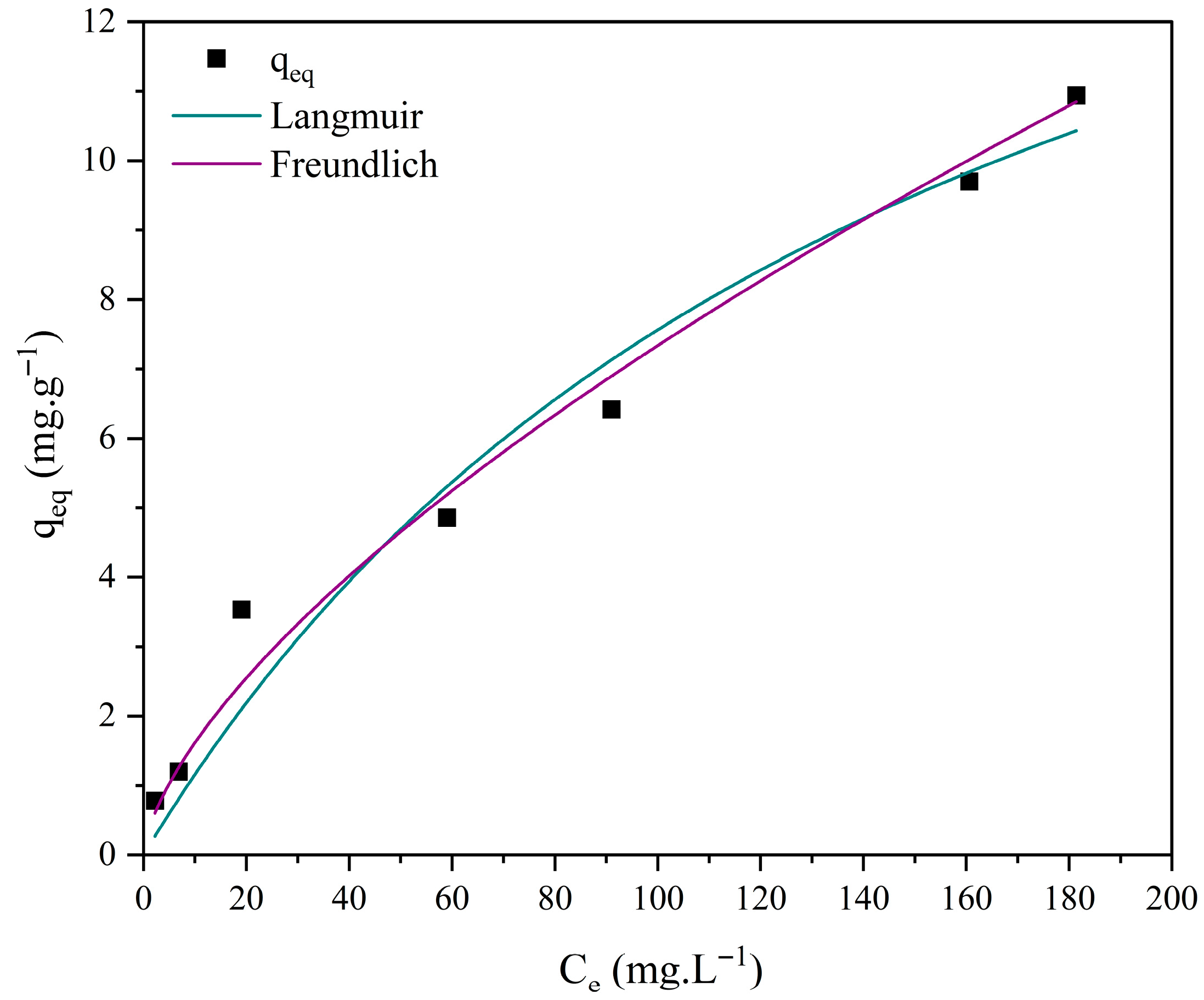
3.7. Adsorption Isotherm Studies
3.8. Mechanism Analysis
3.9. Role of Adsorption and Filtration Mechanisms in Dye Removal
3.10. Preliminary Cost Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- United Nations Sustainable Development Goals: Clean Water and Sanitation. Available online: https://unstats.un.org/sdgs/report/2022/The-Sustainable-Development-Goals-Report-2022.pdf (accessed on 29 February 2024).
- Maab, H.; Touheed, A.; Salman, S.; Khan, M. Novel polytriazole polymer membranes materials developed for the purification and separation of natural gas under high upstream feed pressure. Chin. J. Chem. Eng. 2025. [Google Scholar] [CrossRef]
- Oyekunle, D.T.; Lee, J.; Wang, C.; Chen, Y.; Jang, J.; Choi, S.; Moment, A.J. Surface Modification of Membrane Substrates for Nanofiltration-Based Removal of Essential and Energy-Critical Metals from Industrial Wastewater. Surf. Interfaces 2025, 73, 107486. [Google Scholar] [CrossRef]
- Doukelis, A.; Panopoulos, K.; Koumanakos, A.; Kakaras, E. Palladium Membrane Technology for Hydrogen Production, Carbon Capture and Other Applications: Principles, Energy Production and Other Applications; Woodhead Publishing: Cambridge, UK, 2014. [Google Scholar]
- Usha, Z.R. Surface-modified polymeric membrane-based separation technology with super-wetting properties for sustainable oily wastewater separation: A cutting-edge review. Sep. Purif. Technol. 2025, 380, 135219. [Google Scholar] [CrossRef]
- Samadi, A.; Gao, L.; Kong, L.; Orooji, Y.; Zhao, S. Waste-derived low-cost ceramic membranes for water treatment: Opportunities, challenges and future directions. Resour. Conserv. Recycl. 2022, 185, 106497. [Google Scholar] [CrossRef]
- Hsieh, H.P. Inorganic Membranes for Separation and Reaction; Elsevier: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Bhave, R.R. Inorganic Membranes Synthesis, Characteristics and Applications; Springer: Dordrecht, The Netherlands, 1991. [Google Scholar] [CrossRef]
- Burggraaf, A.J.; Cot, L. Fundamentals of inorganic membrane science and technology. In Membrane Science and Technology/Membrane Science and Technology Series; Elsevier: Amsterdam, The Netherlands, 1996; p. iii. [Google Scholar] [CrossRef]
- Russo, V.; Masiello, D.; Trifuoggi, M.; Di Serio, M.; Tesser, R. Design of an adsorption column for methylene blue abatement over silica: From batch to continuous modeling. Chem. Eng. J. 2016, 302, 287–295. [Google Scholar] [CrossRef]
- Kumar, D.; Gupta, S.K. Sustainable approach for the treatment of dye-containing wastewater—A critical review. Rev. Chem. Eng. 2024, 40, 723–763. [Google Scholar] [CrossRef]
- Drioli, E.; Giorno, L.; Fontananova, E. Comprehensive Membrane Science and Engineering; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Ezugbe, E.O.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Shen, L. Editorial: Advanced Membrane Science and Technology for Sustainable Environmental Applications. Front. Chem. 2020, 8, 609774. [Google Scholar] [CrossRef]
- Zhao, S.; Liao, Z.; Fane, A.; Li, J.; Tang, C.; Zheng, C.; Lin, J.; Kong, L. Engineering antifouling reverse osmosis membranes: A review. Desalination 2020, 499, 114857. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Z.; Gao, B. Ceramic membranes originated from cost-effective and abundant natural minerals and industrial wastes for broad applications—A review. Desalination Water Treat. 2020, 201, 121–138. [Google Scholar] [CrossRef]
- Rani, S.L.S.; Kumar, R.V. Insights on applications of low-cost ceramic membranes in wastewater treatment: A mini-review. Case Stud. Chem. Environ. Eng. 2021, 4, 100149. [Google Scholar] [CrossRef]
- Foorginezhad, S.; Rezvannasab, G.; Asadnia, M. Natural clay membranes: A sustainable and affordable solution for treating dye solutions, coal mine washery waste, and aquaculture wastewater. J. Water Process Eng. 2023, 54, 104012. [Google Scholar] [CrossRef]
- Silva, J.A. Wastewater Treatment and Reuse for Sustainable Water Resources Management: A Systematic Literature review. Sustainability 2023, 15, 10940. [Google Scholar] [CrossRef]
- Bouzerara, F.; Harabi, A.; Condom, S. Porous ceramic membranes prepared from kaolin. Desalination Water Treat. 2009, 12, 415–419. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Matsuura, T.; Ismail, A.F.; Rahman, M.A.; Harun, Z.; Jaafar, J.; Nomura, M. Fabrications and applications of low cost ceramic membrane from kaolin: A comprehensive review. Ceram. Int. 2017, 44, 4538–4560. [Google Scholar] [CrossRef]
- Rekik, S.B.; Bouaziz, J.; Deratani, A.; Beklouti, S. Study of Ceramic Membrane from Naturally Occurring-Kaolin Clays for Microfiltration Applications. Period. Polytech. Chem. Eng. 2017, 61, 206–215. [Google Scholar] [CrossRef]
- Bose, S.; Das, C. Sawdust: From wood waste to pore-former in the fabrication of ceramic membrane. Ceram. Int. 2014, 41, 4070–4079. [Google Scholar] [CrossRef]
- Namaghi, H.A.; Asl, A.H.; Chenar, M.P.; Hesampour, M.; Pihlajamäki, A.; Mänttäri, M. Performance enhancement of thin-film composite membranes in water desalination process by wood sawdust. Polym. Adv. Technol. 2019, 30, 2802–2818. [Google Scholar] [CrossRef]
- Rakcho, Y.; Mouiya, M.; Bouazizi, A.; Abouliatim, Y.; Sehaqui, H.; Mansouri, S.; Benhammou, A.; Hannache, H.; Alami, J.; Abourriche, A. Treatment of seawater and wastewater using a novel low-cost ceramic membrane fabricated with red clay and tea waste. Arab. J. Chem. 2023, 16, 105277. [Google Scholar] [CrossRef]
- Shehata, N.; Egirani, D.; Olabi, A.G.; Inayat, A.; Abdelkareem, M.A.; Chae, K.-J.; Sayed, E.T. Membrane-based water and wastewater treatment technologies: Issues, current trends, challenges, and role in achieving sustainable development goals, and circular economy. Chemosphere 2023, 320, 137993. [Google Scholar] [CrossRef] [PubMed]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Silveira, C.; Mulinari, J.; Junior, A.D.N.; Ambrosi, A.; Hotza, D.; Di Luccio, M. Low-cost ceramic membranes prepared from kaolin and quartz via tape casting using different pore formers. Open Ceram. 2025, 22, 100765. [Google Scholar] [CrossRef]
- Romdhani, M.; Aloulou, W.; Aloulou, H.; Duplay, J.; Charcosset, C.; Amar, R.B. Performance Assessment of a New Flat Sepiolite Clay-Based Ultrafiltration Membrane for the Removal of Paracetamol and Indigo Blue Dyes from Two Synthetic Aqueous Solutions. Sustainability 2024, 16, 1860. [Google Scholar] [CrossRef]
- Barbosa, A.D.S.; Barbosa, A.D.S.; Rodrigues, M.G.F. Low cost membrane used in oil/water removal. Desalination Water Treat. 2024, 317, 100029. [Google Scholar] [CrossRef]
- Carmo, E.S.D.; Silva, W.L.; Barbosa, A.D.S.; Rodrigues, M.G.F. Low-cost ceramic membrane production for dye removal. Desalination Water Treat. 2024, 320, 100726. [Google Scholar] [CrossRef]
- Vasanth, D.; Uppaluri, R.; Pugazhenthi, G. Influence of sintering temperature on the properties of porous ceramic support prepared by uniaxial dry compaction method using Low-Cost raw materials for membrane applications. Sep. Sci. Technol. 2011, 46, 1241–1249. [Google Scholar] [CrossRef]
- Standard Test Methods for Apparent Porosity, Water Absorption, Apparent Specific Gravity, and Bulk Density of Burned Refractory Brick and Shapes by Boiling Water. 2005. Available online: https://dl.azmanco.com/standards/ASTM-C/ASTM-C-Series-Full/C/C20.pdf (accessed on 27 August 2025).
- Ebrahimi, M.; Kujawski, W.; Fatyeyeva, K. Fabrication of Polyamide-6 Membranes—The Effect of Gelation Time towards Their Morphological, Physical and Transport Properties. Membranes 2022, 12, 315. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Golshanikia, P.; Habibi, M.; Jashni, E.; Shen, J.; Ebrahimi, M. Intensifying antibacterial and electrochemical behaviors of CuO induced-ion exchange membrane for water treatment. J. Polym. Res. 2022, 29, 250. [Google Scholar] [CrossRef]
- Yu, F.; Ma, J.; Wang, J.; Zhang, M.; Zheng, J. Magnetic iron oxide nanoparticles functionalized multi-walled carbon nanotubes for toluene, ethylbenzene and xylene removal from aqueous solution. Chemosphere 2015, 146, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wang, N.; Gu, H. The adsorption behavior of niobium (V) on kaolin clay and kaolinite. Appl. Clay Sci. 2023, 235, 106866. [Google Scholar] [CrossRef]
- Andrade, R.M.; Jaques, N.G.; Sousa, J.; Dutra, R.P.S.; Macedo, D.A.; Campos, L.F.A. Preparation of low-cost ceramic membranes for microfiltration using sugarcane bagasse ash as a pore-forming agent. Cerâmica 2019, 65, 620–625. [Google Scholar] [CrossRef]
- Monash, P.; Pugazhenthi, G. Development of Ceramic Supports Derived from Low-Cost Raw Materials for Membrane Applications and its Optimization Based on Sintering Temperature. Int. J. Appl. Ceram. Technol. 2009, 8, 227–238. [Google Scholar] [CrossRef]
- Bergaya, F.; Lagaly, G. Surface modification of clay minerals. Appl. Clay Sci. 2001, 19, 1–3. [Google Scholar] [CrossRef]
- De Souza Santos, P.; Santos, H.S. Ciência e Tecnologia de Argilas, 2nd ed.; Edgard Blücher Ltd.: Sao Paulo, Brazil, 1992. [Google Scholar]
- Gomes, C.F. Argilas: O Que São e Para Que Servem; Fundação Calouste Gulbenkian: Lisboa, Portugal, 1988. [Google Scholar]
- Silva, M.L.P.; Rodrigues, M.G.F.; Silva, M.G.C. Remoção de cádmio a partir da argila de Toritama (estado de Pernambuco) ativada termicamente em sistema de banho finito. Cerâmica 2009, 55, 11–17. [Google Scholar] [CrossRef]
- Grim, R.E. Applied clay mineralogy. Geol. Foereningan I Stockh. Foerhandl. 1962, 84, 533. [Google Scholar] [CrossRef]
- Rashad, A.M. Alkali-activated metakaolin: A short guide for civil Engineer—An overview. Constr. Build. Mater. 2013, 41, 751–765. [Google Scholar] [CrossRef]
- Moore, D.M.; Reynolds, R.C. X-Ray Diffraction and the Identification and Analysis of Clay Minerals; Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Doshi, P.; Srivastava, G.; Pathak, G.; Dikshit, M. Physicochemical and thermal characterization of nonedible oilseed residual waste as sustainable solid biofuel. Waste Manag. 2014, 34, 1836–1846. [Google Scholar] [CrossRef]
- Tan, Z.; Yang, J.; Liang, Y.; Zheng, M.; Hu, H.; Dong, H.; Liu, Y.; Xiao, Y. The changing structure by component: Biomass-based porous carbon for high-performance supercapacitors. J. Colloid Interface Sci. 2020, 585, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Varma, A.K.; Thakur, L.S.; Shankar, R.; Mondal, P. Pyrolysis of wood sawdust: Effects of process parameters on products yield and characterization of products. Waste Manag. 2019, 89, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.G.F. Physical and catalytic characterization of smectites from Boa-Vista, Paraíba, Brazil. Cerâmica 2003, 49, 146–150. [Google Scholar] [CrossRef]
- Ankush, K.; Pugazhenthi, G.; Vasanth, D. Fabrication and properties of polyhydroxybutyrate/kaolin nanocomposites and evaluation of their biocompatibility for biomedical applications. J. Appl. Polym. Sci. 2021, 139, 51803. [Google Scholar] [CrossRef]
- Kumar, A.; Lingfa, P. Sodium bentonite and kaolin clays: Comparative study on their FT-IR, XRF, and XRD. Mater. Today Proc. 2019, 22, 737–742. [Google Scholar] [CrossRef]
- Shaheen, T.I.; Emam, H.E. Sono-chemical synthesis of cellulose nanocrystals from wood sawdust using Acid hydrolysis. Int. J. Biol. Macromol. 2017, 107, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Pereira, K.R.; Hanna, R.A.; Vianna, M.M.G.R.; Pinto, C.A.; Rodrigues, M.G.F.; Valenzuela-Diaz, F.R. Brazilian organoclays as nanostructured sorbents of petroleum-derived hydrocarbons. Mater. Res. 2005, 8, 77–80. [Google Scholar] [CrossRef]
- Barbosa, A.S.; Barbosa, A.S.; Rodrigues, M.G.F. Synthesis of zeolite membrane (MCM-22/α-alumina) and its application in the process of oil-water separation. Desalination Water Treat. 2015, 56, 3665–3672. [Google Scholar] [CrossRef]
- Rafya, M.; Misrar, W.; Saâdi, L.; Mansori, M.; Waqif, M.; Hafidi, A.; Zehhar, N.; Benkhalti, F. Ceramic membrane support based on kaolin and solid waste from hydrodistillation of Rosmarinus officinalis L. Mater. Chem. Phys. 2022, 295, 127030. [Google Scholar] [CrossRef]
- Carmo, E. Produção de Membranas Cerâmicas de Baixo Custo Para Remoção de Corantes. Ph.D. Thesis, Universidade Federal de Campina Grande, Campina Grande, Brazil, 2018. [Google Scholar]
- Li, W.; Wang, Y.; Ji, B.; Jiao, X.; Chen, D. Flexible Pd/CeO2–TiO2 nanofibrous membrane with high efficiency ultrafine particulate filtration and improved CO catalytic oxidation performance. RSC Adv. 2015, 5, 58120–58127. [Google Scholar] [CrossRef]
- Dao, M.U.; Nguyen, T.T.T.; Le, V.T.; Hoang, H.Y.; Le, T.T.N.; Pham, T.N.; Nguyen, T.T.; Akhmadullin, R.M.; Le, H.S.; Tran, H.V.; et al. Non-woven polyester fabric-supported cuprous oxide/reduced graphene oxide nanocomposite for photocatalytic degradation of methylene blue. J. Mater. Sci. 2021, 56, 10353–10366. [Google Scholar] [CrossRef]
- Maruoka, L.M.A.; Pinheiro, I.F.; Freitas, H.S.; Nobre, F.X.; Scalvi, L.V.A. Effect of thermal annealing on kaolin from the Amazon region, aiming at the production of geopolymer. J. Mater. Res. Technol. 2023, 25, 2471–2485. [Google Scholar] [CrossRef]
- ABNT NBR 7171; Ceramic Block for Masonry. Associação Brasileira de Normas Técnicas: Rio de Janeiro, Brazil, 1992.
- Menegazzo, A.P.M.; Paschoal, J.O.A.; Andrade, A.M.; Carvalho, J.C.; Gouvêa, D. Avaliação da resistência mecânica e módulo de Weibull de produtos tipo grês porcelanato e granito. Cerâmica Ind. 2002, 1, 24–32. [Google Scholar]
- Mulder, M. Basic Principles of Membrane Technology; Springer: Amsterdam, The Netherlands, 1996. [Google Scholar] [CrossRef]
- Meghnani, R.; Kumar, M.; Pugazhenthi, G.; Dhakshinamoorthy, V. Synthesis of ceramic membrane using inexpensive precursors and evaluation of its biocompatibility for hemofiltration application. Sep. Purif. Technol. 2020, 256, 117814. [Google Scholar] [CrossRef]
- Guida, M.Y.; Lanaya, S.E.; Rbihi, Z.; Hannioui, A. Thermal degradation behaviors of sawdust wood waste: Pyrolysis kinetic and mechanism. J. Mater. Environ. Sci. 2019, 10, 742–755. [Google Scholar]
- Pecini, E.M.; Avena, M.J. Measuring the isoelectric point of the edges of clay mineral particles: The case of Montmorillonite. Langmuir 2013, 29, 14926–14934. [Google Scholar] [CrossRef] [PubMed]
- Mudzielwana, R.; Gitari, M.W.; Msagati, T.A.M. Characterisation of smectite-rich clay soil: Implication for groundwater defluoridation. S. Afr. J. Sci. 2016, 112, 8. [Google Scholar] [CrossRef]
- Neumann, M.G.; Gessner, F.; Schmitt, C.C.; Sartori, R. Influence of the Layer Charge and Clay Particle Size on the Interactions between the Cationic Dye Methylene Blue and Clays in an Aqueous Suspension. J. Colloid Interface Sci. 2002, 255, 254–259. [Google Scholar] [CrossRef]
- Gessner, F.; Schmitt, C.C.; Neumann, M.G. Time-Dependent Spectrophotometric Study of the Interaction of Basic Dyes with Clays. I. Methylene Blue and Neutral Red on Montmorillonite and Hectorite. Langmuir 1994, 10, 3749–3753. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 1100–1107. [Google Scholar]
- Yenisoy-Karakaş, S.; Aygün, A.; Güneş, M.; Tahtasakal, E. Physical and chemical characteristics of polymer-based spherical activated carbon and its ability to adsorb organics. Carbon 2004, 42, 477–484. [Google Scholar] [CrossRef]
- Sawunyama, L.; Olatunde, O.C.; Oyewo, O.A.; Bopape, M.F.; Onwudiwe, D.C. Application of coal fly ash based ceramic membranes in wastewater treatment: A sustainable alternative to commercial materials. Heliyon 2024, 10, e24344. [Google Scholar] [CrossRef]
- Scheibler, J.R.; Santos, E.R.F.; Barbosa, A.S.; Rodrigues, M.G.F. Performance of zeolite membrane (ZSM-5/γ-Alumina) in the oil/water separation process. Desalination Water Treat. 2014, 56, 3561–3567. [Google Scholar] [CrossRef]
- Barbosa, A.D.S.; Barbosa, A.D.S.; Barbosa, T.L.A.; Rodrigues, M.G.F. Synthesis of zeolite membrane (NaY/alumina): Effect of precursor of ceramic support and its application in the process of oil–water separation. Sep. Purif. Technol. 2018, 200, 141–154. [Google Scholar] [CrossRef]
- Barbosa, T.L.A.; Silva, F.M.N.; Barbosa, A.S.; Lima, E.G.; Rodrigues, M.G.F. Synthesis and application of a composite NaA zeolite/gamma-alumina membrane for oil-water separation process. Cerâmica 2020, 66, 137–144. [Google Scholar] [CrossRef]
- Sarkar, S.; Bandyopadhyay, S.; Larbot, A.; Cerneaux, S. New clay–alumina porous capillary supports for filtration application. J. Membr. Sci. 2011, 392–393, 130–136. [Google Scholar] [CrossRef]
- Samaei, S.M.; Gato-Trinidad, S.; Altaee, A. The application of pressure-driven ceramic membrane technology for the treatment of industrial wastewaters—A review. Sep. Purif. Technol. 2018, 200, 198–220. [Google Scholar] [CrossRef]
- Suresh, K.; Pugazhenthi, G.; Uppaluri, R. Fly ash based ceramic microfiltration membranes for oil-water emulsion treatment: Parametric optimization using response surface methodology. J. Water Process Eng. 2016, 13, 27–43. [Google Scholar] [CrossRef]
- Goswami, K.P.; Pugazhenthi, G. Treatment of starch-rich wastewater using fly ash-based low-cost tubular ceramic membrane. Environ. Prog. Sustain. Energy 2022, 41, e13906. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, H.; Yang, F.; Gray, S. Cost and efficiency perspectives of ceramic membranes for water treatment. Water Res. 2022, 220, 118629. [Google Scholar] [CrossRef] [PubMed]
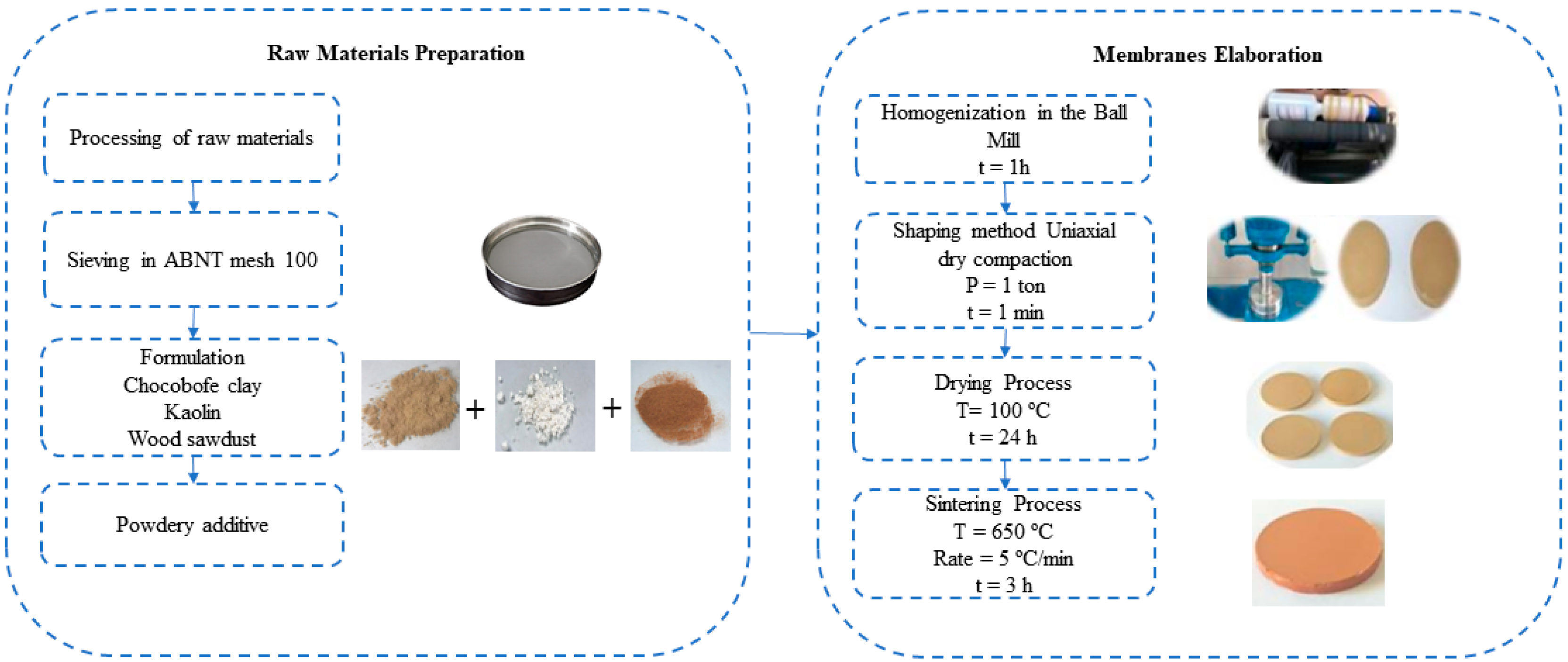
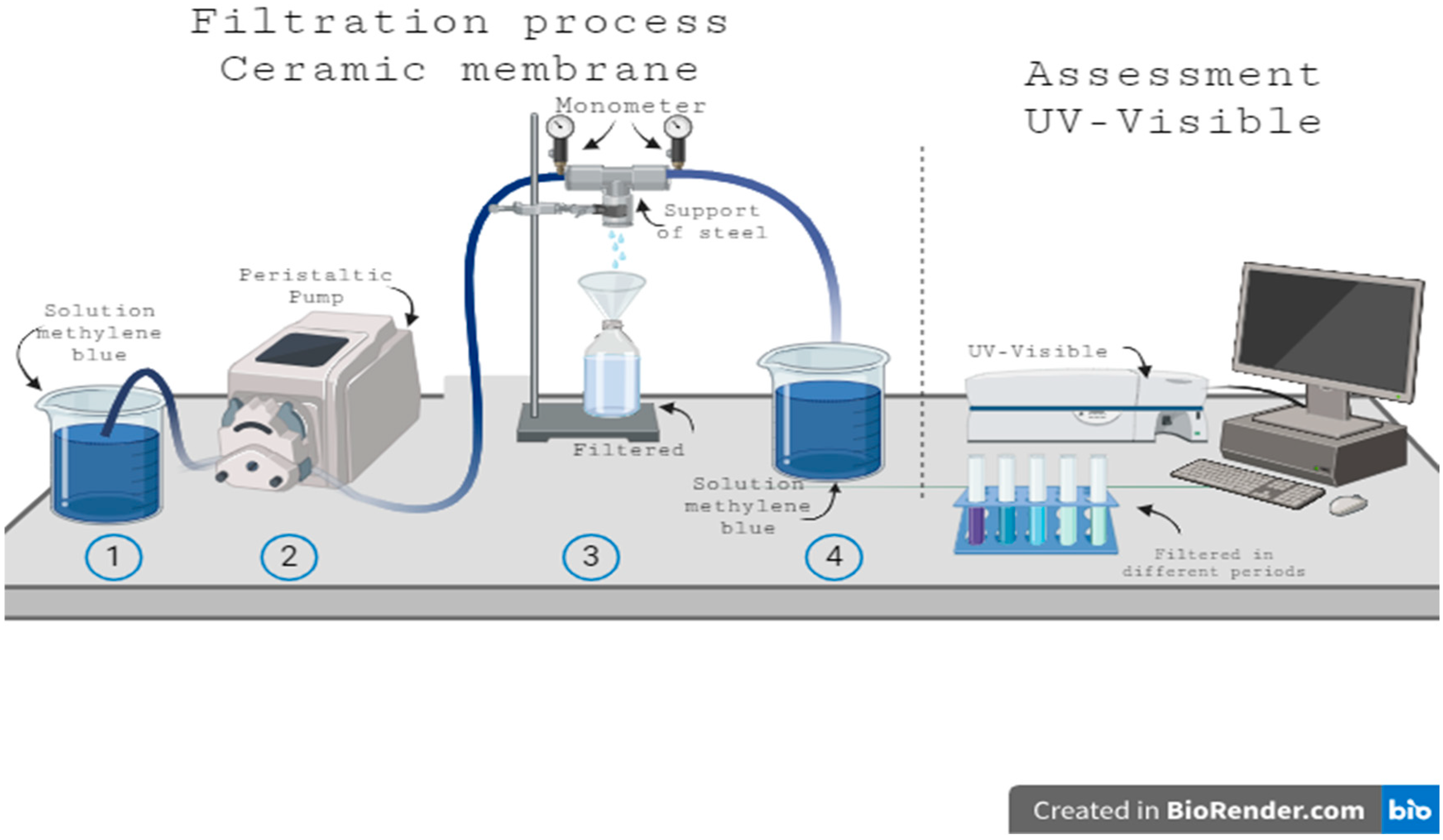
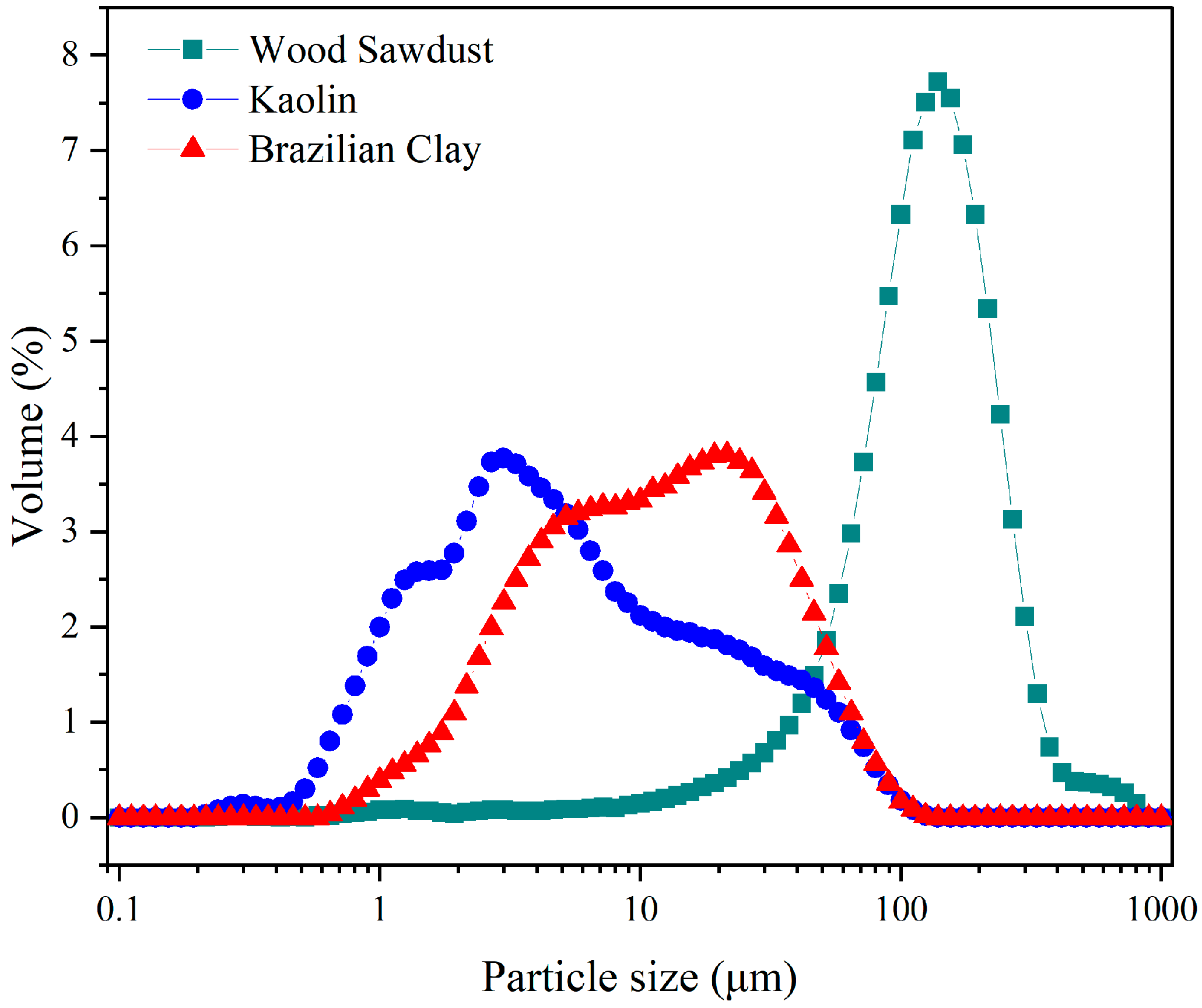
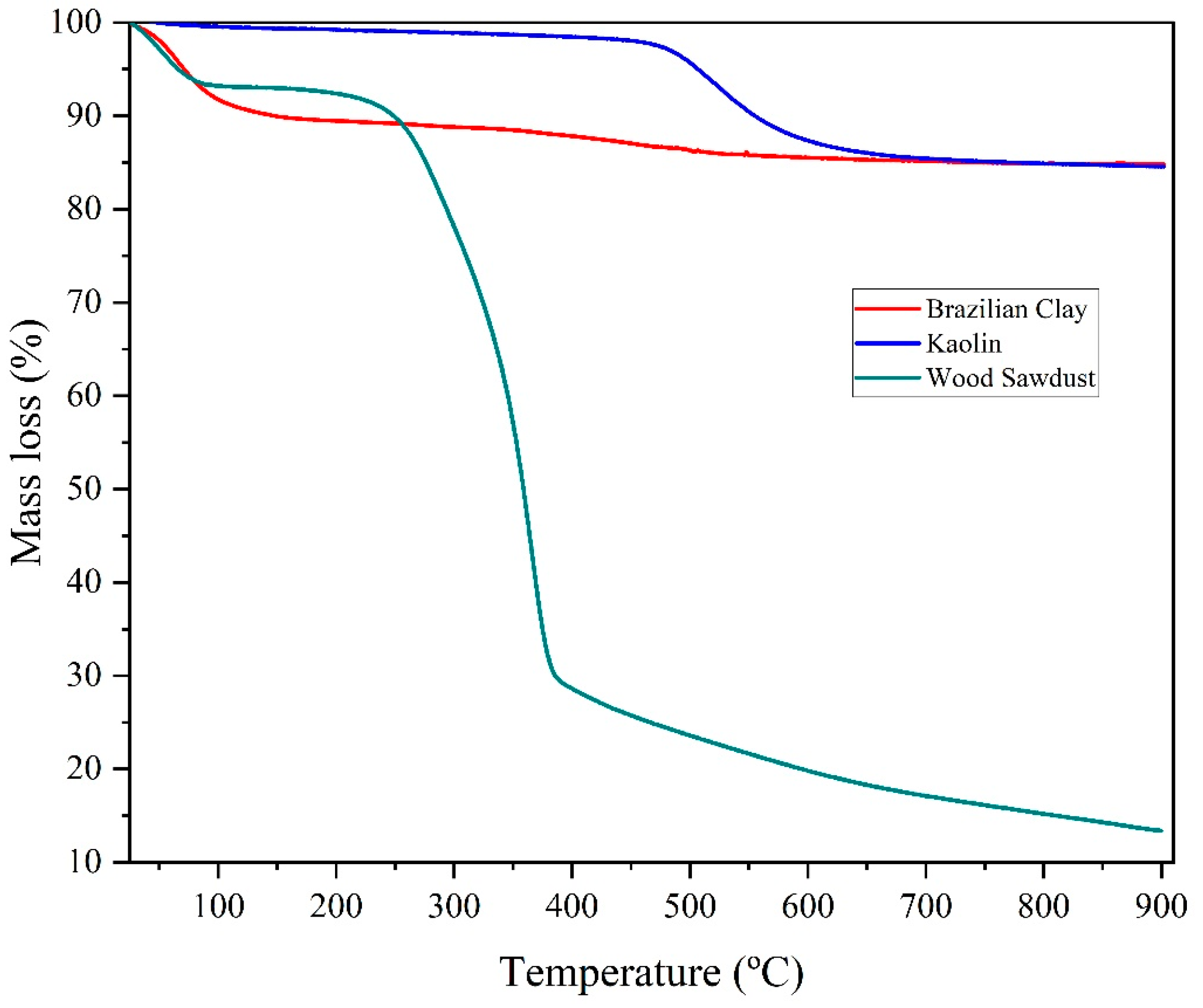



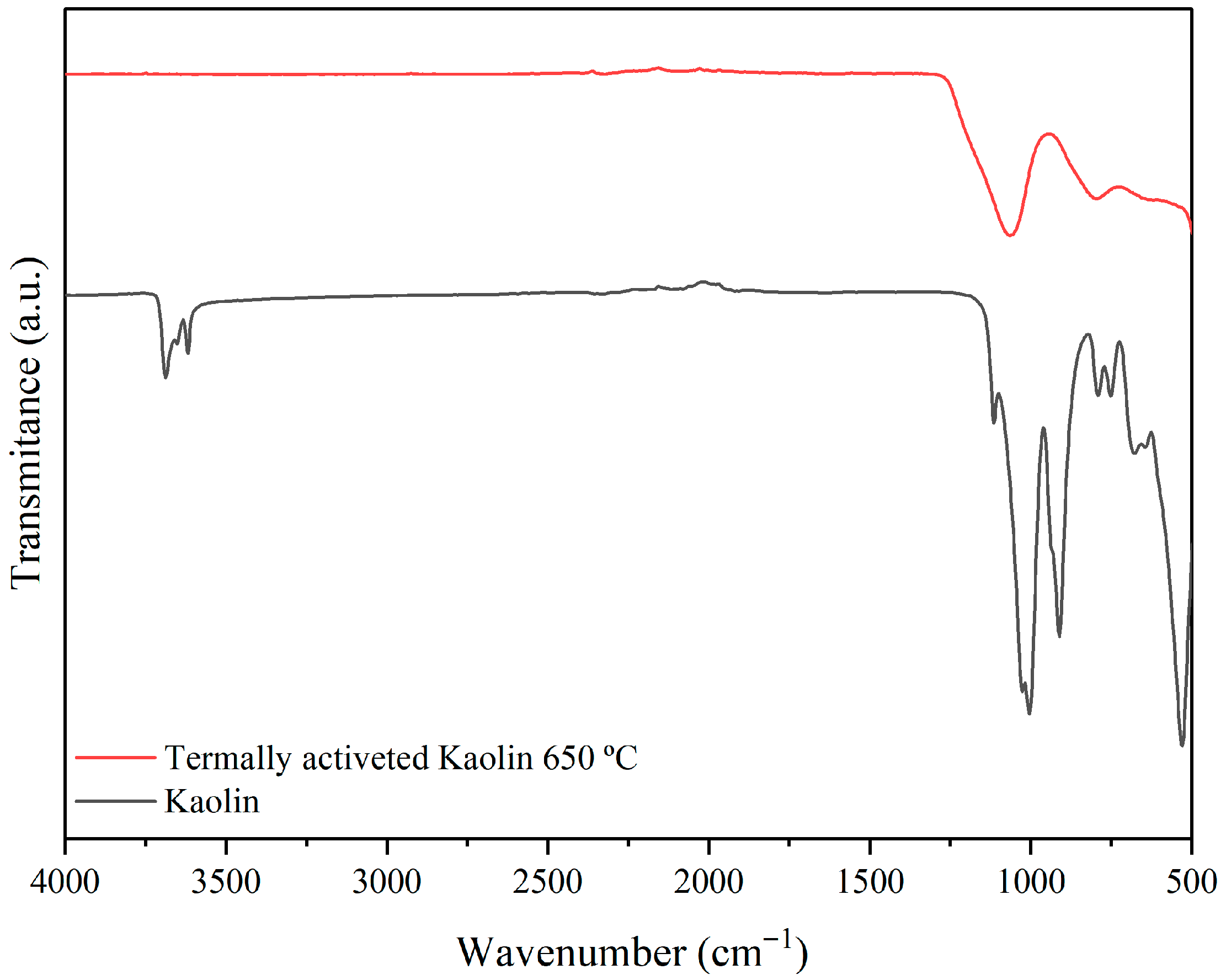
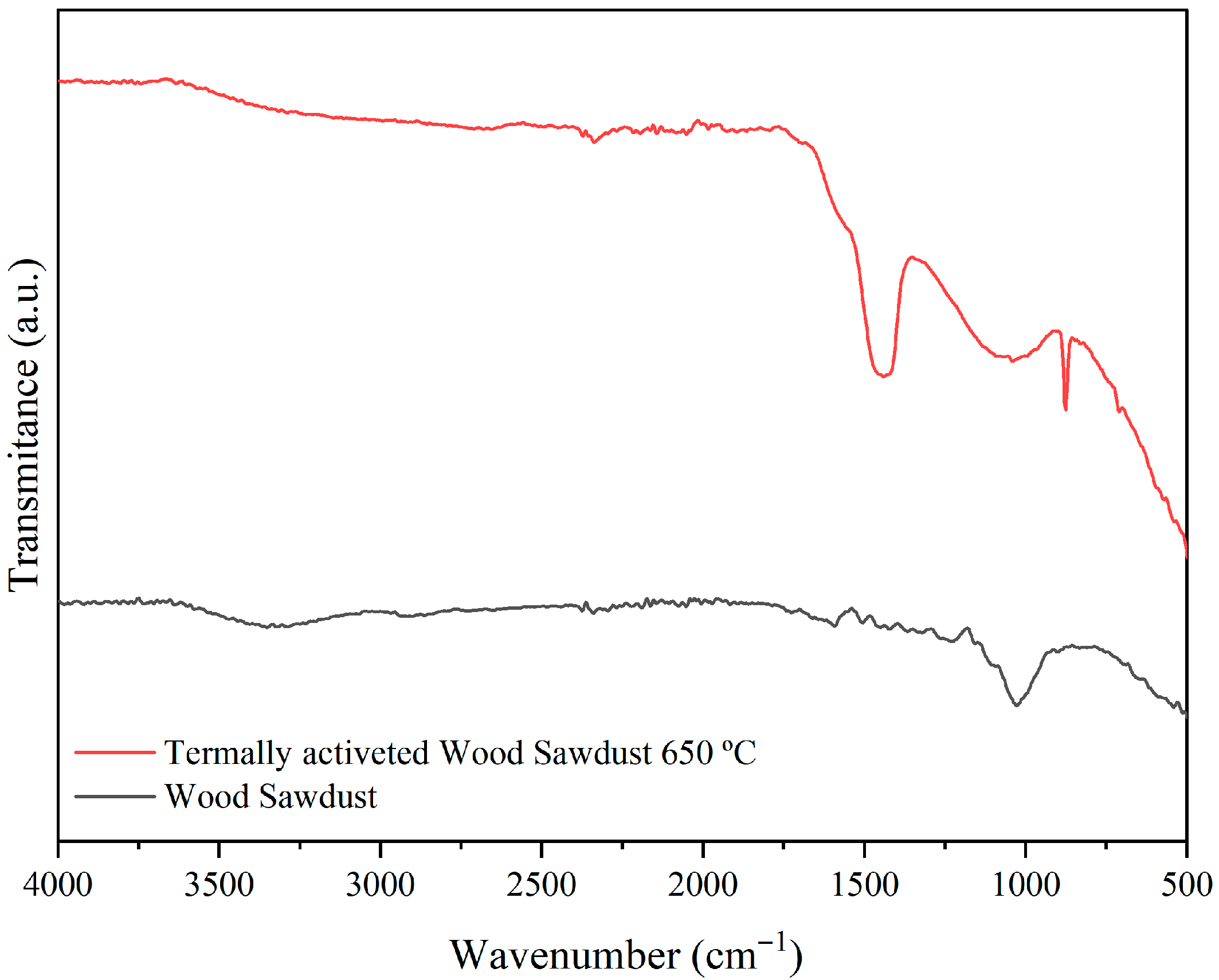
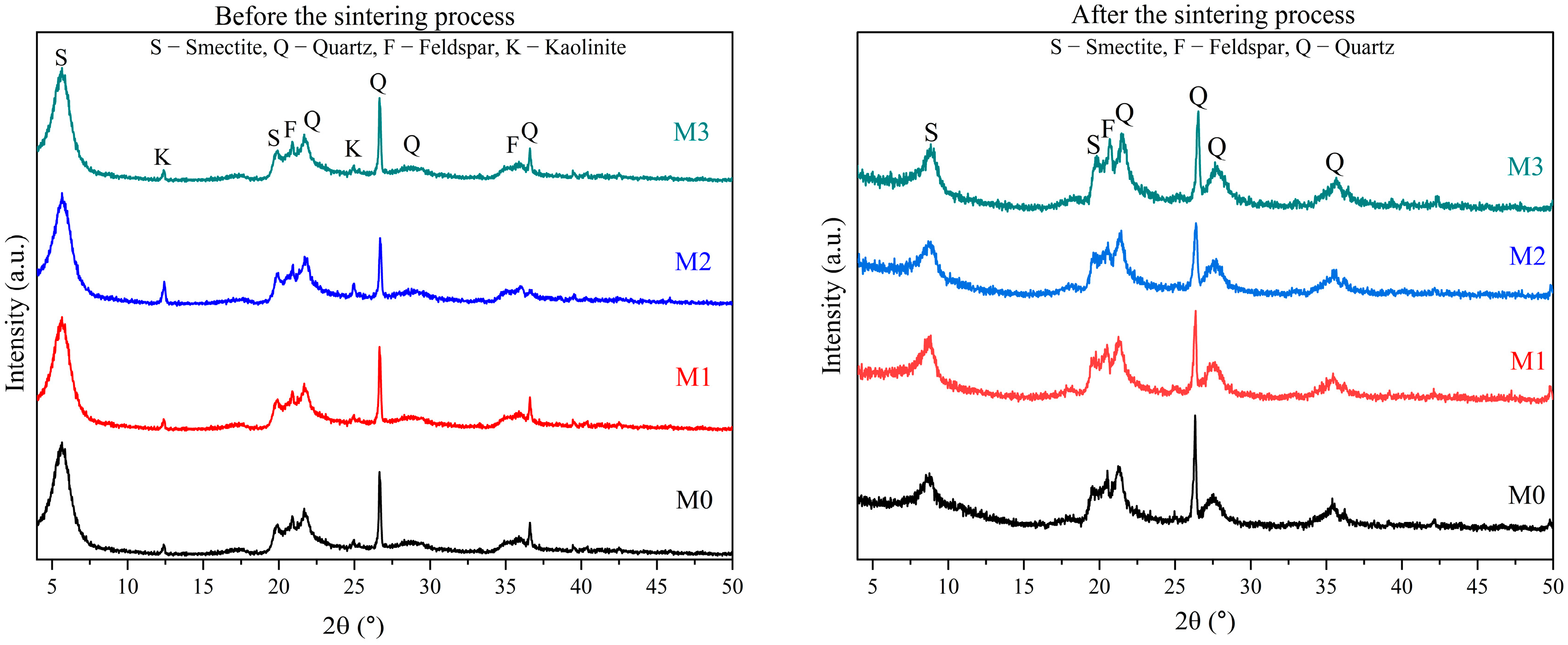
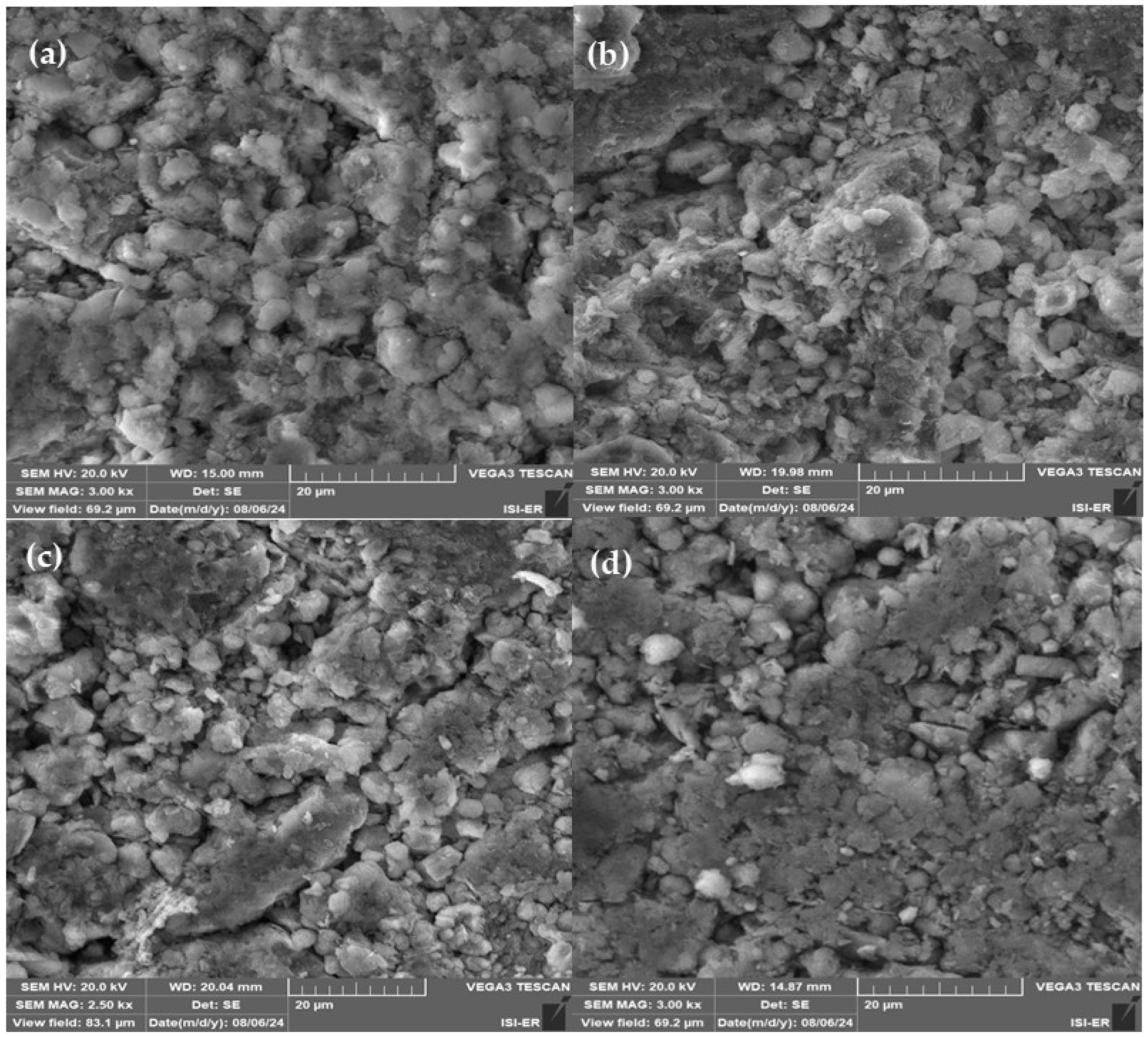

| Formulations | Brazilian Clay (%) | Kaolin (%) | Pore-Forming Agent Wood Sawdust (%) |
|---|---|---|---|
| M0 | 95.0 | 5.0 | 0.0 |
| M1 | 92.5 | 5.0 | 2.5 |
| M2 | 90.0 | 5.0 | 5.0 |
| M3 | 85.0 | 5.0 | 10.0 |
| Sample | SiO2 | Al2O3 | MgO | CaO | Fe2O3 | K2O | LOI |
|---|---|---|---|---|---|---|---|
| Brazilian clay | 71.79 | 14.25 | 2.18 | 1.05 | 14.25 | - | 15.23 |
| Kaolin | 50.25 | 47.62 | 0.70 | - | 0.34 | - | 14.73 |
| Wood sawdust | 5.53 | - | - | 61.11 | 10.62 | 8.19 | 86.62 |
| Membrane Tsintering (°C) | Pore Diameter (μm) | Porosity (%) | Water Uptake (%) | Mechanical Strength (MPa) | Pure Water Flux (L m−2 h−1) | Hydrodynamic Permeability (L m−2 h−1 bar−1) | Ref. |
|---|---|---|---|---|---|---|---|
| M0/650 | 0.346 | 42.96 | 30.21 | 19.92 | 240.73 | 120.36 | This work |
| M1/650 | 0.450 | 40.85 | 29.02 | 15.25 | 382.76 | 190.80 | This work |
| M2/650 | 0.495 | 41.48 | 31.47 | 10.68 | 469.93 | 235.80 | This work |
| M3/650 | 0.622 | 40.99 | 33.22 | 6.18 | 732.89 | 366.48 | This work |
| 1 M/850 | 0.04–0.13 | 34.00–55.00 | - | - | - | - | [23] |
| 2 M1/650 | - | 42.51 | - | - | 546.00 | - | [55] |
| Model | Parameters | M0 | M1 | M2 | M3 |
|---|---|---|---|---|---|
| Langmuir | 0.033 | 7.533 × 10−7 | 1.788 × 10−6 | 0.006 | |
| 4.807 | 7.018 × 104 | 2.833 × 104 | 19.526 | ||
| R2 | 0.981 | 0.965 | 0.950 | 0.963 | |
| χ2 | 0.049 | 0.491 | 0.793 | 0.693 | |
| Freundlich | 0.908 | 0.044 | 0.028 | 0.357 | |
| 0.293 | 1.037 | 1.117 | 0.656 | ||
| n | 3.406 | 0.964 | 0.895 | 1.523 | |
| R2 | 0.939 | 0.966 | 0.952 | 0.994 | |
| χ2 | 0.040 | 0.486 | 0.750 | 0.051 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, A.V.S.; Barbosa, A.d.S.; Maia, L.F.; Rodrigues, M.G.F.; Lins Almeida Barbosa, T.; Luna, C.B.B. Development and Characterization of Sawdust-Based Ceramic Membranes for Textile Effluent Treatment. Membranes 2025, 15, 298. https://doi.org/10.3390/membranes15100298
Marques AVS, Barbosa AdS, Maia LF, Rodrigues MGF, Lins Almeida Barbosa T, Luna CBB. Development and Characterization of Sawdust-Based Ceramic Membranes for Textile Effluent Treatment. Membranes. 2025; 15(10):298. https://doi.org/10.3390/membranes15100298
Chicago/Turabian StyleMarques, Ana Vitória Santos, Antusia dos Santos Barbosa, Larissa Fernandes Maia, Meiry Gláucia Freire Rodrigues, Tellys Lins Almeida Barbosa, and Carlos Bruno Barreto Luna. 2025. "Development and Characterization of Sawdust-Based Ceramic Membranes for Textile Effluent Treatment" Membranes 15, no. 10: 298. https://doi.org/10.3390/membranes15100298
APA StyleMarques, A. V. S., Barbosa, A. d. S., Maia, L. F., Rodrigues, M. G. F., Lins Almeida Barbosa, T., & Luna, C. B. B. (2025). Development and Characterization of Sawdust-Based Ceramic Membranes for Textile Effluent Treatment. Membranes, 15(10), 298. https://doi.org/10.3390/membranes15100298









