Cell Type-Specific Anti- and Pro-Oxidative Effects of Punica granatum L. Ellagitannins
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Chemicals
2.2. Methods
2.2.1. DPPH Assay
2.2.2. ABTS Assay
2.2.3. Superoxide Anion Radical Assay
2.2.4. Nitric Oxide Assay
2.2.5. Preparation of Erythrocytes
2.2.6. Hemolysis Study
2.2.7. Measurement of ROS in Erythrocytes
2.2.8. Detection of Reduced Glutathione
2.2.9. Determination of Erythrocyte Oxidative Hemolysis
2.2.10. HeLa Cell Culture
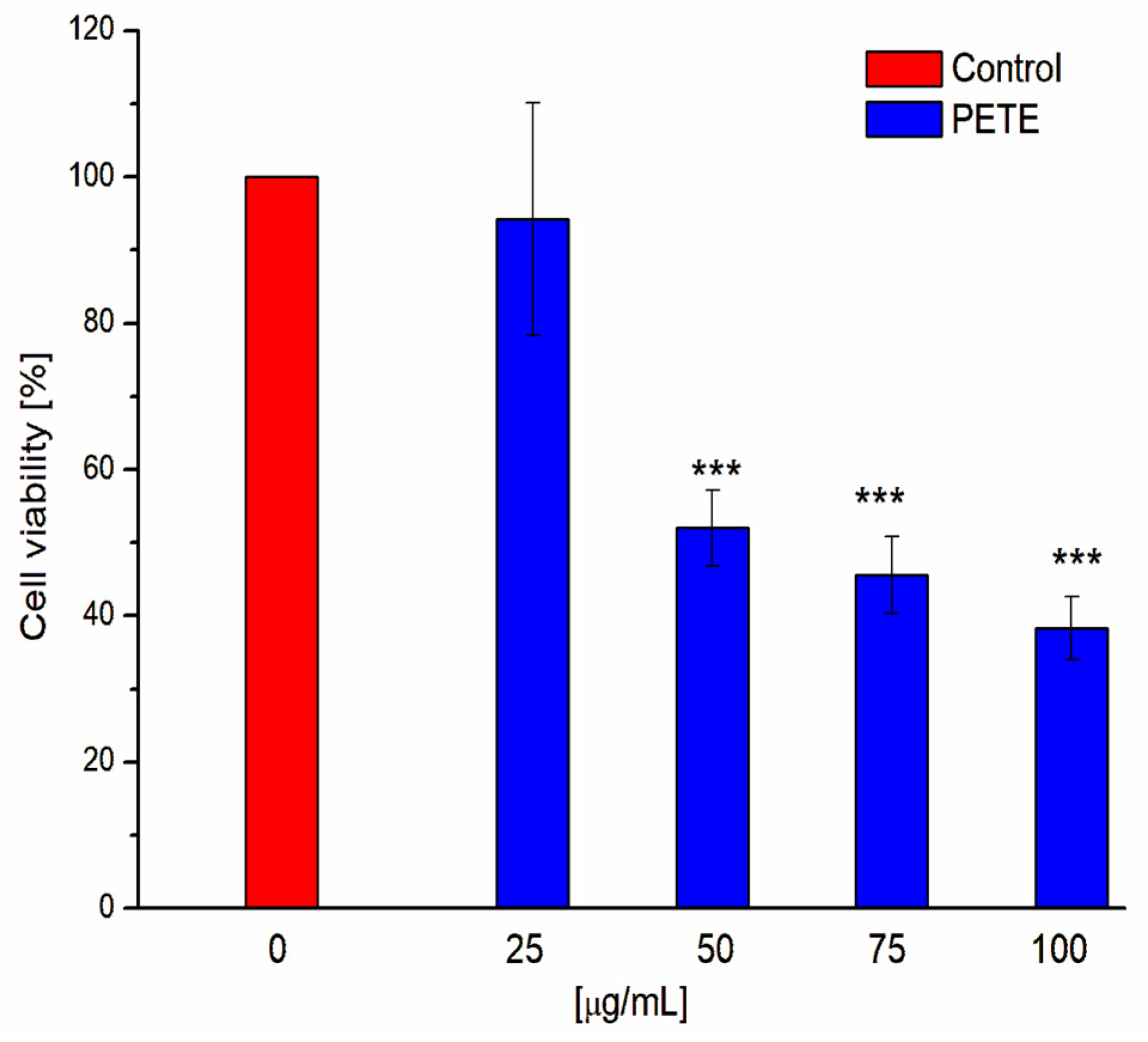
2.2.11. Measurement of HeLa Cells Viability—MTT Assay
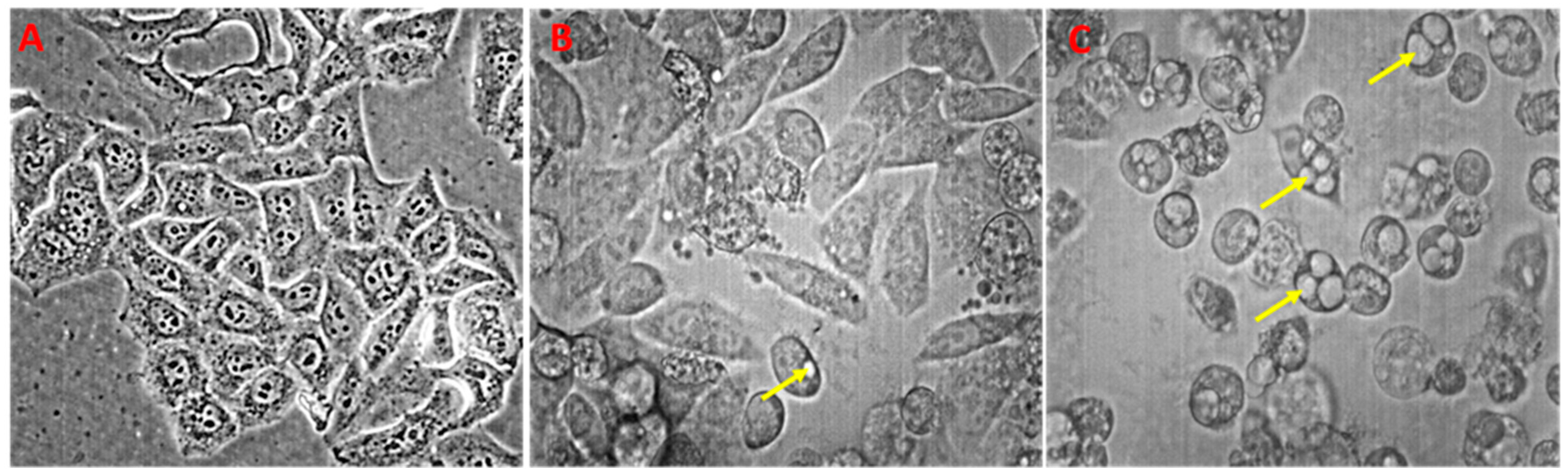
2.2.12. Analyses of HeLa Cells Morphology Changes
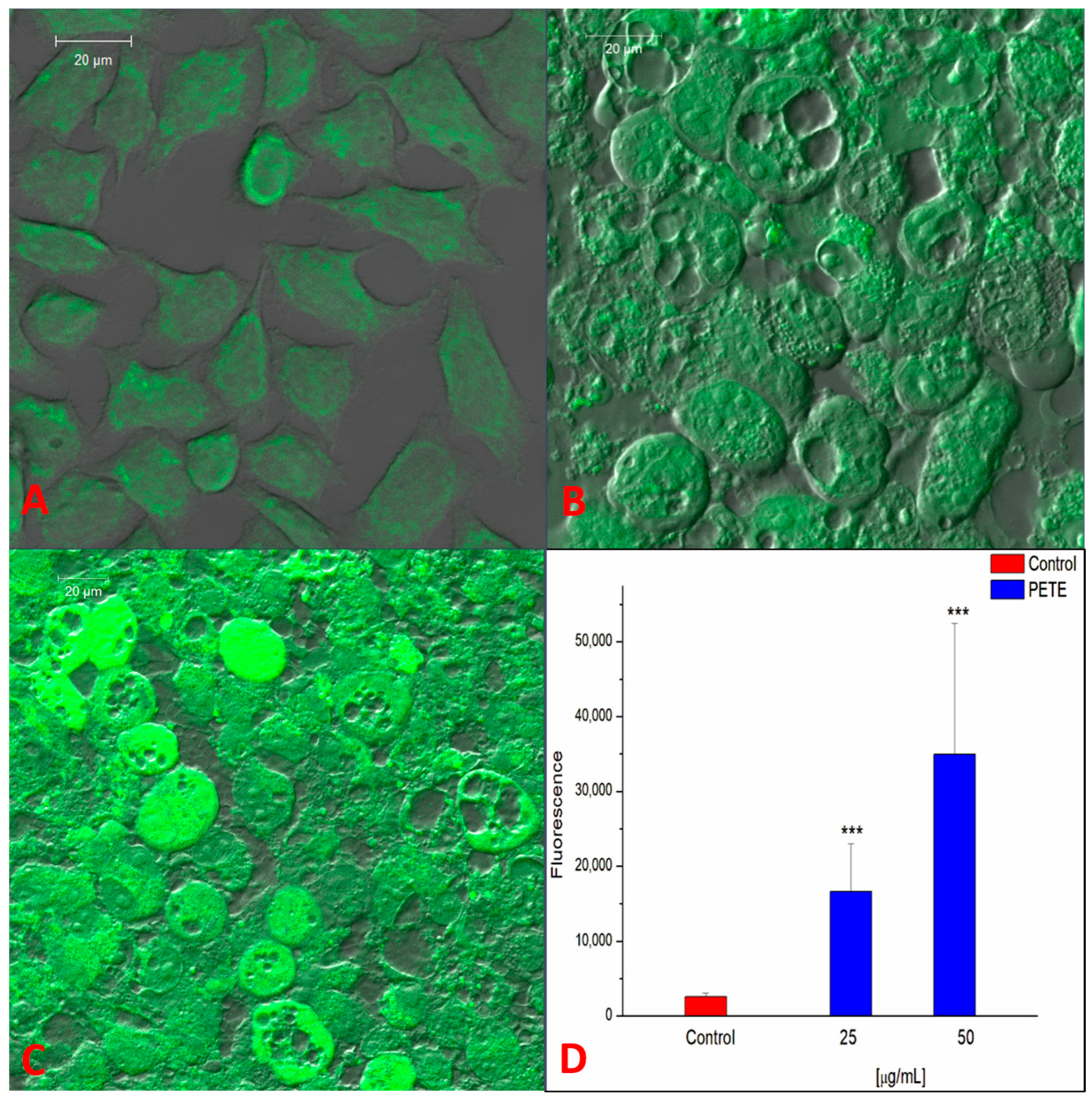
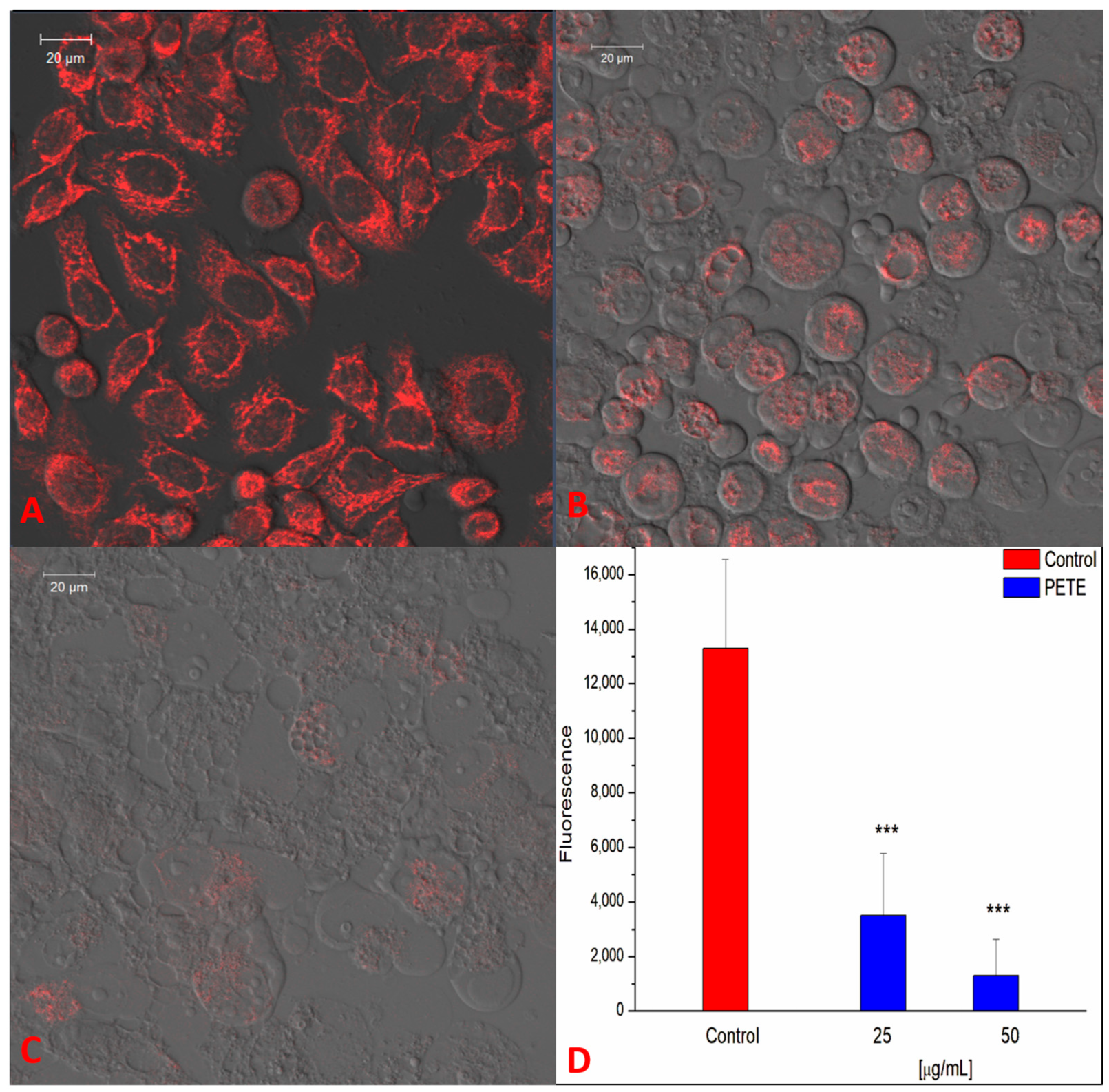
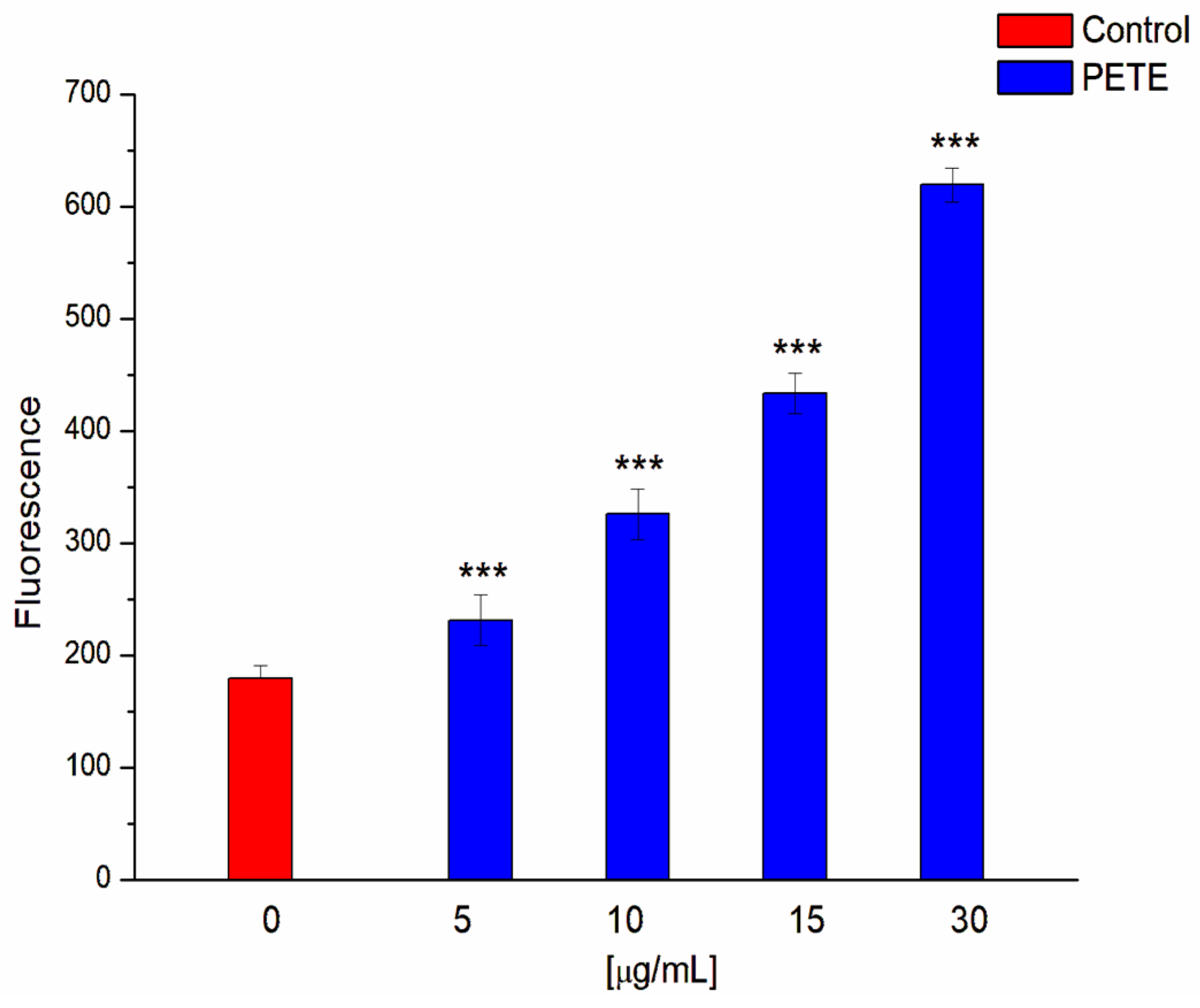
2.2.13. Measurement of ROS—Laser Scanning Confocal Microscopy (LSCM)
2.2.14. Analysis of HeLa Cells’ Mitochondrial Potential
2.2.15. Image Analysis
2.2.16. Bacterial Strain and Growth Conditions
2.2.17. Antimicrobial Activity
2.2.18. Measurement of ROS in S. aureus
2.2.19. Data Analysis
3. Results and Discussion
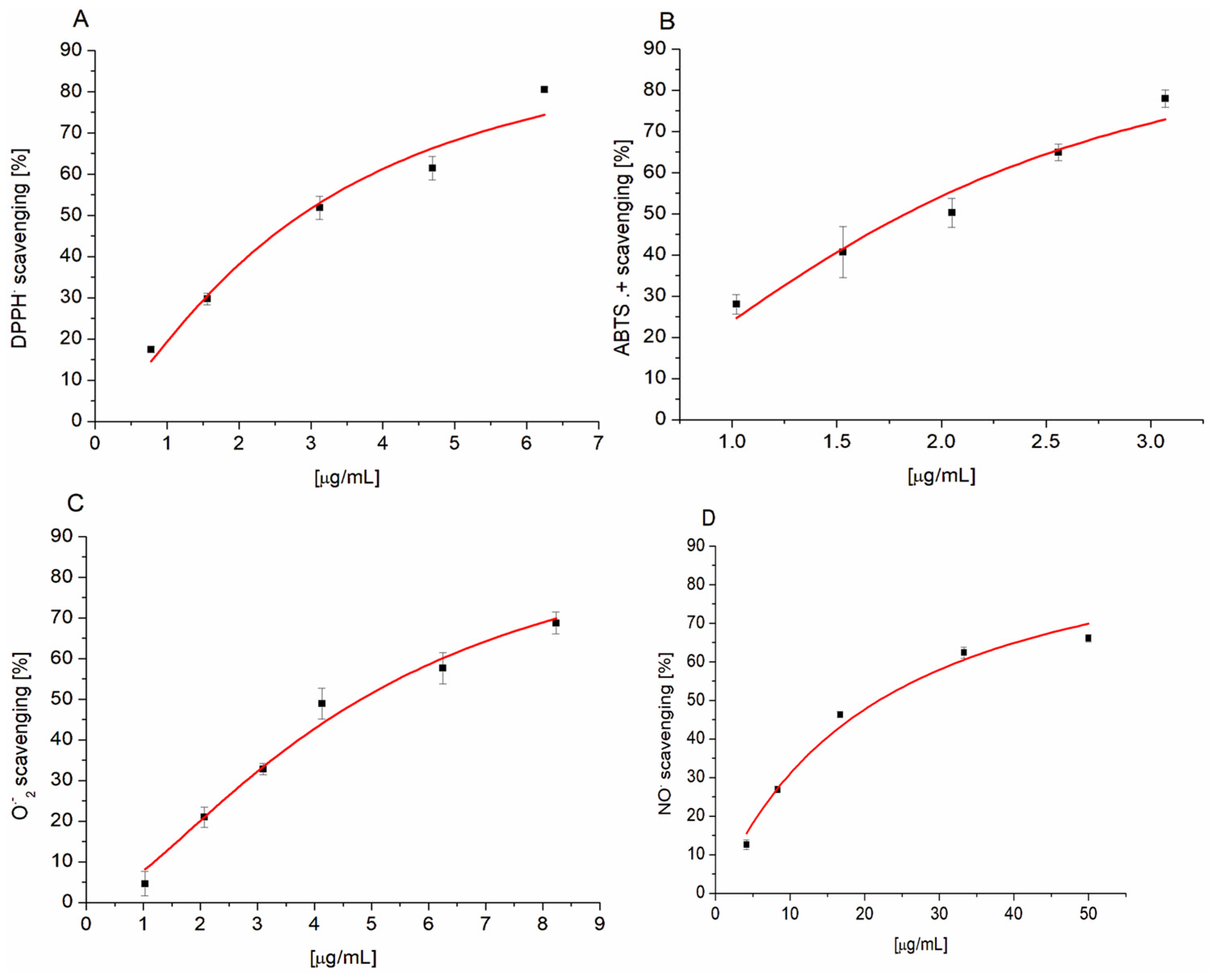
3.1. The Free Radical Scavenging Activity of PETE
3.2. Antioxidative and Pro-Oxidative Effect of PETE in Erythrocyte Model
3.3. Anticancer and Pro-Oxidative Activities of PETE in HeLa Cells
3.4. The Influence of PETE on S. aureus Growth and Viability and on Bacterial ROS Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Babich, H.; Schuck, A.G.; Weisburg, J.H.; Zuckerbraun, H.L. Research Strategies in the Study of the Pro-Oxidant Nature of Polyphenol Nutraceuticals. J. Toxicol. 2011, 2011, 467305. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lan, W.; Xie, J. Natural phenolic compounds: Antimicrobial properties, antimicrobial mechanisms, and potential utilization in the preservation of aquatic products. Food Chem. 2024, 440, 138198. [Google Scholar] [CrossRef] [PubMed]
- Cortez-Trejo, M.C.; Olivas-Aguirre, F.J.; Dufoo-Hurtado, E.; Castaneda-Morena, R.; Villegas-Quintero, H.; Medina-Franco, J.L.; Mendoza, S.; Wall-Medrano, A. Potential anticancer activity of Pomegranate (Punica granatum L.) fruits of different color: In vitro and in silico evidence. Biomolecules 2022, 12, 1649. [Google Scholar] [CrossRef]
- Das, A.K.; Nanda, P.K.; Chowdhury, N.R.; Dandapat, P.; Gagaoua, M.; Chauhan, P.; Peteiro, M.; Lorenzo, J.M. Application of Pomegranate by-products in muscle foods: Oxidative indices, colour stability, shelf life and health benefits. Molecules 2021, 26, 467. [Google Scholar] [CrossRef]
- Khwairakpam, A.D.; Bordoloi, D.; Thakur, K.K.; Monisha, J.; Arfuso, F.; Sethi, G.; Mishra, S.; Kumar, A.P.; Kunnumakkara, A.B. Possible use of Punica granatum (Pomegranate) in cancer therapy. Pharmacol. Res. 2018, 133, 53–64. [Google Scholar] [CrossRef]
- Lavoro, A.; Falzone, L.; Gattuso, G.; Salemi, R.; Cultrera, G.; Leone, G.M.; Scandurra, G.; Candido, S.; Libra, M. Pomegranate: A promising avenue against the most common chronic diseases and their associated risk factors (Review). Int. J. Funct. Nutr. 2021, 2, 6. [Google Scholar] [CrossRef]
- Maphetu, N.; Unuofin, J.O.; Masuku, N.P.; Olisah, C.; Lebelo, S.L. Medicinal uses, pharmacological activities, phytochemistry, and the molecular mechanisms of Punica granatum L. (pomegranate) plant extracts: A review. Biomed. Pharmacother. 2022, 153, 113256. [Google Scholar] [CrossRef]
- Dhawan, V. Reactive Oxygen and Nitrogen Species: General Considerations. Studies on Respiratory Disorders; Springer: New York, NY, USA, 2014; pp. 27–47. [Google Scholar]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell Longev. 2016, 16, 4350965. [Google Scholar] [CrossRef]
- Sieniawska, E. Activities of tannins—From in vitro studies to clinical trials. Nat. Prod. Commun. 2015, 10, 1877–1884. [Google Scholar] [CrossRef]
- Molino, S.; Pilar Francino, M.; Ángel Rufián Henares, J. Why is it important to understand the nature and chemistry of tannins to exploit their potential as nutraceuticals? Food Res. Int. 2023, 173, 113329. [Google Scholar] [CrossRef]
- Quideau, S. (Ed.) Chemistry and Biology of Ellagitannins: An Underestimated Class of Bioactive Plant Polyphenols; World Scientific Publishing Co. Pte. Ltd.: Singapore, 2009; pp. 1–81. [Google Scholar]
- Fukumoto, L.R.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef] [PubMed]
- Maroziene, A.; Nemeikaite-Ceniene, A.; Vidziunaite, R.; Cenas, N. Correlation between mammalian cell cytotoxicity of flavonoids and the redox potential of phenoxyl radical/phenol couple. Acta Biochim. Pol. 2012, 59, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Barbehem, R.V.; Jones, C.P.; Hagerman, A.E.; Karonen, M.; Salminen, J.-P. Ellagitannins have greater oxidative activities than condensed tannins and galloylglucoses at high pH: Potential impact on caterpillars. J. Chem. Ecol. 2006, 32, 2253–2267. [Google Scholar] [CrossRef]
- Salminen, J.-P.; Karonen, M. Chemical ecology of tannins and other phenolics: We need a change in approach. Funct. Ecol. 2011, 25, 325–338. [Google Scholar] [CrossRef]
- Mavlyanov, S.M.; Islambekov, S.Y.; Karimdzhanov, A.K.; Ismailov, A.I. Polyphenols of some varieties of pomegranate growing in Uzbekistan. Chem. Nat. Comp. 1997, 1, 124–127. [Google Scholar] [CrossRef]
- Olchowik, E.; Sciepuk, A.; Mavlyanov, S.; Abdullajanova, N.; Zamaraeva, M. Antioxidant capacities of polyphenols from Sumac (Rhus typhina L.) leaves in protection of erythrocytes against oxidative damage. Biomed. Prev. Nutr. 2012, 2, 99–105. [Google Scholar] [CrossRef]
- Olchowik-Grabarek, E.; Mavlyaonov, S.; Abdullajanova, N.; Gieniusz, R.; Zamaraeva, M. Specificity of hydrolysable tannins from Rhus typhina L. to oxidants in cell and cell-free models. Appl. Biochem. Biotechnol. 2017, 181, 495–510. [Google Scholar] [CrossRef]
- Olchowik, E.; Lotkowski, K.; Mavlyanov, S.; Abdullajanova, N.; Ionov, M.; Bryszewska, M.; Zamaraeva, M. Stabilization of erythrocytes against oxidative and hypotonic stress by tannins isolated from sumac leaves (Rhus typhina L.) and grape seeds (Vitis vinifera L.). Cell. Mol. Biol. Lett. 2012, 17, 333–348. [Google Scholar] [CrossRef]
- Wojtala, A.; Bonora, M.; Malinska, D.; Pinton, P.; Duszynski, J.; Wieckowski, M.R. Chapter Thirteen—Methods to Monitor ROS Production by Fluorescence Microscopy and Fluorometry. Methods Enzymol. 2014, 524, 243–262. [Google Scholar]
- Joshi, D.C.; Bakowska, J.C. Determination of mitochondrial membrane potential and reactive oxygen species in live rat cortical neurons. J. Vis. Exp. 2011, 51, 2704. [Google Scholar]
- Olchowik-Grabarek, E.; Czerkas, K.; Matchanov, A.D.; Esanov, R.S.; Matchanov, U.D.; Zamaraeva, M.; Sekowski, S. Antibacterial and antihemolytic activity of new biomaterial based on glycyrrhizic acid and quercetin (GAQ) against Staphylococcus aureus. J. Funct. Biomater. 2023, 14, 368. [Google Scholar] [CrossRef] [PubMed]
- Elfalleh, W.; Nasri, N.; Marzougui, N.; Thabti, I.; M’rabet, A.; Yahya, Y.; Lachiheb, B.; Guasmi, F.; Ferchichi, A. Physico-chemical properties and DPPH-ABTS scavenging activity of some local pomegranate (Punica granatum) ecotypes. Int. J. Food Sci. Nutr. 2009, 60, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Kowalewska, E.; Litwinienko, G. Phenolic chain-breaking antioxidants—Their activity and mechanisms of action. Postepy Biochemii 2010, 56, 274–283. [Google Scholar] [PubMed]
- Li, X.; Wang, X.; Chen, D.; Chen, S. Antioxidant activity and mechanism of protocatechuic acid in vitro. Funct. Food Health Dis. 2011, 7, 232–244. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M. Erythrocytes as a biological model for screening of xenobiotics toxicity. Chem. Biol. Interact. 2018, 279, 73–83. [Google Scholar] [CrossRef]
- Bettiol, A.; Galora, S.; Argento, F.R.; Fini, E.; Emmi, G.; Mattiolo, I.; Bagni, G.; Fiorillo, C.; Becatti, M. Erythrocyte oxidative stress and thrombosis. Expert. Rev. Mol. Med. 2022, 24, e31. [Google Scholar] [CrossRef]
- Pennathur, S.; Maitra, D.; Byun, J.; Slikovic, I.; Abdulhamid, I.; Saed, G.M.; Diamond, M.P.; Abu-Soud, H.M. Potent antioxidative activity of lycopene: A potential role in scavenging hypochlorous acid. Free Radic. Biol. Med. 2010, 49, 205–213. [Google Scholar] [CrossRef]
- Zavodnik, I.B.; Lapshina, E.A.; Zavodnik, L.B.; Bartosz, G.; Soszynski, M.; Bryszewska, M. Hypochlorous acid damages erythrocyte membrane proteins and alters lipid bilayer structure and fluidity. Free Radic. Biol. Med. 2001, 30, 363–369. [Google Scholar] [CrossRef]
- Suwalsky, M.; Avello, M.; Obreque, J.; Villena, F.; Szymanska, R.; Stojakowska, A.; Strzalka, K. Protective effect of Philesia magellanica (Coicopihue) from Chilean Patagonia against oxidative damage. J. Chil. Chem. Soc. 2015, 60, 2935–2939. [Google Scholar] [CrossRef][Green Version]
- Lu, Y.; Liang, J.-K.; Wang, H.-Y.; Wang, C.; Song, Z.-M.; Hu, Q.; Wu, Q.-Y. Novel chlorinated disinfection byproducts from tannic acid: Nontargeted identification, formation pathways, and computationally predicted toxicity. J. Hazard. Mater. 2022, 425, 127827. [Google Scholar] [CrossRef]
- Koren, E.; Kohen, R.; Ginsburg, I. Polyphenols enhance total oxidant—Scavenging capacities of human blood by binding to red blood cells. Exp. Biol. Med. 2010, 235, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.K.; Gupta, S.K.; Jacob, M.R.; Khan, S.I.; Ferreira, D. Antioxidant, antimalarial and antimicrobial activities of tannin-rich fractions, ellagitannins and phenolic acids from Punica granatum L. Planta Med. 2007, 73, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Barrajon-Catalan, E.; Fernandez-Arroyo, S.; Saura, D.; Guillen, E.; Fernandez-Gutierrez, A.; Segura-Carretero, A.; Micol, V. Cistaceae aqueous extracts containing ellagitannins show antioxidant and antimicrobial capacity, and cytotoxic activity against human cancer cells. Food Chem. Toxicol. 2010, 48, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Sakagami, H.; Jiang, Y.; Kusama, K.; Atsumi, T.; Ueha, T.; Toguchi, M.; Iwakura, I.; Satoh, K.; Ito, H.; Hatano, T.; et al. Cytotoxic activity of hydrolyzable tannins against human oral tumor cell lines—A possible mechanism. Phytomedicine 2000, 7, 39–47. [Google Scholar] [CrossRef]
- Zarin, A.B.; Wan, H.Y.; Isha, A.; Armania, N. Antioxidant, antimicrobial and cytotoxic potential of condensed tannins from Leucaena leucocephala hybrid-Rendang. Food Sci. Hum. Wellness 2016, 5, 65–75. [Google Scholar]
- Sepehr, K.S.; Baradaran, B.; Mazandarani, M.; Khori, V.; Shahneh, F.Z. Studies on the Cytotoxic Activities of Punica granatum L. var. spinosa (Apple Punice) Extract on Prostate Cell Line by Induction of Apoptosis. ISRN Pharm. 2012, 2012, 547942. [Google Scholar]
- Sepehr, K.S.; Baradaran, B.; Mazandarani, M.; Yousefi, B.; Alitappeh, A.M.; Khori, V. Growth-Inhibitory and Apoptosis-Inducing Effects of Punica granatum L. var. spinosa (Apple Punice) on Fibrosarcoma Cell Lines. Adv. Pharm. Bull. 2014, 4, 583–590. [Google Scholar]
- Rajesekar, N.; Sivanantham, A.; Ravikumar, V.; Rajasekaran, S. An overview on the role of plant-derived tannins for the treatment of lung cancer. Phytochemistry 2021, 188, 112799. [Google Scholar] [CrossRef]
- Hasima, N.; Ozpolat, B. Regulation of autophagy by polyphenolic compounds as a potential therapeutic strategy for cancer. Cell Death Dis. 2014, 5, e1509. [Google Scholar] [CrossRef]
- Zhao, W.; Shi, F.; Guo, Z.; Zhao, J.; Song, X.; Yang, H. Metabolite of ellagitannins, urolithin A induces autophagy and inhibits metastasis in human sw620 colorectal cancer cells. Mol. Carcinog. 2018, 57, 193–200. [Google Scholar] [CrossRef]
- Tan, S.; Yu, C.Y.; Sim, Z.W.; Low, Z.S.; Lee, B.; See, F.; Min, N.; Gautam, A.; Chu, J.J.H.; Ng, K.W.; et al. Pomegranate activates TFEB to promote autophagy-lysosomal fitness and mitophagy. Sci. Rep. 2019, 9, 727. [Google Scholar] [CrossRef] [PubMed]
- Elango, S.; Balwas, R.; Padma, V.V. Gallic Acid Isolated from Pomegranate Peel Extract Induces Reactive Oxygen Species Mediated Apoptosis in A549 Cell Line. J. Cancer Ther. 2011, 2, 638–645. [Google Scholar] [CrossRef][Green Version]
- Ślęzak, A.; Moreira, H.; Szyjka, A.; Oszmiański, J.; Gąsorowski, K. Conditions of prooxidant activity of Cistus and Pomegrante polyphenols in V79 cell cultures. Acta Pol. Pharm. 2017, 74, 670–678. [Google Scholar]
- Czarnecka, A.M.; Golik, P.; Bartnik, E. Mitochondria jako integratory apoptozy. Postępy Biol. Komórki 2006, 33, 525–541. [Google Scholar]
- Rambold, A.S.; Lippincott-Schwartz, J. Mechanisms of mitochondria and autophagy crosstalk. Cell Cycle 2011, 10, 4032–4038. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, H.; Zhu, Y.; Liu, S.; Yuan, Z.; Wu, J.; Wen, L. Tannic Acid Induces the Mitochondrial Pathway of Apoptosis and S Phase Arrest in Porcine Intestinal IPEC-J2 Cells. Toxins 2019, 11, 397. [Google Scholar] [CrossRef]
- Sp, N.; Kang, D.Y.; Jo, E.S.; Rugamba, A.; Kim, W.S.; Park, Y.-M.; Hwang, D.-Y.; Yoo, J.-S.; Liu, Q.; Jang, K.-J.; et al. Tannic Acid Promotes TRAIL-Induced Extrinsic Apoptosis by Regulating Mitochondrial ROS in Human Embryonic Carcinoma Cells. Cells 2020, 9, 282. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, J.; Chen, N.G.; Shi, Z.; Qiu, J.; He, C.; Chen, M. Recent Advances in Anticancer Activities and Drug Delivery Systems of Tannins. Med. Res. Rev. 2017, 37, 665–701. [Google Scholar] [CrossRef]
- Toda, K.; Ueyama, M.; Tanaka, S.; Tsukayama, I.; Mega, T.; Konoike, Y.; Tamenobu, A.; Bastian, F.; Akai, I.; Ito, H.; et al. Ellagitannins from Punica granatum leaves suppress microsomal prostaglandin E synthase-1expression and induce lung cancer cells to undergo apoptosis. Biosci. Biotechnol. Biochem. 2020, 84, 757–763. [Google Scholar] [CrossRef]
- Jia, L.; Jin, H.; Zhou, J.; Chen, L.; Lu, Y.; Ming, Y.; Yu, Y. A potential anti-tumor herbal medicine, Corilagin, inhibits ovarian cancer cell growth through blocking the TGF-β signaling pathways. BMC Complement. Altern. Med. 2013, 13, 33. [Google Scholar] [CrossRef]
- Nagesh, P.K.B.; Chowdhury, P.; Hatami, E.; Jain, S.; Dan, N.; Kashyap, V.K.; Chauhan, S.C.; Jaggi, M.; Yallapu, M.M. Tannic acid inhibits lipid metabolism and induce ROS in prostate cancer cells. Sci. Rep. 2020, 10, 980. [Google Scholar] [CrossRef] [PubMed]
- You, B.R.; Moon, H.J.; Han, Y.H.; Park, W.H. Gallic acid inhibits the growth of HeLa cervical cancer cells via apoptosis and/or necrosis. Food Chem. Toxicol. 2010, 48, 1334–1340. [Google Scholar] [CrossRef] [PubMed]
- Labieniec, M.; Gabryelak, T.; Falcioni, G. Antioxidant and pro-oxidant effects of tannins in digestive cells of the freshwater mussel Unio tumidus. Mutat. Res. 2003, 539, 19–28. [Google Scholar] [CrossRef]
- Seremet, D.; Durgo, K.; Kosanovic, J.; Turkovic, A.H.; Jaric, A.M.; Cebin, A.V.; Komes, D. Studying the Functional Potential of Ground Ivy (Glechoma hederacea L.) Extract Using an In Vitro Methodology. Int. J. Mol. Sci. 2023, 24, 16975. [Google Scholar] [CrossRef]
- Moilanen, J.; Salminen, J.-P. Ecologically neglected tannins and their biologically relevant activity: Chemical structures of plant ellagitannins reveal their in vitro oxidative activity at high pH. Chemoecology 2008, 18, 73–83. [Google Scholar] [CrossRef]
- Mileo, A.M.; Miccadei, S. Polyphenols as Modulator of Oxidative Stress in Cancer Disease: New Therapeutic Strategies. Oxid. Med. Cell Longev. 2016, 2016, 6475624. [Google Scholar] [CrossRef]
- Leon-Gonzalez, A.J.; Auger, C.; Schini-Kerth, V.B. Pro-oxidant activity of polyphenols and its implication on cancer chemoprevention and chemotherapy. Biochem. Pharmacol. 2015, 98, 371–380. [Google Scholar] [CrossRef]
- Liou, G.-Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef]
- Howden, B.P.; Giulieri, S.G.; Lung, T.W.F.; Baines, S.L.; Sharkey, L.K.; Jean, Y.; Lee, H.; Hachani, A.; Monk, I.R.; Stinear, T.P. Staphylococcus aureus host interactions and adaptation. Nat. Rev. Microbiol. 2023, 21, 380–395. [Google Scholar] [CrossRef]
- O’Reilly, M.; de Azavedo, J.C.; Kennedy, S.; Foster, T.J. Inactivation of alpha-haemolysin gene of Staphylococcus aureus 8325-4 by site-directed mutagenesis and studies on the expression of its haemolysins. Microb. Pathog. 1986, 1, 125–138. [Google Scholar] [CrossRef]
- Olchowik-Grabarek, E.; Sekowski, S.; Bitiucki, M.; Dobrzynska, I.; Shlyonsky, V.; Ionov, M.; Burzynski, P.; Roszkowska, A.; Swiecicka, I.; Abdulladjanova, N.; et al. Inhibition of interaction between Staphylococcus aureus α-hemolysin and erythrocytes membrane by hydrolysable tannins: Structure-related activity study. Sci. Rep. 2020, 10, 11168. [Google Scholar] [CrossRef] [PubMed]
- Olchowik-Grabarek, E.; Sekowski, S.; Mies, F.; Bitiucki, M.; Swiecicka, I.; Abdulladjanova, N.; Shlyonsky, V.; Zamaraeva, M. Electrophysiological and spectroscopic investigation of hydrolysable tannins interaction with α-hemolysin of S. aureus. Bioelectrochemistry 2023, 150, 108318. [Google Scholar] [CrossRef] [PubMed]
- Olchowik-Grabarek, E.; Mies, F.; Sekowski, S.; Dubis, A.; Laurent, P.; Zamaraeva, M.; Swiecicka, I.; Shlyonsky, V. Enzymatic Synthesis and Characterization of Aryl Iodides of Some Phenolic Acids with Enhanced Antibacterial Properties. Biochim. Biophys. Acta Biomembr. 2022, 1864, 184011. [Google Scholar] [CrossRef] [PubMed]
- Prochazkova, D.; Bousova, I.; Wilhelmova, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Zhao, X.; Drlica, K. Reactive oxygen species and the bacterial response to lethal stress. Curr. Opin. Microbiol. 2014, 21, 1–6. [Google Scholar] [CrossRef]
- Lagage, V.; Chen, V.; Uphoff, S. Adaptation delay causes a burst of mutations in bacteria responding to oxidative stress. EMBO Rep. 2023, 24, e55640. [Google Scholar] [CrossRef]
- Zeng, F.; Shao, S.; Zou, Z.; Guo, S.; Cai, Y.; Yan, C.; Chen, Y.; Wang, M.; Shi, T. Multi-Omics Revealed Antibacterial Mechanisms of Licochalcone a Against Mrsa and its Potential in Fresh Pork Preservation. SSRN preprint. [CrossRef]
- He, L.; Cheng, H.; Chen, F.; Song, S.; Zhang, H.; Sun, W.; Bao, X.; Zhang, H.; He, C. Oxidative Stress-Mediated Antibacterial Activity of the Total Flavonoid Extracted from the Agrimonia pilosa Ledeb. In Methicillin-Resistant Staphylococcus aureus (MRSA). Vet. Sci. 2022, 9, 71. [Google Scholar] [CrossRef]
- Sinsinwar, S.; Vadivel, V. Catechin isolated from cashew nut shell exhibits antibacterial activity against clinical isolates of MRSA through ROS-mediated oxidative stress. Appl. Microbiol. Biotechnol. 2020, 104, 8279–8297. [Google Scholar] [CrossRef]
- Khodade, V.S.; Chandra, M.S.; Banerjee, A.; Lihiri, S.; Pulipeta, M.; Rangarajan, R.; Chakrapani, H. Bio-reductively activated reactive oxygen species (ROS) generators as MRSA inhibitors. ACS Med. Chem. Lett. 2014, 5, 777–781. [Google Scholar] [CrossRef]
- Zhang, L.; Guan, Q.; Jiang, J.; Khan, M.S. Tannin complexation with metal ions and its implication on human health, environment and industry: An overview. Int. J. Biol. Macromol. 2023, 253, 127485. [Google Scholar] [CrossRef] [PubMed]
- Nath, D.; Ghangrekar, M.M. Plant secondary metabolites induced electron flux in microbial fuel cell: Investigation from laboratory-to-field scale. Sci. Rep. 2020, 10, 17185. [Google Scholar] [CrossRef] [PubMed]
- Dadi, P.K.; Ahmad, M.; Ahmad, Z. Inhibition of ATPase activity of Escherichia coli ATP synthase by polyphenols. Int. J. Biol. Macromol. 2009, 45, 72–79. [Google Scholar] [CrossRef]
- Makarewicz, M.; Drożdż, I.; Tarko, T.; Duda-Chodak, A. The Interactions between Polyphenols and Microorganisms, Especially Gut Microbiota. Antioxidants 2021, 10, 188. [Google Scholar] [CrossRef]
- Fakudze, N.T.; Aniogo, E.C.; George, B.P.; Abrahamse, H. The Therapeutic Efficacy of Punica granatum and Its Bioactive Constituents with Special Reference to Photodynamic Therapy. Plants 2022, 11, 2820. [Google Scholar] [CrossRef]

| Radical | PETE | Trolox |
|---|---|---|
| IC50 [µg/mL] | ||
| DPPH | 2.857 ± 0.223 | 4.051 ± 0.962 |
| ABTS+ | 1.829 ± 0.091 * | 2.365 ± 0.137 |
| O2− | 4.745 ± 0.279 *** | 198.668 ± 2.065 |
| NO. | 21.914 ± 1.618 ** | 9.708 ± 1.492 |
| PETE [µg/mL] | Hemolysis [%] | ROS [F/F0] |
|---|---|---|
| 0 | 1.000 ± 0.254 | 1.000 ± 0.039 |
| 25 | 0.980 ± 0.462 | 1.000 ± 0.042 |
| 50 | 1.137 ± 0.497 | 1.008 ± 0.041 |
| 75 | 1.313 ± 0.573 | 1.005 ± 0.027 |
| 100 | 1.921 ± 0.362 * | 1.003 ± 0.050 |
| PETE [µg/mL] | Hemolysis [%] | ROS [F/F0] |
|---|---|---|
| 0 | 2.925 ± 0.601 | 1.000 ± 0.120 |
| 25 | 3.457 ± 0.601 | 1.099 ± 0.062 |
| 50 | 4.137 ± 0.688 | 1.510 ± 0.029 * |
| 75 | 4.048 ± 0.788 | 1.527 ± 0.042 * |
| 100 | 4.373 ± 0.929 | 1.912 ± 0.133 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olchowik-Grabarek, E.; Sekowski, S.; Mierzwinska, I.; Zukowska, I.; Abdulladjanova, N.; Shlyonsky, V.; Zamaraeva, M. Cell Type-Specific Anti- and Pro-Oxidative Effects of Punica granatum L. Ellagitannins. Membranes 2024, 14, 218. https://doi.org/10.3390/membranes14100218
Olchowik-Grabarek E, Sekowski S, Mierzwinska I, Zukowska I, Abdulladjanova N, Shlyonsky V, Zamaraeva M. Cell Type-Specific Anti- and Pro-Oxidative Effects of Punica granatum L. Ellagitannins. Membranes. 2024; 14(10):218. https://doi.org/10.3390/membranes14100218
Chicago/Turabian StyleOlchowik-Grabarek, Ewa, Szymon Sekowski, Iga Mierzwinska, Izabela Zukowska, Nodira Abdulladjanova, Vadim Shlyonsky, and Maria Zamaraeva. 2024. "Cell Type-Specific Anti- and Pro-Oxidative Effects of Punica granatum L. Ellagitannins" Membranes 14, no. 10: 218. https://doi.org/10.3390/membranes14100218
APA StyleOlchowik-Grabarek, E., Sekowski, S., Mierzwinska, I., Zukowska, I., Abdulladjanova, N., Shlyonsky, V., & Zamaraeva, M. (2024). Cell Type-Specific Anti- and Pro-Oxidative Effects of Punica granatum L. Ellagitannins. Membranes, 14(10), 218. https://doi.org/10.3390/membranes14100218






