6FDA-Based Co-Polyimide Membranes Incorporating Modulated MOF-808s for Olefin/Paraffin Gas Separations
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. MOF Synthesis
2.3. Membrane Preparation
2.4. Characterization of MOF and Membrane Samples
2.5. Membrane Performance Analysis
3. Results and Discussion
3.1. Characterization of MOF-808 Samples
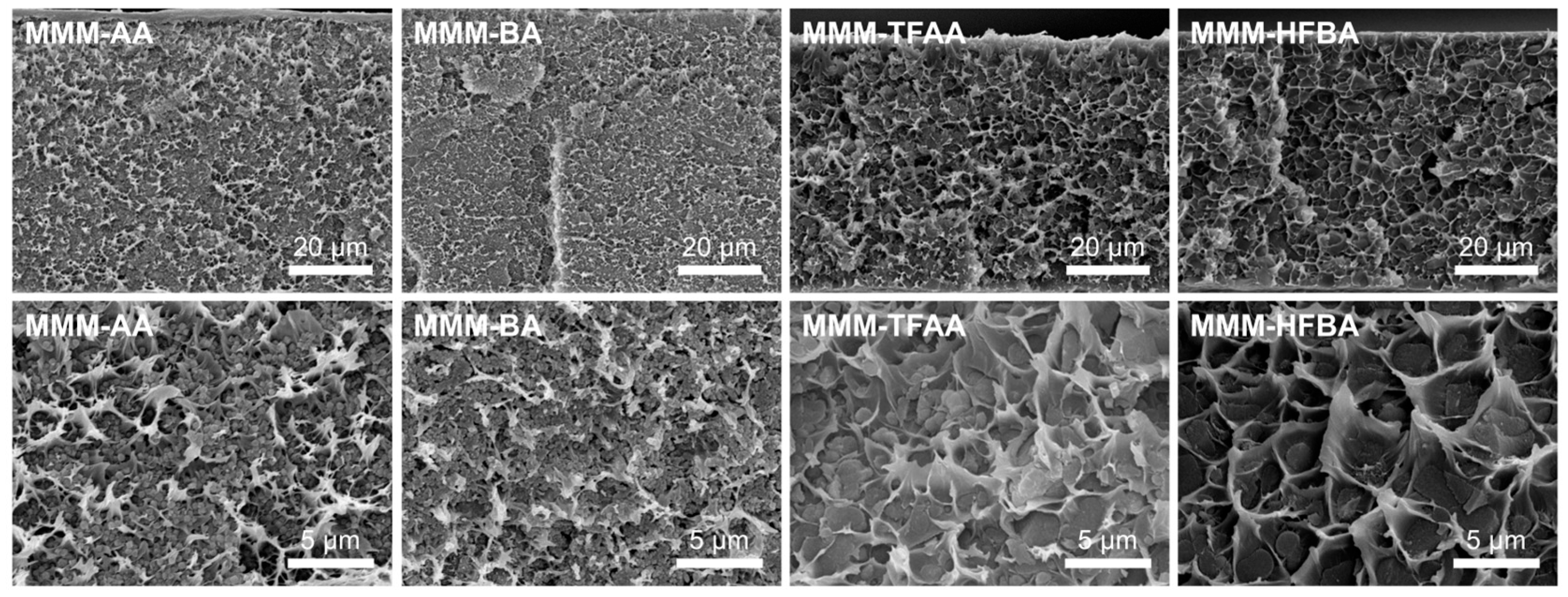
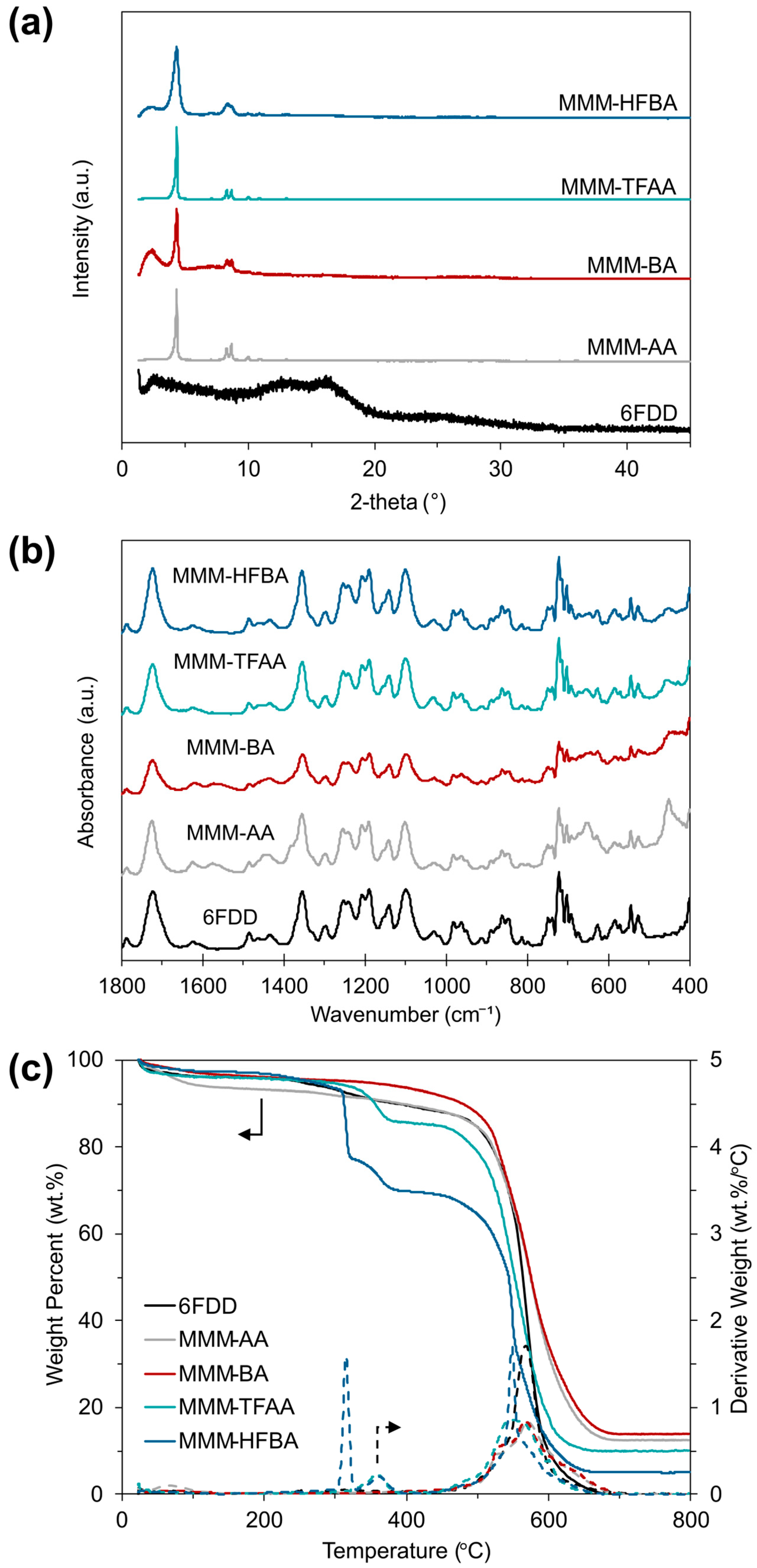
3.2. Characterization of MOF-808/6FDD MMMs
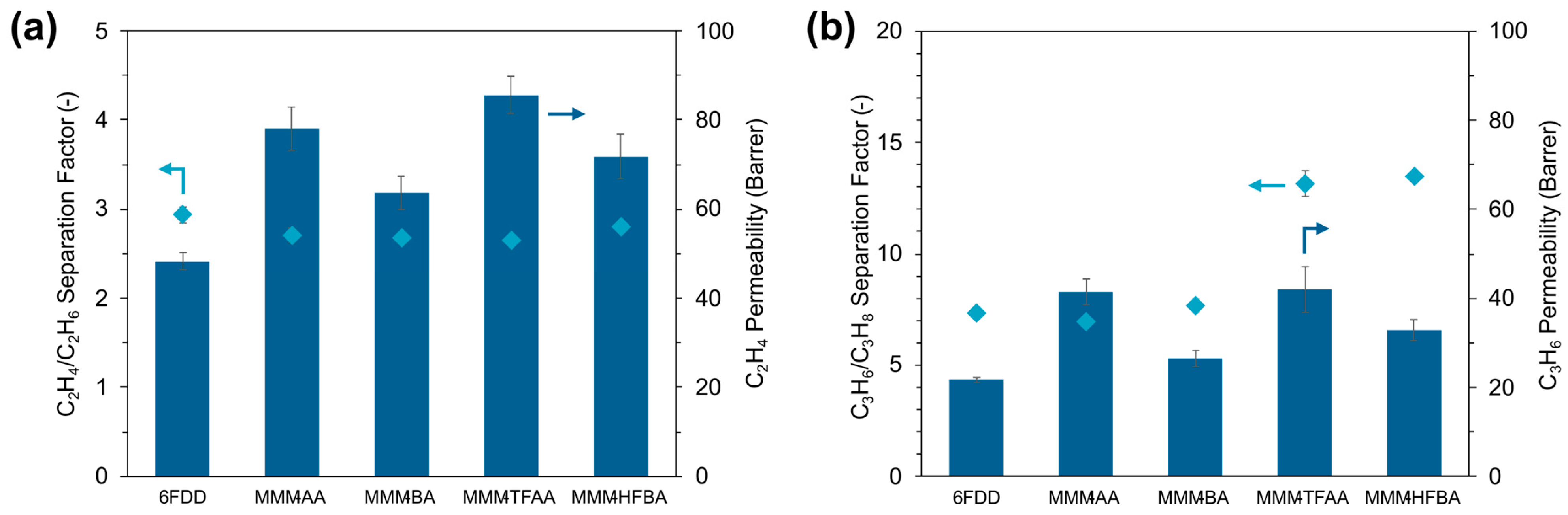
3.3. C2 and C3 Olefin/Paraffin Gas Separation Performance of MOF-808/6FDD MMMs
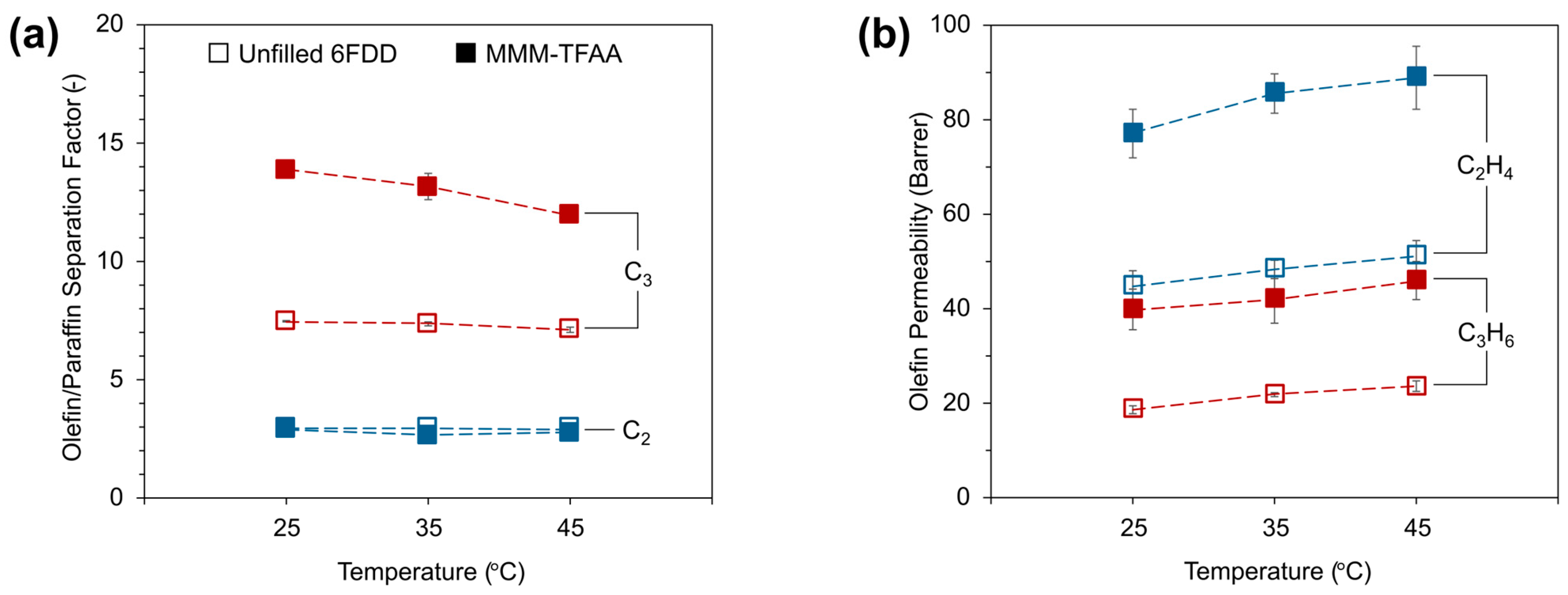
3.4. Effect of Temperature on Olefin/Paraffin Separation Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| 6FDA | 4,4′-(Hexafluoroisopropylidene)diphthalic anhydride |
| DAM | 2,4-Diaminomesitylene |
| DABA | 3,5-Diaminobenzoic acid |
References
- Chernyak, S.A.; Corda, M.; Dath, J.-P.; Ordomsky, V.V.; Khodakov, A.Y. Light olefin synthesis from a diversity of renewable and fossil feedstocks: State-of the-art and outlook. Chem. Soc. Rev. 2022, 51, 7994–8044. [Google Scholar] [CrossRef] [PubMed]
- Galvis, H.M.T.; de Jong, K.P. Catalysts for Production of Lower Olefins from Synthesis Gas: A Review. ACS Catal. 2013, 3, 2130–2149. [Google Scholar] [CrossRef]
- Amghizar, I.; Vandewalle, L.A.; Van Geem, K.M.; Marin, G.B. New Trends in Olefin Production. Engineering 2017, 3, 171–178. [Google Scholar] [CrossRef]
- Bender, M. An Overview of Industrial Processes for the Production of Olefins – C4 Hydrocarbons. ChemBioEng Rev. 2014, 1, 136–147. [Google Scholar] [CrossRef]
- Akah, A.; Williams, J.; Ghrami, M. An Overview of Light Olefins Production via Steam Enhanced Catalytic Cracking. Catal. Surv. Asia 2019, 23, 265–276. [Google Scholar] [CrossRef]
- Hou, J.; Liu, P.; Jiang, M.; Yu, L.; Li, L.; Tang, Z. Olefin/paraffin separation through membranes: From mechanisms to critical materials. J. Mater. Chem. A 2019, 7, 23489–23511. [Google Scholar] [CrossRef]
- Ren, Y.; Liang, X.; Dou, H.; Ye, C.; Guo, Z.; Wang, J.; Pan, Y.; Wu, H.; Guiver, M.D.; Jiang, Z. Membrane-Based Olefin/Paraffin Separations. Adv. Sci. 2020, 7, 2001398. [Google Scholar] [CrossRef]
- Faiz, R.; Li, K. Olefin/paraffin separation using membrane based facilitated transport/chemical absorption techniques. Chem. Eng. Sci. 2012, 73, 261–284. [Google Scholar] [CrossRef]
- Sholl, D.S.; Lively, R.P. Seven chemical separations to change the world. Nature 2016, 532, 435–437. [Google Scholar] [CrossRef]
- Eldridge, R.B. Olefin/paraffin separation technology: A review. Ind. Eng. Chem. Res. 1993, 32, 2208–2212. [Google Scholar] [CrossRef]
- Wang, Y.; Peh, S.B.; Zhao, D. Alternatives to Cryogenic Distillation: Advanced Porous Materials in Adsorptive Light Olefin/Paraffin Separations. Small 2019, 15, e1900058. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Xiong, H.; Zhang, P.; Zhao, Z.; Liu, X.; Tang, S.; Liu, Y.; Zhu, Z.; Zhou, W.; Deng, Z.; et al. Interaction-selective molecular sieving adsorbent for direct separation of ethylene from senary C2-C4 olefin/paraffin mixture. Nat. Commun. 2024, 15, 1–12. [Google Scholar] [CrossRef]
- Safarik, D.J.; Eldridge, R.B. Olefin/Paraffin Separations by Reactive Absorption: A Review. Ind. Eng. Chem. Res. 1998, 37, 2571–2581. [Google Scholar] [CrossRef]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane Gas Separation: A Review/State of the Art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Basu, S.; Khan, A.L.; Cano-Odena, A.; Liu, C.; Vankelecom, I.F.J. Membrane-based technologies for biogas separations. Chem. Soc. Rev. 2010, 39, 750–768. [Google Scholar] [CrossRef]
- Galizia, M.; Chi, W.S.; Smith, Z.P.; Merkel, T.C.; Baker, R.W.; Freeman, B.D. 50th Anniversary Perspective: Polymers and Mixed Matrix Membranes for Gas and Vapor Separation: A Review and Prospective Opportunities. Macromolecules 2017, 50, 7809–7843. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Samarasinghe, S.; Li, W.; Goh, K.; Bae, T.-H. Leveraging Nanocrystal HKUST-1 in Mixed-Matrix Membranes for Ethylene/Ethane Separation. Membranes 2020, 10, 74. [Google Scholar] [CrossRef]
- Zhang, C.; Dai, Y.; Johnson, J.R.; Karvan, O.; Koros, W.J. High performance ZIF-8/6FDA-DAM mixed matrix membrane for propylene/propane separations. J. Membr. Sci. 2012, 389, 34–42. [Google Scholar] [CrossRef]
- Do, X.H.; Nguyen, Q.T.; Kim, S.; Lee, A.S.; Baek, K.-Y. Effect of thermal processing on brominated 6FDA-DAM for effective propylene/propane separation. Sep. Purif. Technol. 2021, 262, 118331. [Google Scholar] [CrossRef]
- Burns, R.L.; Koros, W.J. Defining the challenges for C3H6/C3H8 separation using polymeric membranes. J. Membr. Sci. 2003, 211, 299–309. [Google Scholar] [CrossRef]
- Rungta, M.; Zhang, C.; Koros, W.J.; Xu, L. Membrane-based ethylene/ethane separation: The upper bound and beyond. AIChE J. 2013, 59, 3475–3489. [Google Scholar] [CrossRef]
- Kulak, H.; Swings, W.; Cools, M.; Hannes, L.; Vankelecom, I.F. Developing silver-decorated polyimide asymmetric membranes using green solvents for olefin/paraffin gas separations. J. Membr. Sci. 2025, 734, 124404. [Google Scholar] [CrossRef]
- Qian, Q.; Chi, W.S.; Han, G.; Smith, Z.P. Impact of Post-Synthetic Modification Routes on Filler Structure and Performance in Metal–Organic Framework-Based Mixed-Matrix Membranes. Ind. Eng. Chem. Res. 2019, 59, 5432–5438. [Google Scholar] [CrossRef]
- Zornoza, B.; Tellez, C.; Coronas, J.; Gascon, J.; Kapteijn, F. Metal organic framework based mixed matrix membranes: An increasingly important field of research with a large application potential. Microporous Mesoporous Mater. 2013, 166, 67–78. [Google Scholar] [CrossRef]
- Najari, S.; Saeidi, S.; Gallucci, F.; Drioli, E. Mixed matrix membranes for hydrocarbons separation and recovery: A critical review. Rev. Chem. Eng. 2019, 37, 363–406. [Google Scholar] [CrossRef]
- Tan, X.; Robijns, S.; Thür, R.; Ke, Q.; De Witte, N.; Lamaire, A.; Li, Y.; Aslam, I.; Van Havere, D.; Donckels, T.; et al. Truly combining the advantages of polymeric and zeolite membranes for gas separations. Science 2022, 378, 1189–1194. [Google Scholar] [CrossRef]
- Wei, R.; Liu, X.; Lai, Z. MOF or COF membranes for olefin/paraffin separation: Current status and future research directions. Adv. Membr. 2022, 2, 100035. [Google Scholar] [CrossRef]
- Qian, Q.; Asinger, P.A.; Lee, M.J.; Han, G.; Rodriguez, K.M.; Lin, S.; Benedetti, F.M.; Wu, A.X.; Chi, W.S.; Smith, Z.P. MOF-Based Membranes for Gas Separations. Chem. Rev. 2020, 120, 8161–8266. [Google Scholar] [CrossRef]
- Basu, S.; Cano-Odena, A.; Vankelecom, I.F. MOF-containing mixed-matrix membranes for CO2/CH4 and CO2/N2 binary gas mixture separations. Sep. Purif. Technol. 2011, 81, 31–40. [Google Scholar] [CrossRef]
- Bachman, J.E.; Smith, Z.P.; Li, T.; Xu, T.; Long, J.R. Enhanced ethylene separation and plasticization resistance in polymer membranes incorporating metal–organic framework nanocrystals. Nat. Mater. 2016, 15, 845–849. [Google Scholar] [CrossRef]
- Tan, X.; Robijns, S.; Lamaire, A.; Goeminne, R.; De Witte, N.; Dickmann, M.; Verbeke, R.; Van der Donck, T.; Silva, R.d.O.; Ke, Q.; et al. Design of a Tunable, High-performance Mixed Matrix Membrane Platform for Gas Separations. Adv. Mater. 2025, 37, e2502393. [Google Scholar] [CrossRef]
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to Metal–Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef]
- Meek, S.T.; Greathouse, J.A.; Allendorf, M.D. Metal-Organic Frameworks: A Rapidly Growing Class of Versatile Nanoporous Materials. Adv. Mater. 2010, 23, 249–267. [Google Scholar] [CrossRef]
- Thür, R.; Van Velthoven, N.; Lemmens, V.; Bastin, M.; Smolders, S.; De Vos, D.E.; Vankelecom, I.F.J. Modulator-Mediated Functionalization of MOF-808 as a Platform Tool to Create High-Performance Mixed-Matrix Membranes. ACS Appl. Mater. Interfaces 2019, 11, 44792–44801. [Google Scholar] [CrossRef]
- Reinsch, H.; Waitschat, S.; Chavan, S.M.; Lillerud, K.P.; Stock, N. A Facile “Green” Route for Scalable Batch Production and Continuous Synthesis of Zirconium MOFs. Eur. J. Inorg. Chem. 2016, 2016, 4490–4498. [Google Scholar] [CrossRef]
- Najafi, M.; Kulak, H.; Landa, H.O.R.; Vankelecom, I.F.; Denayer, J.F. Appraising separation performance of MOF-808-based adsorbents for light olefins and paraffins. Microporous Mesoporous Mater. 2024, 367, 112961. [Google Scholar] [CrossRef]
- Kulak, H.; Thür, R.; Vankelecom, I.F.J. MOF/Polymer Mixed-Matrix Membranes Preparation: Effect of Main Synthesis Parameters on CO2/CH4 Separation Performance. Membranes 2022, 12, 425. [Google Scholar] [CrossRef]
- Khan, A.L.; Basu, S.; Cano-Odena, A.; Vankelecom, I.F. Novel high throughput equipment for membrane-based gas separations. J. Membr. Sci. 2010, 354, 32–39. [Google Scholar] [CrossRef]
- Jin, L.; Zhou, C.; Chen, S.; Liu, P. Controlled synthesis of defective MOF-808 and its application in levulinic acid esterification. Appl. Catal. A: Gen. 2024, 681, 119773. [Google Scholar] [CrossRef]
- Liu, X.; Kirlikovali, K.O.; Chen, Z.; Ma, K.; Idrees, K.B.; Cao, R.; Zhang, X.; Islamoglu, T.; Liu, Y.; Farha, O.K. Small Molecules, Big Effects: Tuning Adsorption and Catalytic Properties of Metal–Organic Frameworks. Chem. Mater. 2021, 33, 1444–1454. [Google Scholar] [CrossRef]
- Pascual-Colino, J.; Artetxe, B.; Beobide, G.; Castillo, O.; Fidalgo-Mayo, M.L.; Isla-López, A.; Luque, A.; Mena-Gutiérrez, S.; Pérez-Yáñez, S. The Chemistry of Zirconium/Carboxylate Clustering Process: Acidic Conditions to Promote Carboxylate-Unsaturated Octahedral Hexamers and Pentanuclear Species. Inorg. Chem. 2022, 61, 4842–4851. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Zhu, Y.; Wang, S.; Su, S.; Zhou, L.; Zhang, H. Combining Coordination Modulation with Acid–Base Adjustment for the Control over Size of Metal–Organic Frameworks. Chem. Mater. 2012, 24, 444–450. [Google Scholar] [CrossRef]
- Ardila-Suárez, C.; Rodríguez-Pereira, J.; Baldovino-Medrano, V.G.; Ramírez-Caballero, G.E. An analysis of the effect of zirconium precursors of MOF-808 on its thermal stability, and structural and surface properties. CrystEngComm 2019, 21, 1407–1415. [Google Scholar] [CrossRef]
- Pan, Y.; Li, L.; Yang, B.; Ji, G.; Zhao, Z.; Zhang, H.; Wang, F.; Xia, M.; Tao, Y. Adaptive structural modification of Zr-based MOF-808 via solvent and ligand engineering for enhanced fluoride ion adsorption efficiency. Sep. Purif. Technol. 2024, 348, 127731. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, F.; Wu, Y. Fluorinated Metal–Organic Framework–Polymer Mixed Matrix Membrane with Tunable Hydrophobic Channel for Efficient Pervaporation of Butanol/Water. Small Struct. 2023, 5, 2300333. [Google Scholar] [CrossRef]
- Pretsch, E.; Buhlmann, P.; Badertscher, M. Structure Determination of Organic Compounds; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Ardila-Suárez, C.; Molina V., D.R.; Alem, H.; Baldovino-Medrano, V.G.; Ramírez-Caballero, G.E. Synthesis of ordered microporous/macroporous MOF-808 through modulator-induced defect-formation, and surfactant self-assembly strategies. Phys. Chem. Chem. Phys. 2020, 22, 12591–12604. [Google Scholar] [CrossRef]
- Qiu, W.; Xu, L.; Chen, C.-C.; Paul, D.R.; Koros, W.J. Gas separation performance of 6FDA-based polyimides with different chemical structures. Polymer 2013, 54, 6226–6235. [Google Scholar] [CrossRef]
- Jain, A.; Ahmad, M.Z.; Linkès, A.; Martin-Gil, V.; Castro-Muñoz, R.; Izak, P.; Sofer, Z.; Hintz, W.; Fila, V. 6FDA-DAM:DABA Co-Polyimide Mixed Matrix Membranes with GO and ZIF-8 Mixtures for Effective CO2/CH4 Separation. Nanomaterials 2021, 11, 668. [Google Scholar] [CrossRef]
- Breitenbach (nee Stastny), C.; Christoffels, R.; Mattick, T.; Edelmann, A.; Pierkes, T.; König, S.; Fröba, M.; Ruschewitz, U. UoC-2: A Fluorinated Derivative of the Robust Metal-Organic Framework MFM-300. Eur. J. Inorg. Chem. 2024, 27, e202400231. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, Q.; Geng, C.; Zhang, G.-R.; Zhang, Z.; Qiao, Z.; Zhong, C. Ethane-selective permeation in mixed matrix membranes containing fluorinated carboxylic acid functionalized metal-organic frameworks. Chem. Eng. J. 2023, 479, 147656. [Google Scholar] [CrossRef]
- Budd, P.M.; Ghanem, B.S.; Makhseed, S.; McKeown, N.B.; Msayib, K.J.; Tattershall, C.E. Polymers of intrinsic microporosity (PIMs): Robust, solution-processable, organic nanoporous materials. Chem. Commun. 2003, 230–231. [Google Scholar] [CrossRef]
- An, H.; Park, S.; Kwon, H.T.; Jeong, H.-K.; Lee, J.S. A new superior competitor for exceptional propylene/propane separations: ZIF-67 containing mixed matrix membranes. J. Membr. Sci. 2017, 526, 367–376. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Liu, G.; Belmabkhout, Y.; Adil, K.; Eddaoudi, M.; Koros, W. Conformation-Controlled Molecular Sieving Effects for Membrane-Based Propylene/Propane Separation. Adv. Mater. 2019, 31, e1807513. [Google Scholar] [CrossRef] [PubMed]
- Moghadam, F.; Lee, T.H.; Park, I.; Park, H.B. Thermally annealed polyimide-based mixed matrix membrane containing ZIF-67 decorated porous graphene oxide nanosheets with enhanced propylene/propane selectivity. J. Membr. Sci. 2020, 603, 118019. [Google Scholar] [CrossRef]
- Wang, Y.; Li, T.; Li, L.; Lin, R.; Jia, X.; Chang, Z.; Wen, H.; Chen, X.; Li, J. Construction of Fluorinated Propane-Trap in Metal–Organic Frameworks for Record Polymer-Grade Propylene Production under High Humidity Conditions. Adv. Mater. 2023, 35, e2207955. [Google Scholar] [CrossRef]
- Chen, F.; Prasetyo, N.; Sakaki, S.; Otake, K.; Kitagawa, S. Benchmark Paraffin Adsorption in a Super-Hydrophobic Porous Coordination Polymer with Blade-Like Circular Phenyl Nanotraps. Angew. Chem. Int. Ed. Engl. 2025, 64, e202423371. [Google Scholar] [CrossRef]
- Chen, X.; Chen, G.; Liu, G.; Liu, G.; Jin, W. UTSA-280 metal–organic framework incorporated 6FDA-polyimide mixed-matrix membranes for ethylene/ethane separation. AIChE J. 2022, 68, e17688. [Google Scholar] [CrossRef]
- Lin, R.-B.; Li, L.; Zhou, H.-L.; Wu, H.; He, C.; Li, S.; Krishna, R.; Li, J.; Zhou, W.; Chen, B. Molecular sieving of ethylene from ethane using a rigid metal–organic framework. Nat. Mater. 2018, 17, 1128–1133. [Google Scholar] [CrossRef]
- Das, M.; Koros, W.J. Performance of 6FDA–6FpDA polyimide for propylene/propane separations. J. Membr. Sci. 2010, 365, 399–408. [Google Scholar] [CrossRef]
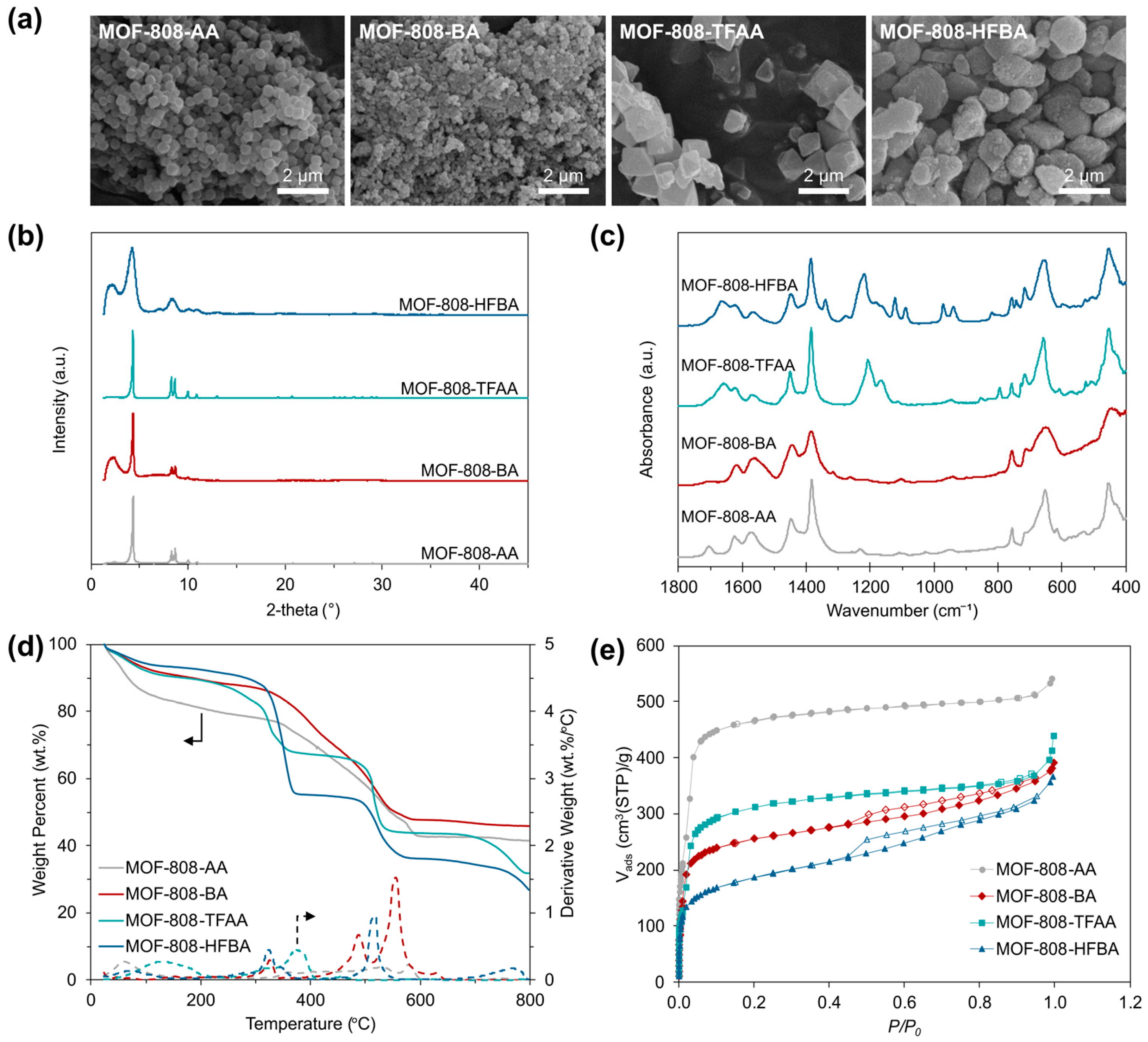

| Sample | Surface Area * (m2/g) | Pore Volume (cm3/g) | Pore Size (Å) |
|---|---|---|---|
| MOF-808-AA | 1518 | 0.54 | 16.6 |
| MOF-808-BA | 846 | 0.24 | 14.8 |
| MOF-808-TFAA | 1031 | 0.29 | 16.6 |
| MOF-808-HFBA | 637 | 0.11 | 13.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulak, H.; Hannes, L.; Vankelecom, I.F.J. 6FDA-Based Co-Polyimide Membranes Incorporating Modulated MOF-808s for Olefin/Paraffin Gas Separations. Membranes 2025, 15, 290. https://doi.org/10.3390/membranes15100290
Kulak H, Hannes L, Vankelecom IFJ. 6FDA-Based Co-Polyimide Membranes Incorporating Modulated MOF-808s for Olefin/Paraffin Gas Separations. Membranes. 2025; 15(10):290. https://doi.org/10.3390/membranes15100290
Chicago/Turabian StyleKulak, Harun, Lore Hannes, and Ivo F. J. Vankelecom. 2025. "6FDA-Based Co-Polyimide Membranes Incorporating Modulated MOF-808s for Olefin/Paraffin Gas Separations" Membranes 15, no. 10: 290. https://doi.org/10.3390/membranes15100290
APA StyleKulak, H., Hannes, L., & Vankelecom, I. F. J. (2025). 6FDA-Based Co-Polyimide Membranes Incorporating Modulated MOF-808s for Olefin/Paraffin Gas Separations. Membranes, 15(10), 290. https://doi.org/10.3390/membranes15100290







