Recycled Polystyrene as a Sustainable Material for Hollow Fiber Membranes in Dye Filtration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polystyrene Sulfonation
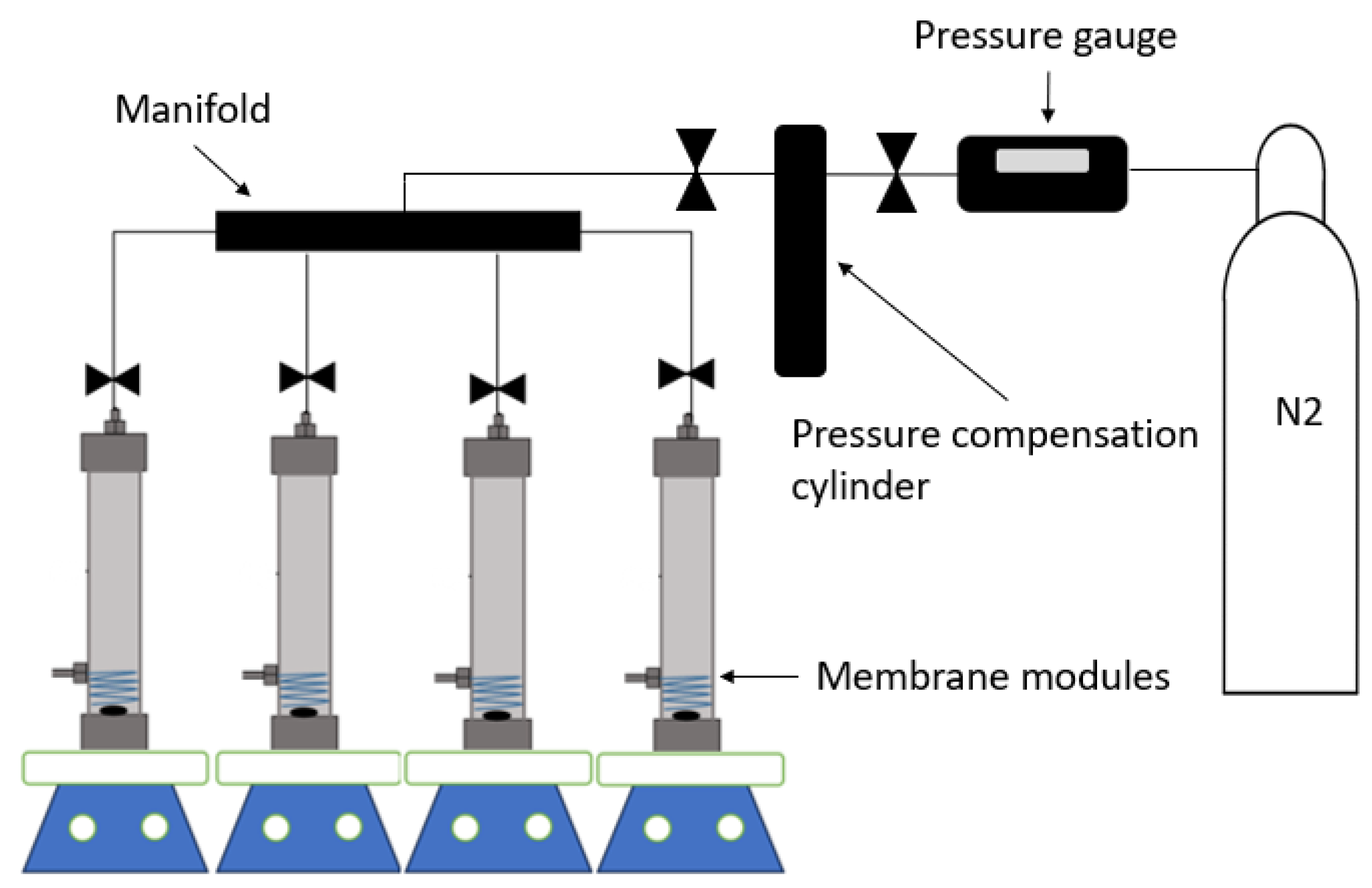
2.3. PPSU/sEPS Membrane Elaboration
2.4. Characterization
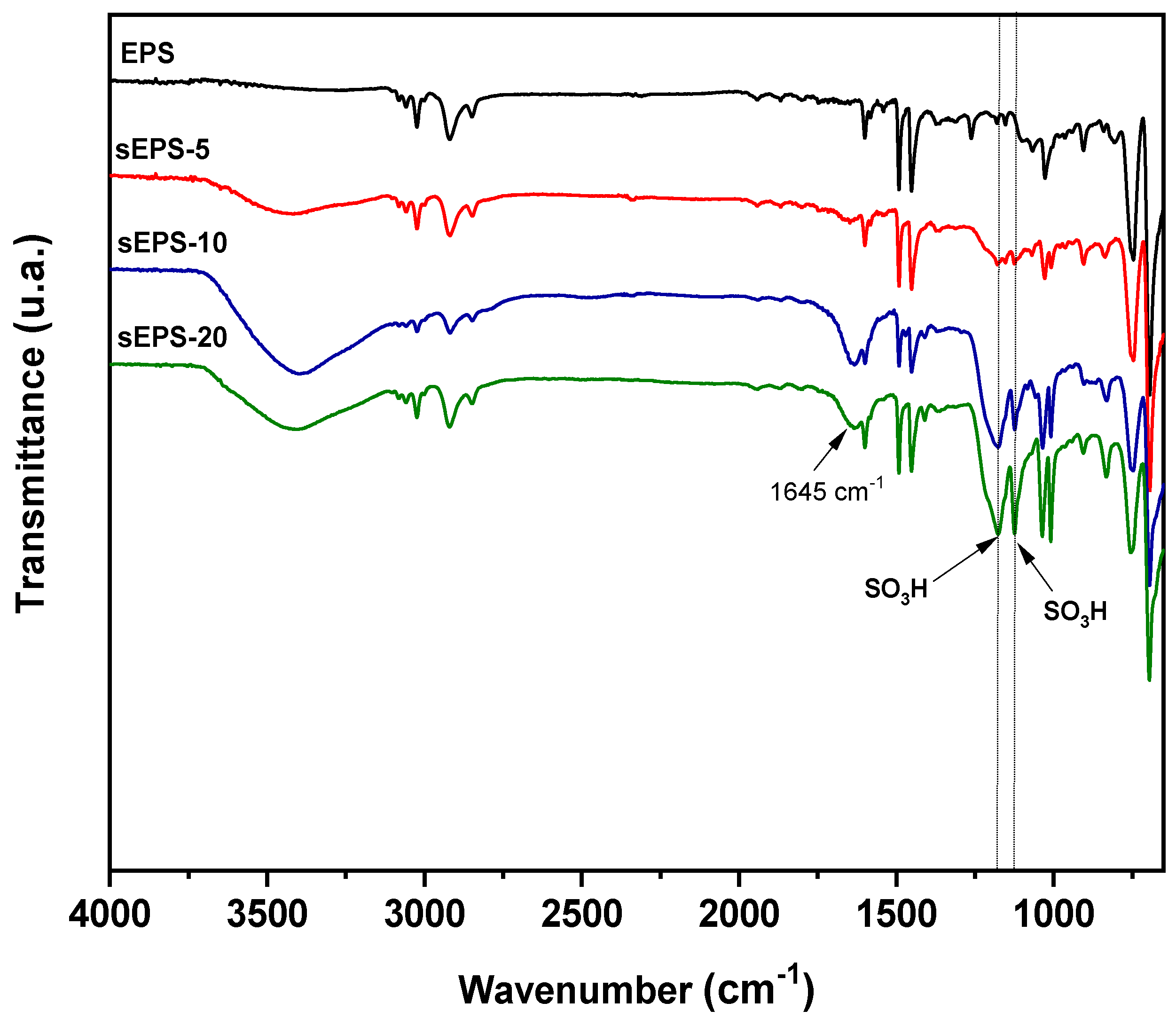
2.4.1. Characterization of Sulfonated EPS
2.4.2. Solubility Parameter Difference and Polymer–Polymer Miscibility
2.4.3. Membrane Morphology and Mechanical Properties
2.4.4. Pore Size Radius, Porosity, Water Contact Angle, and Membrane Separation Performance
3. Results and Discussion
3.1. HFM Morphology
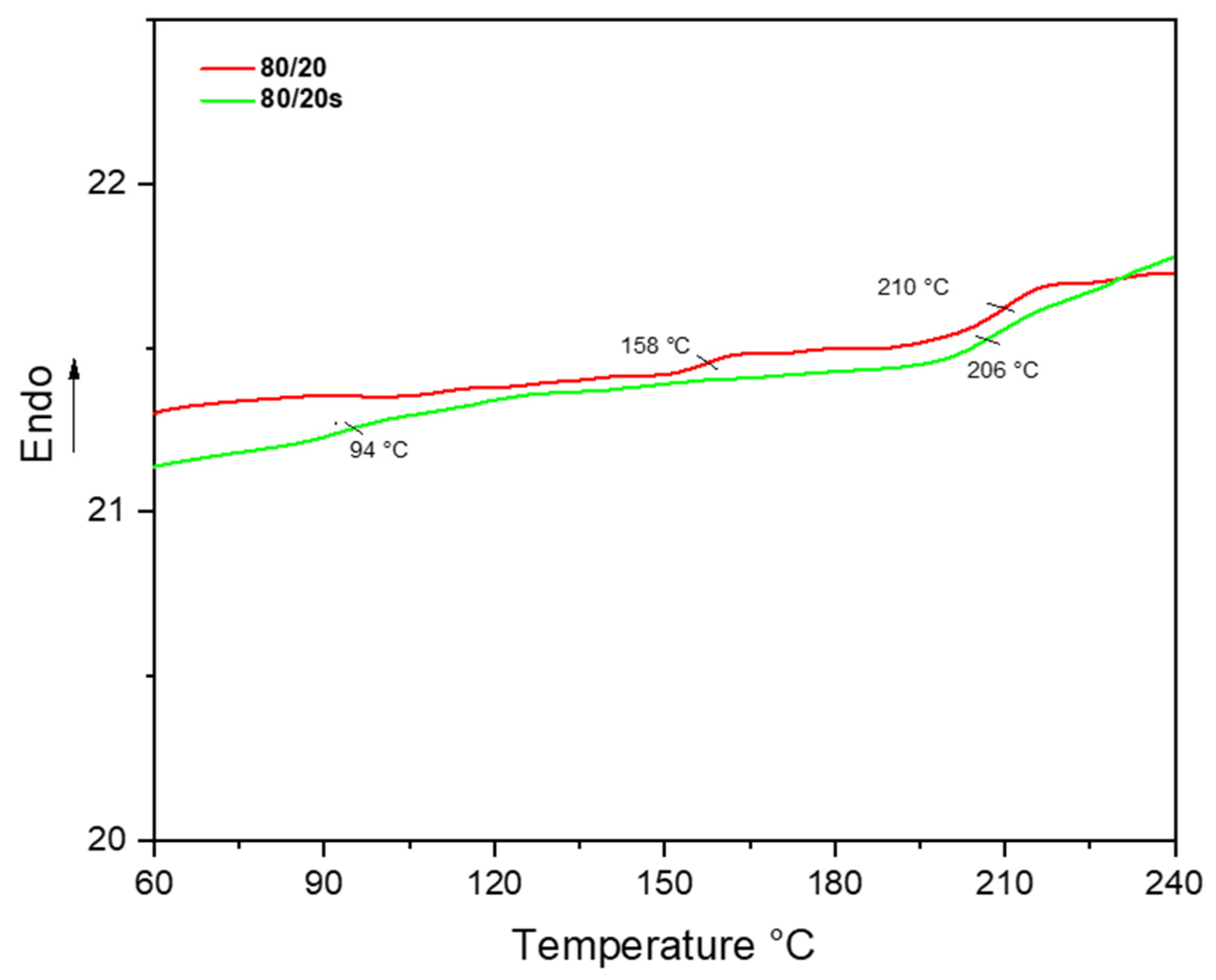
3.2. Solubility Parameter and PPSU/sEPS Miscibility
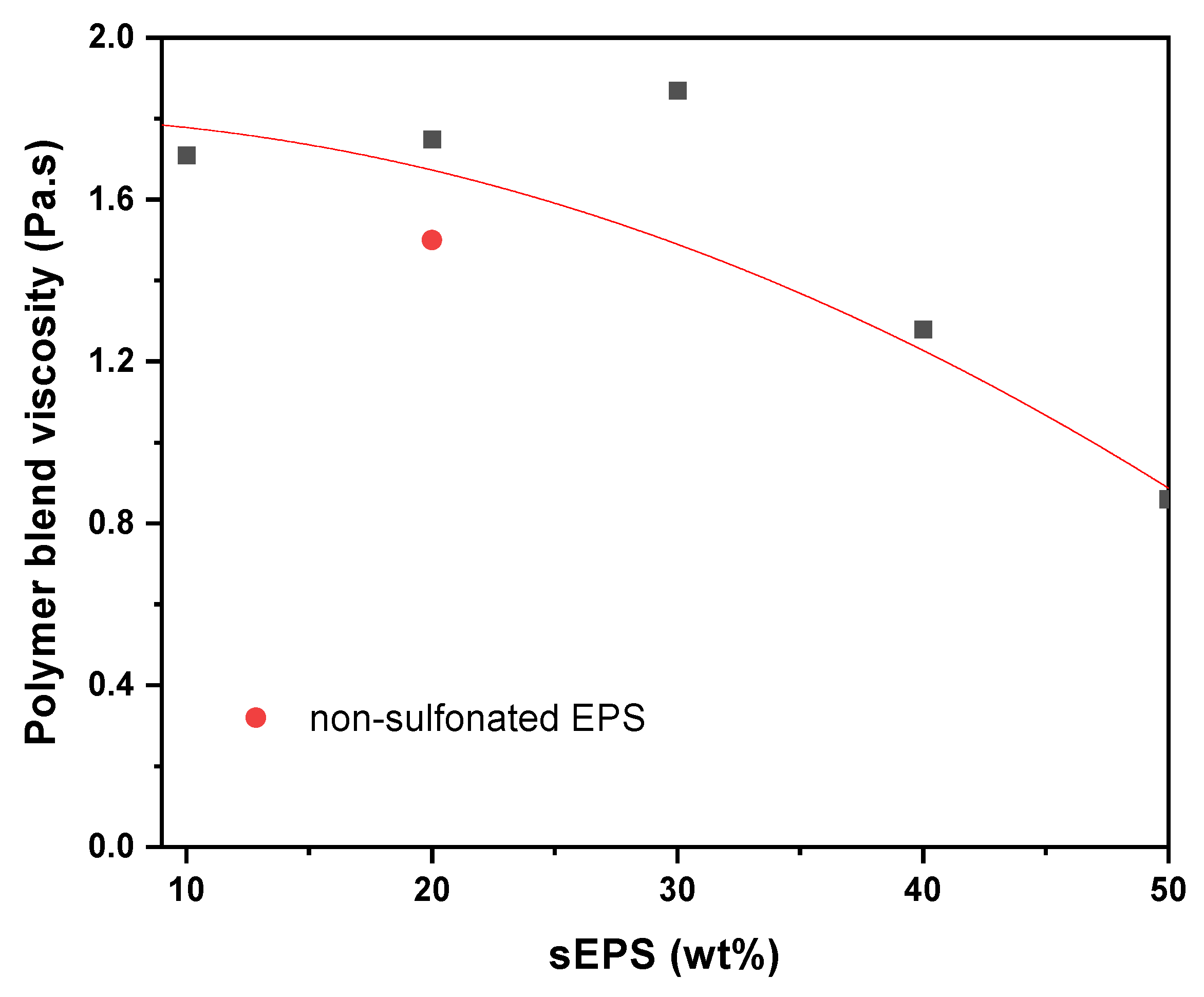
3.3. Dynamical Viscosity
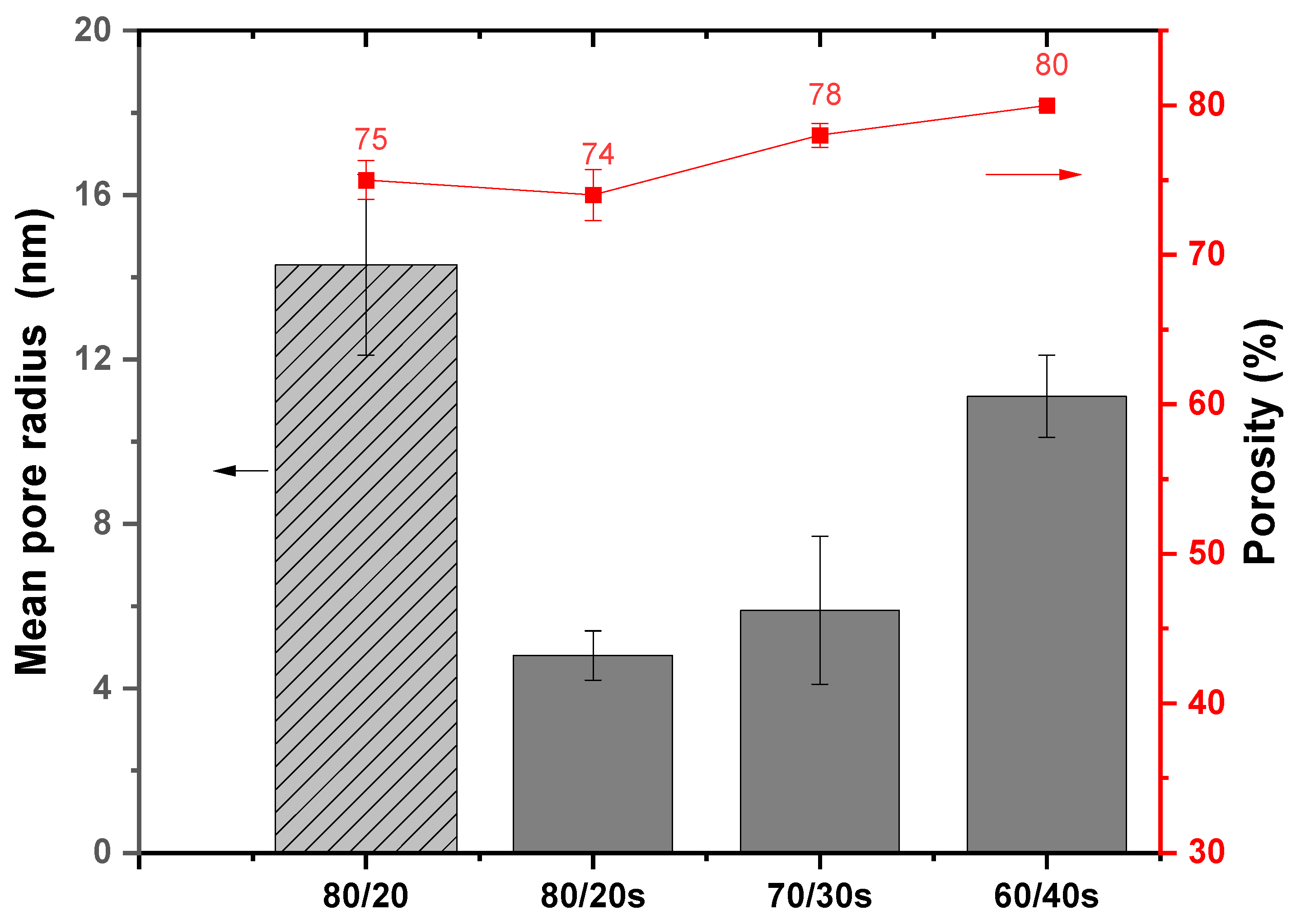
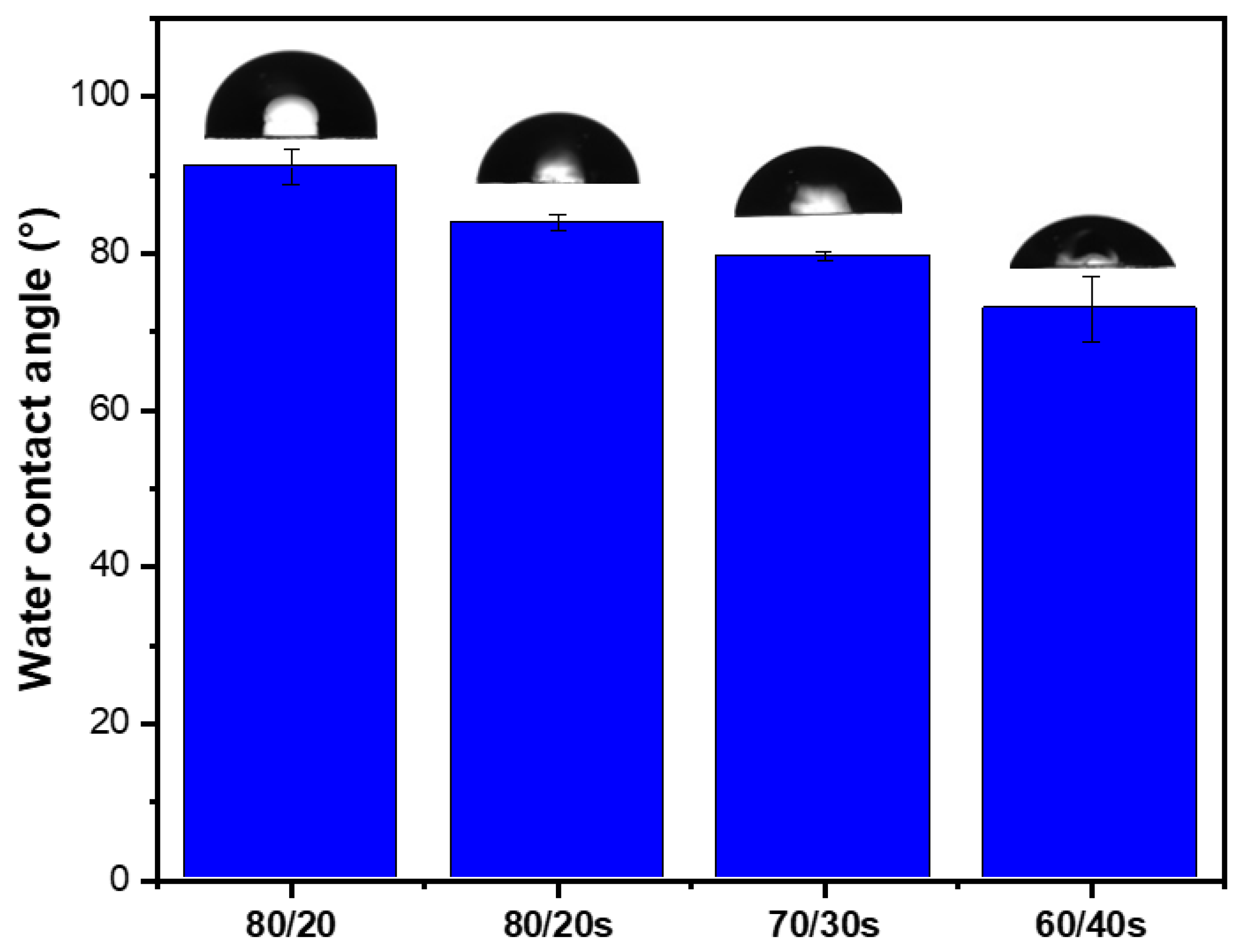
3.4. Mean Pore Size and Porosity
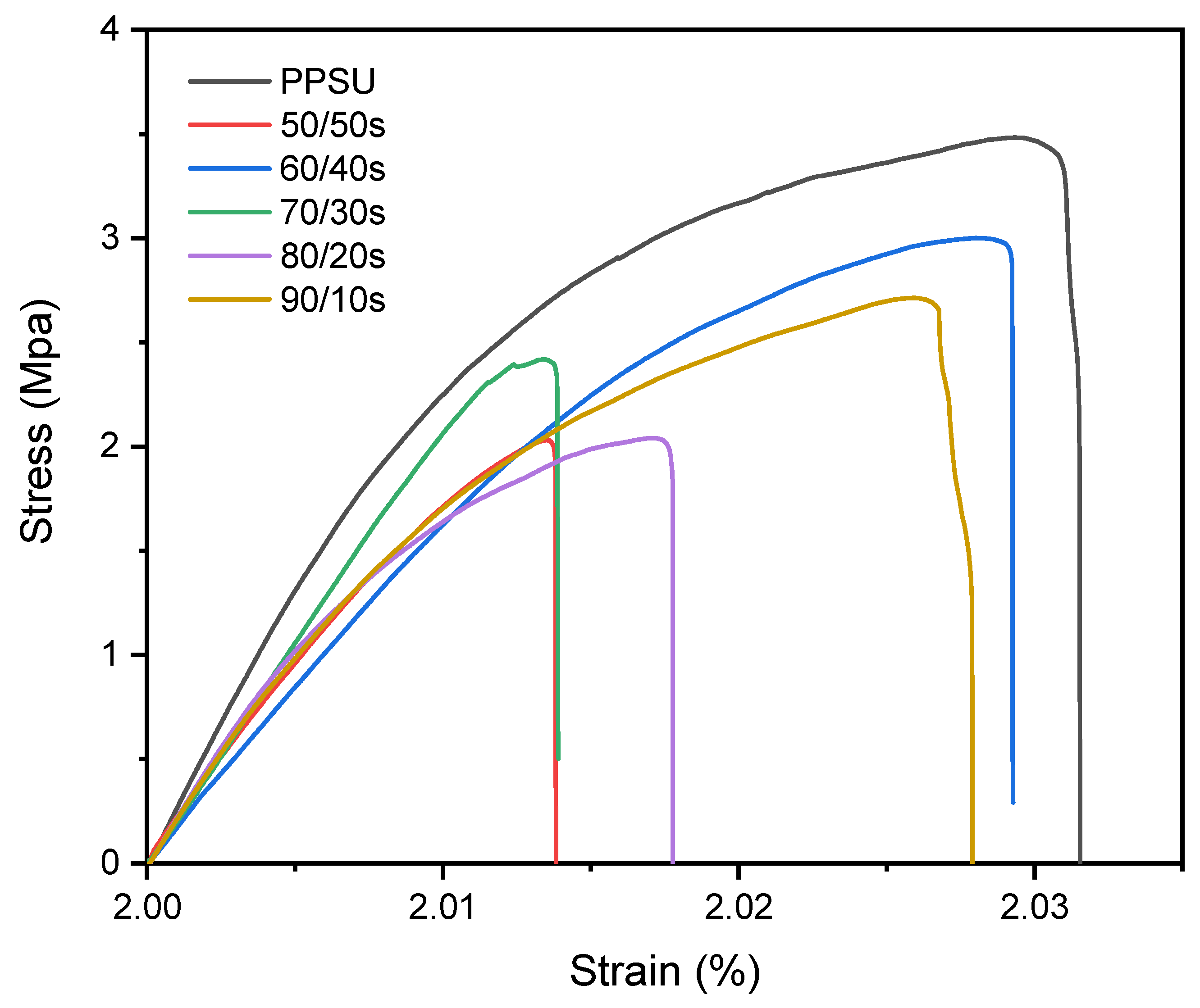
3.5. Mechanical Properties
3.6. Permeability and Flux Recovery Ratio (FRR %)
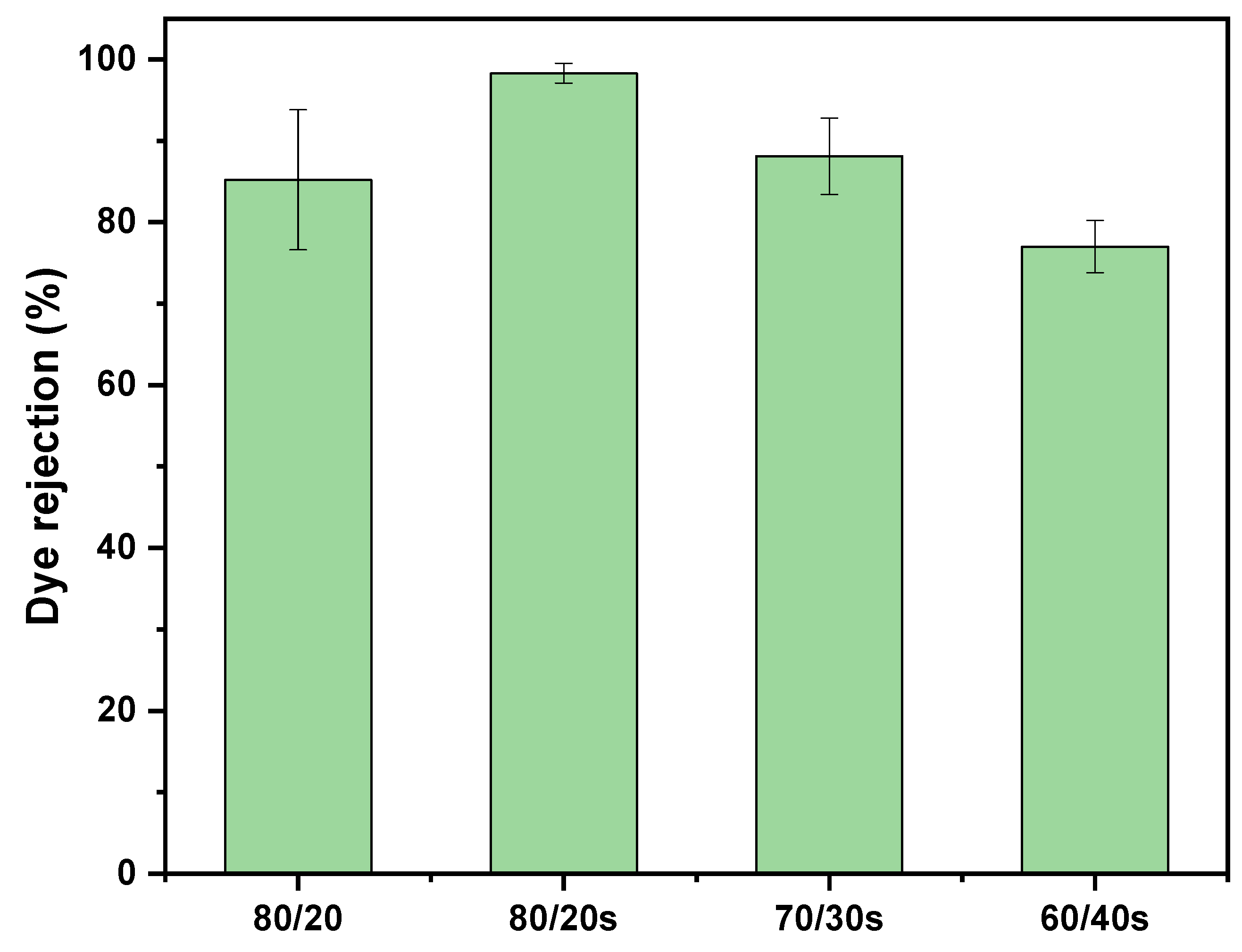
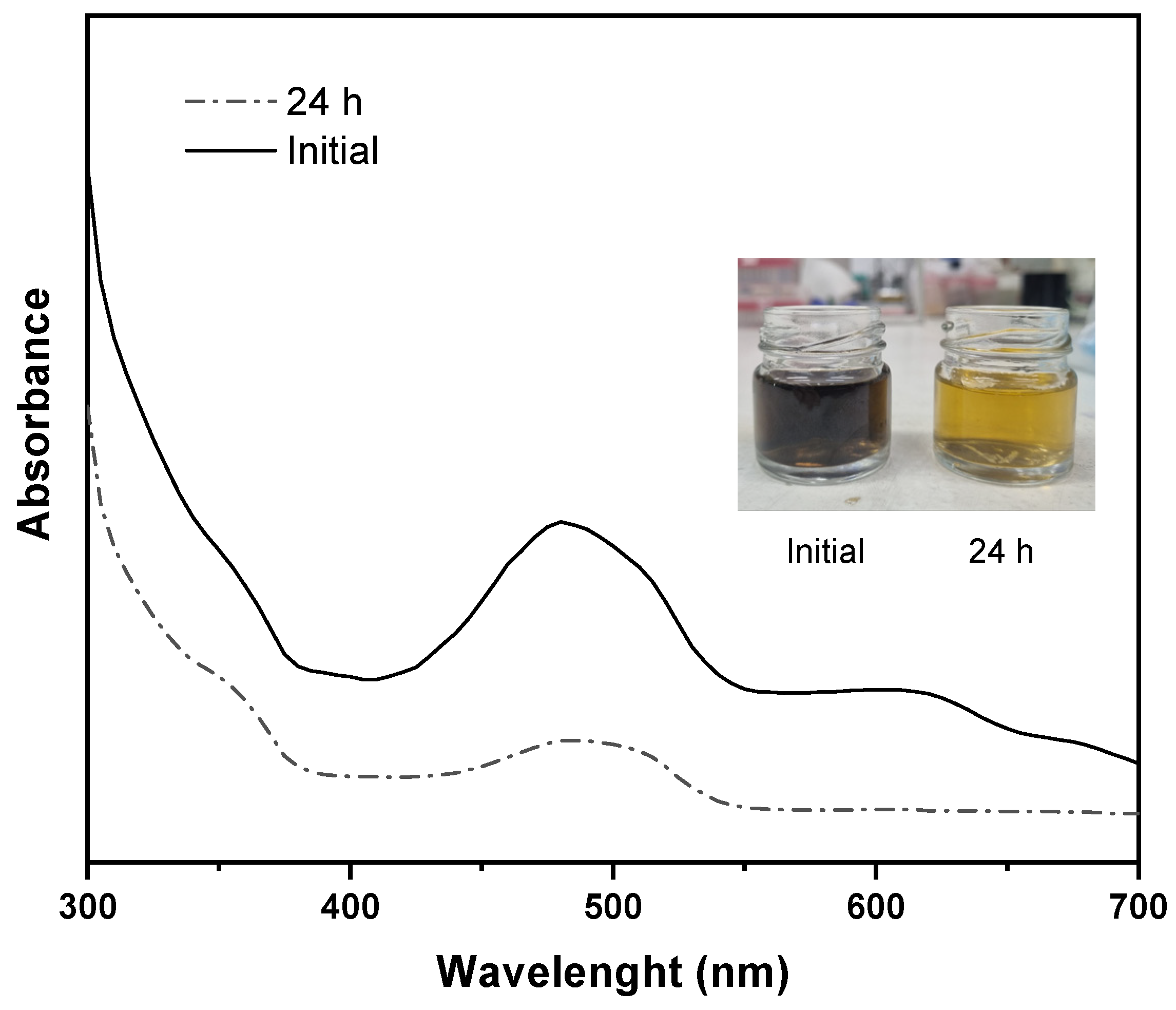
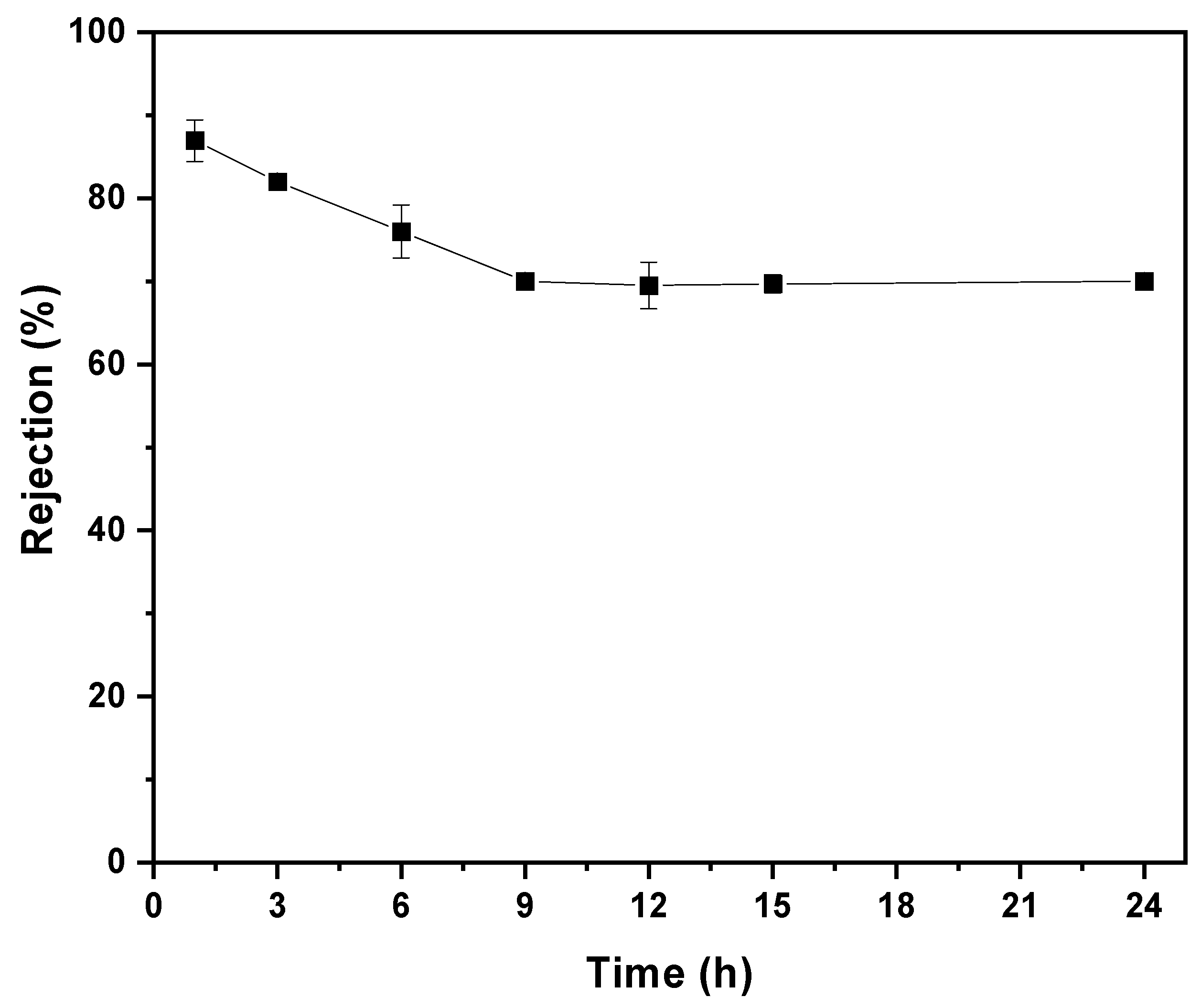
3.7. Membrane Dye Separation Performance
3.8. Energy Consumption and Sustainability of the sEPS Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dorigato, A. Recycling of polymer blends. Adv. Ind. Eng. Polym. Res. 2021, 4, 53–69. [Google Scholar] [CrossRef]
- Nguyen, Q.H.; Tran, A.T.K.; Hoang, N.T.T.; Tran, Y.T.H.; Nguyen, P.X.; Pham, T.T.; Nguyen, M.K.; Van der Bruggen, B. Plastic waste as a valuable resource: Strategy to remove heavy metals from wastewater in bench scale application. Environ. Sci. Pollut. Res. 2022, 29, 42074–42089. [Google Scholar] [CrossRef]
- Ruziwa, D.; Chaukura, N.; Gwenzi, W.; Pumure, I. Removal of Zn 2+ and Pb 2+ ions from aqueous solution using sulphonated waste polystyrene. J. Environ. Chem. Eng. 2015, 3, 2528–2537. [Google Scholar] [CrossRef]
- Chaukura, N.; Gwenzi, W.; Bunhu, T.; Ruziwa, D.T.; Pumure, I. Potential uses and value-added products derived from waste polystyrene in developing countries: A review. Resour. Conserv. Recycl. 2016, 107, 157–165. [Google Scholar] [CrossRef]
- Tran, A.T.; Pham, T.T.; Nguyen, Q.H.; Hoang, N.T.; Bui, D.T.; Nguyen, M.T.; Nguyen, M.K.; Van der Bruggen, B. From waste disposal to valuable material: Sulfonating polystyrene waste for heavy metal removal. J. Environ. Chem. Eng. 2020, 8, 104302. [Google Scholar] [CrossRef]
- Liu, S.-H.; Lu, C.-C.; Lin, C.-W.; Chang, S.-H. Rapid modification of waste expanded polystyrene with H2SO4/trace persulfate in one pot for effective adsorption of fluoroquinolone antibiotic and its regeneration. Chemosphere 2021, 271, 129529. [Google Scholar] [CrossRef]
- Ramos-Olmos, R.; Rogel-Hernández, E.; Flores-López, L.Z.; Lin, S.W.; Espinoza-Gómez, H. SYNTHESIS AND CHARACTERIZATION OF ASYMMETRIC ULTRAFILTRATION MEMBRANE MADE WITH RECYCLED POLYSTYRENE FOAM AND DIFFERENT ADDITIVES. J. Chil. Chem. Soc. 2008, 53, 1705–1708. [Google Scholar] [CrossRef]
- Ke, H.; Feldman, E.; Guzman, P.; Cole, J.; Wei, Q.; Chu, B.; Alkhudhiri, A.; Alrasheed, R.; Hsiao, B.S. Electrospun polystyrene nanofibrous membranes for direct contact membrane distillation. J. Membr. Sci. 2016, 515, 86–97. [Google Scholar] [CrossRef]
- Adamczak, M.; Kamińska, G.; Bohdziewicz, J. Application of Waste Polymers as Basic Material for Ultrafiltration Membranes Preparation. Water 2020, 12, 179. [Google Scholar] [CrossRef]
- Nurherdiana, S.D.; Wahyudi, B.; Stefanny, M.J.; Karlina, A.; Yogaswara, R.R.; Jalil, M.J.; Fansuri, H. CHARACTERISTICS OF STYROFOAM WASTE-BASED MEMBRANE THROUGH VAPOR AND LIQUID-INDUCED PHASE INVERSION PROCESS. J. Kim. Ris. 2023, 8, 37–48. [Google Scholar] [CrossRef]
- Arrosyid, B.H.; Zulfi, A.; Nur’AIni, S.; Hartati, S.; Rafryanto, A.F.; Noviyanto, A.; Hapidin, D.A.; Feriyanto, D.; Khairurrijal, K. High-Efficiency Water Filtration by Electrospun Expanded Polystyrene Waste Nanofibers. ACS Omega 2023, 8, 23664–23672. [Google Scholar] [CrossRef]
- Ghaly, S.T.; Noby, H.; Hayashi, J.; El-Shazly, A. Various waste polystyrene for useful membrane fabrication: Comparative experimental study. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Lima, F.d.A.; Chagas, P.A.M.; Honorato, A.C.S.; da Silva, E.N.; Aguiar, M.L.; Guerra, V.G. Multifactorial evaluation of an ultra-fast process for electrospinning of recycled expanded polystyrene to manufacture high-efficiency membranes for nanoparticle air filtration. J. Environ. Manag. 2024, 362, 121352. [Google Scholar] [CrossRef]
- A, S.N.; Alhelaly, A.; Saleh, N.J.; Alsalhy, Q. Effect of Alcohol as Additives on the Morphology and Separation Performance of Polyethersulfone (PES) Hollow Fiber Ultrafiltration Membranes. Eng. Technol. J. 2008, 26, 1263–1273. [Google Scholar] [CrossRef]
- Inoue, Y.; Okamoto, H. Estimation method to achieve desired mechanical properties with minimum virgin polymer in plastics recycling. Resour. Conserv. Recycl. 2024, 211, 107856. [Google Scholar] [CrossRef]
- Berradi, M.; Berradi, O.; Chellouli, M.; Hsissou, R.; El Bouchti, M.; El Gouri, M.; Sallek, B.; El Bachiri, A.; El Harfi, A. Optimization of the synthesis of ultrafiltration asymmetric membranes based on organic polymers. Results Eng. 2020, 6, 100116. [Google Scholar] [CrossRef]
- Alsalhy, Q.; Merza, A.; Rashid, K.; Adam, A.; Figoli, A.; Simone, S.; Drioli, E. Preparation and characterization of poly(vinyl chloride)/polystyrene/poly(ethylene glycol) hollow-fiber ultrafiltration membranes. J. Appl. Polym. Sci. 2013, 130, 989–1004. [Google Scholar] [CrossRef]
- Mansor, E.S.; Abdallah, H.; Shaban, A.M. Highly effective ultrafiltration membranes based on plastic waste for dye removal from water. Water Environ. Res. 2024, 96, e11018. [Google Scholar] [CrossRef] [PubMed]
- Šišková, A.O.; Peer, P.; Andicsová, A.E.; Jordanov, I.; Rychter, P. Circulatory Management of Polymer Waste: Recycling into Fine Fibers and Their Applications. Materials 2021, 14, 4694. [Google Scholar] [CrossRef]
- Bower, D.I. An Introduction to Polymer Physics; Cambridge University Press: New York, NY, USA, 2002. [Google Scholar] [CrossRef]
- Sawitri, N.; Ghufira; Fitriani, D.; Banon, C.; Triawan, D.A.; Gustian, I. Synthesis of proton-conducting membranes based on sulfonated polystyrene and bacterial cellulose. J. Phys. Conf. Ser. 2021, 1940, 012041. [Google Scholar] [CrossRef]
- Yao, J.; Wang, K.; Ren, M.; Liu, J.Z.; Wang, H. Phase inversion spinning of ultrafine hollow fiber membranes through a single orifice spinneret. J. Membr. Sci. 2012, 421-422, 8–14. [Google Scholar] [CrossRef]
- Suresh, R.; Antony, J.V.; Vengalil, R.; Kochimoolayil, G.E.; Joseph, R. Esterification of free fatty acids in non- edible oils using partially sulfonated polystyrene for biodiesel feedstock. Ind. Crop. Prod. 2017, 95, 66–74. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Kujawski, W.; Fatyeyeva, K. Fabrication of Polyamide-6 Membranes—The Effect of Gelation Time towards Their Morphological, Physical and Transport Properties. Membranes 2022, 12, 315. [Google Scholar] [CrossRef]
- Van Krevelen, D.W.; Te Nijenhuis, K. Chapter 7—Cohesive Properties and Solubility. In Properties of Polymers. Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contributions, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 189–227. [Google Scholar] [CrossRef]
- Othman, R.; Mun, G.; Sinnathamby, N.; Gopinanth, S.C.B.; Ekanem, E. Compatible organic and natural solvent mixture of synthesising biodegradable polymeric nanoparticles. J. Phys. Conf. Ser. 2021, 2080, 012028. [Google Scholar] [CrossRef]
- Maniruzzaman, M.; Pang, J.; Morgan, D.J.; Douroumis, D. Molecular Modeling as a Predictive Tool for the Development of Solid Dispersions. Mol. Pharm. 2015, 12, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Vatanpour, V.; Madaeni, S.S.; Moradian, R.; Zinadini, S.; Astinchap, B. Fabrication and characterization of novel antifouling nanofiltration membrane prepared from oxidized multiwalled carbon nanotube/polyethersulfone nanocomposite. J. Membr. Sci. 2011, 375, 284–294. [Google Scholar] [CrossRef]
- Arockiasamy, D.L.; Alhoshan, M.; Alam, J.; Muthumareeswaran; Figoli, A.; Kumar, S.A. Separation of proteins and antifouling properties of polyphenylsulfone based mixed matrix hollow fiber membranes. Sep. Purif. Technol. 2017, 174, 529–543. [Google Scholar] [CrossRef]
- Heu, R.; Ateia, M.; Yoshimura, C. Photocatalytic Nanofiltration Membrane Using Zr-MOF/GO Nanocomposite with High-Flux and Anti-Fouling Properties. Catalysts 2020, 10, 711. [Google Scholar] [CrossRef]
- Oulad, F.; Zinadini, S.; Zinatizadeh, A.A.; Derakhshan, A.A. Preparation and characterization of loose antifouling nanofiltration membrane using branched aniline oligomers grafted onto polyether sulfone and application for real algal dye removal. Chem. Eng. J. 2020, 401, 125861. [Google Scholar] [CrossRef]
- He, H.-L.; Liang, F.-X. Interfacial Engineering of Polymer Blend with Janus Particle as Compatibilizer. Chin. J. Polym. Sci. 2022, 41, 500–515. [Google Scholar] [CrossRef]
- Mural, P.K.S.; Madras, G.; Bose, S. Polymeric membranes derived from immiscible blends with hierarchical porous structures, tailored bio-interfaces and enhanced flux: Potential and key challenges. Nano-Structures Nano-Objects 2018, 14, 149–165. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, K.-H. Effect of PEG additive on membrane formation by phase inversion. J. Membr. Sci. 1998, 138, 153–163. [Google Scholar] [CrossRef]
- Im, S.; Park, C.; Cho, W.; Kim, J.; Jeong, M.; Kim, J.H. Synthesis of Solution-Stable PEDOT-Coated Sulfonated Polystyrene Copolymer PEDOT:P(SS-co-St) Particles for All-Organic NIR-Shielding Films. Coatings 2019, 9, 151. [Google Scholar] [CrossRef]
- Li, S.; Cui, Z.; Zhang, L.; He, B.; Li, J. The effect of sulfonated polysulfone on the compatibility and structure of polyethersulfone-based blend membranes. J. Membr. Sci. 2016, 513, 1–11. [Google Scholar] [CrossRef]
- Brown, S.B. Reactive Compatibilization. In Polymer Blends Handbook; Springer: Dordrecht, The Netherlands, 2014; pp. 517–675. [Google Scholar] [CrossRef]
- Peng, N.; Chung, T.-S.; Wang, K.Y. Macrovoid evolution and critical factors to form macrovoid-free hollow fiber membranes. J. Membr. Sci. 2008, 318, 363–372. [Google Scholar] [CrossRef]
- Johari, A.; Razmjouei, M.; Mansourizadeh, A.; Emadzadeh, D. Fabrication of blend hydrophilic polyamide imide (Torlon®)-sulfonated poly (ether ether ketone) hollow fiber membranes for oily wastewater treatment. Polym. Test. 2020, 91, 106733. [Google Scholar] [CrossRef]
- Feng, Y.; Han, G.; Zhang, L.; Chen, S.-B.; Chung, T.-S.; Weber, M.; Staudt, C.; Maletzko, C. Rheology and phase inversion behavior of polyphenylenesulfone (PPSU) and sulfonated PPSU for membrane formation. Polymer 2016, 99, 72–82. [Google Scholar] [CrossRef]
- Ollé, E.P.; Casals-Terré, J.; Martínez, J.A.L.; Farré-Lladós, J. Hansen Solubility Parameters (HSPs): A Reliable Tool for Assessing the Selectivity of Pristine and Hybrid Polymer Nanocomposites in the Presence of Volatile Organic Compounds (VOCs) Mixtures. Macromol. Mater. Eng. 2022, 308, 2200511. [Google Scholar] [CrossRef]
- Lin, C.-C. A Mathematical Model for Viscosity in Capillary Extrusion of Two-Component Polyblends. Polym. J. 1979, 11, 185–192. [Google Scholar] [CrossRef][Green Version]
- Okoroafor, E.; Villemaire, J.-P.; Agassant, J.-F. The viscosity of immiscible polymer blends: Influences of the interphase and deformability. Polymer 1992, 33, 5264–5271. [Google Scholar] [CrossRef]
- Hu, C.-H.; Weber, M.; Huang, Y.-H.; Lai, J.-Y.; Chung, T.-S. Investigating the impact of the sulfonation degree in sulfonated polyphenylsulfone (sPPSU) on PES/sPPSU polymer blend membranes. J. Membr. Sci. 2024, 705, 122890. [Google Scholar] [CrossRef]
- Turken, T.; Sengur-Tasdemir, R.; Ates-Genceli, E.; Tarabara, V.V.; Koyuncu, I. Progress on reinforced braided hollow fiber membranes in separation technologies: A review. J. Water Process. Eng. 2019, 32, 100938. [Google Scholar] [CrossRef]
- Koroteeva, L.I.; Khozina, E.N.; Korolev, P.A. Determination of Mechanical Strength of Hollow-Fiber Membranes to Optimize Technological Processes. Fibre Chem. 2021, 53, 20–24. [Google Scholar] [CrossRef]
- Fang, L.-F.; Yang, H.-Y.; Cheng, L.; Kato, N.; Jeon, S.; Takagi, R.; Matsuyama, H. Effect of Molecular Weight of Sulfonated Poly(ether sulfone) (SPES) on the Mechanical Strength and Antifouling Properties of Poly(ether sulfone)/SPES Blend Membranes. Ind. Eng. Chem. Res. 2017, 56, 11302–11311. [Google Scholar] [CrossRef]
- Xix-Rodriguez, C.; Varguez-Catzim, P.; Alonzo-García, A.; Rodriguez-Fuentes, N.; Vázquez-Torres, H.; González-Diaz, A.; Aguilar-Vega, M.; González-Díaz, M.O. Amphiphilic poly(lactic acid) membranes with low fouling and enhanced hemodiafiltration. Sep. Purif. Technol. 2021, 259, 118124. [Google Scholar] [CrossRef]
- Abdelhamid, A.E.; Selim, S.E.; Meligi, G.A.; Hussain, A.I.; Mabrouk, M.A. Antifouling ultrafiltration membranes based on acrylic fibers waste/nanochitosan for Congo red and crystal violet removal. Waste Dispos. Sustain. Energy 2024, 6, 511–527. [Google Scholar] [CrossRef]
- Al-Nahari, A.; Li, S.; Su, B. Negatively charged nanofiltration membrane with high performance via the synergetic effect of benzidinedisulfonic acid and trimethylamine during interfacial polymerization. Sep. Purif. Technol. 2022, 291, 120947. [Google Scholar] [CrossRef]
- Doblhoff-Dier, K.; Koper, M.T.M. Modeling the Gouy–Chapman Diffuse Capacitance with Attractive Ion–Surface Interaction. J. Phys. Chem. C 2021, 125, 16664–16673. [Google Scholar] [CrossRef]
- Nakao, A.; Suzuki, Y.; Iwaki, M. Water Wettability and Zeta-Potential of Polystyrene Surface Modified by Ne or Na Implantation. J. Colloid Interface Sci. 1998, 197, 257–262. [Google Scholar] [CrossRef]
- Ye, W.; Lin, J.; Borrego, R.; Chen, D.; Sotto, A.; Luis, P.; Liu, M.; Zhao, S.; Tang, C.Y.; Van der Bruggen, B. Advanced desalination of dye/NaCl mixtures by a loose nanofiltration membrane for digital ink-jet printing. Sep. Purif. Technol. 2018, 197, 27–35. [Google Scholar] [CrossRef]
- Meevasana, K.; Pavasant, P. Quantitative measurement techniques for binary dye mixtures: A case study in an adsorption system. Sci. Asia 2008, 34, 390–394. [Google Scholar] [CrossRef]
- Thong, Z.; Gao, J.; Lim, J.X.Z.; Wang, K.-Y.; Chung, T.-S. Fabrication of loose outer-selective nanofiltration (NF) polyethersulfone (PES) hollow fibers via single-step spinning process for dye removal. Sep. Purif. Technol. 2018, 192, 483–490. [Google Scholar] [CrossRef]
- Nayak, M.C.; Isloor, A.M.; Moslehyani, A.; Ismail, N.; Ismail, A. Fabrication of novel PPSU/ZSM-5 ultrafiltration hollow fiber membranes for separation of proteins and hazardous reactive dyes. J. Taiwan Inst. Chem. Eng. 2018, 82, 342–350. [Google Scholar] [CrossRef]
- Kolangare, I.M.; Isloor, A.M.; Karim, Z.A.; Kulal, A.; Ismail, A.F.; Inamuddin; Asiri, A.M. Antibiofouling hollow-fiber membranes for dye rejection by embedding chitosan and silver-loaded chitosan nanoparticles. Environ. Chem. Lett. 2018, 17, 581–587. [Google Scholar] [CrossRef]
- Senol-Arslan, D.; Gul, A.; Dizge, N.; Ocakoglu, K.; Uzal, N. The different impacts of g-C3N4 nanosheets on PVDF and PSF ultrafiltration membranes for Remazol black 5 dye rejection. J. Appl. Polym. Sci. 2023, 140, 54514. [Google Scholar] [CrossRef]
- Yao, L.; Zhang, L.; Wang, R.; Chou, S.; Dong, Z. A new integrated approach for dye removal from wastewater by polyoxometalates functionalized membranes. J. Hazard. Mater. 2016, 301, 462–470. [Google Scholar] [CrossRef]
- Maurya, S.; Parashuram, K.; Singh, P.; Ray, P.; Reddy, A. Preparation of polysulfone–polyamide thin film composite hollow fiber nanofiltration membranes and their performance in the treatment of aqueous dye solutions. Desalination 2012, 304, 11–19. [Google Scholar] [CrossRef]
- Semiz, L. Removal of reactive black 5 from wastewater by membrane filtration. Polym. Bull. 2019, 77, 3047–3059. [Google Scholar] [CrossRef]
- Marczak, H. Energy Inputs on the Production of Plastic Products. J. Ecol. Eng. 2022, 23, 146–156. [Google Scholar] [CrossRef]
- Nicholson, S.R.; Rorrer, N.A.; Carpenter, A.C.; Beckham, G.T. Manufacturing energy and greenhouse gas emissions associated with plastics consumption. Joule 2021, 5, 673–686. [Google Scholar] [CrossRef]
- Bachmann, M.; Marxen, A.; Schomäcker, R.; Bardow, A. High performance, but low cost and environmental impact? Integrated techno-economic and life cycle assessment of polyoxazolidinone as a novel high-performance polymer. Green Chem. 2022, 24, 9143–9156. [Google Scholar] [CrossRef]



| HFM | PPSU (g) | sEPS (g) | Viscosity (Pa.s) |
|---|---|---|---|
| 90/10s | 4.5 | 0.5 | 1.71 ± 0.08 |
| 80/20s | 4.0 | 1.0 | 1.69 ± 0.05 |
| 80/20 * | 4.0 | 1.0 | 1.50 ± 0.04 |
| 70/30s | 3.5 | 1.5 | 1.87 ± 0.05 |
| 60/40s | 3.0 | 2.0 | 1.28 ± 0.10 |
| 50/50s | 2.5 | 2.5 | 0.86 ± 0.09 |
| Spinning Parameters | Operating Conditions |
|---|---|
| Dope flow rate (mL/min) | 2.8 |
| Bore fluid flow rate (mL/min) | 0.8 |
| Bore fluid | Deionized water |
| External coagulant | Tap water |
| External coagulant temperature | 23 °C |
| Dope temperature | 23 °C |
| Air gap distance (cm) | 3.5 |
| Concentration of dope solution (%) | 18 |
| Spinneret outer/inner diameter of needle (mm) | 1.6/0.337 |
| Polymer | δv | Ra(v) (MPa1/2) | |||
|---|---|---|---|---|---|
| EPS | 20.2 | 1.2 | 0 | 1.2 | 10.7 |
| sEPS | 20.1 | 3.8 | 4.2 | 5.7 | 4.7 |
| PPSU | 18.7 | 5 | 7.4 | 9.0 | - |
| HFM | E (MPa) | TS (MPa) | ε (%) |
|---|---|---|---|
| PPSU | 547.3 ± 38.96 | 4.04 ± 0.67 | 2.04 ± 0.01 |
| 90/10s | 243.7 ± 13.26 | 3.19 ± 0.53 | 2.03 ± 0.01 |
| 80/20 * | 183.0 ± 5.67 | 2.52 ± 0.18 | 2.04 ± 0.01 |
| 80/20s | 241.2 ± 11.64 | 2.98 ± 0.71 | 2.05 ± 0.02 |
| 70/30s | 235.9 ± 21.87 | 2.96 ± 0.99 | 2.04 ± 0.02 |
| 60/40s | 234.5 ± 27.24 | 3.66 ± 1.27 | 2.02 ± 0.01 |
| 50/50s | 207.2 ± 23.83 | 3.34 ± 0.42 | 2.03 ± 0.01 |
| Membrane | BSA Solution | XG Solution | ||||
|---|---|---|---|---|---|---|
| Water Flux Jw1 (L m−2 h−1) | Flux Recovery Ratio (FRR%) | Flux Recovery Ratio (FRR%) | ||||
| Pressure (bar) | 2 | 3 | 2 | 3 | 2 | 3 |
| 80/20 | 31.0 ± 8.6 | - | 47.2 ± 5.6 | - | 69.8 ± 9.5 | - |
| 80/20s | 4.7 ± 1.2 | 6.9 ± 0.8 | 79.9 ± 4.9 | 80.7 ± 2.0 | 91.9 ± 1.1 | 66.8 ± 2.7 |
| 70/30s | 6.4 ± 2.9 | 24.1 ± 7.1 | 78.1 ± 0.5 | 64.2 ± 6.7 | 99.3 ± 0.2 | 76.1 ± 13 |
| 60/40s | 21.2 ± 3.4 | - | 80.9 ± 6.0 | - | 74.8 ± 16 | - |
| Membrane | Dye Concentration (ppm) | RB5 Rejection (%) | PWP (L m−2 h−1) | P (Bar) | Ref. |
|---|---|---|---|---|---|
| POMs-M | 20 | 100 | 19 | 0.5 | [59] |
| Polysulfone-polyamide | 500–2000 | 60–97 | 0.0059–0.0206 | 1.7 | [60] |
| PAN(92)-co-P2EHA(8) | 100 | 45.1 | 85.8 | 2 | [61] |
| PES | 50 | 96.7 | 66 | 5 | [55] |
| PPSU/ZSM-5 | 100 | 90.8 | 113.9 | 3 | [56] |
| HF 0.30 | 10 | 89.2 | 96.8 | 2 | [57] |
| PZ-0 (PPSU/2%PVP) | 100 | 58 | 26.2 | 4 | [58] |
| PSF | 40 | <60 | 26.8 | 25 | [56] |
| 80/20s | 50 | 98.3 | 4.7 | 2 | This work |
| 70/30s | 50 | 88.1 | 6.4 | 2 | This work |
| 60/40s | 50 | 77.0 | 21.2 | 2 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huhn-Ibarra, M.; Itza-Uitzil, L.M.; Yam-Cervantes, M.; González-Díaz, A.; Zapata-Catzin, F.J.; Cauich-Cupul, J.I.; Aguilar-Vega, M.; González-Díaz, M.O. Recycled Polystyrene as a Sustainable Material for Hollow Fiber Membranes in Dye Filtration. Membranes 2025, 15, 285. https://doi.org/10.3390/membranes15100285
Huhn-Ibarra M, Itza-Uitzil LM, Yam-Cervantes M, González-Díaz A, Zapata-Catzin FJ, Cauich-Cupul JI, Aguilar-Vega M, González-Díaz MO. Recycled Polystyrene as a Sustainable Material for Hollow Fiber Membranes in Dye Filtration. Membranes. 2025; 15(10):285. https://doi.org/10.3390/membranes15100285
Chicago/Turabian StyleHuhn-Ibarra, Mauricio, Libia Madai Itza-Uitzil, Marcial Yam-Cervantes, Abigail González-Díaz, Fernando José Zapata-Catzin, Javier Ivan Cauich-Cupul, Manuel Aguilar-Vega, and Maria Ortencia González-Díaz. 2025. "Recycled Polystyrene as a Sustainable Material for Hollow Fiber Membranes in Dye Filtration" Membranes 15, no. 10: 285. https://doi.org/10.3390/membranes15100285
APA StyleHuhn-Ibarra, M., Itza-Uitzil, L. M., Yam-Cervantes, M., González-Díaz, A., Zapata-Catzin, F. J., Cauich-Cupul, J. I., Aguilar-Vega, M., & González-Díaz, M. O. (2025). Recycled Polystyrene as a Sustainable Material for Hollow Fiber Membranes in Dye Filtration. Membranes, 15(10), 285. https://doi.org/10.3390/membranes15100285







