Experimental Study of a Sequential Membrane Process of Ultrafiltration and Nanofiltration for Efficient Polyphenol Extraction from Wine Lees
Abstract
1. Introduction
1.1. Polyphenols: Antioxidants for the Industry
1.2. Revalorization of Residues from the Wine Industry
1.3. Membrane Technology for Polyphenol Extraction
1.4. Membrane Fouling Models in UF
1.4.1. Complete Blocking Model
1.4.2. Intermediate Blocking Model
1.4.3. Standard Blocking Model
1.4.4. Cake Layer Formation
1.5. Mathematical Model for NF Processes
2. Materials and Methods
2.1. Determination of Phenolic Compounds
2.2. Wine Lees Residues
2.3. Membranes and Experimental Set-Up
2.4. Pretreatment and Conservation of Wine Lees
2.5. UF and NF Membrane Permeability Characterisation Experiments
2.6. UF and NF Filtration Experiments with Wine Lees
2.7. Cleaning Experiments
3. Results and Discussion
3.1. UF and NF Membrane Characterisation
3.2. Polyphenols Selectivity in Sequential Membrane Filtration Process
3.3. UF and NF Membrane Recovery Analysis
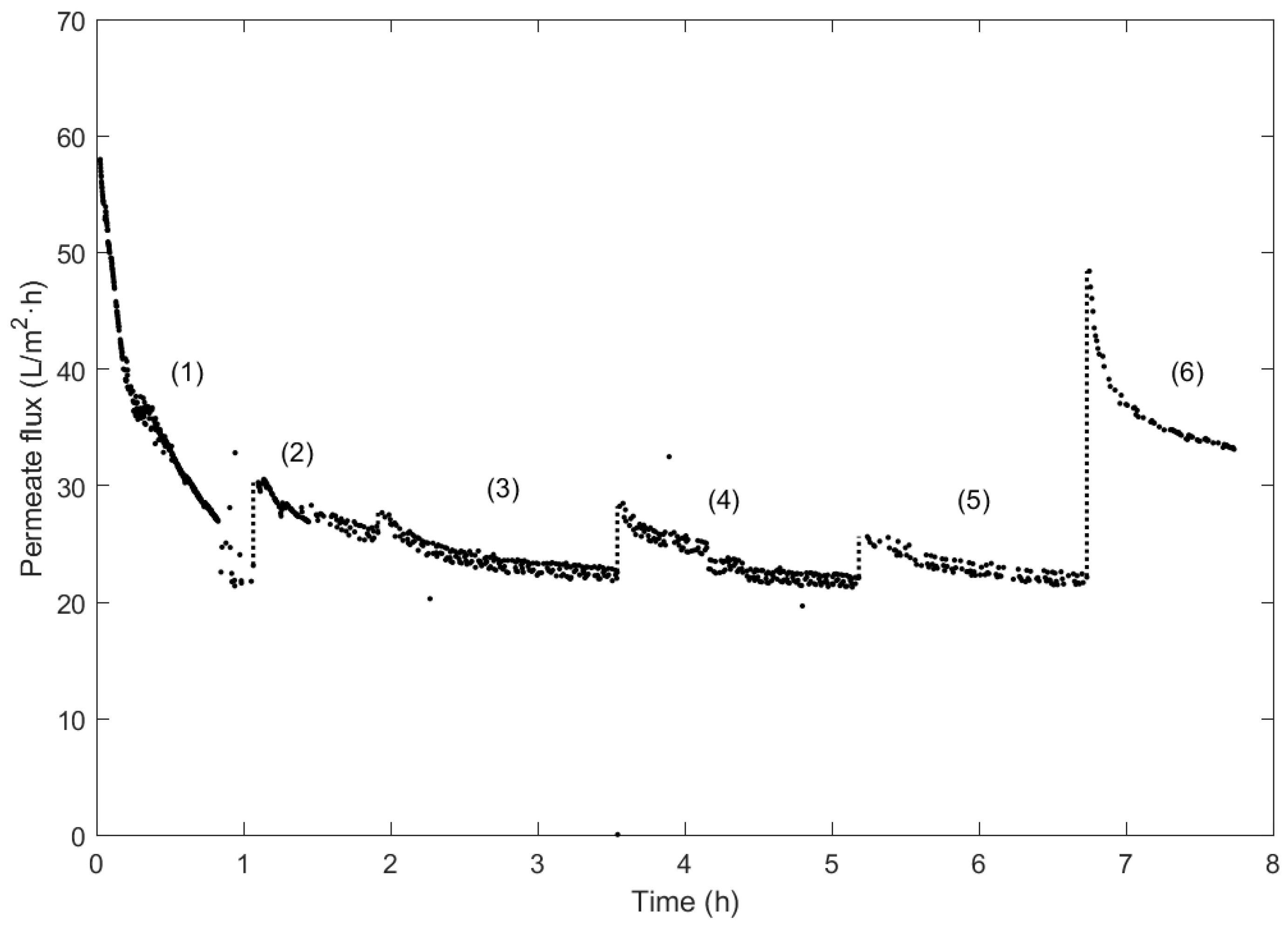
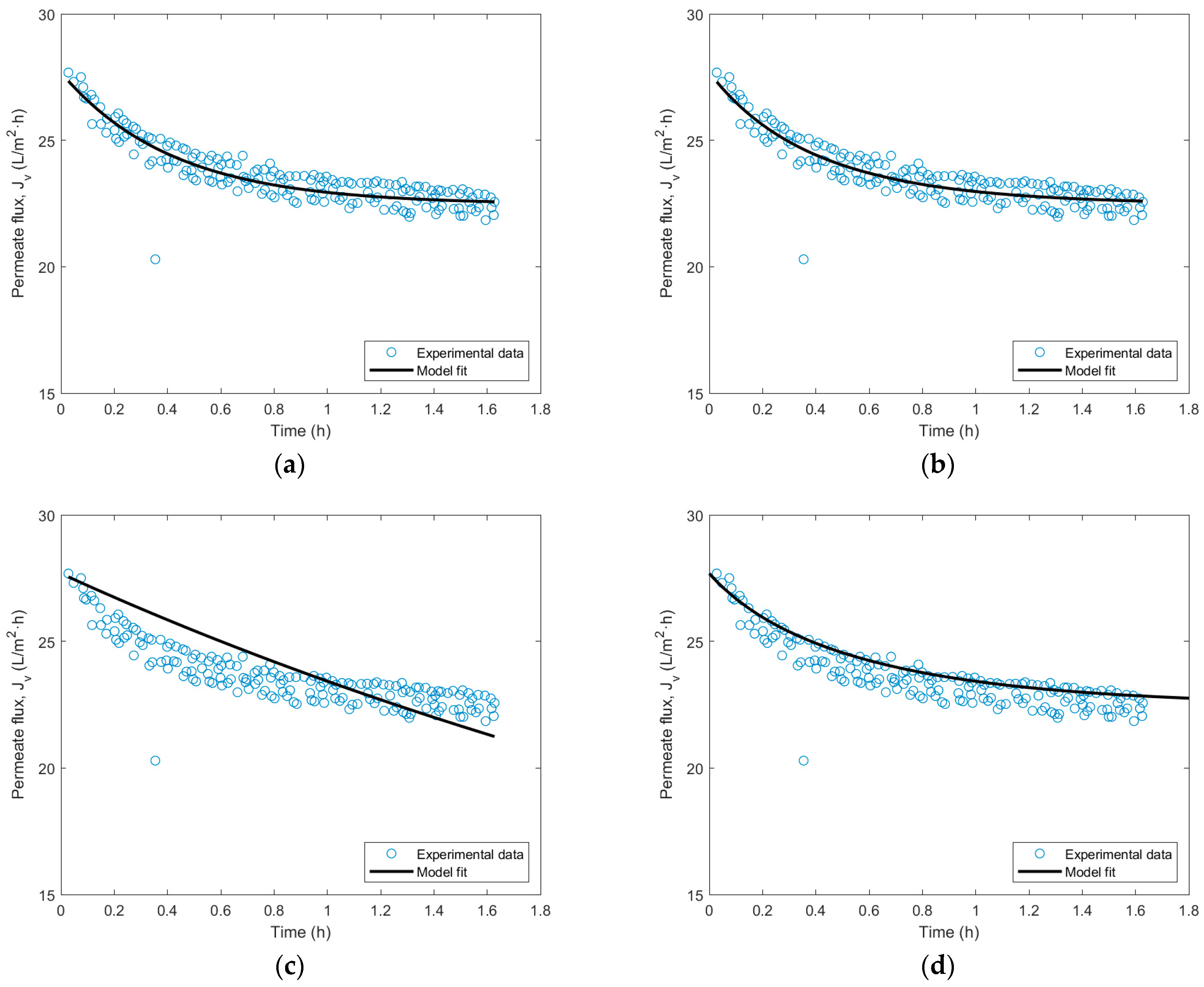
3.4. Fouling Identification Type in UF Membrane with Wine Lees Filtration
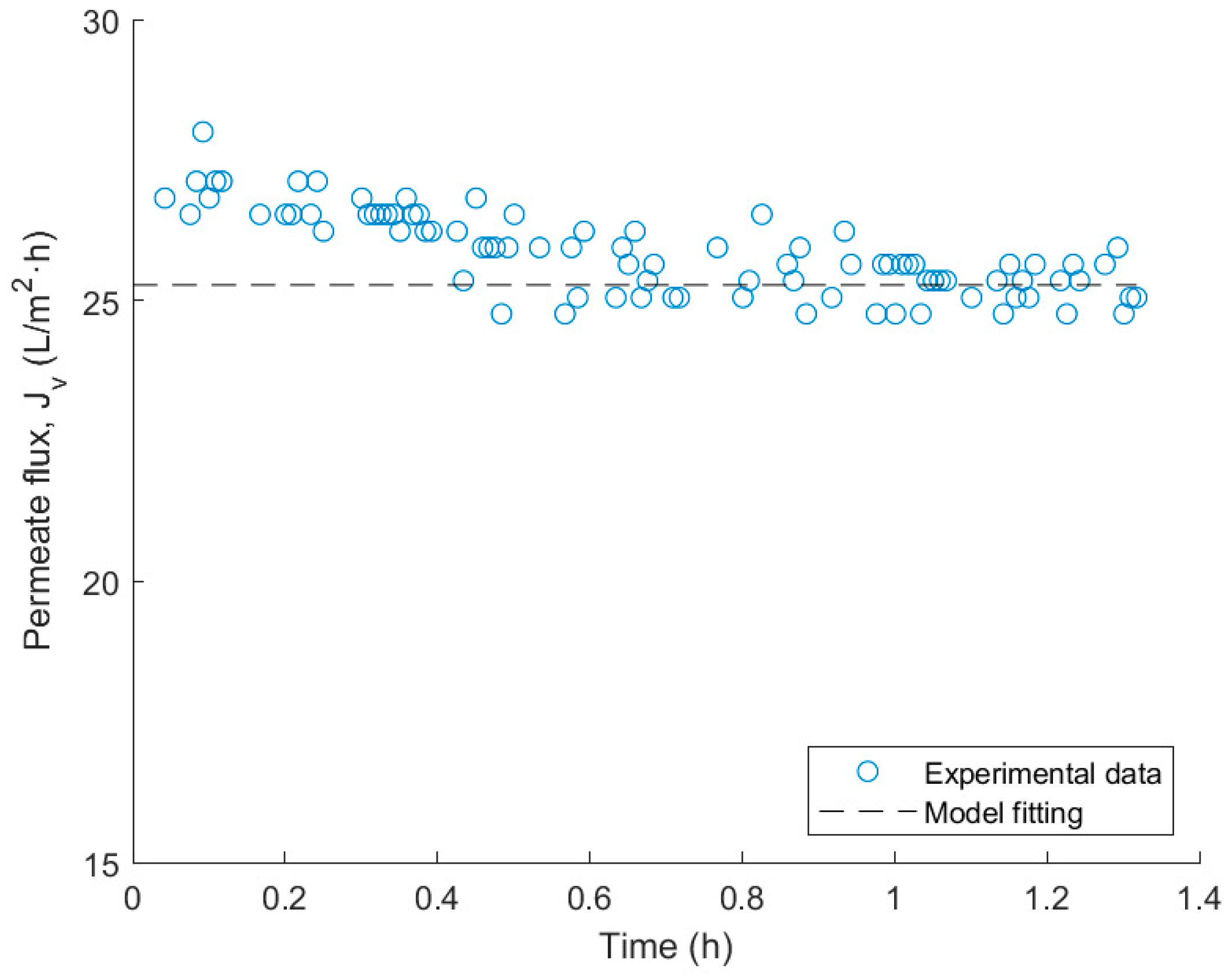
3.5. Fitting of the Spiegler–Kedem NF Model
3.6. Polyphenol Content in Wine Lees Analysis for Industrial Application
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COD | Chemical Oxygen Demand |
| FCR | Folin-Ciocalteu Reagent |
| FSS | Fixed Suspended Solids |
| MF | Microfiltration |
| NF | Nanofiltration |
| RO | Reverse Osmosis |
| TMP | Transmembrane Pressure |
| TSS | Total Suspended Solids |
| UF | Ultrafiltration |
| VSS | Volatile Suspended Solids |
Nomenclature
| Membrane’s active area, m2 | |
| Concentration of polyphenols in feed solution, mol/L | |
| Concentration of polyphenols in permeate solution, mol/L | |
| Initial permeate flux, m/s | |
| Steady-state permeate flux, m/s | |
| Permeate flux, m/s | |
| Mass transfer coefficient parameter in L/m2·h. | |
| Complete blocking model constant for crossflow filtration, 1/m | |
| Cake layer formation blocking model constant for crossflow filtration, s/m2 | |
| Intermediate blocking model constant for crossflow filtration, 1/m | |
| Standard blocking model constant for crossflow filtration, 1/s | |
| Membrane hydraulic permeability, L/bar·m2·h | |
| Universal gas constant, J/mol·K | |
| Rejection index | |
| Intrinsic resistance of the membrane, m2 | |
| Temperature, K | |
| Time, s | |
| Permeate volume obtained, L | |
| Greek | |
| Transmembrane pressure, bar | |
| Dynamic viscosity, bar·h | |
| Reflection coefficient |
References
- Csepregi, K.; Neugart, S.; Schreiner, M.; Hideg, É. Comparative Evaluation of Total Antioxidant Capacities of Plant Polyphenols. Molecules 2016, 21, 208. [Google Scholar] [CrossRef]
- Mojzer, E.B.; Hrnčič, M.K.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Durgo, K.; Huđek, A.; Bačun-Družina, V.; Komes, D. Overview of polyphenols and their properties. Polyphen. Prop. Recovery Appl. 2018, 3–44. [Google Scholar] [CrossRef]
- El Gharras, H. Polyphenols: Food sources, properties and applications—A review. Int. J. Food Sci. Technol. 2009, 44, 2512–2518. [Google Scholar] [CrossRef]
- Bertin, L.; Ferri, F.; Scoma, A.; Marchetti, L.; Fava, F. Recovery of high added value natural polyphenols from actual olive mill wastewater through solid phase extraction. Chem. Eng. J. 2011, 171, 1287–1293. [Google Scholar] [CrossRef]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Putnik, P.; Lorenzo, J.M.; Barba, F.J.; Roohinejad, S.; Režek Jambrak, A.; Granato, D.; Montesano, D.; Bursać Kovačević, D. Novel Food Processing and Extraction Technologies of High-Added Value Compounds from Plant Materials. Foods 2018, 7, 106. [Google Scholar] [CrossRef]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant Polyphenols: Chemical Properties, Biological Activities, and Synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Xia, E.Q.; Deng, G.F.; Guo, Y.J.; Li, H.B. Biological Activities of Polyphenols from Grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Piñeiro, Z.; Cantos-Villar, E.; Palma, M.; Puertas, B. Direct liquid chromatography method for the simultaneous quantification of hydroxytyrosol and tyrosol in red wines. J. Agric Food Chem. 2011, 59, 11683–11689. [Google Scholar] [CrossRef]
- Concluye Una Desafiante Vendimia de 2023, Con Poca Producción y Muchos Retos Por Delante—Federación Española de Enología—FEAE. Available online: https://federacionenologia.com/eno-2341/ (accessed on 8 February 2024).
- La OIV Estima que la Producción Mundial de Vino de 2023 será la Más Baja de los Últimos 60 Años—Revista Enólogos. Available online: https://revistaenologos.es/la-oiv-estima-que-la-produccion-mundial-de-vino-de-2023-sera-la-mas-baja-de-los-ultimos-60-anos/ (accessed on 8 February 2024).
- Maicas, S.; Mateo, J.J. Sustainability of Wine Production. Sustainability 2020, 12, 559. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J. Wine Lees as a Source of Antioxidant Compounds. Antioxidants 2019, 8, 45. [Google Scholar] [CrossRef]
- Khan, K.R.; Rajbhar, K.; Dawda, H.; Mukundan, U. Polyphenols: Methods of Extraction. Sci. Revs. Chem. Commun. 2015, 5, 1–6. Available online: https://www.sadgurupublications.com (accessed on 21 January 2024).
- Le, N.L.; Nunes, S.P. Materials and membrane technologies for water and energy sustainability. Sustain. Mater. Technol. 2016, 7, 1–28. [Google Scholar] [CrossRef]
- Ezugbe, E.O.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A Review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2004. [Google Scholar] [CrossRef]
- Carbonell-Alcaina, C.; Álvarez-Blanco, S.; Bes-Piá, M.A.; Mendoza-Roca, J.A.; Pastor-Alcañiz, L. Ultrafiltration of residual fermentation brines from the production of table olives at different operating conditions. J. Clean Prod. 2018, 189, 662–672. [Google Scholar] [CrossRef]
- Cifuentes-Cabezas, M.; Carbonell-Alcaina, C.; Vincent-Vela, M.C.; Mendoza-Roca, J.A.; Álvarez-Blanco, S. Comparison of different ultrafiltration membranes as first step for the recovery of phenolic compounds from olive-oil washing wastewater. Process Saf. Environ. Prot. 2021, 149, 724–734. [Google Scholar] [CrossRef]
- Conidi, C.; Cassano, A. Recovery of phenolic compounds from bergamot juice by nanofiltration membranes. Desalination Water Treat 2015, 56, 3510–3518. [Google Scholar] [CrossRef]
- Zagklis, D.P.; Paraskeva, C.A. Purification of grape marc phenolic compounds through solvent extraction, membrane filtration and resin adsorption/desorption. Sep. Purif. Technol. 2015, 156, 328–335. [Google Scholar] [CrossRef]
- Crespo, J.G.; Brazinha, C. Membrane processing: Natural antioxidants from winemaking by-products. Filtr. Sep. 2010, 47, 32–35. [Google Scholar] [CrossRef]
- Arboleda Mejia, J.A.; Ricci, A.; Figueiredo, A.S.; Versari, A.; Cassano, A.; Parpinello, G.P.; De Pinho, M.N. Recovery of Phenolic Compounds from Red Grape Pomace Extract through Nanofiltration Membranes. Foods 2020, 9, 1649. [Google Scholar] [CrossRef]
- Mir-Cerdà, A.; Carretero, I.; Coves, J.R.; Pedrouso, A.; Castro-Barros, C.M.; Alvarino, T.; Cortina, J.L.; Saurina, J.; Granados, M.; Sentellas, S. Recovery of phenolic compounds from wine lees using green processing: Identifying target molecules and assessing membrane ultrafiltration performance. Sci. Total Environ. 2023, 857, 159623. [Google Scholar] [CrossRef]
- López-Borrell, A.; López-Pérez, M.-F.; Cardona, S.C.; Lora-García, J. Experimental Study and Mathematical Modeling of a Nanofiltration Membrane System for the Recovery of Polyphenols from Wine Lees. Membranes 2022, 12, 240. [Google Scholar] [CrossRef]
- Li, C.; Ma, Y.; Li, H.; Peng, G. Concentration of polyphenolic compounds from grape seed by nanofiltration technology. Int. J. Food Eng. 2018, 14. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Ng, C.Y.; Lim, Y.P.; Ng, G.H. Ultrafiltration in Food Processing Industry: Review on Application, Membrane Fouling, and Fouling Control. Food Bioproc. Technol. 2012, 5, 1143–1156. [Google Scholar] [CrossRef]
- Sousa, M.R.S.; Lora-Garcia, J.; López-Pérez, M.F. Modelling approach to an ultrafiltration process for the removal of dissolved and colloidal substances from treated wastewater for reuse in recycled paper manufacturing. J. Water Process Eng. 2018, 21, 96–106. [Google Scholar] [CrossRef]
- Vela, M.C.V.; Blanco, S.Á.; García, J.L.; Rodríguez, E.B. Analysis of membrane pore blocking models adapted to crossflow ultrafiltration in the ultrafiltration of PEG. Chem. Eng. J. 2009, 149, 232–241. [Google Scholar] [CrossRef]
- Susanto, H.; Feng, Y.; Ulbricht, M. Fouling behavior of aqueous solutions of polyphenolic compounds during ultrafiltration. J. Food Eng. 2009, 91, 333–340. [Google Scholar] [CrossRef]
- Cai, M.; Lv, Y.; Luo, S.; Liu, Y.; Sun, P. Fouling Behavior of Polyphenols during Model Juice Ultrafiltration: Effect of Membrane Properties. Food Bioproc. Technol. 2018, 11, 1787–1793. [Google Scholar] [CrossRef]
- Sousa, L.D.S.; Cabral, B.V.; Madrona, G.S.; Cardoso, V.L.; Reis, M.H.M. Purification of polyphenols from green tea leaves by ultrasound assisted ultrafiltration process. Sep. Purif. Technol. 2016, 168, 188–198. [Google Scholar] [CrossRef]
- Hermia, J. Constant Pressure Blocking Filtration Laws—Application To Power-Law Non-Newtonian Fluids. Trans. Inst. Chem. Eng. 1982, 60, 183–187. [Google Scholar]
- Chen, C.; Qin, H. A Mathematical Modeling of the Reverse Osmosis Concentration Process of a Glucose Solution. Processes 2019, 7, 271. [Google Scholar] [CrossRef]
- Al-Zoubi, H.; Hilal, N.; Darwish, N.A.; Mohammad, A.W. Rejection and modelling of sulphate and potassium salts by nanofiltration membranes: Neural network and Spiegler–Kedem model. Desalination 2007, 206, 42–60. [Google Scholar] [CrossRef]
- Agbor, G.A.; Vinson, J.A.; Donnelly, P.E. Folin-Ciocalteau Reagent for Polyphenolic Assay. Int. J. Food Sci. Nutr. Diet. 2014, 3, 147–156. [Google Scholar] [CrossRef]
- Kabsch-Korbutowicz, M.; Urbanowska, A. Comparison of Polymeric and Ceramic Ultrafiltration Membranes for Separation of Natural Organic Matter from Water. J. Environ. Prot. Eng. 2010, 36, 125–135. [Google Scholar]
- Ricceri, F.; Malaguti, M.; Derossi, C.; Zanetti, M.; Riggio, V.; Tiraferri, A. Microalgae biomass concentration and reuse of water as new cultivation medium using ceramic membrane filtration. Chemosphere 2022, 307, 135724. [Google Scholar] [CrossRef]
- Sabaté, J.; Pujolà, M.; Llorens, J. Comparison of Polysulfone and Ceramic Membranes for the Separation of Phenol in MicellarEnhanced Ultrafiltration. J. Colloid Interface Sci. 2002, 246, 157–163. [Google Scholar] [CrossRef]
- Aguiar, A.O.; Andrade, L.H.; Ricci, B.C.; Pires, W.L.; Miranda, G.A.; Amaral, M.C.S. Gold acid mine drainage treatment by membrane separation processes: An evaluation of the main operational conditions. Sep. Purif. Technol. 2016, 170, 360–369. [Google Scholar] [CrossRef]
- Crossley, O.P.; Thorpe, R.B.; Peus, D.; Lee, J. The effect of salinity on the pressure susceptibility of the NF270 membrane. Desalination 2023, 564, 116804. [Google Scholar] [CrossRef]
- Giacobbo, A.; Bernardes, A.M.; Rosa, M.J.F.; De Pinho, M.N. Concentration Polarization in Ultrafiltration/Nanofiltration for the Recovery of Polyphenols from Winery Wastewaters. Membranes 2018, 8, 46. [Google Scholar] [CrossRef]
- Ferreira, R.B.; Piçarra-Pereira, M.A.; Monteiro, S.; Loureiro, V.B.; Teixeira, A.R. The wine proteins. Trends Food Sci. Technol. 2001, 12, 230–239. [Google Scholar] [CrossRef]
- Polyphenols—Phenol-Explorer. Available online: http://phenol-explorer.eu/compounds (accessed on 13 February 2024).
- Arribas, A.S.; Borrell-Fernández, M.; Chicharro, M. The role of electroanalytical techniques in analysis of polyphenols in wine. TrAC Trends Anal. Chem. 2012, 34, 78–96. [Google Scholar] [CrossRef]
- Cancino-Madariaga, B.; Ruby, R.; Castro, C.A.; Torrico, J.S.; Riquelme, M.L. Analysis of the membrane fouling mechanisms involved in clarified grape juice ultrafiltration using statistical tools. Ind. Eng. Chem. Res. 2012, 51, 4017–4024. [Google Scholar] [CrossRef]
- Rodrigues, R.P.; Gando-Ferreira, L.M.; Quina, M.J. Micellar enhanced ultrafiltration for the valorization of phenolic compounds and polysaccharides from winery wastewaters. J. Water Process Eng. 2020, 38, 101565. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of Temperatures on Polyphenols during Extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Sólyom, K.; Solá, R.; Cocero, M.J.; Mato, R.B. Thermal degradation of grape marc polyphenols. Food Chem. 2014, 159, 361–366. [Google Scholar] [CrossRef]
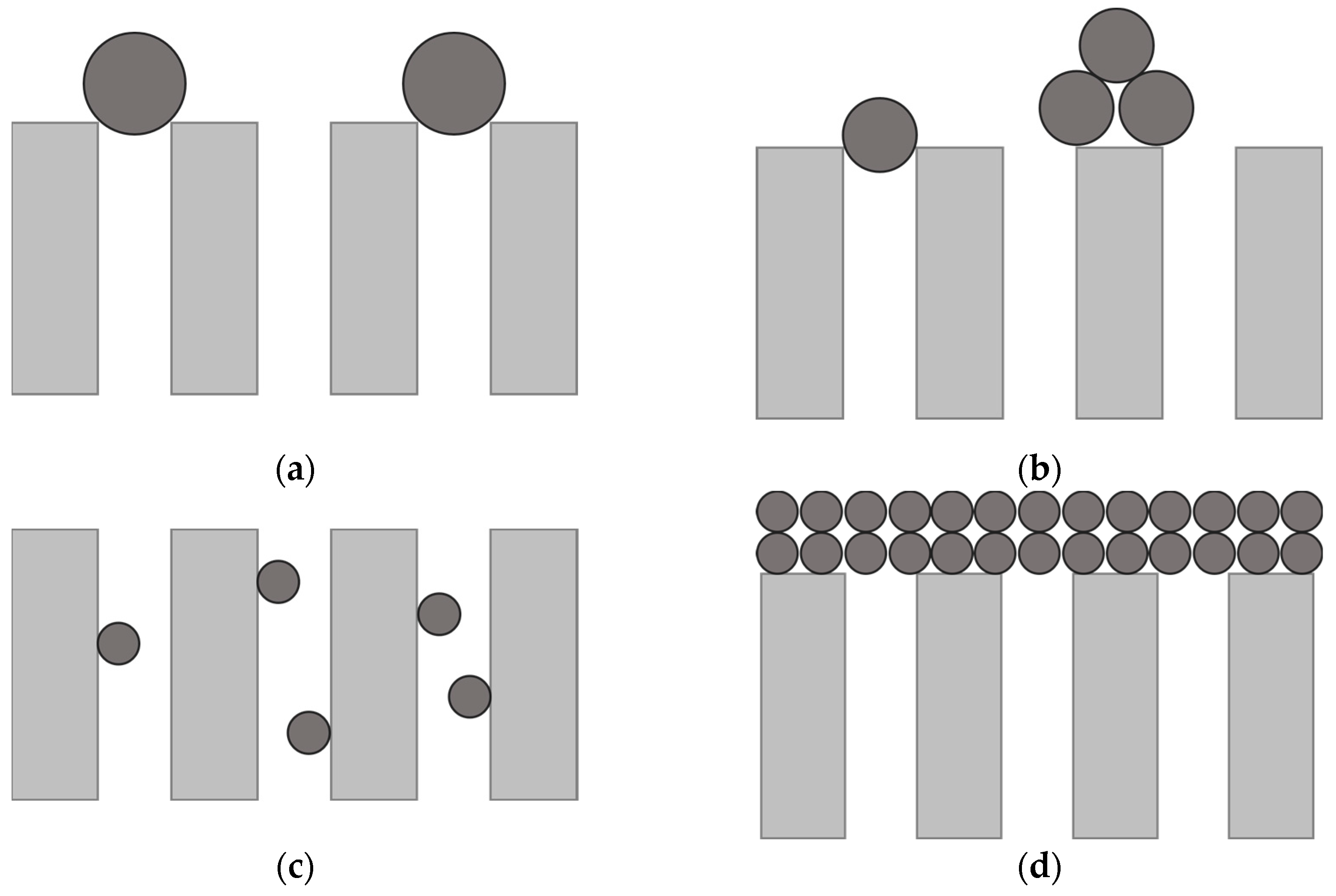


| Parameter | Value |
|---|---|
| pH | 3.75 ± 0.01 1 |
| Conductivity (mS/cm) | 4.8 ± 0.9 |
| COD (g/L) | 176.2 ± 0.5 |
| Turbidity (NTU) | 25,275 ± 301 |
| TSS (g/L) | 66.2 ± 4.7 |
| VSS (g/L) | 56.6 ± 5.2 |
| FSS (g/L) | 17.2 ± 2.8 |
| Total polyphenols (mg Tyrosol eq/L) | 2320 ± 109 |
| Membrane | INSIDE CéRAM™ | NF270 |
|---|---|---|
| Membrane type | Ultrafiltration | Nanofiltration |
| Material | TiO2 | Thin film composite polyamide |
| MWCO (Da) | 15,000 | 340 |
| Active area (cm2) | 2500 | 42 |
| Stabilized salt rejection (%) | - a | >97.0 |
| Free chlorine tolerance (ppm) | - | <0.1 |
| Maximum operation pressure (bar) | 4 | 41 |
| Maximum operating temperature (°C) | 350 | 45 |
| pH range | 2–12 | 3–10 (Continuous operation) 1–12 (Short-term cleaning) |
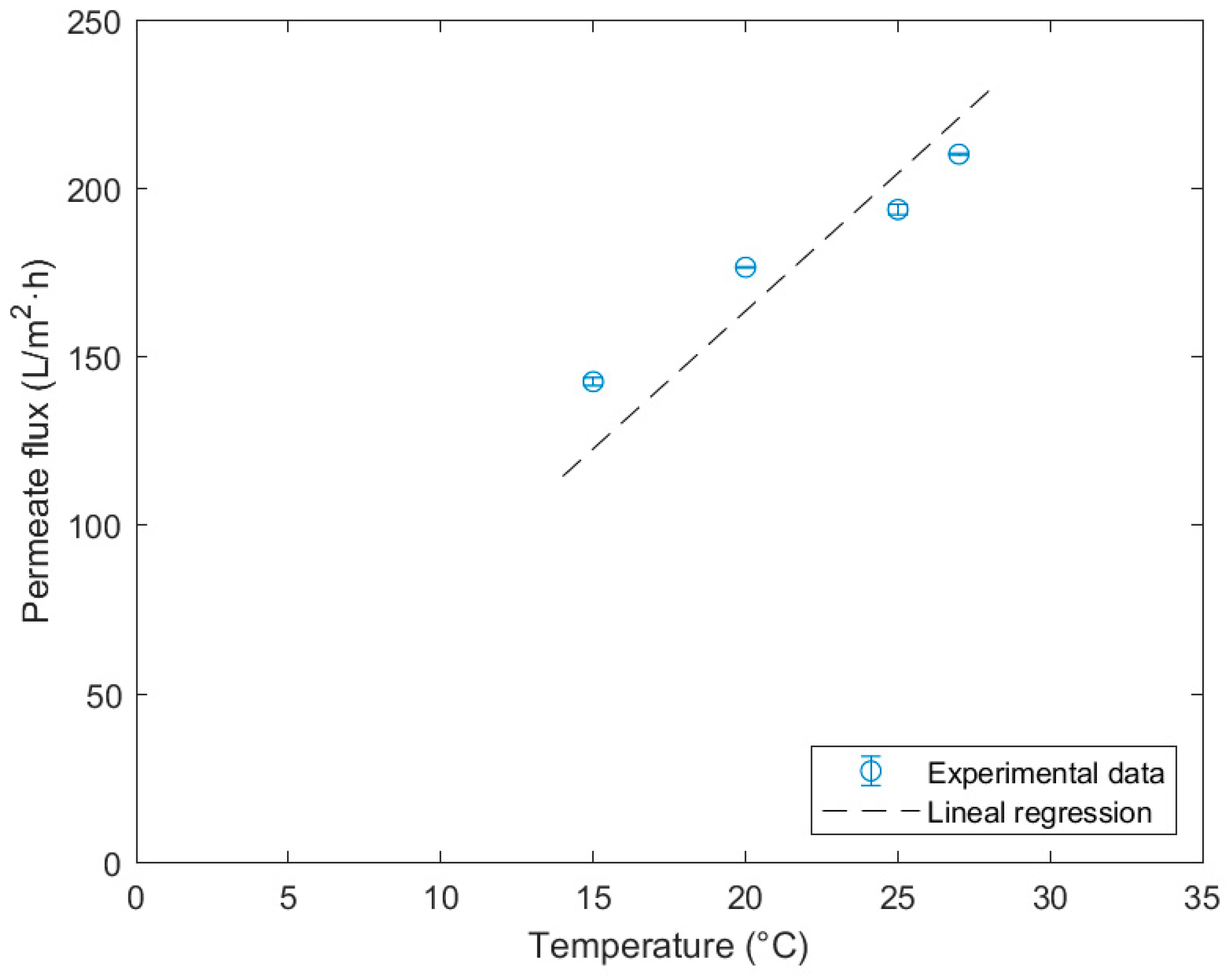
| Temperature (°C) | (L/m2·h) |
|---|---|
| 15 | 142.7 ± 1.2 |
| 20 | 176.7 ± 0.2 |
| 25 | 193.8 ± 1.5 |
| 27 | 210.2 ± 0.2 |
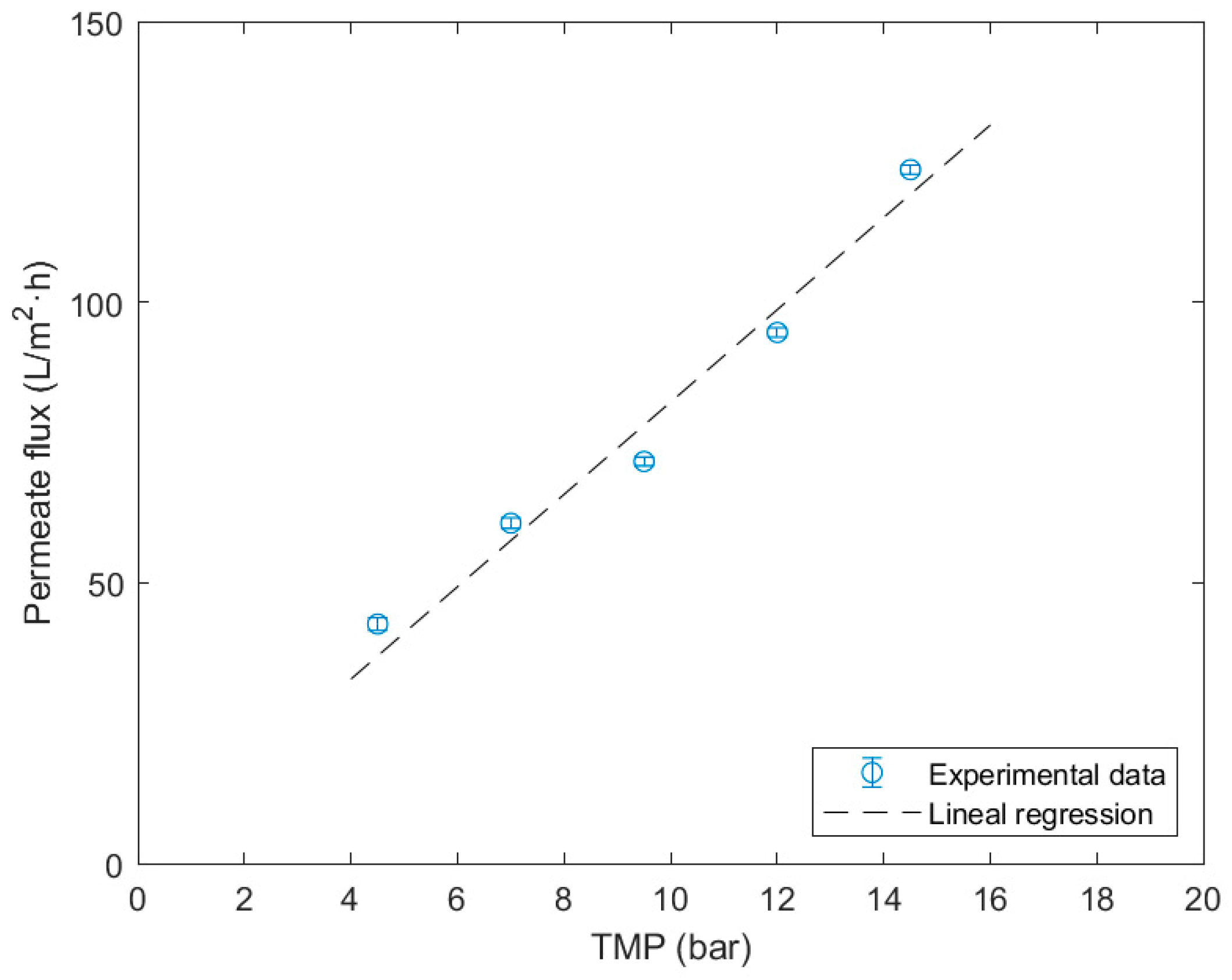
| TMP (bar) | (L/m2·h) |
|---|---|
| 4.5 | 42.7 ± 1.1 |
| 7.0 | 60.7 ± 1.0 |
| 9.5 | 71.7 ± 0.8 |
| 12.0 | 94.7 ± 0.8 |
| 14.5 | 123.9 ± 0.8 |
| Filtration Step | Experiment | Rejection (%) |
|---|---|---|
| UF | 1 | 57.6 ± 6.5 |
| 2 | 52.1 ± 4.8 | |
| 3 | 53.0 ± 4.2 | |
| NF | 1 | 90.0 ± 1.2 |
| 2 | 91.2 ± 0.6 | |
| 3 | 90.4 ± 1.0 | |
| 4 | 90.4 ± 1.2 | |
| 5 | 88.1 ± 2.0 | |
| 6 | 90.8 ± 0.8 |
| Experiment | Recovery (%) |
|---|---|
| 2 | 52.31 ± 0.03 |
| 4 | 48.79 ± 0.03 |
| 5 | 44.28 ± 0.02 |
| 6 | 83.68 ± 0.03 |
| Experiment | Recovery (%) |
|---|---|
| 3 | 120.91 ± 0.08 |
| 5 | 118.71 ± 0.08 |
| Filtration Step | Experiment | (L/m2·h) | Recovery (%) |
|---|---|---|---|
| UF | Initial flux | 170.6 ± 0.3 | - |
| Cleaning with NaOH | 157.8 ± 0.2 | 92.5 ± 0.3 | |
| Cleaning with distilled water | 61.4 ± 0.2 | 36.0 ± 0.2 | |
| NF | Initial flux | 71.8 ± 0.9 | - |
| Cleaning with NaOH | 96.7 ± 1.0 | 134.8 ± 0.7 | |
| Cleaning with distilled water | 53.8 ± 2.4 | 75.0 ± 0.3 |
| Fouling Model | Fitting Parameter | Value |
|---|---|---|
| Complete blocking | (1/m) | 86.5 ± 5.1 |
| Intermediate blocking | (1/m) | 95.3 ± 6.2 |
| Standard blocking | (1/s)·104 | 87.3 ± 3.8 |
| Cake layer formation | (s/m2)·10−7 | 1.07 ± 0.04 |
| Sampling Time | Experiment | Total Polyphenols (mg Tyrosol eq/L) |
|---|---|---|
| Before filtration | 1 | 2320 ± 109 |
| 2 | 2482 ± 304 | |
| 3 | 2231 ± 246 | |
| After filtration | 1 | 1585 ± 95 |
| 2 | 1588 ± 81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reig-Valor, M.-J.; Rozas-Martínez, J.; López-Borrell, A.; Lora-García, J.; López-Pérez, M.-F. Experimental Study of a Sequential Membrane Process of Ultrafiltration and Nanofiltration for Efficient Polyphenol Extraction from Wine Lees. Membranes 2024, 14, 82. https://doi.org/10.3390/membranes14040082
Reig-Valor M-J, Rozas-Martínez J, López-Borrell A, Lora-García J, López-Pérez M-F. Experimental Study of a Sequential Membrane Process of Ultrafiltration and Nanofiltration for Efficient Polyphenol Extraction from Wine Lees. Membranes. 2024; 14(4):82. https://doi.org/10.3390/membranes14040082
Chicago/Turabian StyleReig-Valor, Miguel-Jorge, Javier Rozas-Martínez, Alexis López-Borrell, Jaime Lora-García, and María-Fernanda López-Pérez. 2024. "Experimental Study of a Sequential Membrane Process of Ultrafiltration and Nanofiltration for Efficient Polyphenol Extraction from Wine Lees" Membranes 14, no. 4: 82. https://doi.org/10.3390/membranes14040082
APA StyleReig-Valor, M.-J., Rozas-Martínez, J., López-Borrell, A., Lora-García, J., & López-Pérez, M.-F. (2024). Experimental Study of a Sequential Membrane Process of Ultrafiltration and Nanofiltration for Efficient Polyphenol Extraction from Wine Lees. Membranes, 14(4), 82. https://doi.org/10.3390/membranes14040082








