Electro-Spun Waste Polystyrene/Steel Slag Composite Membrane for Water Desalination: Modelling and Photothermal Activity Evaluation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Characterization
2.3. Waste Polystyrene/Slag Composite Membrane Preparation
2.4. Electrospinning Process Optimization Using Response Surface Methodology
2.5. Membrane Distillation Performance
2.5.1. Direct Contact Membrane Distillation (DCMD) System Performance
2.5.2. Photothermal Activity Evaluation
2.5.3. Photothermal Membrane Distillation Evaluation
3. Results and Discussion
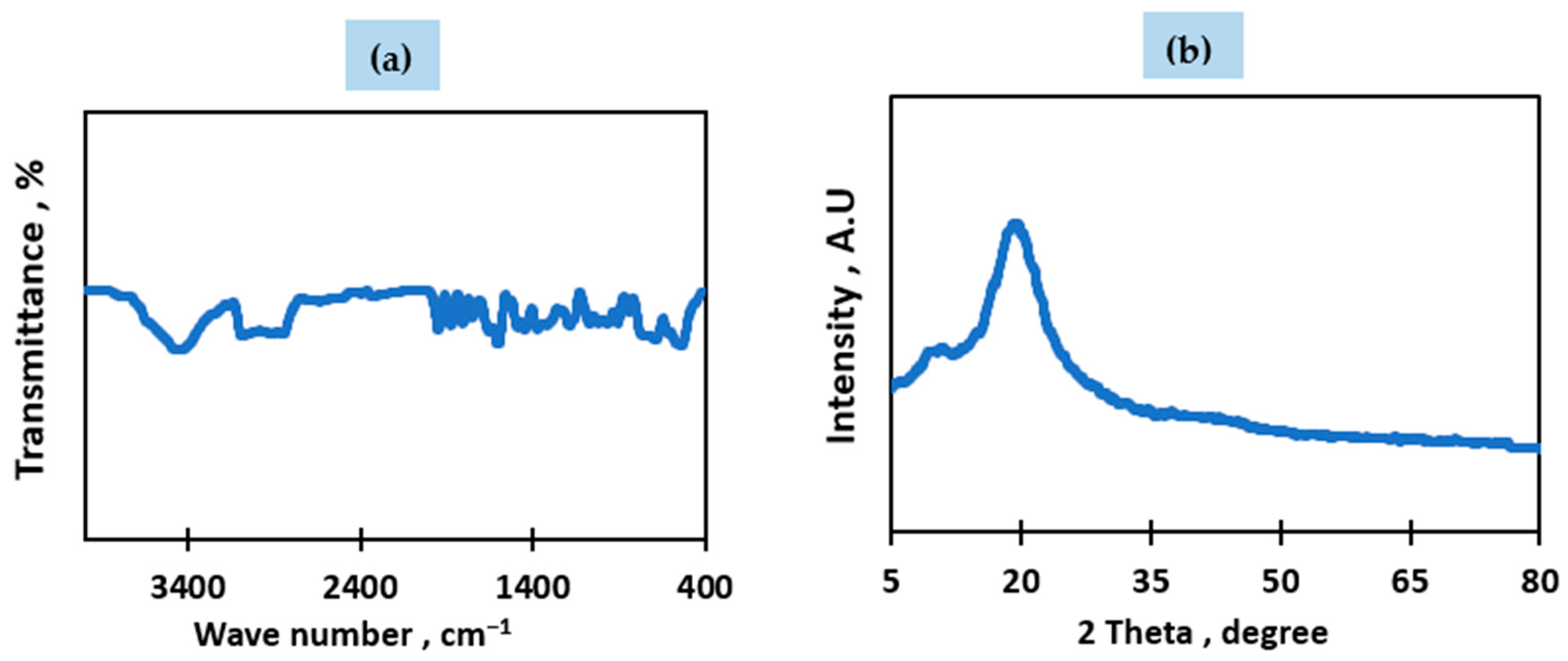
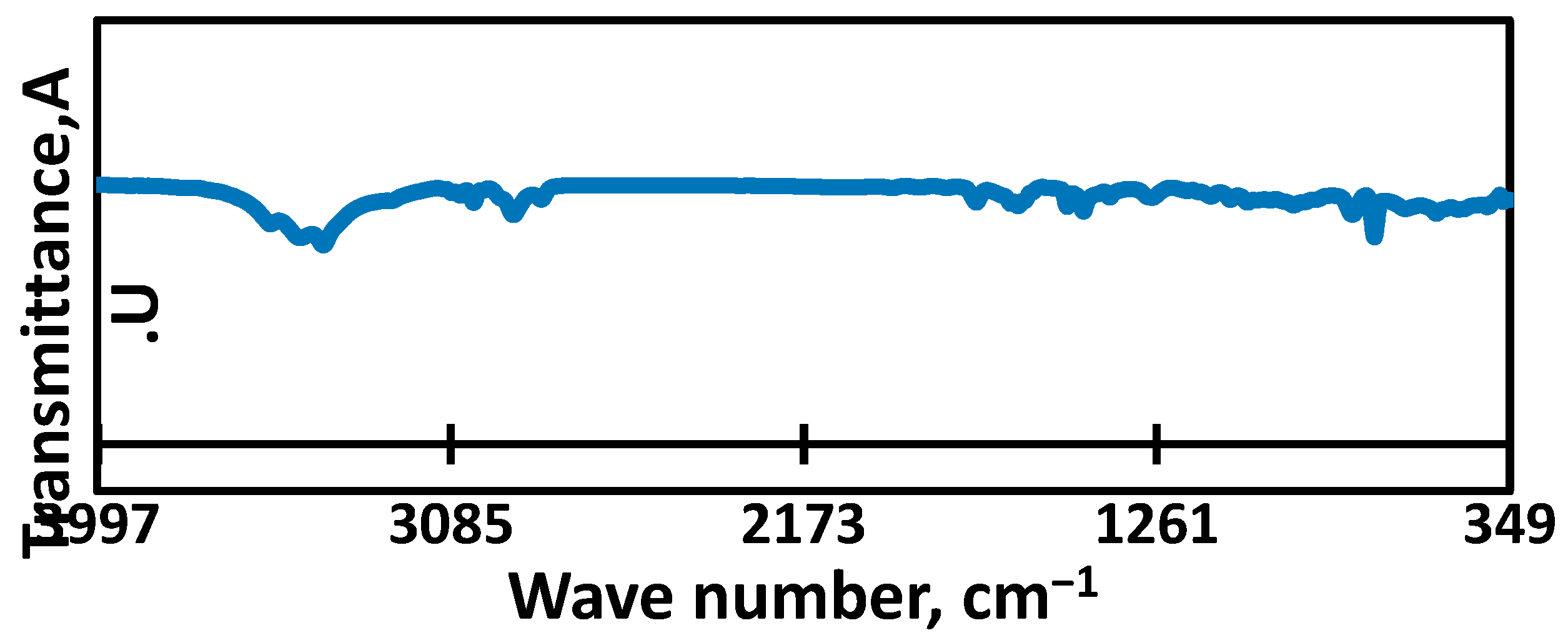
3.1. Raw Material Characterization
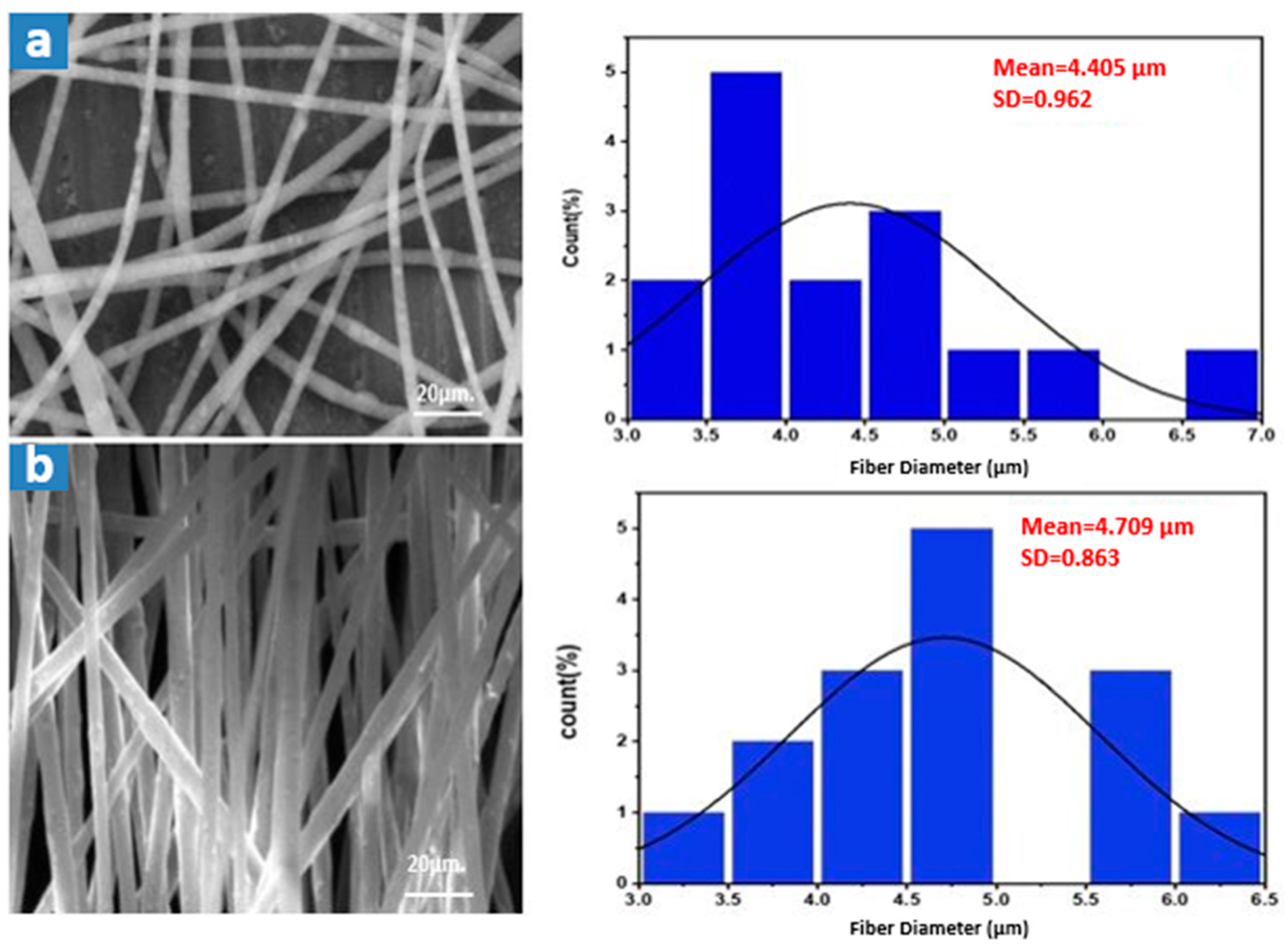
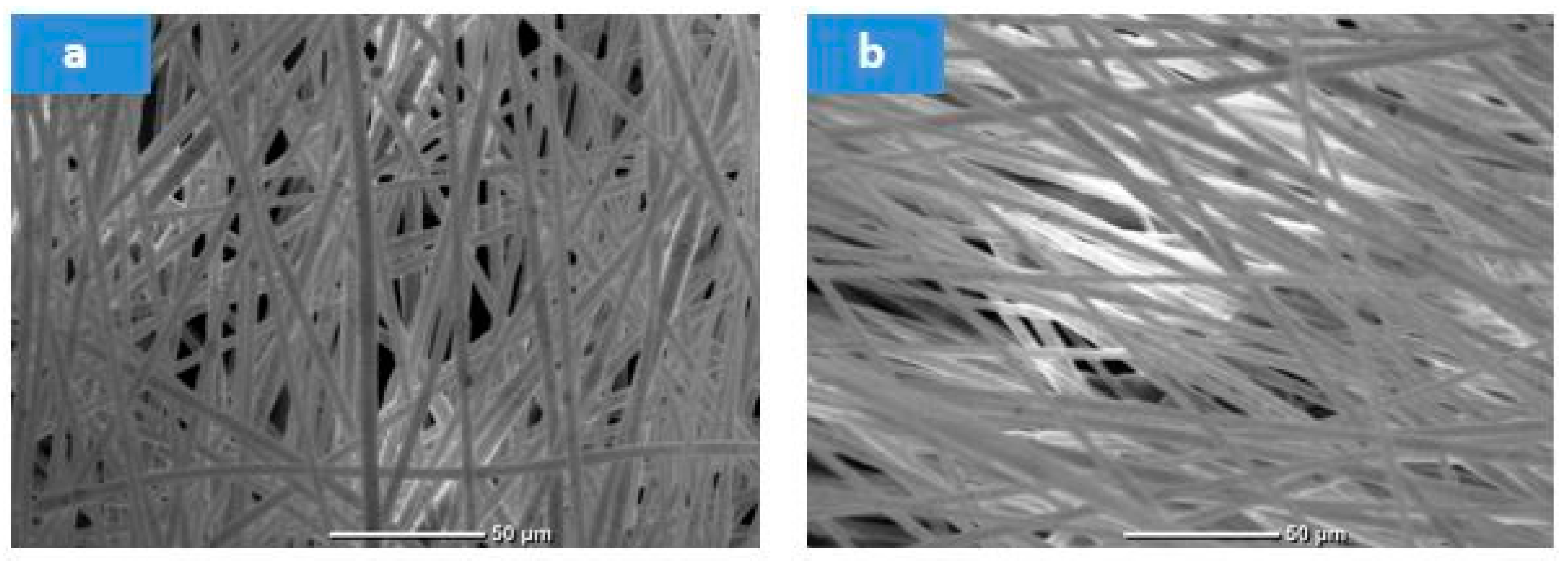
3.2. Waste Polystyrene Electrospun Membrane Results
3.3. Composite Waste Polystyrene/Slag Electrospinning Optimization Using RSM
3.4. Direct Contact Membrane Distillation Testing Results
3.5. Photothermal Evaluation
3.5.1. Membranes’ Temperature Profile
3.5.2. Evaporation Rate Experimental Results
3.5.3. Photothermal Membrane Distillation (PMD) Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank Group: Washington, DC, USA, 2015. [Google Scholar]
- Kotb, M. Egypt’s Plastic Production Industry Is Growing Fast, But So Is Recycling. 2024. Available online: https://egyptianstreets.com/2024/09/24/egypts-plastic-production-industry-is-growing-fast-but-so-is-recycling/ (accessed on 24 September 2024).
- Steel Slag Production in Egypt; Egyptian Ministry of Trade and Industry: Cairo, Egypt, 2023.
- Food and Agriculture Organization of the United Nations. AQUASTAT—FAO’s Global Information System on Water and Agriculture. Available online: https://www.fao.org/aquastat/en/ (accessed on 1 October 2024).
- World Bank Group. The World Bank in Egypt. Available online: https://www.worldbank.org/en/country/egypt/overview (accessed on 3 October 2024).
- Zhou, S.; Jiang, W.; Wang, T.; Lu, Y. Highly Hydrophobic, Compressible, and Magnetic Polystyrene/Fe3O4/Graphene Aerogel Composite for Oil-Water Separation. Ind. Eng. Chem. Res. 2015, 54, 5460–5467. [Google Scholar] [CrossRef]
- Anvari, A.; Kekre, K.M.; Yancheshme, A.A.; Yao, Y.; Ronen, A. Membrane distillation of high salinity water by induction heated thermally conducting membranes. J. Memb. Sci. 2019, 589, 117253. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.; Yao, L.; Ren, X.; Li, Y.; Deng, L. Fe3O4/PVDF-HFP photothermal membrane with in-situ heating for sustainable, stable and efficient pilot-scale solar-driven membrane distillation. Desalination 2020, 478, 114288. [Google Scholar] [CrossRef]
- De León-Condés, C.A.; Roa-Morales, G.; Martínez-Barrera, G.; Balderas-Hernández, P.; Menchaca-Campos, C.; Ureña-Núñez, F. A novel sulfonated waste polystyrene/iron oxide nanoparticles composite: Green synthesis, characterization and applications. J. Environ. Chem. Eng. 2019, 7, 102841. [Google Scholar] [CrossRef]
- Ray, S.S.; Chen, S.S.; Li, C.W.; Nguyen, N.C.; Nguyen, H.T. A comprehensive review: Electrospinning technique for fabrication and surface modification of membranes for water treatment application. RSC Adv. 2016, 6, 85495–85514. [Google Scholar] [CrossRef]
- Nayak, V.; Shivanna, J.M.; Ramu, S.; Radoor, S.; Balakrishna, R.G. Efficacy of Electrospun Nanofiber Membranes on Fouling Mitigation: A Review. ACS Omega 2022, 7, 43346–43363. [Google Scholar] [CrossRef] [PubMed]
- Nadaf, A.; Gupta, A.; Hasan, N.; Fauziya; Ahmad, S.; Kesharwani, P.; Ahmad, F.J. Recent update on electrospinning and electrospun nanofibers: Current trends and their applications. RSC Adv. 2022, 12, 23808–23828. [Google Scholar] [CrossRef]
- Ji, K.; Liu, C.; He, H.; Mao, X.; Wei, L.; Wang, H.; Zhang, M.; Shen, Y.; Sun, R.; Zhou, F. Research Progress of Water Treatment Technology Based on Nanofiber Membranes. Polymers 2023, 15, 741. [Google Scholar] [CrossRef]
- Khatri, M.; Francis, L.; Hilal, N. Modified Electrospun Membranes Using Different Nanomaterials for Membrane Distillation. Membranes 2023, 13, 338. [Google Scholar] [CrossRef]
- Arrosyid, B.H.; Zulfi, A.; Nur’AIni, S.; Hartati, S.; Rafryanto, A.F.; Noviyanto, A.; Hapidin, D.A.; Feriyanto, D.; Khairurrijal, K. High-Efficiency Water Filtration by Electrospun Expanded Polystyrene Waste Nanofibers. ACS Omega 2023, 8, 23664–23672. [Google Scholar] [CrossRef]
- Keirouz, A.; Galiano, F.; Russo, F.; Fontananova, E.; Castro-Dominguez, B.; Figoli, A.; Mattia, D.; Leese, H.S. Cyrene-Enabled Green Electrospinning of Nanofibrous Graphene-Based Membranes for Water Desalination via Membrane Distillation. ACS Sustain. Chem. Eng. 2024, 12, 17713–17725. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yin, Y.; Zhou, H.; Fan, Y.; Yang, Y.; Gao, Q.; Li, P.; Gao, G.; Li, J. Recent Advances in Electrospinning Techniques for Precise Medicine. Cyborg Bionic Syst. 2024, 5, 0101. [Google Scholar] [CrossRef] [PubMed]
- Borah, A.R.; Hazarika, P.; Duarah, R.; Goswami, R.; Hazarika, S. Biodegradable Electrospun Membranes for Sustainable Industrial Applications. ACS Omega 2024, 9, 11129–11147. [Google Scholar] [CrossRef]
- Elrasheedy, A.; Rabie, M.; El-Shazly, A.; Bassyouni, M.; Abdel-Hamid, S.M.S.; El Kady, M.F. Numerical Investigation of Fabricated MWCNTs/Polystyrene Nanofibrous Membrane for DCMD. Polymers 2021, 13, 160. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, D.C.; Runger, G.C. Applied Statistics and Probability for Engineers, 5th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; ISBN 978-0-470-05304-1. [Google Scholar]
- Abdullah, A.; Al-Qahatani, A.; Alquraish, M.; Bailey, C.; El-Shazly, A.; El-Mofty, S. Modeling and Simulation of Fabricated Graphene Nanoplates/Polystyrene Nanofibrous Membrane for DCMD. Polymers 2021, 13, 2987. [Google Scholar] [CrossRef]
- Qasim, M.; Samad, I.; Darwish, N.; Hilal, N. Comprehensive review of membrane design and synthesis for membrane distillation. Desalination 2021, 518, 115168. [Google Scholar] [CrossRef]
- Elrasheedy, A.; Rabie, M.; El-Shazly, A.; Bassyouni, M.; El-Moneim, A.; Kady, M. Investigation of Different Membrane Porosities on the Permeate Flux of Direct Contact Membrane Distillation. Key Eng. Mater. 2021, 889, 85–90. [Google Scholar] [CrossRef]
- Mohsenpour, S.; Safekordi, A.; Tavakolmoghadam, M.; Rekabdar, F.; Hemmati, M. Comparison of the membrane morphology based on the phase diagram using PVP as an organic additive and TiO2 as an inorganic additive. Polymer 2016, 97, 559–568. [Google Scholar] [CrossRef]
- Ke, H.; Feldman, E.; Guzman, P.; Cole, J.; Wei, Q.; Chu, B.; Alkhudhiri, A.; Alrasheed, R.; Hsiao, B.S. Electrospun polystyrene nanofibrous membranes for direct contact membrane distillation. J. Memb. Sci. 2016, 515, 86–97. [Google Scholar] [CrossRef]
- Tijing, L.D.; Choi, J.; Lee, S.; Kim, S.; Kyong, H. Recent progress of membrane distillation using electrospun nano fi brous membrane. J. Memb. Sci. 2014, 453, 435–462. [Google Scholar] [CrossRef]
- Gorji, M.; Sadeghian Maryan, A. Breathable-windproof membrane via simultaneous electrospinning of PU and P(AMPS-GO) hybrid nanofiber: Modeling and optimization with response surface methodology. J. Ind. Text. 2018, 47, 1645–1663. [Google Scholar] [CrossRef]
- Chen, X.; Vanangamudi, A.; Wang, J.; Jegatheesan, J.; Mishra, V.; Sharma, R.; Gray, S.R.; Kujawa, J.; Kujawski, W.; Wicaksana, F.; et al. Direct contact membrane distillation for effective concentration of perfluoroalkyl substances—Impact of surface fouling and material stability. Water Res. 2020, 182, 116010. [Google Scholar] [CrossRef]
- Salem, M.S.; El-Shazly, A.H.; Nady, N.; Elmarghany, M.R.; Sabry, M.N. PES/PVDF blend membrane and its composite with graphene nanoplates: Preparation, characterization, and water desalination via membrane distillation. Desalin. Water Treat. 2019, 166, 9–23. [Google Scholar] [CrossRef]
- Rabie, M.; Ali, A.Y.; Abo-Zahhad, E.M.; Elqady, H.I.; Elkady, M.; Ookawara, S.; El-Shazly, A.; Salem, M.S.; Radwan, A. Thermal analysis of a hybrid high concentrator photovoltaic/membrane distillation system for isolated coastal regions. Sol. Energy 2021, 215, 220–239. [Google Scholar] [CrossRef]
- Qtaishat, M.; Matsuura, T.; Kruczek, B.; Khayet, M. Heat and mass transfer analysis in direct contact membrane distillation. Desalination 2008, 219, 272–292. [Google Scholar] [CrossRef]
- Rochd, S.; Zerradi, H.; Mizani, S.; Dezairi, A.; Ouaskit, S. Modelisation of Membrane Distillation: Mass and Heat Transfer in Air Gap Membrane Distillation. J. Membr. Sci. Technol. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Phattaranawik, J.; Jiraratananon, R.; Fane, A.G. Heat transport and membrane distillation coefficients in direct contact membrane distillation. J. Memb. Sci. 2003, 212, 177–193. [Google Scholar] [CrossRef]
- Soukane, S.; Naceur, M.W.; Francis, L.; Alsaadi, A.; Ghaffour, N. Effect of feed flow pattern on the distribution of permeate fluxes in desalination by direct contact membrane. Distillation 2017, 418, 43–59. [Google Scholar] [CrossRef]
- Lawal, D.U.; Khalifa, A.E. Flux Prediction in Direct Contact Membrane Distillation. Int. J. Mater. Mech. Manuf. 2014, 2, 302–308. [Google Scholar] [CrossRef]
- Bahmanyar, A.; Asghari, M.; Khoobi, N. Numerical simulation and theoretical study on simultaneously effects of operating parameters in direct contact membrane distillation. Chem. Eng. Process. Process Intensif. 2012, 61, 42–50. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, J.; Han, Y.; Xu, H.; Wang, Y.; Qi, D.; Wang, W. A simple and universal strategy to deposit Ag/polypyrrole on various substrates for enhanced interfacial solar evaporation and antibacterial activity. Chem. Eng. J. 2020, 384, 123379. [Google Scholar] [CrossRef]
- Fang, K.; Du, C.; Zhang, J.; Zhou, C.; Yang, S. Molecular engineering of a synergistic photocatalytic and photothermal membrane for highly efficient and durable solar water purification. J. Memb. Sci. 2022, 663, 121037. [Google Scholar] [CrossRef]
- Ghim, D.; Wu, X.; Suazo, M.; Jun, Y. Achieving maximum recovery of latent heat in photothermally driven multi-layer stacked membrane distillation. Nano Energy 2020, 80, 105444. [Google Scholar] [CrossRef]
- Zhuang, G.; Tseng, H.; Wey, M. Feasibility of using waste polystyrene as amembrane material for gas separation. Chem. Eng. Res. Des. 2016, 111, 204–217. [Google Scholar] [CrossRef]
- Haile, L. Study of Recycling of Waste High Impact Polystyrene (PS-HI) and Polystyrene with Flame Retardant Additives (PS-FR) Co-Polymers. J. Text. Sci. Fash. Technol. 2021, 7, 1–11. [Google Scholar] [CrossRef]
- Noby, H.; El-Shazly, A.; Elkady, M.; Ohshima, M. Novel Preparation of a Self-assembled HCl-doped Polyaniline Nanotubes using Compressed CO2-assisted Polymerization. Polymer 2018, 156, 71–75. [Google Scholar] [CrossRef]
- Acuña, P.; Morales, G.; de León, R. Synthesis and Characterization of High-Impact Polystyrene Using a Multifunctional Cyclic Peroxide as the Initiator. J. Appl. Polym. Sci. 2009, 114, 3198–3210. [Google Scholar] [CrossRef]
- Kim, K.; Seo, M.G.; Jung, J.; Ahn, J.; Chang, T.; Jeon, H.B.; Paik, H.-J. Direct introduction of hydroxyl groups in polystyrene chain ends prepared by atom-transfer radical polymerization. Polym. J. 2020, 52, 57–64. [Google Scholar] [CrossRef]
- Hussen, S.A. Structural and optical characterization of pure and SnZrO3 doped PS based polymer nanocomposite. Mater. Res. Express 2020, 7, 105302. [Google Scholar] [CrossRef]
- Demirel, E.; Zhang, B.; Papakyriakou, M.; Xia, S.; Chen, Y. Fe2O3 nanocomposite PVC membrane with enhanced properties and separation performance. J. Memb. Sci. 2017, 529, 170–184. [Google Scholar] [CrossRef]
- Moatmed, S.; Khedr, M.H.; El-Dek, S.I.; Kim, H.Y.; El-Deen, A.G. Highly efficient and reusable superhydrophobic/superoleophilic polystyrene@ Fe3O4 nanofiber membrane for high-performance oil/water separation. J. Environ. Chem. Eng. 2019, 7, 103508. [Google Scholar] [CrossRef]
- Teli, D.; Nadathut, G.T.; Shinde, A.; Pandit, P. Electrospinning of Polystyrene and Modification in Physical properties. J. Text. Assoc. 2018, 79, 173–179. [Google Scholar]
- Sallakh, A.; Saeed, N. Mechanically improved superhydrophobic nanofibrous polystyrene/high-impact polystyrene membranes for promising membrane distillation application. J. Appl. Polym. Sci. 2021, 138, 50917. [Google Scholar] [CrossRef]
- Lee, M.W.; An, S.; Latthe, S.S.; Lee, C.; Hong, S.; Yoon, S.S. Electrospun polystyrene nanofiber membrane with superhydrophobicity and superoleophilicity for selective separation of water and low viscous oil. ACS Appl. Mater. Interfaces 2013, 5, 10597–10604. [Google Scholar] [CrossRef] [PubMed]
- Esteves, R.J.A. Activated carbon-doped polystyrene fibers for direct contact membrane desalination. Emergent Mater. 2020, 3, 807–814. [Google Scholar] [CrossRef]
- Youssef, A.; Bujdosó, T.; Hornok, V.; Papp, S.; Hakim, A.; Dekany, I. Structural and thermal properties of polystyrene nanocomposites containing hydrophilic and hydrophobic layered double hydroxides. Appl. Clay Sci. 2013, 77–78, 46–51. [Google Scholar] [CrossRef]
- McArthur, S.; Baird, M. Oxyfunctionalization of Polystyrene by Hydrogen Peroxide using Non-Heme Iron Catalysts. Eur. Polym. J. 2014, 55, 170–178. [Google Scholar] [CrossRef]
- Nanditha, A.; Manokaran, J.; Balasubramanian, N. Fabrication of Lys-PVA-Fe3O4 modified electrode for the electrochemical determination of uric acid. Res. J. Chem. Environ. 2014, 18, 54–61. [Google Scholar]
- Safo, K.; Noby, H.; Matatoshi, M.; Naragino, H.; El-Shazly, A.H. Statistical optimization modeling of organic dye photodegradation process using slag nanocomposite. Res. Chem. Intermed. 2022, 48, 4183–4208. [Google Scholar] [CrossRef]
- Devaraj, K.; Veerasamy, M.; Aathika, S.; Mani, Y.; Thanarasu, A.; Dhanasekaran, A.; Subramanian, S. Study on effectiveness of activated calcium oxide in pilot plant biodiesel production. J. Clean. Prod. 2019, 225, 18–26. [Google Scholar] [CrossRef]
- Ghaly, S.T.; Eldemerdash, U.N.; El-Shazly, A.H. Morphology and Thermodynamic Study of a Novel Composite Membrane from Waste Polystyrene/Slag: Experimental Investigation. ACS Omega 2024, 9, 23512–23522. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.; Noh, S.; Park, Y. Enhancing solar absorption and reversibility in calcium looping-based energy storage via salt-promoted CaO. Chem. Eng. J. 2023, 470, 144036. [Google Scholar] [CrossRef]
- Da, Y.; Zhou, J. Decorating Calcium-Based Materials with Transition Metal Elements for Directly Capturing and Storing Solar Energy. ACS Omega 2022, 7, 47202–47213. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, C.; Yu, H.; Qian, G.; Zheng, X.; Zhou, H.; Huang, L.; Zhang, F.; Zhong, Y. Research on the Properties of Steel Slag with Different Preparation Processes. Materials 2024, 17, 1555. [Google Scholar] [CrossRef]
- Li, W.; Deng, L.; Huang, H.; Zhou, J.; Liao, Y.; Qiu, L.; Yang, H.; Yao, L. Janus Photothermal Membrane as an Energy Generator and a Mass-Transfer Accelerator for High-Efficiency Solar-Driven Membrane Distillation. ACS Appl. Mater. Interfaces 2021, 13, 26861–26869. [Google Scholar] [CrossRef]
- Shokrollahi, M.; Asadollahi, M.; Mousavi, S.A.; Rajabi-ghahnavieh, A.; Behzadi-Sarok, M.; Khayet, M. Photothermally heated and mesh-gridded solar-driven direct contact membrane distillation for high saline water desalination. Int. J. Heat Mass Transf. 2022, 199, 123442. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.; Zhou, J.; Chen, X. Photothermal Janus PPy-SiO2@PAN/F-SiO2@PVDF-HFP membrane for high-efficient, low energy and stable desalination through solar membrane distillation. Chem. Eng. J. 2023, 451 Pt 2, 138473. [Google Scholar] [CrossRef]










| Element, wt% | C | Si |
|---|---|---|
| 99.31 | 0.69 |
| Element, wt% | CaO | TiO2 | Fe2O3 | ZrO2 | SiO2 | Al2O3 | MgO | MnO |
|---|---|---|---|---|---|---|---|---|
| 45.5000 | 0.4500 | 35.5000 | - | 9.0900 | 3.0300 | 2.0900 | 2.5200 |
| Independent Variable | Symbol | Coded Levels | References | ||
|---|---|---|---|---|---|
| −1 | 0 | +1 | |||
| Slag Dosage, wt% | X1 | 0 | 5 | 10 | [8,46,47] |
| Applied voltage, kV. | X2 | 15 | 22.5 | 30 | [25,48,49] |
| Feed flow rate, mL·h−1 | X3 | 0.18 | 5.09 | 10 | [50,51] |
| Independent Variables | Response | |||||
|---|---|---|---|---|---|---|
| Run Order | Slag Dosage(wt%) | Applied Voltage (kV) | Spinning Rate (mL·h−1) | Average Fiber Diameter (µm) | ||
| Actual Value | Predicted Value | Residual | ||||
| 1 | 5 | 22.5 | 5.09 | 2.96 | 2.92 | 0.0381 |
| 2 | 10 | 15 | 5.09 | 2.01 | 2.01 | −0.0057 |
| 3 | 10 | 30 | 5.09 | 1.53 | 1.54 | −0.0102 |
| 4 | 5 | 22.5 | 5.09 | 2.82 | 2.92 | −0.105 |
| 5 | 5 | 22.5 | 5.09 | 2.91 | 2.92 | −0.0164 |
| 6 | 5 | 15 | 0.18 | 1.69 | 1.73 | −0.0344 |
| 7 | 5 | 30 | 0.18 | 1.3 | 1.33 | −0.0298 |
| 8 | 5 | 30 | 10 | 3.4 | 3.36 | 0.0344 |
| 9 | 5 | 22.5 | 5.09 | 2.98 | 2.92 | 0.0611 |
| 10 | 10 | 22.5 | 0.18 | 1.31 | 1.27 | 0.0401 |
| 11 | 0 | 15 | 5.09 | 3.42 | 3.41 | 0.0102 |
| 12 | 5 | 15 | 10 | 4.02 | 3.99 | 0.0298 |
| 13 | 0 | 30 | 5.09 | 2.86 | 2.86 | 0.0057 |
| 14 | 10 | 22.5 | 10 | 3.98 | 4 | −0.0241 |
| 15 | 0 | 22.5 | 10 | 4.74 | 4.78 | −0.0401 |
| 16 | 5 | 22.5 | 5.09 | 2.95 | 2.92 | 0.0221 |
| 17 | 0 | 22.5 | 0.18 | 3.23 | 3.21 | 0.0241 |
| Source | Lack of Fit p-Value | Adjusted R2 | Predicted R2 | |
|---|---|---|---|---|
| Linear | 0.0008 | 0.8314 | 0.7171 | |
| 2FI | 0.0005 | 0.8168 | 0.4275 | |
| Quadratic | 0.6034 | 0.9962 | 0.9893 | Suggested |
| Cubic | 0.9957 | Aliased |
| Factor | Coefficient Estimate |
|---|---|
| Intercept | 2.92 |
| A-filler dosage (slag) | −0.6779 |
| B-Applied voltage | −0.2567 |
| C-Flow rate | 1.08 |
| AB | 0.0189 |
| AC | 0.2899 |
| BC | −0.0571 |
| A2 | 0.1208 |
| B2 | −0.5919 |
| C2 | 0.2702 |
| R2 | 0.9983 |
| Independent Variables | Response | ||||
|---|---|---|---|---|---|
| Slag Dosage (wt%) | Applied Voltage (kV) | Spinning Rate (mL·h−1) | Average Fiber Diameter (µm) | ||
| Actual Value | Predicted Value | Residual | |||
| 10 | 29.189 | 0.18 | 0.65 | 0.639 | 0.011 |
| 10 | 21 | 0.18 | 1.492 | 1.284 | 0.208 |
| 10 | 15 | 0.429 | 0.935 | 0.905 | 0.03 |
| Optimum Conditions | Coded Levels | Actual Levels | |
|---|---|---|---|
| Slag dosage, (wt%) | +1 | 10 | |
| Applied voltage, (kV) | 0 | 15 | |
| Spinning rate, (mL·h−1) | −0.786 | 1.229 | |
| Response | Experimental values | Predicted values | Residual |
| Fiber diameter, (µm) | 1.172 | 1.061 | 0.111 |
| %Element | C | Si | Ca | Ti | Fe | Zr | Mg | Al | Na | S |
|---|---|---|---|---|---|---|---|---|---|---|
| Waste PS | 96.302 | 1.202 | 0.392 | 0.000 | 0.629 | 1.475 | 0.000 | 0.000 | 0.000 | 0.000 |
| Waste PS+ optimized Slag | 60.208 | 5.210 | 18.071 | 0.000 | 13.568 | 0.000 | 1.489 | 1.454 | 0.000 | 0.000 |
| Type | 35wt% Waste PS Membrane | 35wt% waste PS/Optimized Slag Composite Membrane |
|---|---|---|
| Average pore diameter, µm. | 2.562 ± 0.225 | 0.736 ± 0.107 |
| Porosity, % | 71.764 | 82.331 |
| Contact angle, ° | 99.448 | 102.179 |
| Membrane Type | Thickness, µm | LEP, bar | Tensile Strength, MPa |
|---|---|---|---|
| Transparent waste PS | 320.00 | 0.12 | 1.020 |
| PS +optimized slag | 384.00 | 0.38 | 1.144 |
| Membrane Type | Porosity, % | Pore Size, µm | Thickness, µm | Flux (kg·m−2·h−1) | |||
|---|---|---|---|---|---|---|---|
| Experimental | Simulated | Error, % | Salt Rejection, % | ||||
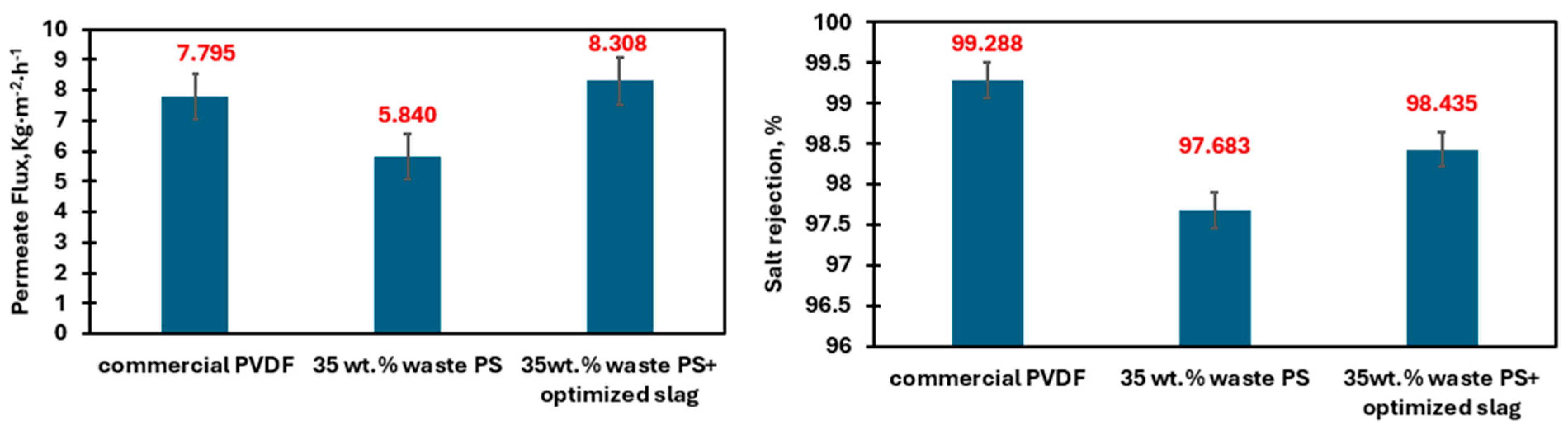
| Waste PS | 72 | 1.281 | 320 | 5.840 | 5.867 | 0.460 | 97.683 |
| Waste PS + Optimized slag | 82 | 0.368 | 384 | 8.308 | 8.328 | 0.240 | 98.435 |
| Tf = 29 °C, Tꝏ = 21 °C, | Qf = 500 mL·min−1, | S = 15, | ɛ = 0.95 |
| A = 0.062 × 0.062 m2 | |||
| L = 0.0155 m | |||
| Feed bulk parameters | ρw(kg·m−3) = 1017.665692, ρs (kg·m−3) = 1028.830059 | ||
| β(°C−1) = 0.001152551, µ(kg·m−1·s−1) = 0.001234 ∆Lvap.(KJ kg−1) = 2432.708064 | |||
| K(W·m−1·°C−1) = 0.619434, Cp(J·kg−1 ·°C−1) = 4103.956233 | |||
| Re = 186.942384, Pr = 8.174362, Nu = 8.214267 | |||
| Heat flux (W·m−2) values using initial value of (Tmf = Tf = 29 °C) | |||
| Qe | QR | Qconv-feed | Qconv-membrane |
| 111.840160 | 45.694920 | 2626.165073 | 2783.700154 |
| Tmf0pt (°C) = 34.079747 | |||
| Flux (kg·m−2·h−1) | Reference | ||
|---|---|---|---|
| Experimental | Simulated | ||
| PVDF | 2.77 | 2.960 | This Study |
| JPTM | 1.290 | 1.421 | [61] |
| PAN-CB/PVDF/PP | 0.430 | 0.450 | [62] |
| PSP-SPH | 44.400 | 45.104 | [63] |
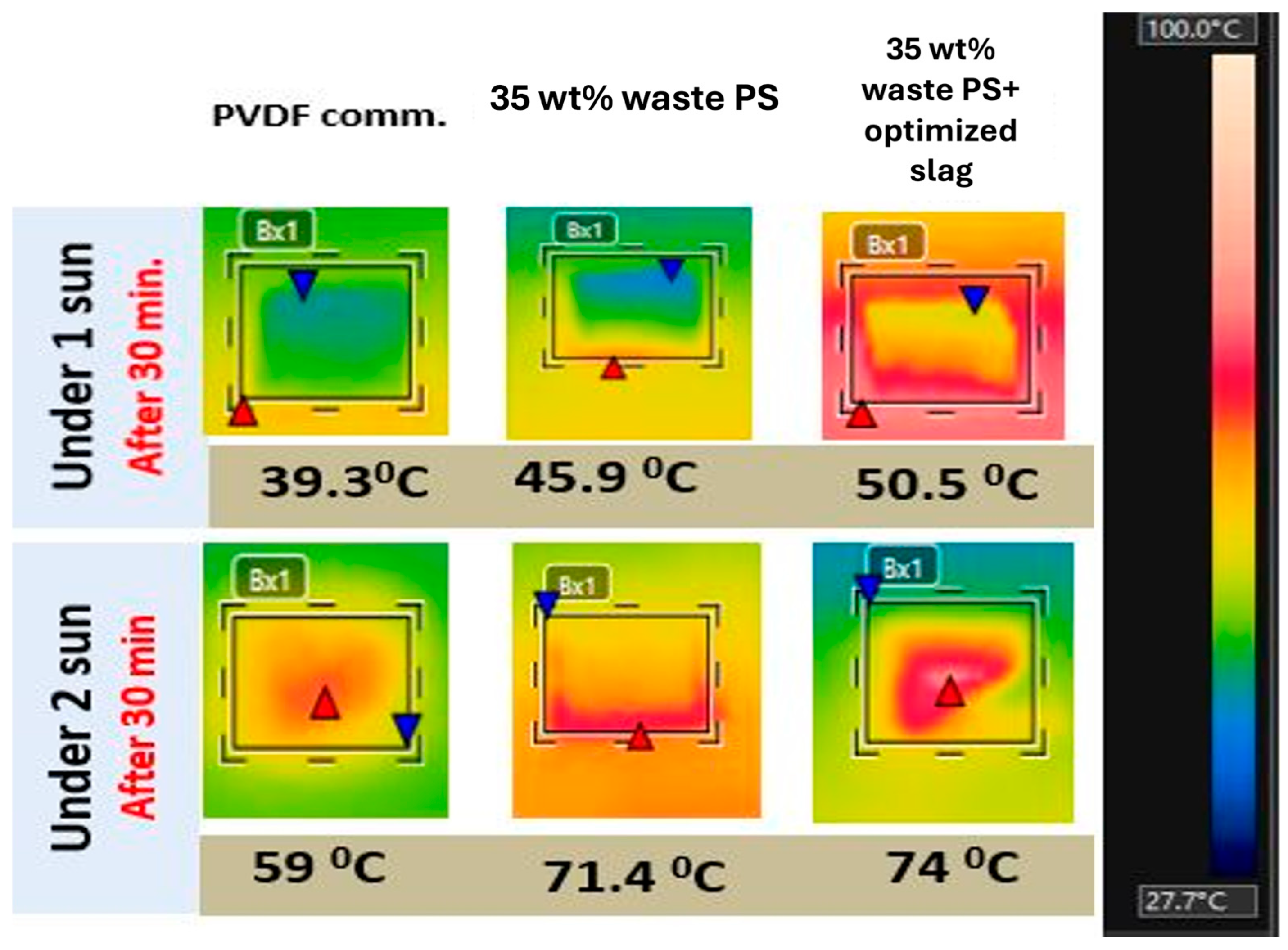
| Type | Tmf | PMD Flux, kg·m−2·h−1 | DCMD Flux, kg·m−2·h−1 |
|---|---|---|---|
| Commercial PVDF | 34.080 | 2.960 | 0.607 |
| 35 wt% waste PS | 34.285 | 3.159 | 0.474 |
| 35 wt% waste PS+ optimized slag | 34.542 | 3.181 | 0.700 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghaly, S.T.; Eldemerdash, U.N.; El-Shazly, A.H. Electro-Spun Waste Polystyrene/Steel Slag Composite Membrane for Water Desalination: Modelling and Photothermal Activity Evaluation. Membranes 2025, 15, 294. https://doi.org/10.3390/membranes15100294
Ghaly ST, Eldemerdash UN, El-Shazly AH. Electro-Spun Waste Polystyrene/Steel Slag Composite Membrane for Water Desalination: Modelling and Photothermal Activity Evaluation. Membranes. 2025; 15(10):294. https://doi.org/10.3390/membranes15100294
Chicago/Turabian StyleGhaly, Salma Tarek, Usama Nour Eldemerdash, and Ahmed H. El-Shazly. 2025. "Electro-Spun Waste Polystyrene/Steel Slag Composite Membrane for Water Desalination: Modelling and Photothermal Activity Evaluation" Membranes 15, no. 10: 294. https://doi.org/10.3390/membranes15100294
APA StyleGhaly, S. T., Eldemerdash, U. N., & El-Shazly, A. H. (2025). Electro-Spun Waste Polystyrene/Steel Slag Composite Membrane for Water Desalination: Modelling and Photothermal Activity Evaluation. Membranes, 15(10), 294. https://doi.org/10.3390/membranes15100294






