Optimization of Membrane Condenser Process with PTFE Hollow Fiber Membrane
Abstract
1. Introduction
2. Experiment
2.1. Materials
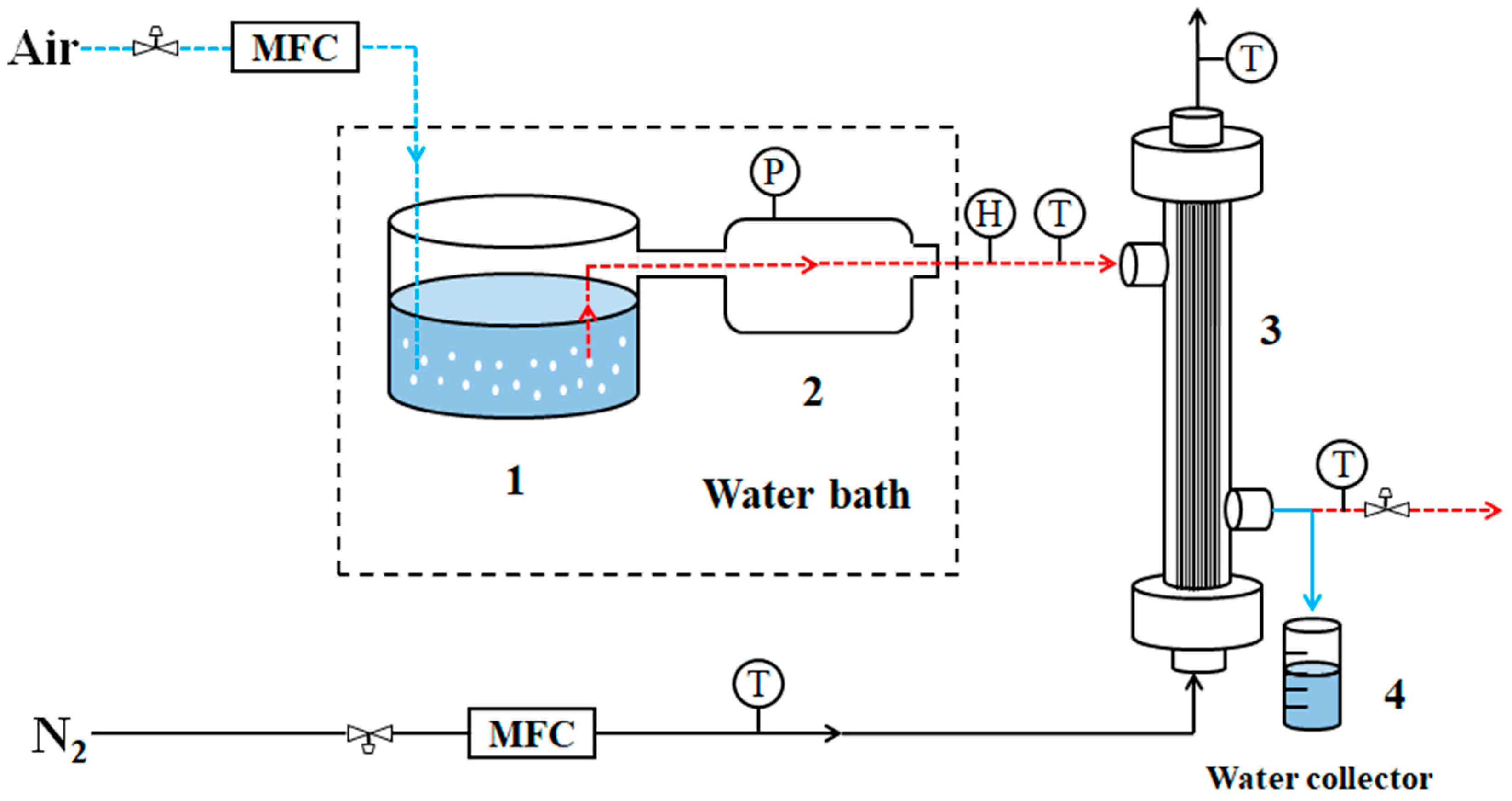
2.2. Membrane Condenser Experiments
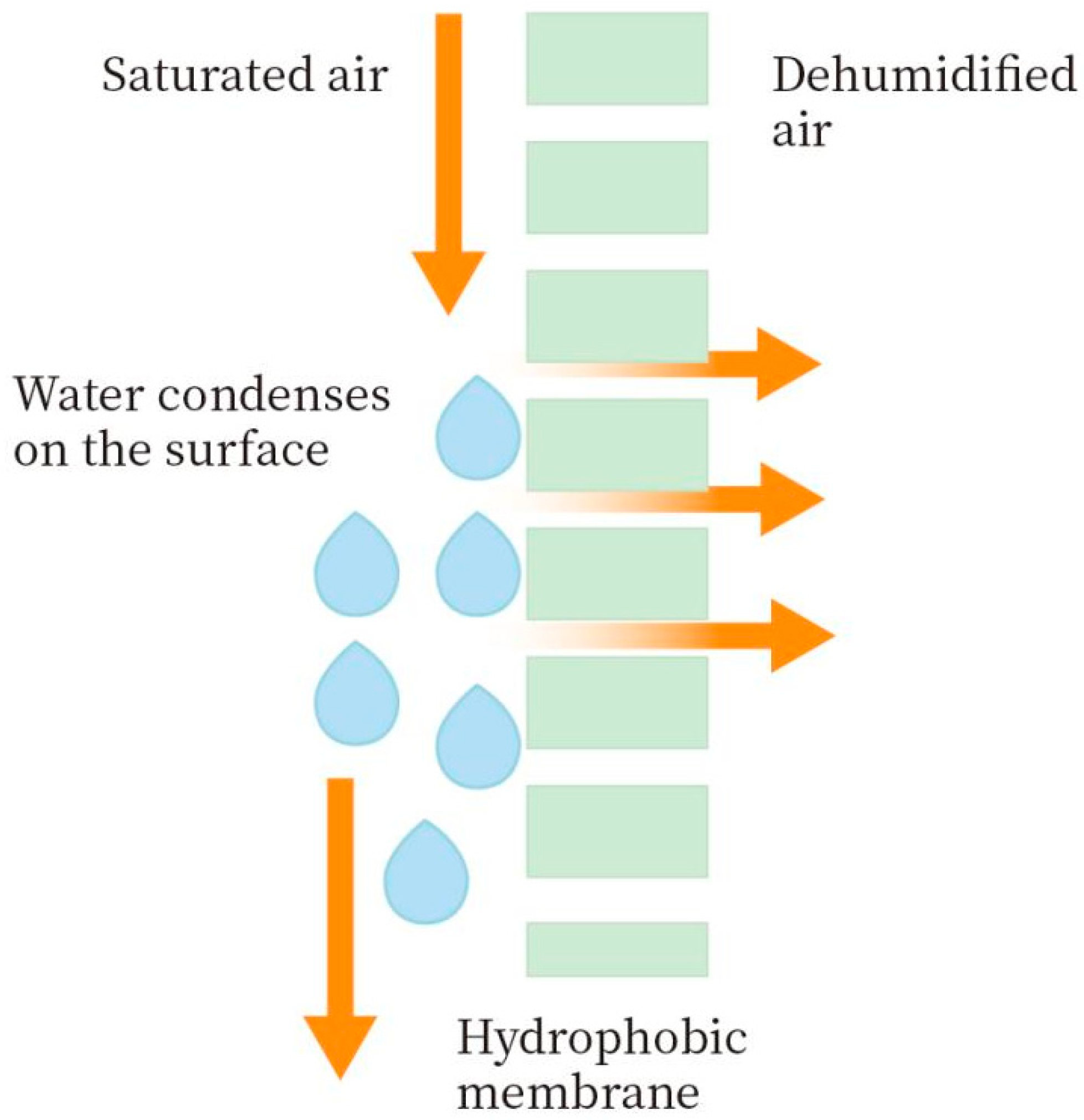
2.3. Experimental Process of Membrane Condenser
2.4. Structure and Characterization of Porous Hydrophobic Membranes
2.4.1. Measurement of Membrane Porosity
2.4.2. Measurement of Membrane Pore Size Distribution
2.4.3. Water Contact Angle Measurement
3. Results and Discussion
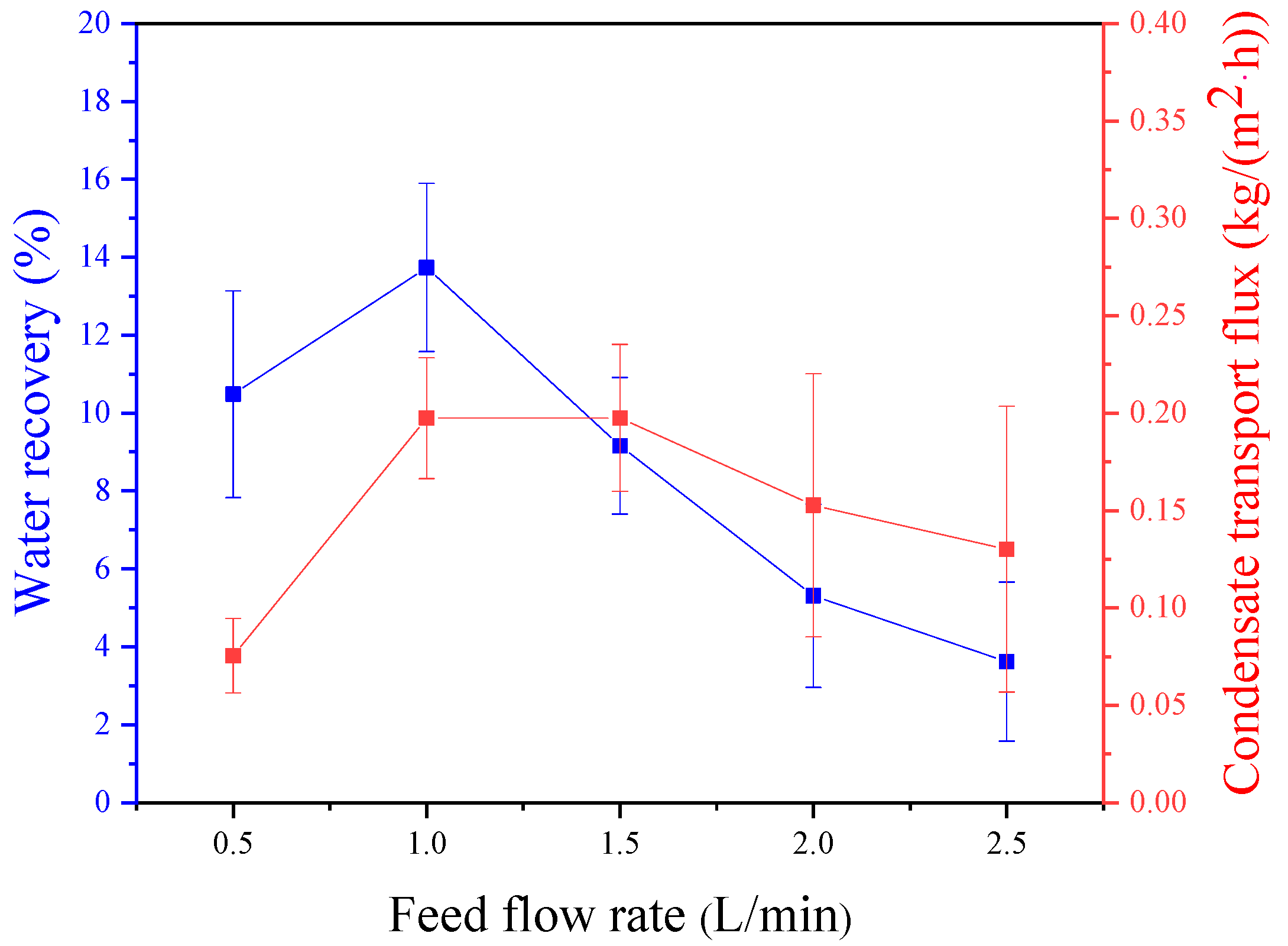
3.1. Effect of Feed Gas Flux
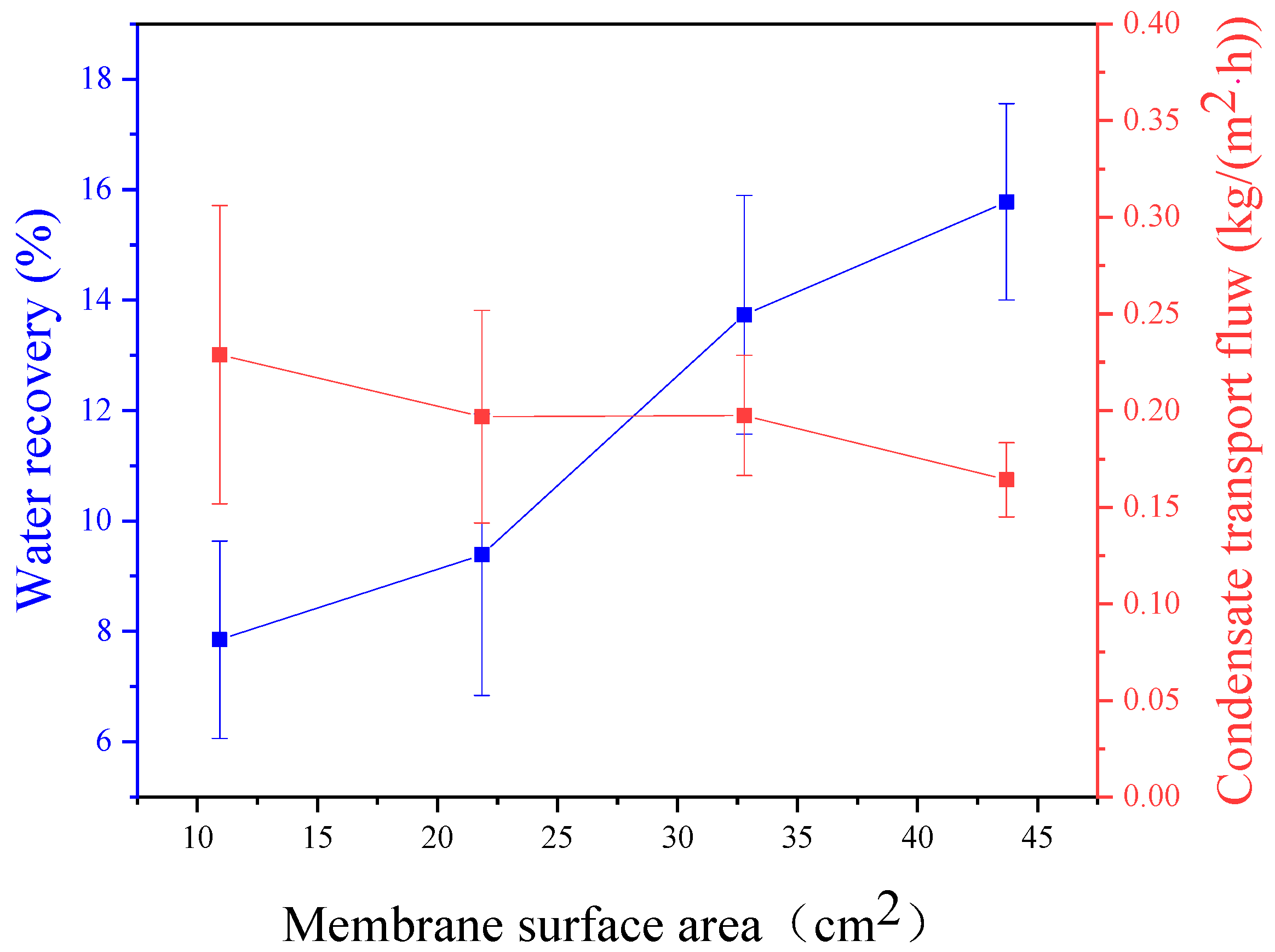
3.2. Effect of Membrane Area
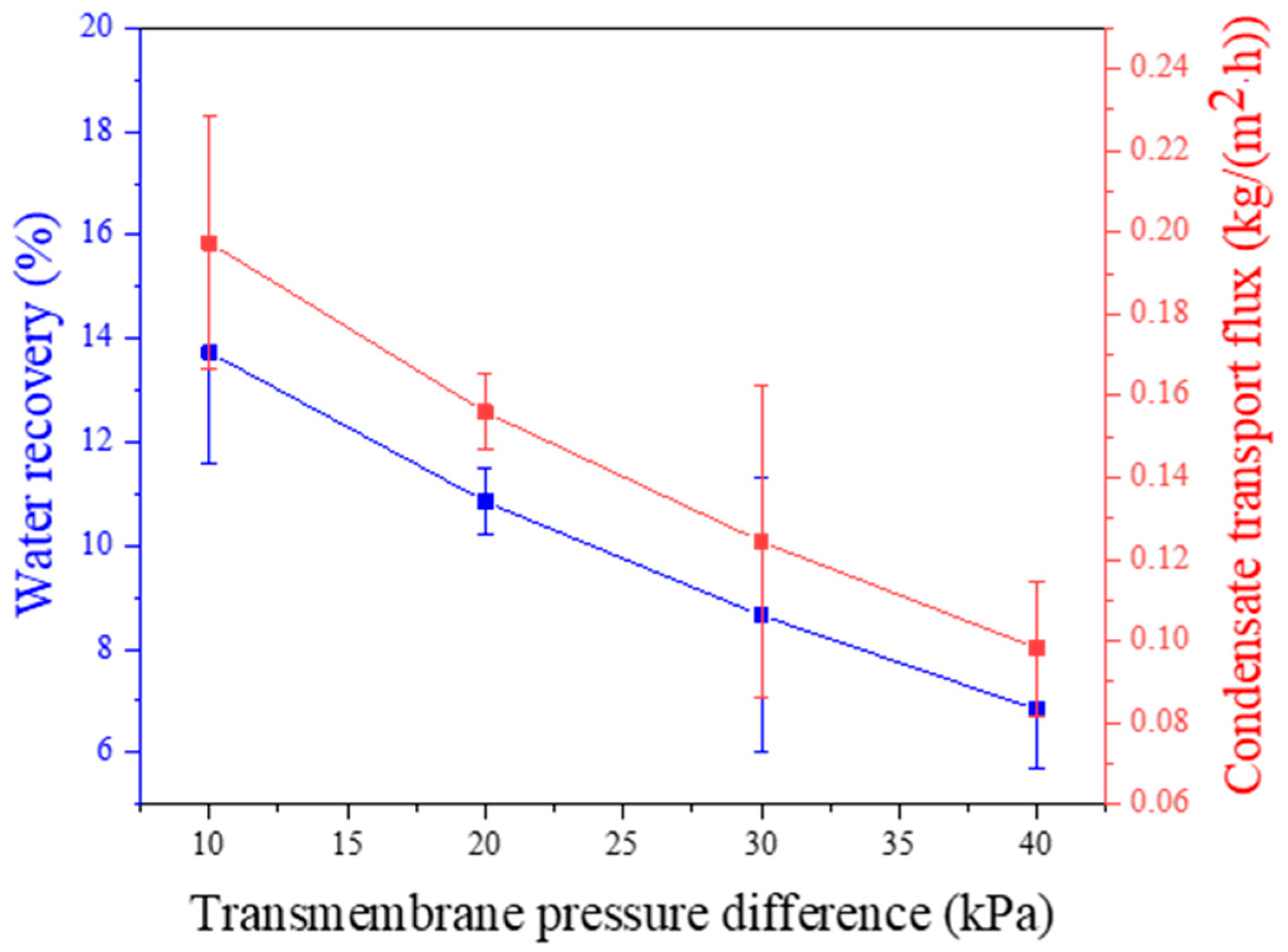
3.3. Effect of Feed Gas Pressure
3.4. Effect of Feed Gas Temperature
3.5. Effect of Sweeping Gas Flux
4. Conclusions
- All investigated operational parameters have an impact on the membrane condenser process. Among them, the influence of feed gas temperature and sweeping gas flux is more significant compared to the effect of water recovery rate. At a temperature of 60 °C, the water recovery rate reaches 24.7%, while with a sweeping gas flux of 4 L/min, the water recovery rate reaches 22.7%;
- When the intake flow rate is 1 L/min and 0.5 L/min, the water recovery rate exhibits an inverse relationship with the feed pressure. However, taking into account both the water recovery rate and condensing flow, the process parameters with a feed gas flux of 1 L/min and the feed pressure of 10 kPa are deemed optimal;
- When the membrane area is held constant, the efficiency of the membrane condenser decreases with an increase in feed gas flux. Conversely, when the feed gas flux is constant, the efficiency of the membrane condenser increases with an increase in membrane area. This indicates a direct relationship between feed gas flux and membrane area. Experimental results suggest that the optimal relationship between the feed gas flux and membrane area falls within the range of 0.51–0.67 cm/s.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Qaterji, A.M.; Salilih, E.M.; Siddiqui, M.E.; Almatrafi, E.; Nurrohman, N.; Abulkhair, H.; Alsaiari, A.; Macedonio, F.; Wang, Z.; Albeirutty, M.; et al. Development of an integrated membrane condenser system with LNG cold energy for water recovery from humid flue gases in power plants. Int. J. Hydrogen Energy 2023, 48, 30791–30803. [Google Scholar] [CrossRef]
- Luo, P.; Shen, G.; Sun, M.; Teng, D.; Jia, X.; Zhang, S. Research on water and waste heat recovery from flue gas using micron ceramic membranes. Therm. Sci. Eng. Prog. 2023, 43, 102004. [Google Scholar] [CrossRef]
- Ma, S.; Chai, J.; Jiao, K.; Ma, L.; Zhu, S.; Wu, K. Environmental influence and countermeasures for high humidity flue gas discharging from power plants. Renew. Sustain. Energy Rev. 2017, 73, 225–235. [Google Scholar] [CrossRef]
- Liu, F.; Tan, Q.; Jiang, X.; Yang, F.; Jiang, W. Effects of relative humidity and PM(2.5) chemical compositions on visibility impairment in Chengdu, China. J. Environ. Sci. 2019, 86, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Cao, M.; Shan, H.; Du, C.; Li, B. Polyhedral oligomeric silsesquioxane functionalized carbon nanotubes for high thermal conductive poly(vinylidene fluoride) composite membrane. Mater. Des. 2018, 156, 242–251. [Google Scholar] [CrossRef]
- Rongwong, W.; Goh, K. Resource recovery from industrial wastewaters by hydrophobic membrane contactors: A review. J. Environ. Chem. Eng. 2020, 8, 104242. [Google Scholar] [CrossRef]
- Koronaki, I.P.; Christodoulaki, R.I.; Papaefthimiou, V.D.; Rogdakis, E.D. Thermodynamic analysis of a counter flow adiabatic dehumidifier with different liquid desiccant materials. Appl. Therm. Eng. 2013, 50, 361–373. [Google Scholar] [CrossRef]
- Preißinger, M.; Pöllinger, S.; Brüggemann, D. Ionic liquid based absorption chillers for usage of low grade waste heat in industry. Int. J. Energy Res. 2013, 37, 1382–1388. [Google Scholar] [CrossRef]
- Racha, S.M.; Mitra, S.; Shown, B.; Mandal, S.; Das, A.K. Emerging membrane technologies for low-cost desalination. Proc. Inst. Civil. Eng.-Water Manag. 2023, 177, 112–122. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, H.; Shi, L.; Wu, Z.; Zhang, S.; Wang, S.; Sun, H. Photocatalysis coupling with membrane technology for sustainable and continuous purification of wastewater. Sep. Purif. Technol. 2024, 329, 125225. [Google Scholar] [CrossRef]
- Hu, H.W.; Tang, G.H.; Niu, D. Wettability modified nanoporous ceramic membrane for simultaneous residual heat and condensate recovery. Sci. Rep. 2016, 6, 27274. [Google Scholar] [CrossRef]
- Guo, Q.; Huang, Y.; Xu, M.; Huang, Q.; Cheng, J.; Yu, S.; Zhang, Y.; Xiao, C. PTFE porous membrane technology: A comprehensive review. J. Membr. Sci. 2022, 664, 121115. [Google Scholar] [CrossRef]
- Cao, J.; Pan, J.; Cui, Z.; Wang, Z.; Wang, X.; Drioli, E. Improving efficiency of PVDF membranes for recovering water from humidified gas streams through membrane condenser. Chem. Eng. Sci. 2019, 210, 115234. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Z.; Yang, X.; Ru, K.; Song, J. Simulation of water recovery in membrane condenser dehumidification process. Appl. Therm. Eng. 2021, 193, 117018. [Google Scholar] [CrossRef]
- Brennan, B.; Briciu-Burghina, C.; Hickey, S.; Abadie, T.; al Ma Awali, S.M.; Delaure, Y.; Durkan, J.; Holland, L.; Quilty, B.; Tajparast, M.; et al. Pilot Scale Study: First Demonstration of Hydrophobic Membranes for the Removal of Ammonia Molecules from Rendering Condensate Wastewater. Int. J. Mol. Sci. 2020, 21, 3914. [Google Scholar] [CrossRef]
- Brunetti, A.; Santoro, S.; Macedonio, F.; Figoli, A.; Drioli, E.; Barbieri, G. Waste Gaseous Streams: From Environmental Issue to Source of Water by Using Membrane Condensers. Clean-Soil Air Water 2014, 42, 1145–1153. [Google Scholar] [CrossRef]
- Drioli, E.; Santoro, S.; Simone, S.; Barbieri, G.; Brunetti, A.; Macedonio, F.; Figoli, A. ECTFE membrane preparation for recovery of humidified gas streams using membrane condenser. React. Funct. Polym. 2014, 79, 1–7. [Google Scholar] [CrossRef]
- Macedonio, F.; Brunetti, A.; Barbieri, G.; Drioli, E. Membrane Condenser as a New Technology for Water Recovery from Humidified “Waste” Gaseous Streams. Ind. Eng. Chem. Res. 2012, 52, 1160–1167. [Google Scholar] [CrossRef]
- Macedonio, F.; Cersosimo, M.; Brunetti, A.; Barbieri, G.; Drioli, E. Water recovery from humidified waste gas streams: Quality control using membrane condenser technology. Chem. Eng. Process. 2014, 86, 196–203. [Google Scholar] [CrossRef]
- Macedonio, F.; Frappa, M.; Brunetti, A.; Barbieri, G.; Drioli, E. Recovery of water and contaminants from cooling tower plume. Environ. Eng. Res. 2019, 25, 222–229. [Google Scholar] [CrossRef]
- Huang, Q.L.; Huang, Y.; Gao, S.P.; Zhang, M.Y.; Xiao, C.F. Novel Ultrafine Fibrous Poly(tetrafluoroethylene) Hollow Fiber Membrane Fabricated by Electrospinning. Polymers 2018, 10, 464. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.L.; Tian, K.X.; Li, Y.P.; Su, C.L.; Duan, F.; Xu, Y. Super-hydrophobic PTFE hollow fiber membrane fabricated by electrospinning of Pullulan/PTFE emulsion for membrane deamination. Sep. Purif. Technol. 2021, 274, 118186. [Google Scholar] [CrossRef]
- Rosner, F.; Chan, C.Y.; Paul, B.; Samuelsen, S. Nano-porous transport membrane condenser for flue gas water recovery: Modeling and parametric membrane design analysis. Int. J. Therm. Sci. 2023, 192, 108365. [Google Scholar] [CrossRef]
- Kim, J.F.; Park, A.; Kim, S.J.; Lee, P.S.; Cho, Y.H.; Park, H.; Nam, S.E.; Park, Y.I. Harnessing Clean Water from Power Plant Emissions Using Membrane Condenser Technology. ACS Sustain. Chem. Eng. 2018, 6, 6425–6433. [Google Scholar] [CrossRef]



| Membrane | Average Pore Size (nm) | Porosity (%) | Contact Angle (°) | Outer Diameter (mm) |
|---|---|---|---|---|
| PTFE | 227.4 ± 7.1 | 42.12 ± 0.31 | 122.3 ± 1.6 | 1.74 ± 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Long, S.; Wang, Z.; Drioli, E.; Zhang, F.; Cui, Z. Optimization of Membrane Condenser Process with PTFE Hollow Fiber Membrane. Membranes 2024, 14, 141. https://doi.org/10.3390/membranes14060141
Zhou Y, Long S, Wang Z, Drioli E, Zhang F, Cui Z. Optimization of Membrane Condenser Process with PTFE Hollow Fiber Membrane. Membranes. 2024; 14(6):141. https://doi.org/10.3390/membranes14060141
Chicago/Turabian StyleZhou, Yue, Susu Long, Zhaohui Wang, Enrico Drioli, Feng Zhang, and Zhaoliang Cui. 2024. "Optimization of Membrane Condenser Process with PTFE Hollow Fiber Membrane" Membranes 14, no. 6: 141. https://doi.org/10.3390/membranes14060141
APA StyleZhou, Y., Long, S., Wang, Z., Drioli, E., Zhang, F., & Cui, Z. (2024). Optimization of Membrane Condenser Process with PTFE Hollow Fiber Membrane. Membranes, 14(6), 141. https://doi.org/10.3390/membranes14060141








