Comprehensive Investigation of Iron Salt Effects on Membrane Bioreactor from Perspective of Controlling Iron Leakage
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
2.2.1. Continuous Flow Experiments
2.2.2. MBR Long-Term Operation Test
2.3. Analytical Methods
2.3.1. Routine Chemical Test Items and Methods
2.3.2. Method for the Determination of Fe3+
2.3.3. Classification of Different Forms of Fe3+
2.3.4. Determination of Phosphorus Release and Phosphorus Absorption Capacity
2.3.5. Determination of Sludge-Specific Nitrification Rate
2.3.6. Determination of Sludge-Specific Oxygen Uptake Rate
2.3.7. Testing of Molecular Weight Distribution of Organic Matter
2.3.8. Statistical Analysis
3. Results and Discussions
3.1. Optimization of Iron Salt Dosing in MBR
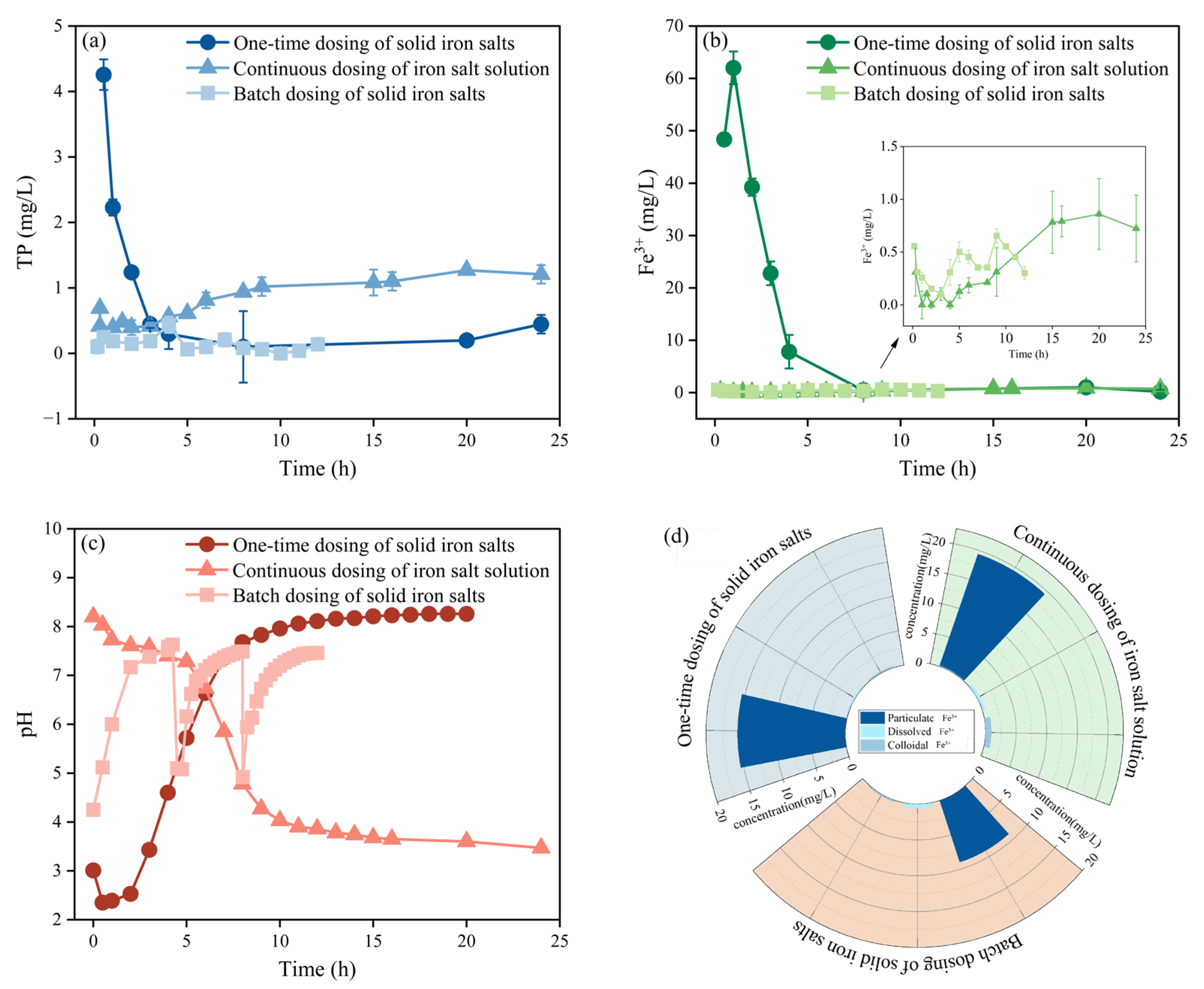
3.1.1. Optimization of Iron Salt Addition in Influent
3.1.2. Optimization of Iron Salt Dosing in Activated Sludge
3.2. Effect of Iron Salts on Pollutant Removal in MBR Systems
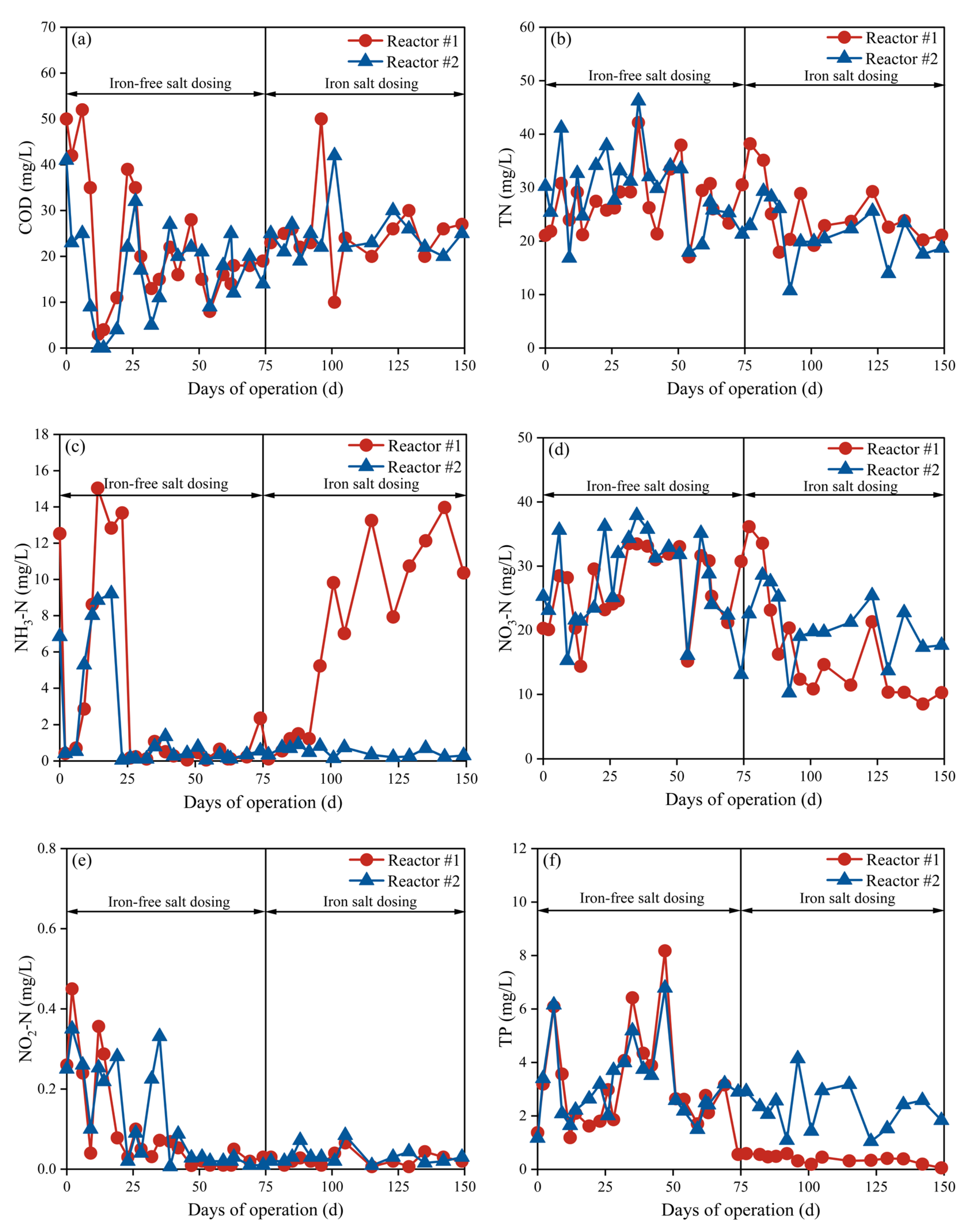
3.2.1. Water Quality Analysis
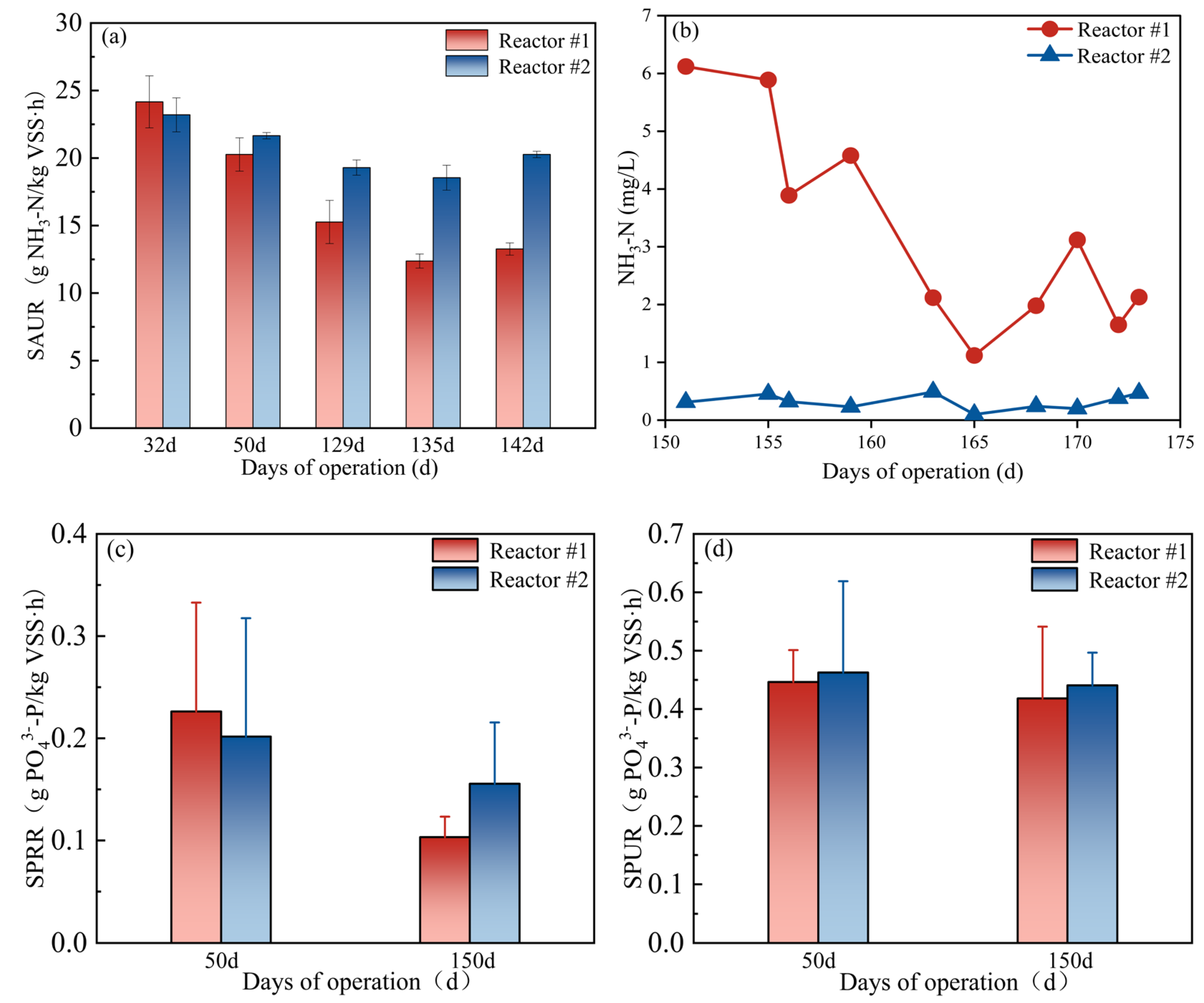
3.2.2. Nitrogen Removal
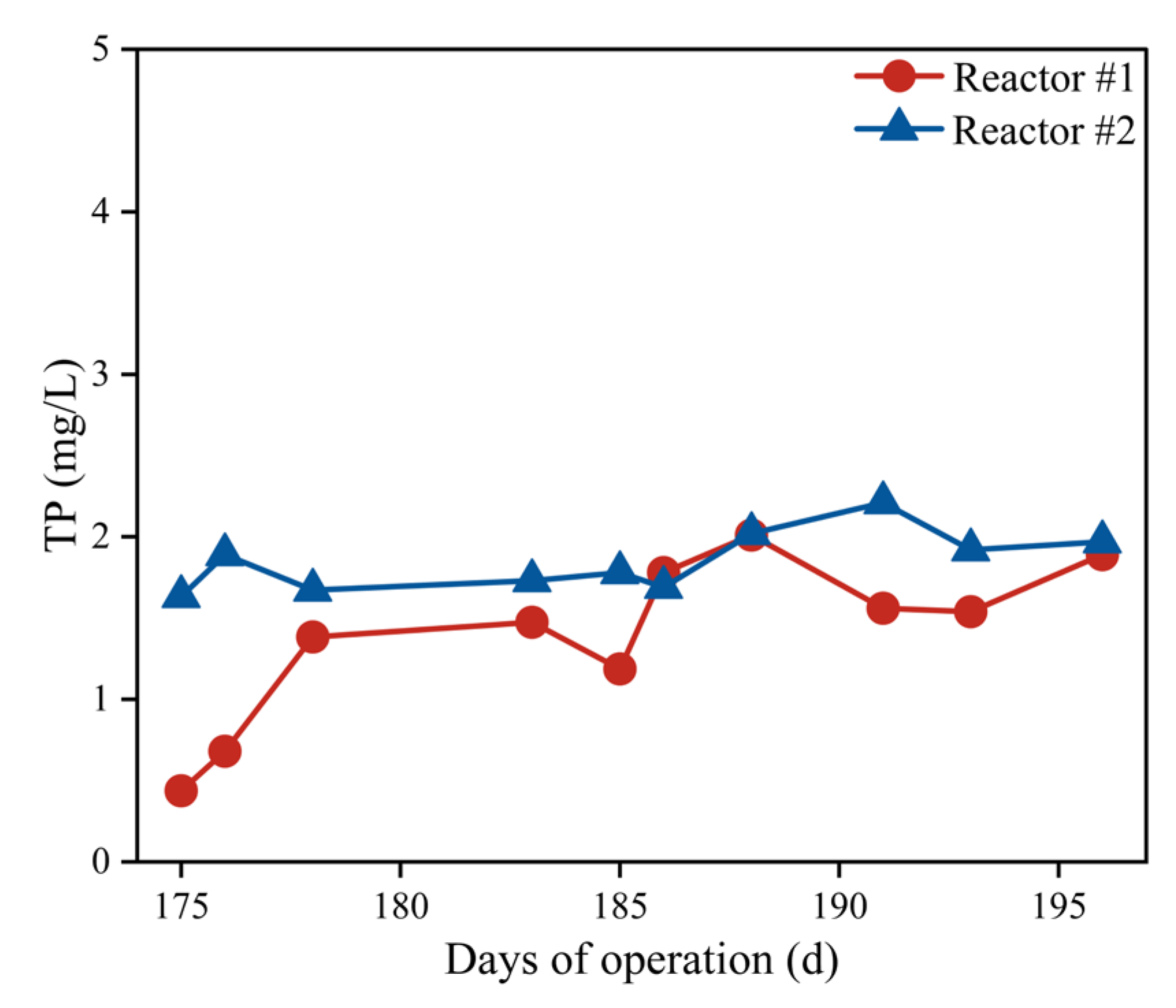
3.2.3. TP Removal
3.3. Contribution of Membrane Retention to Effluent Water Quality
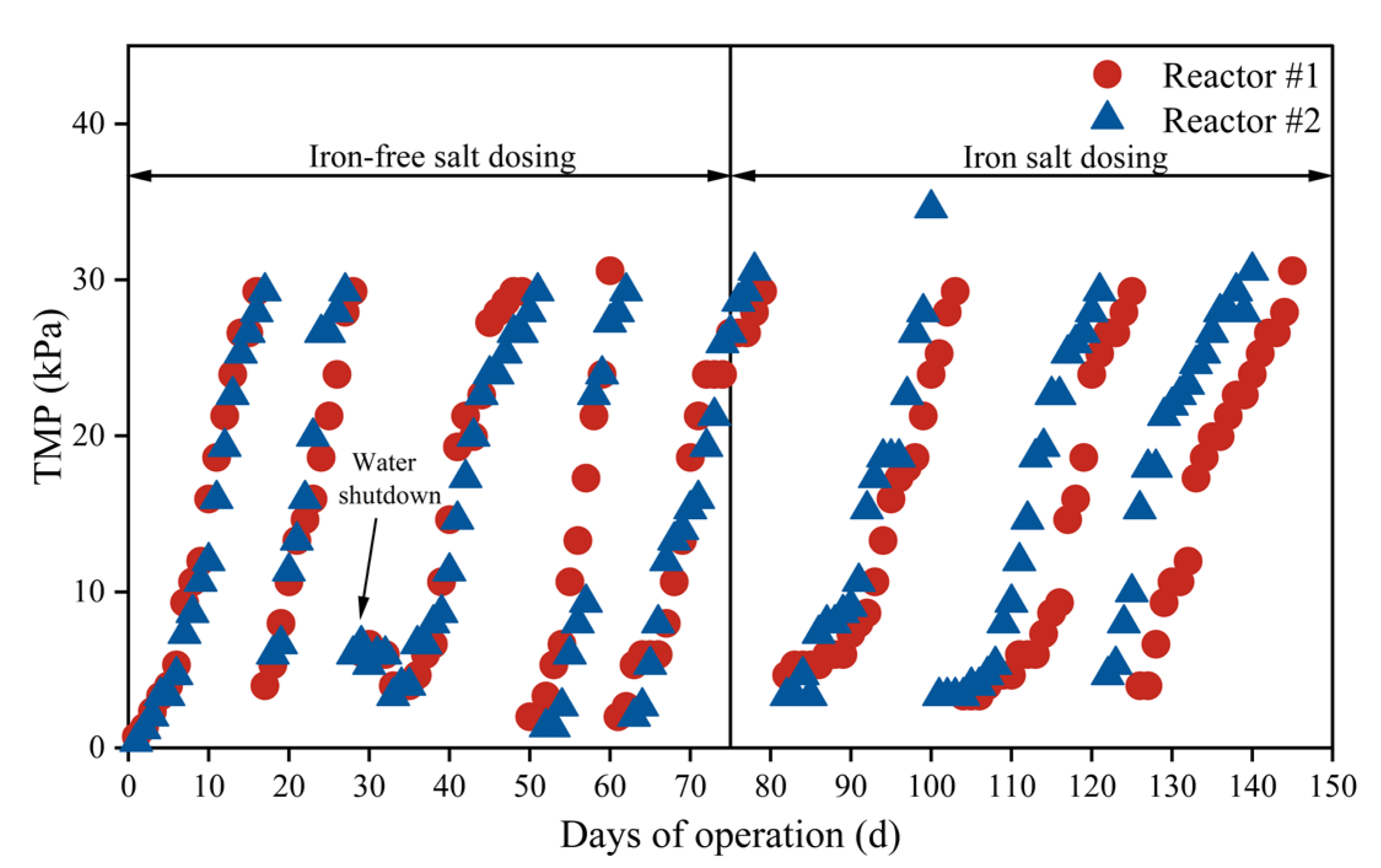
3.4. The Effect of Iron Salt Addition on Membrane Fouling
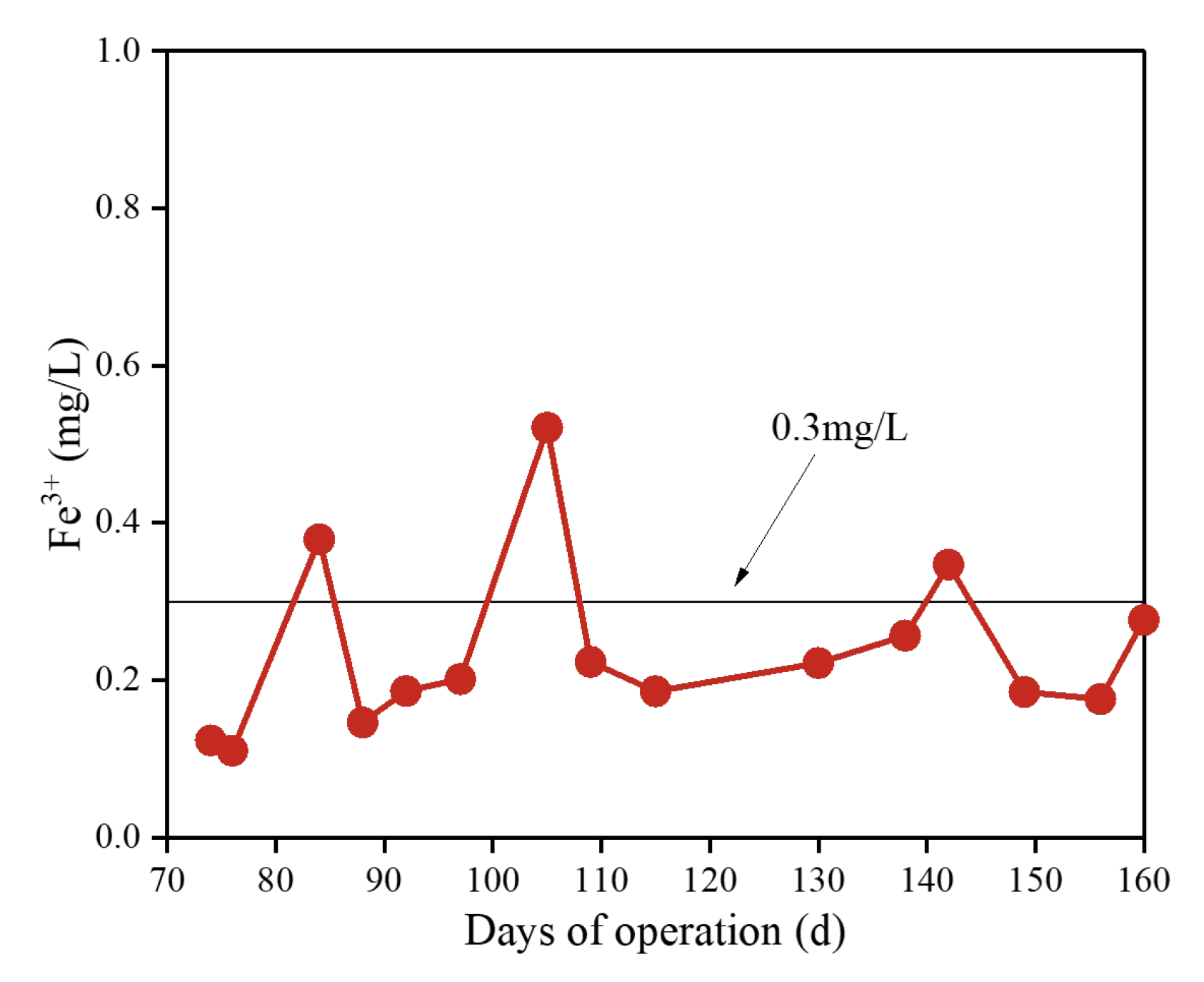
3.5. Changes in Fe3+ Concentration in Membrane Effluent
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Judd, S. The MBR book: Principles and Applications of Membrane Bioreactors for Water and Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Wang, C.; Ng, T.C.A.; Ng, H.Y. Comparison between novel vibrating ceramic MBR and conventional air-sparging MBR for domestic wastewater treatment: Performance, fouling control and energy consumption. Water Res. 2021, 203, 117521. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; You, H.; Li, Z.P.; Xie, B.H.; Qi, S.J.; Zhu, J.; Qin, Q.Q.; Wang, H.; Sun, J.X.; Ding, Y.; et al. A novel reduced graphene oxide/polypyrrole conductive ceramic membrane enhanced electric field membrane bioreactor: Mariculture wastewater treatment performance and membrane fouling mitigation. Bioresour. Technol. 2023, 376, 128917. [Google Scholar] [CrossRef]
- Lopes, T.S.D.; Hessler, R.; Bohner, C.; Athayde, G.B.; de Sena, R.F. Pesticides removal from industrial wastewater by a membrane bioreactor and post-treatment with either activated carbon, reverse osmosis or ozonation. J. Environ. Chem. Eng. 2020, 8, 104538. [Google Scholar] [CrossRef]
- Sun, J.S.; Liang, P.; Yan, X.X.; Zuo, K.C.; Xiao, K.; Xia, J.L.; Qiu, Y.; Wu, Q.; Wu, S.J.; Huang, X.; et al. Reducing aeration energy consumption in a large-scale membrane bioreactor: Process simulation and engineering application. Water Res. 2016, 93, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Li, R.H.; Wang, W.J.; Li, B.; Zhang, J.Y.; Liu, J.; Zhang, G.J.; Guo, X.C.; Zhang, X.H.; Li, X.Y. Acidogenic phosphorus recovery from the wastewater sludge of the membrane bioreactor systems with different iron-dosing modes. Bioresour. Technol. 2019, 280, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Li, R.H.; Cui, J.L.; Li, X.D.; Li, X.Y. Phosphorus removal and recovery from wastewater using Fe-dosing bioreactor and cofermentation: Investigation by X-ray absorption near-edge structure spectroscopy. Environ. Sci. Technol. 2018, 52, 14119–14128. [Google Scholar] [CrossRef]
- De-Bashan, L.E.; Bashan, Y. Recent advances in removing phosphorus from wastewater and its future use as fertilizer (1997–2003). Water Res. 2004, 38, 4222–4246. [Google Scholar] [CrossRef]
- Li, R.H.; Wang, X.M.; Li, X.Y. A membrane bioreactor with iron dosing and acidogenic co-fermentation for enhanced phosphorus removal and recovery in wastewater treatment. Water Res. 2018, 129, 402–412. [Google Scholar] [CrossRef]
- Yang, X.L.; Song, H.L.; Chen, M.; Cheng, B. Characterizing membrane foulants in MBR with addition of polyferric chloride to enhance phosphorus removal. Bioresour. Technol. 2011, 102, 9490–9496. [Google Scholar] [CrossRef]
- Haldar, D.; Duarah, P.; Purkait, M.K. MOFs for the treatment of arsenic, fluoride and iron contaminated drinking water: A review. Chemosphere 2020, 251, 126388. [Google Scholar] [CrossRef]
- Kumar, V.; Bharti, P.K.; Talwar, M. Studies on high iron content in water resources of Moradabad district (UP), India. Water Sci. 2017, 31, 44–51. [Google Scholar] [CrossRef]
- Abed, E.A.M.; Alaboudi, K.A.N.; Abbas, M.H.H.; Attia, T.M.S.; Abdelhafez, A.A. Iron Contamination in groundwater: Risk assessment and remediation techniques in egypt’s new valley. Water 2024, 16, 1834. [Google Scholar] [CrossRef]
- Rahman, M.A.; Hashem, M.A. Arsenic, iron and chloride in drinking water at primary school, Satkhira, Bangladesh. Phys. Chem. Earth 2019, 109, 49–58. [Google Scholar]
- Chen, M.; Li, X.H.; He, Y.H.; Song, N.; Cai, H.Y.; Wang, C.H.; Li, Y.T.; Chu, H.Y.; Krumholz, L.R.; Jiang, H.L. Increasing sulfate concentrations result in higher sulfide production and phosphorous mobilization in a shallow eutrophic freshwater lake. Water Res. 2016, 96, 94–104. [Google Scholar] [CrossRef]
- Xu, W.H.; Wang, W.W.; Deng, B.B.; Liu, Q.X. A review of the formation conditions and assessment methods of black and odorous water. Environ. Monit. Assess. 2024, 196, 42. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.S.; Ding, S.M.; Liu, L.; Xu, D.; Han, C.; Zhang, C.S. Iron-coupled inactivation of phosphorus in sediments by macrozoobenthos (chironomid larvae) bioturbation: Evidences from high-resolution dynamic measurements. Environ. Pollut. 2015, 204, 241–247. [Google Scholar]
- Rong, N.; Lu, W.Z.; Zhang, C.Y.; Wang, Y.S.; Zhu, J.L.; Zhang, W.Q.; Lei, P. In situ high-resolution measurement of phosphorus, iron and sulfur by diffusive gradients in thin films in sediments of black-odorous rivers in the Pearl River Delta region, South China. Environ. Res. 2020, 189, 109918. [Google Scholar] [CrossRef]
- Liu, J.J.; Lu, J.; Zhu, G.W. Occurrence characteristics of black patch events and their influencing factors in Lake Taihu during 2009 and 2017. J. Lake Sci. 2018, 30, 1196–1205. [Google Scholar] [CrossRef]
- Zhuang, Y.; Chen, R.; Shi, B.Y. Iron particle formation under chlorine disinfection considering effects of deoxidizers in drinking water. J. Hazard. Mater. 2021, 420, 126581. [Google Scholar] [CrossRef]
- Yang, L.; Cai, X.; Li, R. Ferroptosis induced by pollutants: An emerging mechanism in environmental toxicology. Environ. Sci. Technol. 2024, 58, 2166–2184. [Google Scholar] [CrossRef]
- Zhuang, Y.; Li, D.H.; Shi, B.Y. Perfluorooctanoic acid (PFOA) incorporated into iron particles promoted the formation of disinfection byproducts under drinking water conditions. Environ. Sci. Technol. 2023, 57, 4863–4869. [Google Scholar] [CrossRef]
- Wang, Y.; Tng, K.H.; Wu, H.; Leslie, G.; Waite, T.D. Removal of phosphorus from wastewaters using ferrous salts—A pilot scale membrane bioreactor study. Water Res. 2014, 57, 140–150. [Google Scholar]
- Gkotsis, P.K.; Mitrakas, M.M.; Tolkou, A.K.; Zouboulis, A.I. Batch and continuous dosing of conventional and composite coagulation agents for fouling control in a pilot-scale MBR. Chem. Eng. J. 2017, 311, 255–264. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.L.; Wang, Z.Z.; Zheng, Z.M.; Zhang, J. Assessing biomass activities, sludge characteristics and membrane fouling in the University of Cape Town membrane bioreactor under ferric chloride addition. Environ. Technol. Innov. 2021, 23, 101796. [Google Scholar] [CrossRef]
- Jiang, L.Y.; Liu, Y.; Guo, F.J.; Zhou, Z.; Jiang, J.; You, Z.C.; Wang, Q.Y.; Wang, Z.W.; Wu, Z.C. Evaluation of nutrient removal performance and resource recovery potential of anaerobic/anoxic/aerobic membrane bioreactor with limited aeration. Bioresour. Technol. 2021, 340, 125728. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.Y.; Li, Y.L.; Wang, Z.W.; Liu, Y.Y.; Zhou, Z.; Hu, W.J.; Lin, L.F.; Wu, Z.C. Effects of microplastics accumulation on performance of membrane bioreactor for wastewater treatment. Chemosphere 2022, 287, 131968. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, J.; Wang, Q.Y.; Zhang, H.; Wang, Z.W.; Wu, Z.C. Evaluating of the performance of natural mineral vermiculite modified PVDF membrane for oil/water separation by membrane fouling model and XDLVO theory. J. Membr. Sci. 2022, 641, 119886. [Google Scholar] [CrossRef]
- Torapava, N.; Radkevich, A.; Persson, I.; Davydov, D.; Eriksson, L. Formation of a heteronuclear hydrolysis complex in the Th IV–Fe III system. Dalton Trans. 2012, 41, 4451–4459. [Google Scholar]
- Cornell, R.M.; Giovanoli, R.; Schneider, W. Review of the hydrolysis of iron (III) and the crystallization of amorphous iron (III) hydroxide hydrate. J. Chem. Technol. Biotechnol. 1989, 46, 115–134. [Google Scholar] [CrossRef]
- Wang, Y.; Leslie, G.L.; Waite, T.D. Impact of iron dosing of membrane bioreactors on membrane fouling. Chem. Eng. J. 2014, 252, 239–248. [Google Scholar] [CrossRef]
- Zhang, Y.; Bu, D.; Liu, C.G.; Luo, X.; Gu, P. Study on retarding membrane fouling by ferric salts dosing in membrane bioreactors. In Proceedings of the IWA Water Environment-Membrane Technology, Seoul, Republic of Korea, 7–10 June 2004; Volume 753, pp. 7–10. [Google Scholar]
- Sun, J.C.; Zhu, D.F.; Fang, R.; Zhang, G.F. Arsenic-containing waste acid treatment—Experimental study on the removal of arsenic from copper smelting wwaste acid by lime-iron salt-ion exchange. JOM 2025, 77, 5632–5642. [Google Scholar] [CrossRef]
- Shi, W.; Li, J.; Gao, F.; Meng, L.J.; Su, X.; Wang, Z.W. Strongly coordinating mediator enables single-step resource recovery from heavy metal-organic complexes in wastewater. Nat. Commun. 2024, 15, 10828. [Google Scholar] [CrossRef]
- Sarah, P.; Korneel, R.; Willy, V. Impact of iron salts on activated sludge and interaction with nitrite or nitrate. Bioresour. Technol. 2003, 88, 229–239. [Google Scholar] [CrossRef]
- Guo, W.; Ngo, H.H.; Vigneswaran, S.; Dharmawan, F.; Nguyen, T.T.; Aryal, R. Effect of different flocculants on short-term performance of submerged membrane bioreactor. Sep. Purif. Technol. 2010, 70, 274–279. [Google Scholar] [CrossRef]
- Clark, T.; Stephenson, T.; Pearce, P.A. Phosphorus removal by chemical precipitation in a biological aerated filter. Water Res. 1997, 31, 2557–2563. [Google Scholar] [CrossRef]
- Huang, X.; Yao, K.; Yu, J.H.; Dong, W.Y.; Zhao, Z.L. Nitrogen removal performance and microbial characteristics during simultaneous chemical phosphorus removal process using Fe3+. Bioresour. Technol. 2022, 363, 127972. [Google Scholar] [CrossRef]
- Wang, Z.W.; Wu, Z.C. Distribution and transformation of molecular weight of organic matters in membrane bioreactor and conventional activated sludge process. Chem. Eng. J. 2004, 150, 396–402. [Google Scholar] [CrossRef]
- Kimura, K.; Yamato, N.; Yamamura, H.; Watanabe, Y. Membrane fouling in pilot-scale membrane bioreactors (MBRs) treating municipal wastewater. Environ. Sci. Technol. 2005, 39, 6293–6299. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Kakuda, T.; Iwasaki, H. Membrane fouling caused by lipopolysaccharides: A suggestion for alternative model polysaccharides for MBR fouling research. Sep. Purif. Technol. 2019, 223, 224–233. [Google Scholar] [CrossRef]
- Wang, X.M.; Waite, T.D. Iron speciation and iron species transformation in activated sludge membrane bioreactors. Water Res. 2010, 44, 3511–3521. [Google Scholar] [CrossRef]
- Li, H.S.; Wen, Y.; Cao, A.S.; Huang, J.S.; Zhou, Q.; Somasundaran, P. The influence of additives (Ca2+, Al3+, and Fe3+) on the interaction energy and loosely bound extracellular polymeric substances (EPS) of activated sludge and their flocculation mechanisms. Bioresour. Technol. 2012, 114, 188–194. [Google Scholar] [CrossRef]


| No. | COD (mg/L) | NH3-N (mg/L) | TN (mg/L) | TP (mg/L) | NO3-N (mg/L) | NO2-N (mg/L) |
| #1 | 18.31 ± 6.92 | 0.31 ± 0.39 | 28.65 ± 6.67 | 3.60 ± 1.86 | 28.38 ± 5.77 | 0.04 ± 0.03 |
| #2 | 18.38 ± 7.60 | 0.39 ± 0.37 | 29.49 ± 7.17 | 3.33 ± 1.43 | 29.80 ± 6.26 | 0.07 ± 0.10 |
| No. | Organic Matter | Mn (kDa) | Mw (kDa) | Mz (kDa) | Mw/Mn |
|---|---|---|---|---|---|
| #1 | P1 | 70.1 | 72.8 | 75.8 | 1.0 |
| P2 | 336.3 | 378.8 | 443.2 | 1.1 | |
| P3 | 2722.9 | 4521.1 | 8496.9 | 1.7 | |
| #2 | P1 | 79.2 | 85.5 | 92.6 | 1.1 |
| P2 | 361.0 | 405.0 | 474.0 | 1.1 | |
| P3 | 4905.8 | 6649.8 | 9582.8 | 1.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Zhang, B.; Sun, J.; Zheng, W.; Zhang, J.; Wu, Z. Comprehensive Investigation of Iron Salt Effects on Membrane Bioreactor from Perspective of Controlling Iron Leakage. Membranes 2025, 15, 297. https://doi.org/10.3390/membranes15100297
Wang Q, Zhang B, Sun J, Zheng W, Zhang J, Wu Z. Comprehensive Investigation of Iron Salt Effects on Membrane Bioreactor from Perspective of Controlling Iron Leakage. Membranes. 2025; 15(10):297. https://doi.org/10.3390/membranes15100297
Chicago/Turabian StyleWang, Qiaoying, Bingbing Zhang, Jicheng Sun, Wenjia Zheng, Jie Zhang, and Zhichao Wu. 2025. "Comprehensive Investigation of Iron Salt Effects on Membrane Bioreactor from Perspective of Controlling Iron Leakage" Membranes 15, no. 10: 297. https://doi.org/10.3390/membranes15100297
APA StyleWang, Q., Zhang, B., Sun, J., Zheng, W., Zhang, J., & Wu, Z. (2025). Comprehensive Investigation of Iron Salt Effects on Membrane Bioreactor from Perspective of Controlling Iron Leakage. Membranes, 15(10), 297. https://doi.org/10.3390/membranes15100297







