Hydrogen (H2)/Toluene (TOL) Separation via One and Two Stages of the Bis(triethoxysily)ethane (BTESE) Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of BTESE-Derived Sols and Membranes
2.2. Single-Gas Permeation (SGP)
2.3. H2/TOL Separation Test
3. Results and Discussion
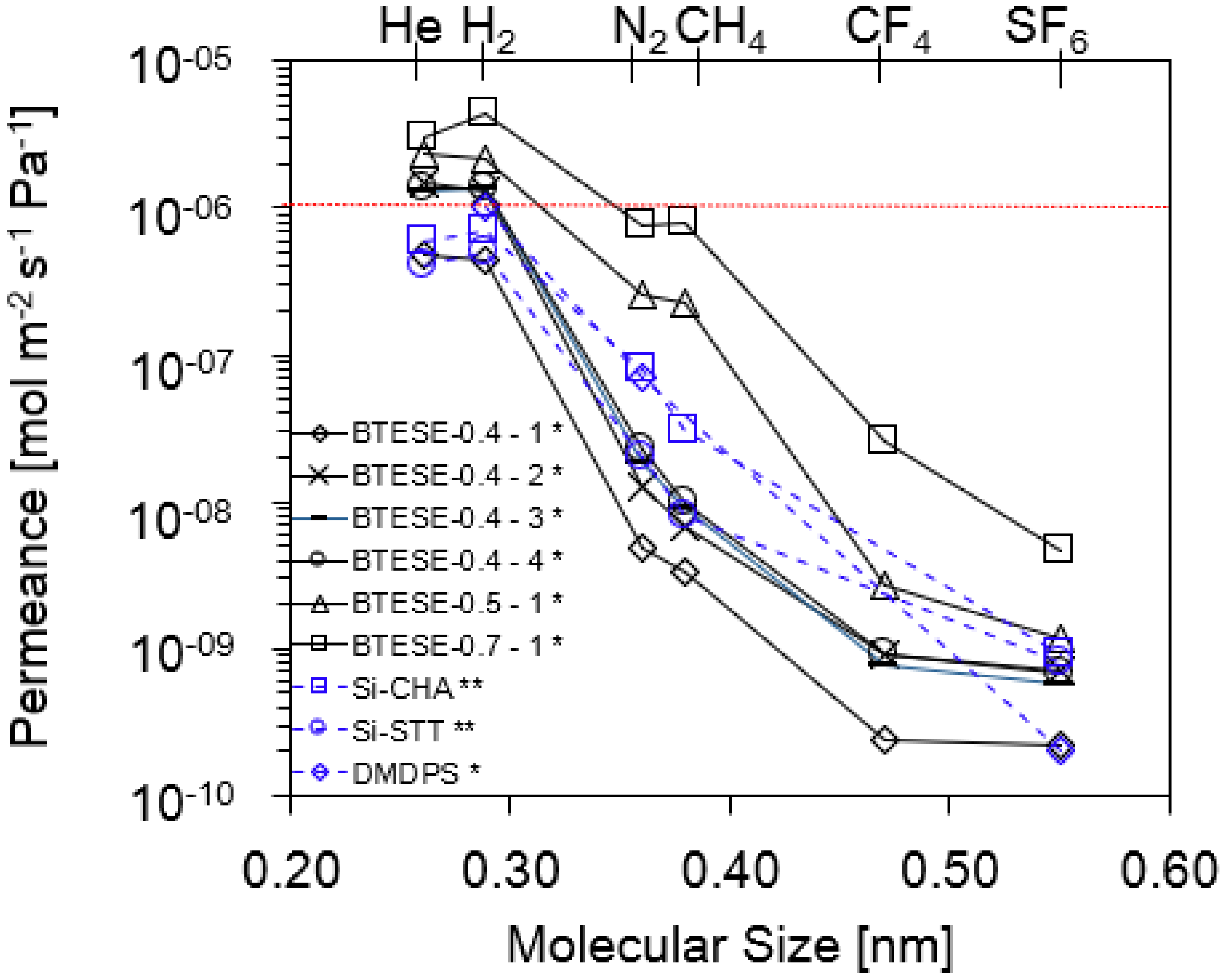
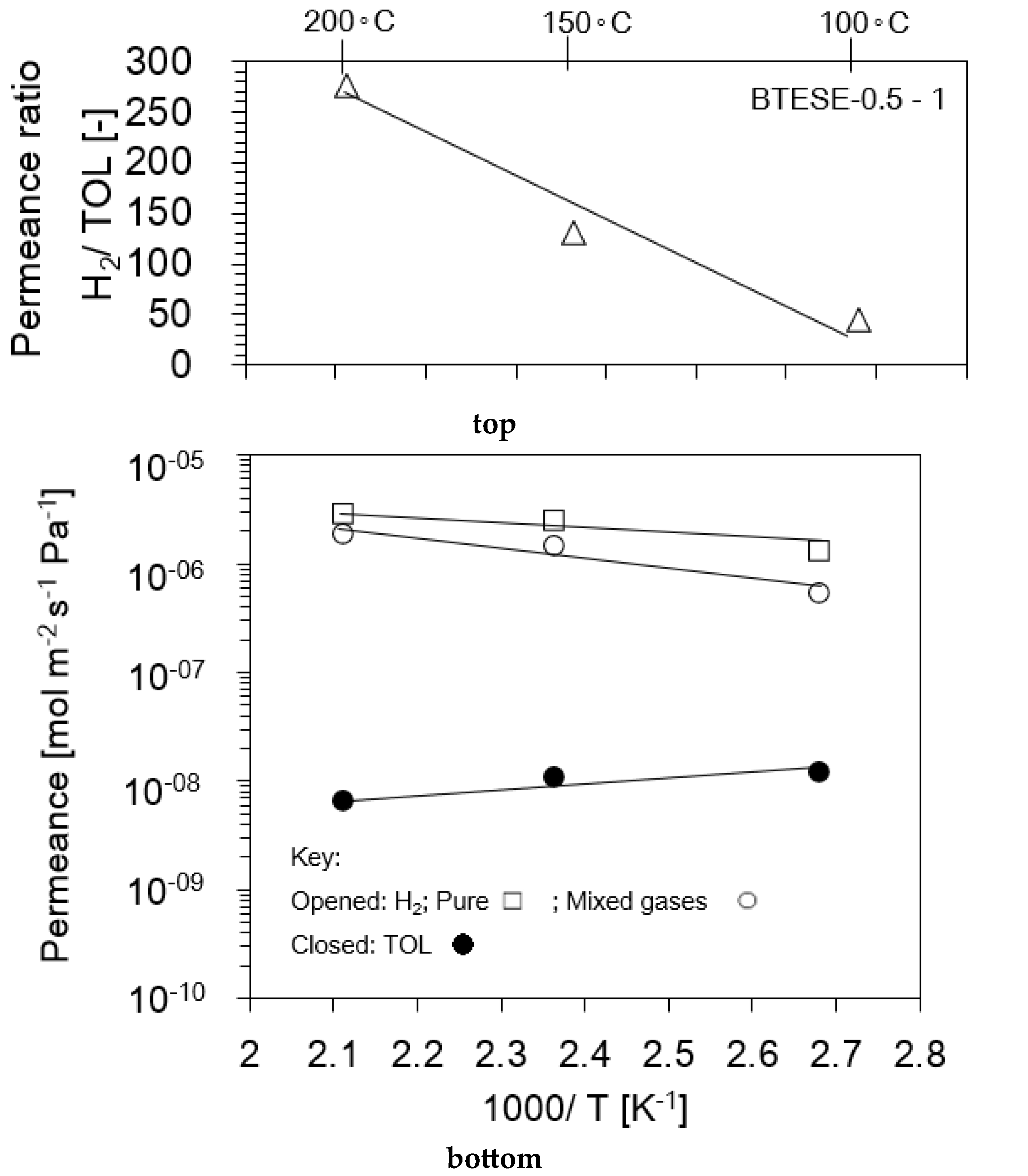
3.1. Single-Gas Permeation (SGP) and Pore Size Evaluation
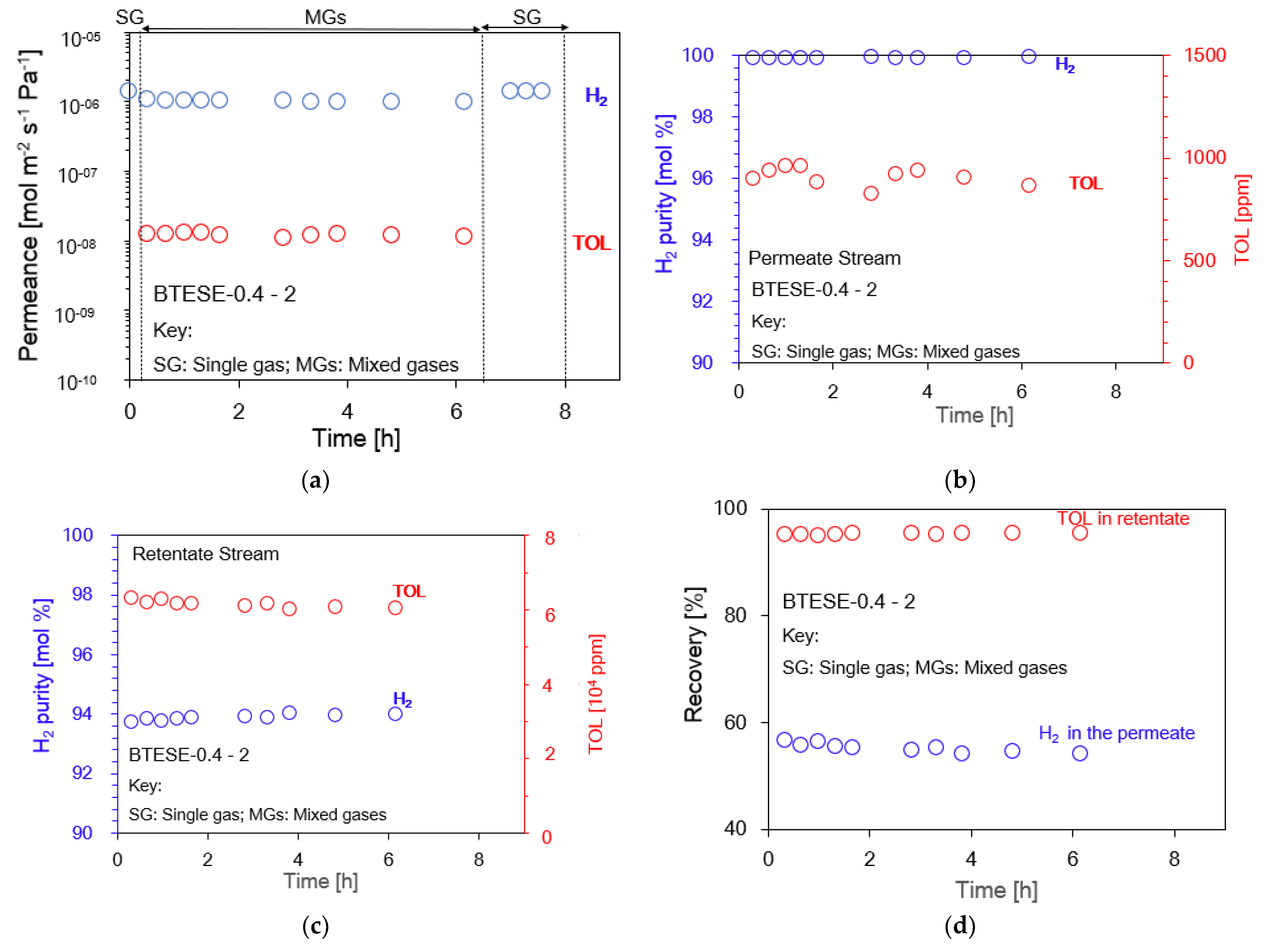
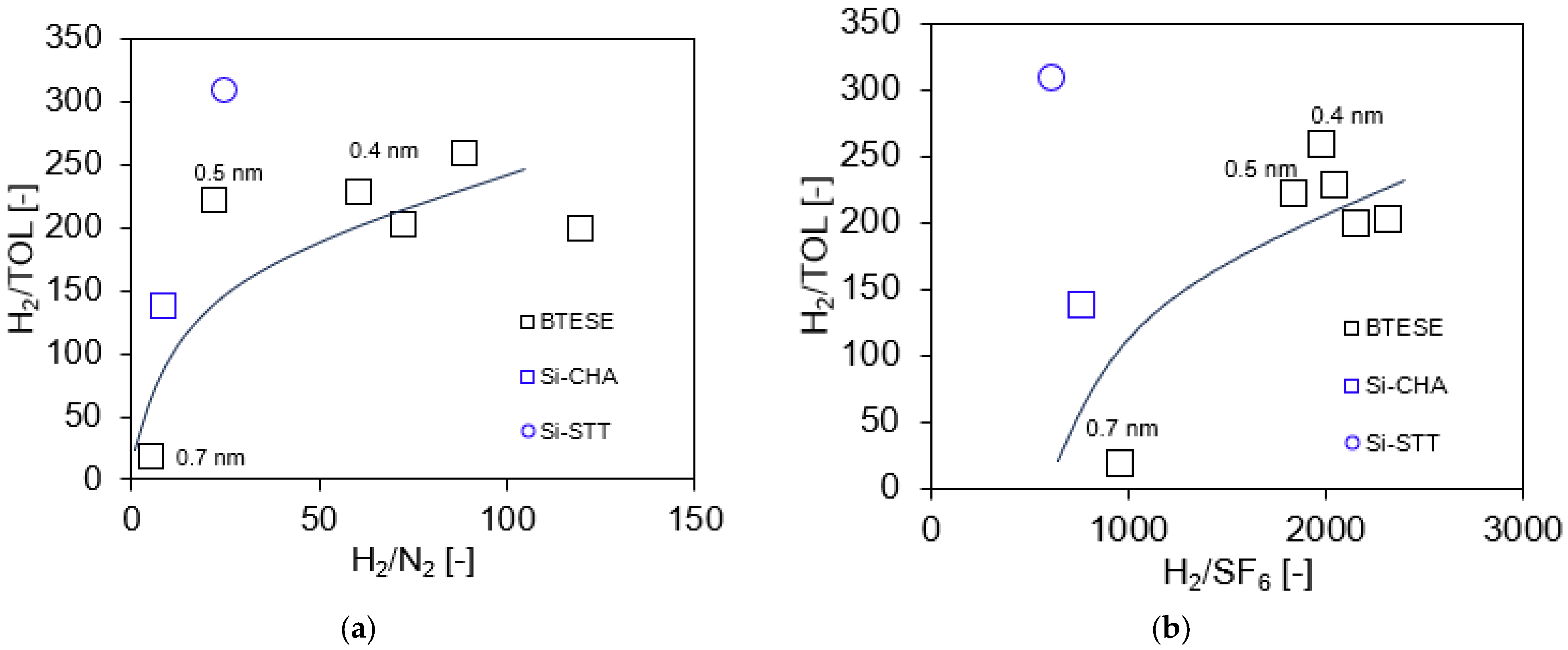
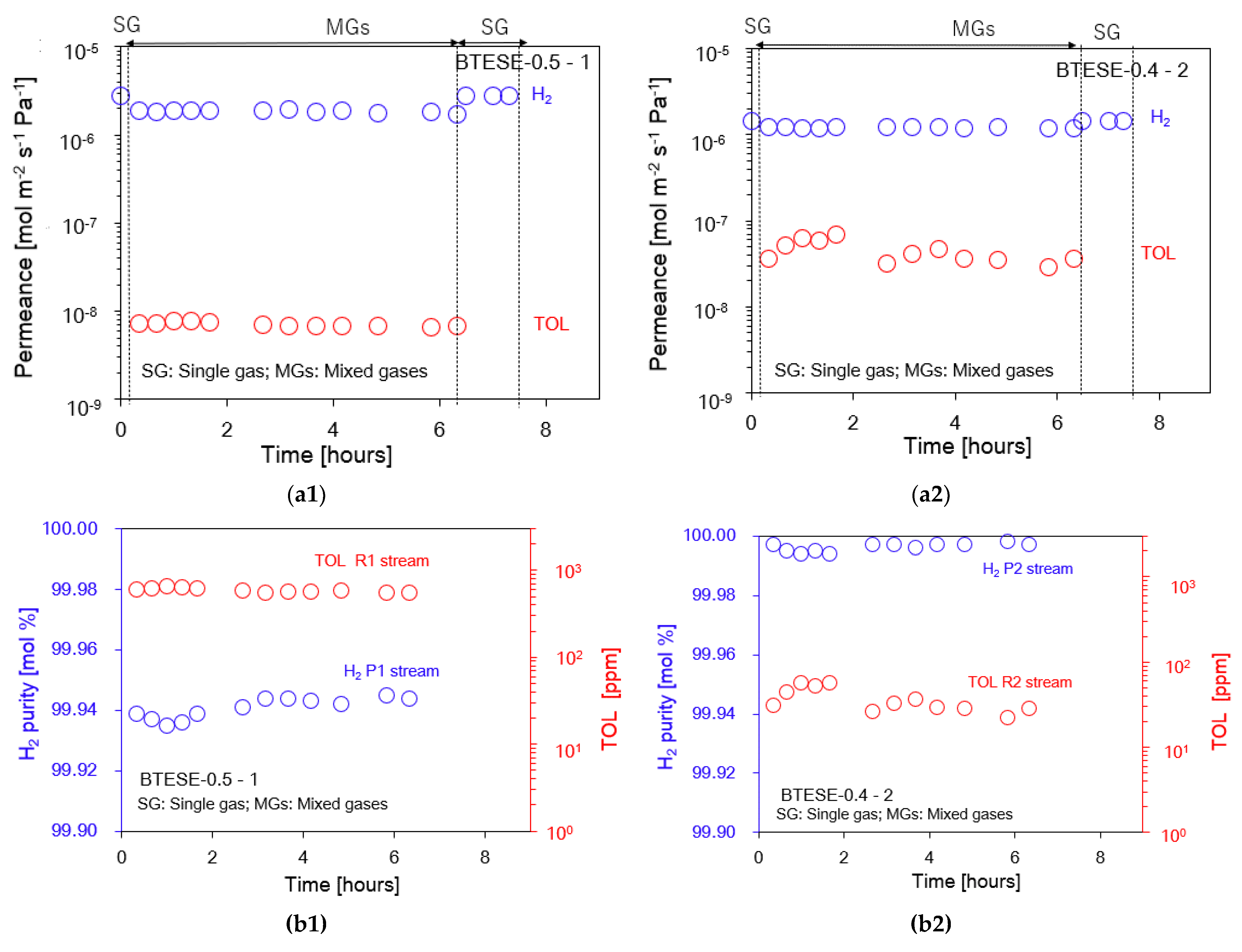
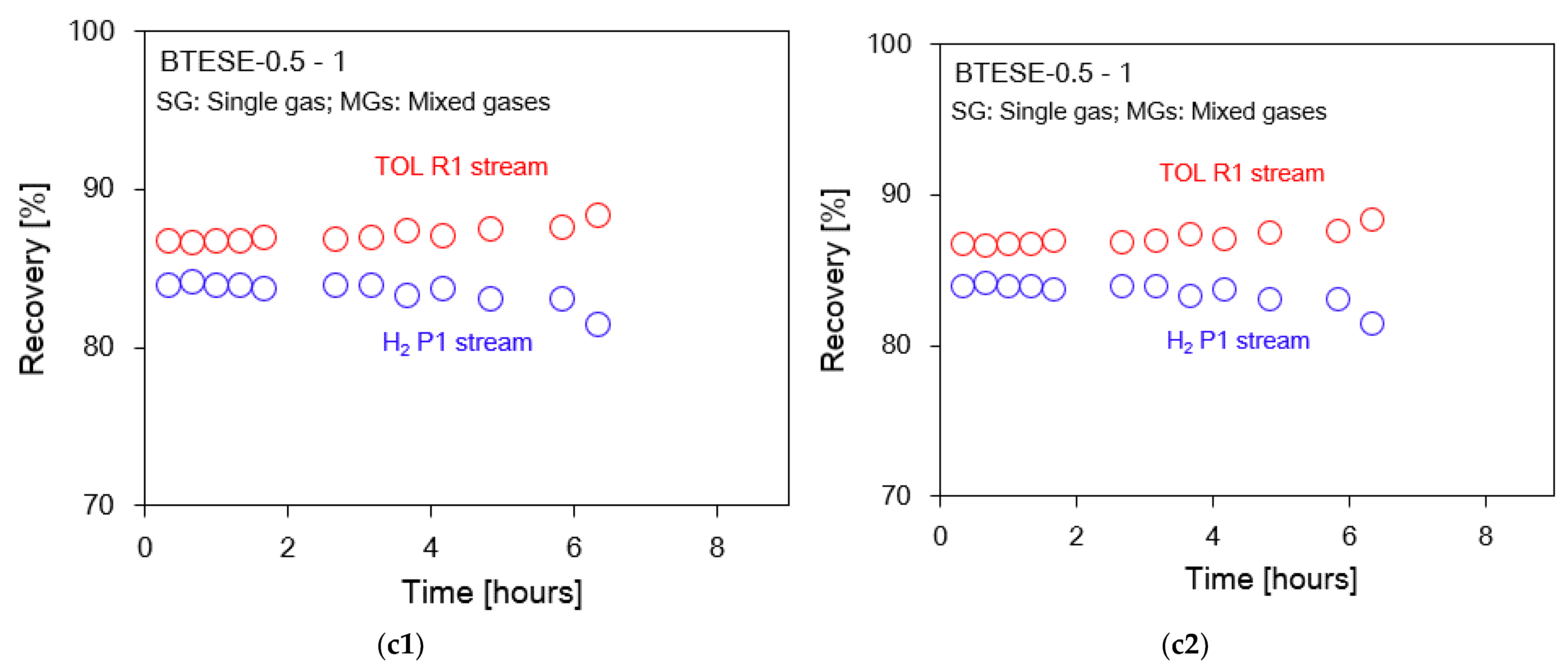
3.2. Hydrogen (H2)/Toluene (TOL) Binary Separation in a One-Stage Membrane Configuration System
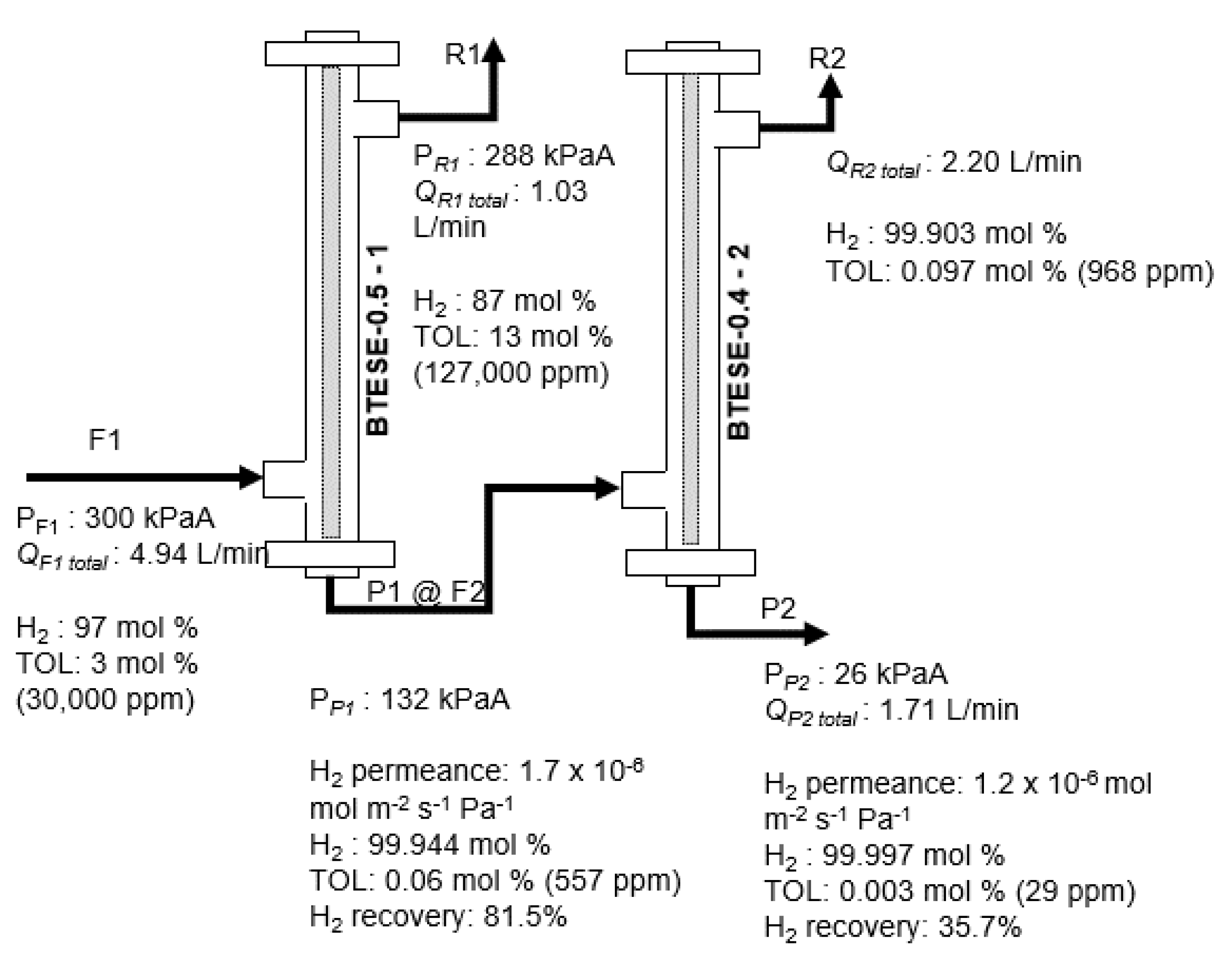
3.3. Hydrogen (H2)/Toluene (TOL) Binary Separation in a Two-Stage Membrane Configuration System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EU. Hydrogen and Fuel Cells—A Vision of Our Future. High Level Group Draft Report v4.8. Publications Office. 2003. Available online: https://inis.iaea.org/search/search.aspx?orig_q=RN:37121708 (accessed on 22 July 2024).
- Levin, D.B.; Chahine, R. Challenges for renewable hydrogen production from biomass. Int. J. Hydrogen Energy 2010, 35, 4962–4969. [Google Scholar] [CrossRef]
- Rambhujun, N.; Salman, M.S.; Wang, T.; Pratthana, C.; Sapkota, P.; Costalin, M.; Lai, Q.; Aguey-Zinsou, K.F. Renewable hydrogen for the chemical industry. MRS Energy Sustain. 2020, 7, E33. [Google Scholar] [CrossRef] [PubMed]
- IEA. The Future of Hydrogen. 2019. Available online: https://www.iea.org/reports/the-future-of-hydrogen (accessed on 22 July 2024).
- Giuli, M. Geopolitics of the Energy Transition. In Handbook of Energy Transitions; CRC Press: Boca Raton, FL, USA, 2022; pp. 41–59. [Google Scholar]
- Liu, C.; Zhang, X.; Zhai, J.; Li, X.; Guo, X.; He, G. Research progress and prospects on hydrogen separation membranes. Clean Energy 2023, 7, 217–241. [Google Scholar] [CrossRef]
- Bernado, G.; Arau’jo, T.; da Silva Lopes, T.; Sousa, J.; Mendes, A. Recent advances in membrane technologies for hydrogen purification. Int. J. Hydrogen Energy 2020, 45, 7313–7338. [Google Scholar] [CrossRef]
- Adhikari, S.; Fernando, S. Hydrogen Membrane Separation Techniques. Ind. Eng. Chem. Res. 2006, 45, 875–881. [Google Scholar] [CrossRef]
- Freeman, B.D.; Pinnau, I. Gas and liquid separations using membranes: An overview. In Advanced Materials for Membrane Separations; Pinnau, I., Freeman, B.D., Eds.; ACS Symposium Series; ACS: Washington, DC, USA, 2004; Volume 876, pp. 1–21. [Google Scholar]
- Kayı, H.; Kaya, P.; Kurt, T.; Mirza, E.S.; Topuz, B. Ti-substituted organosilica membranes for H2 sieving: Sol-gel and DFT insights. Int. J. Hydrogen Energy 2024, 65, 496–504. [Google Scholar] [CrossRef]
- Moriyama, N.; Nagasawa, H.; Kanezashi, M.; Tsuru, T. Improved performance of organosilica membranes for steam recovery at moderate-to-high temperatures via the use of a hydrothermally stable intermediate layer. J. Membr. Sci. 2021, 620, 118895. [Google Scholar] [CrossRef]
- Sawamura, K.; Okamoto, S.; Todokoro, Y. Development of Mass Production Technology of Highly Permeable Nano-Porous 534 Supports for Silica-Based Separation Membranes. Membranes 2019, 9, 103. [Google Scholar] [CrossRef]
- Ibrahim, S.M.; Sawamura, K.; Mishina, K.; Yu, X.; Salak, F.; Miyata, S.; Moriyama, N.; Nagasawa, H.; Kanezashi, M.; Tsuru, T. Bis(triethoxysilyl)ethane (BTESE)–Organosilica Membranes for H2O/DMF Separation in Reverse Osmosis (RO): Evaluation and Correlation of Subnanopores via Nanopermporometry (NPP), Modified Gas Translation (mGT) and RO Performance. Membranes 2024, 14, 8. [Google Scholar] [CrossRef]
- Niimi, T.; Nagasawa, H.; Kanezashi, M.; Yoshioka, T.; Ito, K.; Tsuru, T. Preparation of BTESE-derived organosilica membranes for catalytic membrane reactors of methylcyclohexane dehydrogenation. J. Membr. Sci. 2014, 455, 375–383. [Google Scholar] [CrossRef]
- Raza, W.; Yang, J.; Wang, J.; Saulat, H.; He, G.; Lu, J.; Zhang, Y. HCl modification and pervaporation performance of BTESE membrane for the dehydration of acetic acid/water mixture. Sep. Purif. Technol. 2020, 235, 116102. [Google Scholar] [CrossRef]
- Ibrahim, S.M.; Nagasawa, H.; Kanezashi, M.; Tsuru, T. Organosilica bis(triethoxysilyl)ethane (BTESE) membranes for gas permeation (GS) and reverse osmosis (RO): The effect of preparation conditions on structure, and the correlation between gas and liquid permeation properties. J. Membr. Sci. 2017, 526, 242–251. [Google Scholar] [CrossRef]
- Xu, R.; Liu, Q.; Ren, X.; Lin, P.; Zhong, J. Tuning the Pore Structures of Organosilica Membranes for Enhanced Desalination Performance via the Control of Calcination Temperatures. Membranes 2020, 10, 392. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Wei, Y.; Qi, H. Tailoring pore structures to improve the permselectivity of organosilica membranes by tuning calcination parameters. J. Mater. Chem. A 2017, 5, 24657–24666. [Google Scholar] [CrossRef]
- Ren, Y.; He, D.; Wang, T.; Qi, H. Effect of ZIF-7 doping content on H2/CO2 separation performance of 1,2-bis(triethoxysilyl)ethane-derived organosilica membranes. Sep. Purif. Technol. 2023, 311, 123347. [Google Scholar] [CrossRef]
- Zhang, H.; He, D.; Niu, S.; Qi, H. Tuning the microstructure of organosilica membranes with improved gas permselectivity via the co-polymerization of 1,2-bis(triethoxysilyl) ethane and 1,2-bis(triethoxysilyl)methane. Int. J. Hydrogen Energy 2021, 46, 17221–17230. [Google Scholar] [CrossRef]
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Hefny, M.; Al-Hinai, A.; Al-Muhtaseb, A.H.; Rooney, D.W. Hydrogen production, storage, utilisation and environmental impacts: A review. Environ. Chem. Lett. 2022, 20, 153–188. [Google Scholar] [CrossRef]
- Massarweh, O.; Al-Khuzaei, M.; Al-Shafi, M.; Bicer, Y.; Abushaikha, A.S. Blue hydrogen production from natural gas reservoirs: A review of application and feasibility. J. CO2 Util. 2023, 70, 102438. [Google Scholar] [CrossRef]
- Noussan, M.; Raimondi, P.P.; Scita, R.; Hafner, M. The role of green and blue hydrogen in the energy transition—A technological and geopolitical perspective. Sustainability 2021, 13, 298. [Google Scholar] [CrossRef]
- Alhumaidan, F.; Cresswell, D.; Garforth, A. Hydrogen Storage in Liquid Organic Hydride: Producing Hydrogen Catalytically from Methylcyclohexane. Energy Fuels 2011, 25, 4217–4234. [Google Scholar] [CrossRef]
- Seshimo, M.; Urai, H.; Sasa, K.; Nishino, H.; Yamaguchi, Y.; Nishida, R.; Nakao, S. Bench-Scale Membrane Reactor for Methylcyclohexane Dehydrogenation using Silica Membrane Module. Membranes 2021, 11, 326. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.K.; Baiker, A. Dehydrogenation of methylcyclohexane to toluene in a pilot-scale membrane reactor. Appl. Catal. A-Gen. 1997, 155, 41–57. [Google Scholar] [CrossRef]
- Kreuder, H.; Boeltken, T.; Cholewa, M.; Meier, J.; Pfeifer, P.; Dittmeyer, R. Heat storage by the dehydrogenation of methylcyclohexane—Experimental studies for the design of a microstructured membrane reactor. Int. J. Hydrogen Energy 2016, 41, 12082–12092. [Google Scholar] [CrossRef]
- Li, G.; Niimi, T.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Equilibrium shift of methylcyclohexane dehydrogenation in a thermally stable organosilica membrane reactor for high-purity hydrogen production. Int. J. Hydrogen Energy 2013, 38, 15302–15306. [Google Scholar] [CrossRef]
- Acharya, D.; Ng, D.; Xie, Z. Recent Advances in Catalysts and Membranes for MCH Dehydrogenation: A Mini Review. Membranes 2021, 11, 955. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Akamatsu, K.; Nakao, S. Hyrogen Separation in Hydrogen-Methylcylohexane-Toluene Gaseous Mixtures through Triphenylmethoxysilane-derived Silica Membranes Prepared by Chemical Vapor Deposition. Ind. Eng. Chem. Res. 2016, 55, 5395–5402. [Google Scholar] [CrossRef]
- Kida, K.; Maeta, Y.; Kuno, T.; Yogo, K. Hydrogen Purification from Chemical Hydride Using Pure Silica Zeolite Membranes. Chem. Lett. 2017, 46, 1724–1727. [Google Scholar] [CrossRef]
- Seshimo, M.; Saito, T.; Akamatsu, K.; Segawa, A.; Nakao, S. Influence of toluene vapor on the H2-selective performance of dimethoxydiphenylsilane-derived silica membranes prepared by the chemical vapor deposition method. J. Membr. Sci. 2012, 415–416, 51–56. [Google Scholar] [CrossRef]
- Lee, H.L.; Kanezashi, M.; Shimomura, Y.; Yoshioka, T.; Tsuru, T. Evaluation and fabrication of pore size tune silica membranes with tetraethoxydimetyl disiloxane for gas separation. AIChE J. 2011, 57, 2755–2765. [Google Scholar] [CrossRef]
- Xiao, J.; Wei, J. Diffusion mechanism of hydrocarbons in zeolites-I. Theory. Chem. Eng. Sci. 1992, 47, 1123–1142. [Google Scholar] [CrossRef]
- Shelekhin, A.B.; Dixon, A.G.; Ma, Y.H. Theory of gas diffusion and permeation in inorganic molecular-sieve membranes. AIChE J. 1995, 41, 58–67. [Google Scholar] [CrossRef]
- Kumakiri, I.; Qiu, L.; Liu, N.; Tanaka, K.; Kita, H.; Sato, T.; Nishida, R. Application of MFI Zeolite Membrane Prepared with Fluoride Ions to Hydrogen/Toluene. Sep. J. Chem. Eng. Jpn. 2016, 49, 753–755. [Google Scholar] [CrossRef]
- Hirota, Y.; Maeda, Y.; Yamamoto, Y.; Miyamoto, M.; Nishiyama, N. Organosilica membrane with ionic liquid properties for separation of toluene/H2 mixture. Materials 2017, 10, 901. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Kanezashi, M.; Yoshioka, T.; Tsuru, T. Ammonia decomposition in catalystic membrane reactors: Simulation and experimental studies. AIChE J. 2013, 59, 168–179. [Google Scholar] [CrossRef]
- Imasaka, S.; Nakai, A.; Araki, S.; Yamamoto, H. Syntesis and Gas Permeation Properties of STT-type Zeolite Membranes. J. Jpn. Pet. Inst. 2018, 61, 263–271. [Google Scholar] [CrossRef]
- Camblor, M.A.; Diaz-Cabanas, M.J.; Perez-Pariente, J.; Teat, S.J.; Clegg, W.; Shannon, J.; Lightfoot, P.; Wright, P.A.; Morris, R.E. SSZ-23: An Odd Zeolite with Pore Openings of Seven and Nine Tetrahedral Atoms. Angew. Chem. Int. Ed. 1998, 37, 2122–2126. [Google Scholar] [CrossRef]
- Funke, H.H.; Argo, A.M.; Falconer, J.L.; Noble, R.D. Separations of Cyclic, Branched and Linear Hydrocarbon Mixtures through Silicate Membranes. Ind. Eng. Chem. Res. 1997, 36, 137–143. [Google Scholar] [CrossRef]

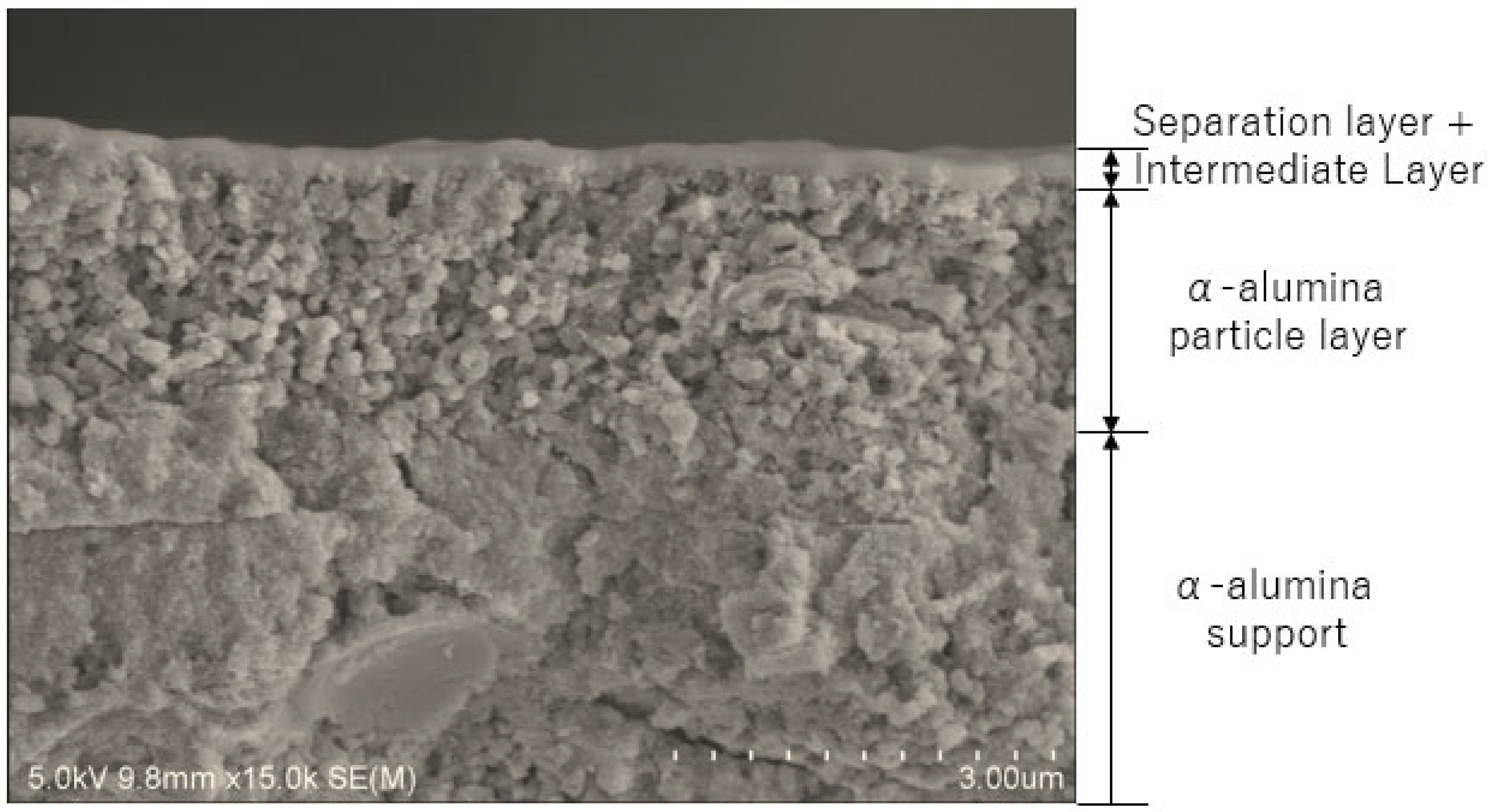






| Membrane Lists | Coating Times of Top Layers | BTESE Sols for Top Layers |
|---|---|---|
| BTESE-0.7-1 | 4 | 1 wt.% BTESE sol after aging for 8 days at 50 °C |
| BTESE-0.5-1 | 5 | |
| BTESE-0.4-1 | 6 | |
| BTESE-0.4-2 | ||
| BTESE-0.4-3 | ||
| BTESE-0.4-4 |
| Membrane Numbers | H2 Permeance [10−6 mol m−2 s−1 Pa−1] | Permeance Ratio | NKP [nm] | |
|---|---|---|---|---|
| H2/N2 | H2/SF6 | |||
| BTESE-0.4-1 * | 0.43 | 89 | 1991 | ~0.46 |
| BTESE-0.4-2 * | 1.33 | 106 | 1908 | ~0.46 |
| BTESE-0.4-3 * | 1.36 | 73 | 2321 | ~0.47 |
| BTESE-0.4-4 * | 1.38 | 61 | 2051 | ~0.47 |
| BTESE-0.5-1 * | 2.15 | 8 | 1846 | ~0.54 |
| BTESE-0.7-1 * | 4.45 | 6 | 972 | ~0.73 |
| Si-CHA [31] ** | 0.70 | 9 | 778 | ~0.55 |
| Si-STT [31] ** | 0.50 | 25 | 625 | ~0.53 |
| DMDPS [32] * | 1.00 | 14 | 5000 | N/A |
| Membrane Code | Pressure [kPaA] | Temperature [°C] | H2/TOL Concentration Feed mol [%] | Permeate Stream | Retentate Stream | Refs | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Permeance [10−6 mol m−2 s−1 Pa−1] | H2/ TOL [-] | H2 Purity [mol %] | H2 Recovery [%] | TOL Concentration [ppm] | TOL Recovery [%] | TOL Concentration [ppm] | |||||||
| Feed | Permeate | H2 | TOL | ||||||||||
| BTESE-0.4-1 | 200 | 101.3 | 200 | 97/3 | 0.32 | 0.00123 | 258 | 99.972 | 8.8 | 280 | 98.2 | 32,668 | [This work] |
| BTESE-0.4-2 | 200 | 101.3 | 200 | 97/3 | 1.16 | 0.00586 | 198 | 99.957 | 30.2 | 428 | 98.5 | 41,730 | [This work] |
| BTESE-0.4-2 | 300 | 111.0 | 200 | 97/3 | 0.96 | 0.01104 | 87 | 99.914 | 53.9 | 859 | 95.3 | 60,149 | [This work] |
| BTESE-0.4-3 | 200 | 101.3 | 200 | 97/3 | 1.21 | 0.00602 | 200 | 99.958 | 34.1 | 418 | 95.9 | 43,977 | [This work] |
| BTESE-0.4-4 | 200 | 101.3 | 200 | 97/3 | 1.31 | 0.00579 | 227 | 99.963 | 38.7 | 375 | 100.1 | 46,972 | [This work] |
| BTESE-0.5-1 | 200 | 101.3 | 200 | 97/3 | 1.83 | 0.00830 | 221 | 99.956 | 50.2 | 441 | 96.1 | 56,490 | [This work] |
| BTESE-0.5-1 | 300 | 101.3 | 200 | 97/3 | 1.73 | 0.00681 | 253 | 99.944 | 81.5 | 557 | 88.0 | 127,303 | [This work] |
| BTESE-0.7-1 | 200 | 101.3 | 200 | 97/3 | 2.15 | 0.12300 | 17 | 99.456 | 60.0 | 5441 | 92.0 | 62,293 | [This work] |
| Si-CHA | 300 | 101.3 | 90 | 98/2 | 0.88 | 0.00500 | 176 | 99.989 | N/A | N/A | N/A | N/A | [31] |
| Si-CHA | 300 | 101.3 | 120 | 98/2 | 0.77 | 0.00520 | 148 | 99.987 | N/A | N/A | N/A | N/A | [31] |
| Si-CHA | 300 | 101.3 | 150 | 98/2 | 0.75 | 0.00550 | 136 | 99.984 | N/A | N/A | N/A | N/A | [31] |
| Si-STT | 300 | 101.3 | 90 | 98/2 | 0.18 | 0.00060 | 300 | 99.992 | N/A | N/A | N/A | N/A | [31] |
| Si-STT | 300 | 101.3 | 120 | 98/2 | 0.19 | 0.00062 | 306 | 99.992 | N/A | N/A | N/A | N/A | [31] |
| Si-STT | 300 | 101.3 | 150 | 98/2 | 0.20 | 0.00065 | 308 | 99.991 | N/A | N/A | N/A | N/A | [31] |
| DMDPS | N/A | N/A | 200 | 98/2 | 0.90 | N/A | N/A | 99.996 | [32] | ||||
| MFI | 300 | 101.3 | 120 | 98/2 | 0.04 | 0.00940 | 4 | N/A | N/A | N/A | N/A | N/A | [36] |
| MFI | 300 | 101.3 | 200 | 98/2 | 0.16 | 0.02500 | 6 | N/A | N/A | N/A | N/A | N/A | [36] |
| SILM | 220 | 200 | 70 | N/A | 0.0002 | 0.10 | 0.0020 | N/A | N/A | N/A | N/A | N/A | [37] |
| ILOS | 220 | 200 | 70 | N/A | 0.00001 | 0.10 | 0.0001 | N/A | N/A | N/A | N/A | N/A | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, S.M.; Yu, X.; Miyata, S.; Mishina, K.; Salak, F.; Lawal, S.O.; Tsuru, T.; Sawamura, K.-i. Hydrogen (H2)/Toluene (TOL) Separation via One and Two Stages of the Bis(triethoxysily)ethane (BTESE) Membranes. Membranes 2024, 14, 165. https://doi.org/10.3390/membranes14080165
Ibrahim SM, Yu X, Miyata S, Mishina K, Salak F, Lawal SO, Tsuru T, Sawamura K-i. Hydrogen (H2)/Toluene (TOL) Separation via One and Two Stages of the Bis(triethoxysily)ethane (BTESE) Membranes. Membranes. 2024; 14(8):165. https://doi.org/10.3390/membranes14080165
Chicago/Turabian StyleIbrahim, Suhaina Mohd, Xin Yu, Shigeru Miyata, Kengo Mishina, Feridoun Salak, Sulaiman Oladipo Lawal, Toshinori Tsuru, and Ken-ichi Sawamura. 2024. "Hydrogen (H2)/Toluene (TOL) Separation via One and Two Stages of the Bis(triethoxysily)ethane (BTESE) Membranes" Membranes 14, no. 8: 165. https://doi.org/10.3390/membranes14080165
APA StyleIbrahim, S. M., Yu, X., Miyata, S., Mishina, K., Salak, F., Lawal, S. O., Tsuru, T., & Sawamura, K.-i. (2024). Hydrogen (H2)/Toluene (TOL) Separation via One and Two Stages of the Bis(triethoxysily)ethane (BTESE) Membranes. Membranes, 14(8), 165. https://doi.org/10.3390/membranes14080165





