Electroformation of Giant Unilamellar Vesicles from Damp Films in Conditions Involving High Cholesterol Contents, Charged Lipids, and Saline Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
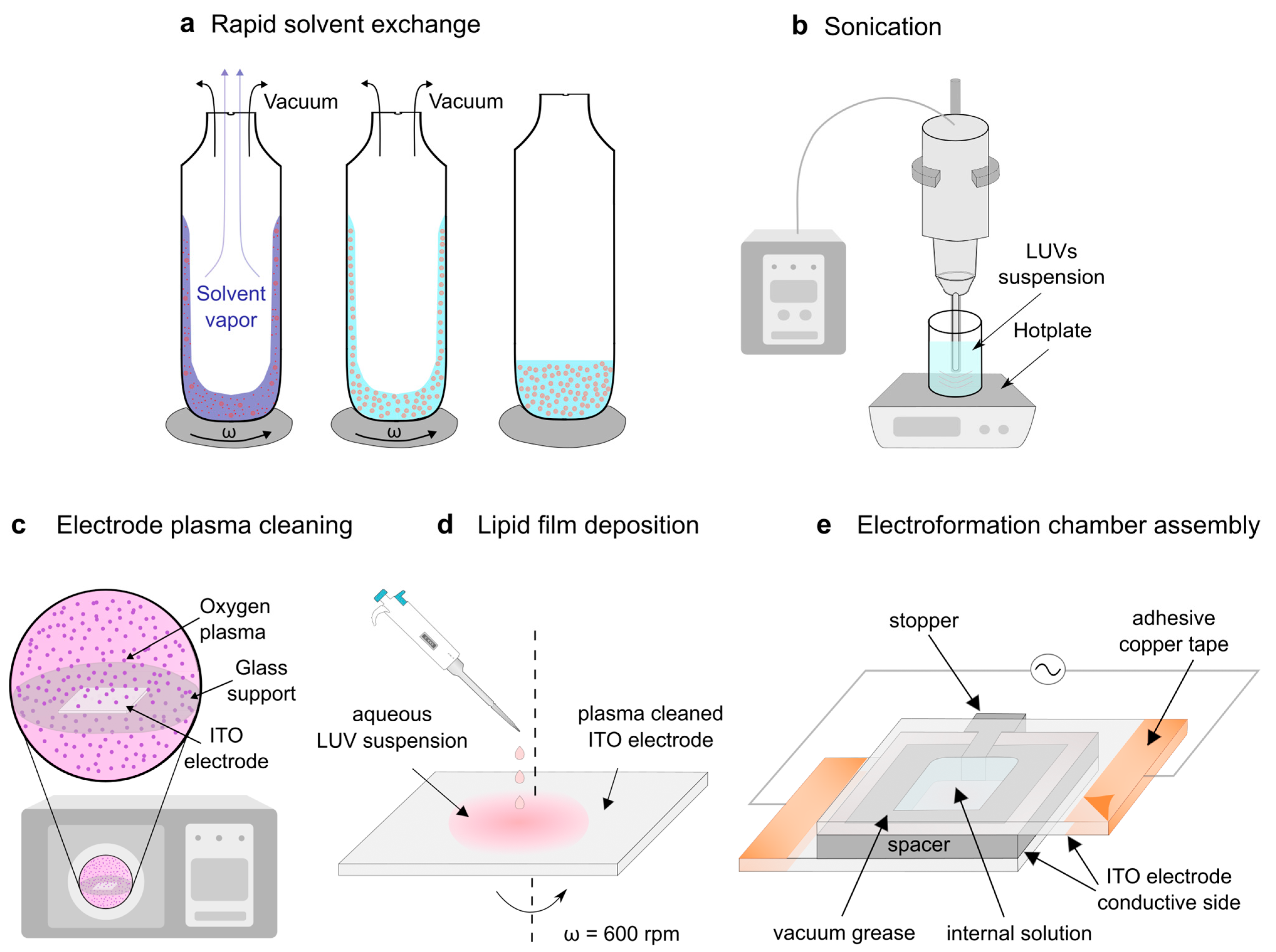
2.2. Preparation of Multilamellar Vesicles Using the Rapid Solvent Exchange Method
2.3. Preparation of Unilamellar Vesicles by Sonication
2.4. Preparation of the Damp Lipid Film
2.5. Electroformation Protocol
2.6. Dynamic Light Scattering
2.7. Fluorescence Imaging and Data Analysis
3. Results and Discussion
3.1. The Protocol
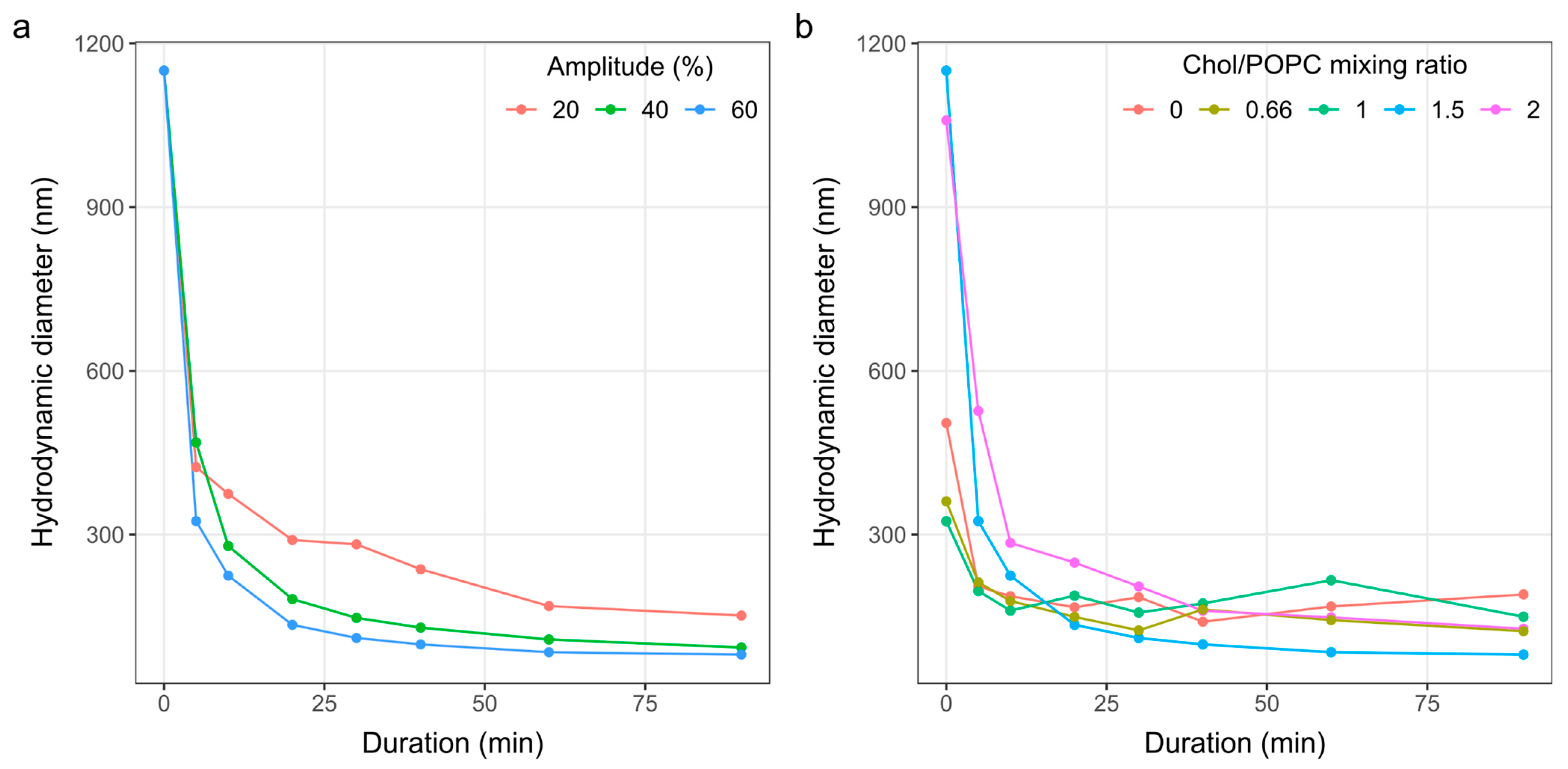
3.2. Determination of the Sonication Parameters
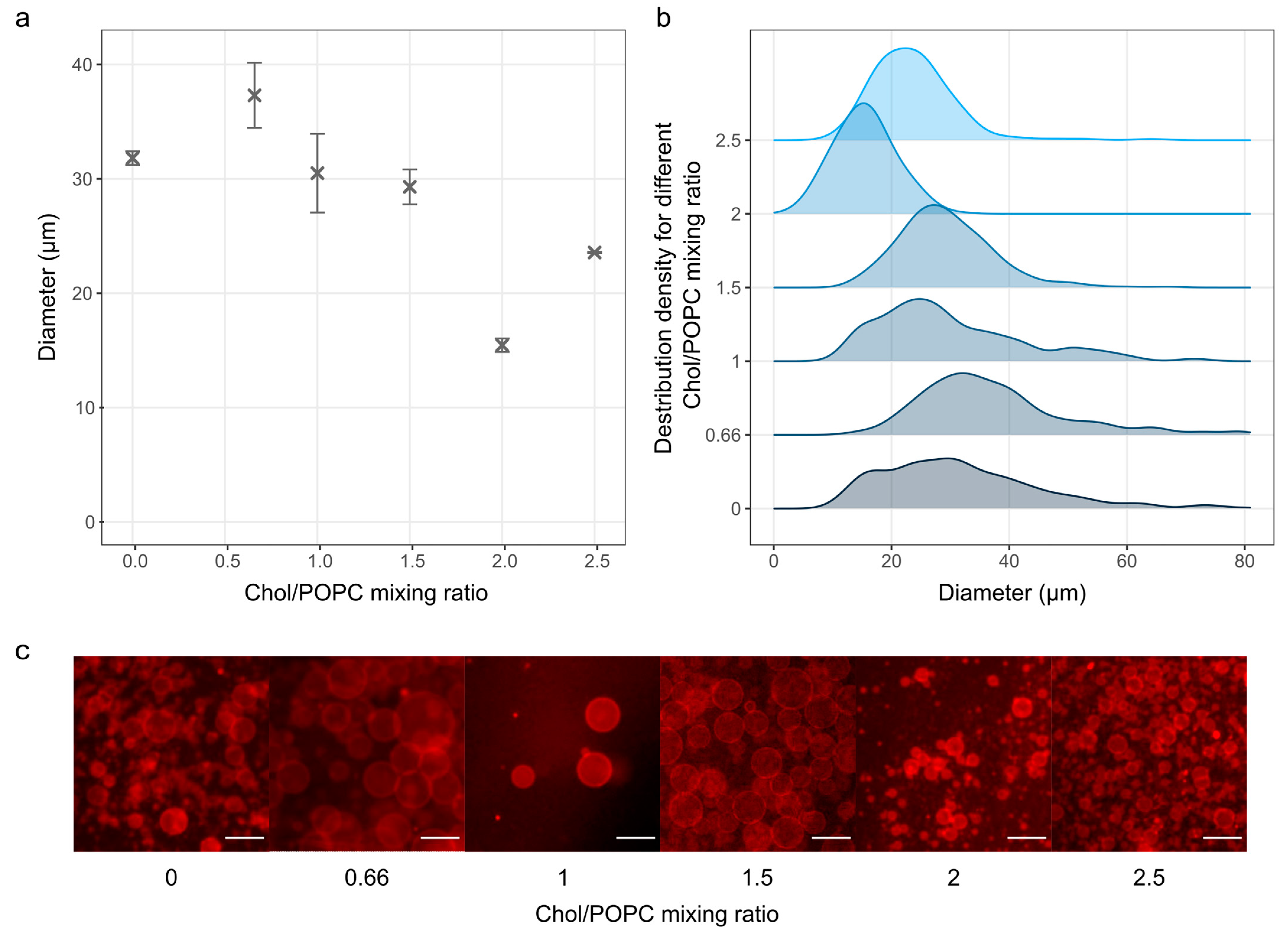
3.3. The Effect of Chol Content
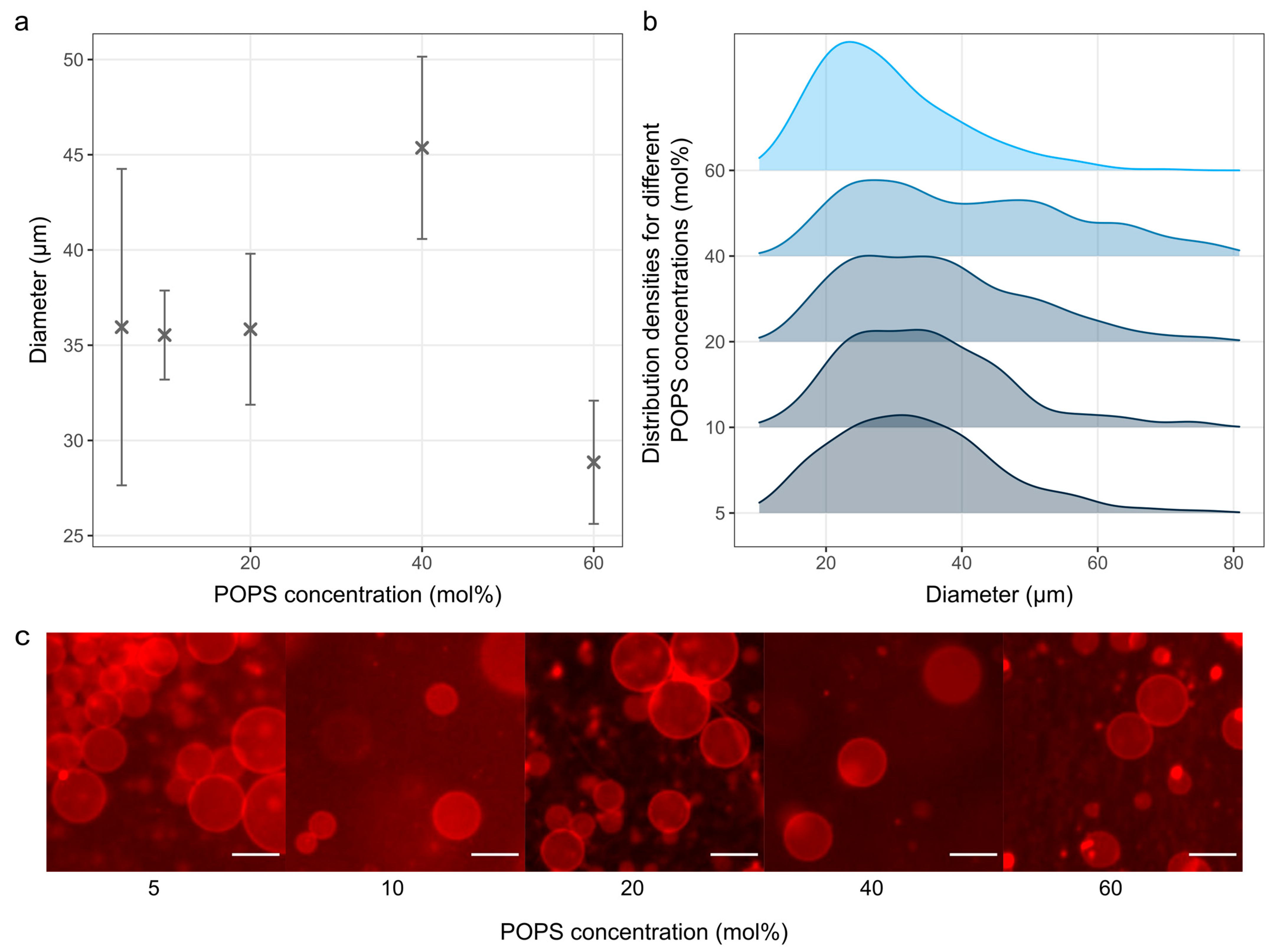
3.4. The Effect of Charged Lipids
3.5. The Effect of Using Saline Solutions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rideau, E.; Dimova, R.; Schwille, P.; Wurm, F.R.; Landfester, K. Liposomes and Polymersomes: A Comparative Review towards Cell Mimicking. Chem. Soc. Rev. 2018, 47, 8572–8610. [Google Scholar] [CrossRef] [PubMed]
- Gudheti, M.V.; Mlodzianoski, M.; Hess, S.T. Imaging and Shape Analysis of GUVs as Model Plasma Membranes: Effect of Trans DOPC on Membrane Properties. Biophys. J. 2007, 93, 2011–2023. [Google Scholar] [CrossRef] [PubMed]
- Angelova, M.I.; Dimitrov, D.S. Liposome Electroformation. Faraday Discuss. Chem. Soc. 1986, 81, 303. [Google Scholar] [CrossRef]
- Angelova, M.; Dimitrov, D.S. Angelova, M.; Dimitrov, D.S. A Mechanism of Liposome Electroformation. In Trends in Colloid and Interface Science II; Steinkopff: Darmstadt, Germany, 1988; Volume 67, pp. 59–67. [Google Scholar]
- Veatch, S.L. Electro-Formation and Fluorescence Microscopy of Giant Vesicles with Coexisting Liquid Phases. Methods Mol Biol. 2007, 398, 59–72. [Google Scholar] [PubMed]
- Morales-Penningston, N.F.; Wu, J.; Farkas, E.R.; Goh, S.L.; Konyakhina, T.M.; Zheng, J.Y.; Webb, W.W.; Feigenson, G.W. GUV Preparation and Imaging: Minimizing Artifacts. Biochim. Biophys. Acta—Biomembr. 2010, 1798, 1324–1332. [Google Scholar] [CrossRef]
- Boban, Z.; Mardešić, I.; Subczynski, W.K.; Raguz, M. Giant Unilamellar Vesicle Electroformation: What to Use, What to Avoid, and How to Quantify the Results. Membranes 2021, 11, 860. [Google Scholar] [CrossRef]
- Estes, D.J.; Mayer, M. Electroformation of Giant Liposomes from Spin-Coated Films of Lipids. Colloids Surf. B Biointerfaces 2005, 42, 115–123. [Google Scholar] [CrossRef]
- Boban, Z.; Puljas, A.; Kovač, D.; Subczynski, W.K.; Raguz, M. Effect of Electrical Parameters and Cholesterol Concentration on Giant Unilamellar Vesicles Electroformation. Cell Biochem. Biophys. 2020, 78, 157–164. [Google Scholar] [CrossRef]
- Boban, Z.; Mardešić, I.; Jozić, S.P.; Šumanovac, J.; Subczynski, W.K.; Raguz, M. Electroformation of Giant Unilamellar Vesicles from Damp Lipid Films Formed by Vesicle Fusion. Membranes 2023, 13, 352. [Google Scholar] [CrossRef]
- Boban, Z.; Mardešić, I.; Subczynski, W.K.; Jozić, D.; Raguz, M. Optimization of Giant Unilamellar Vesicle Electroformation for Phosphatidylcholine/Sphingomyelin/Cholesterol Ternary Mixtures. Membranes 2022, 12, 525. [Google Scholar] [CrossRef]
- Mardešić, I.; Boban, Z.; Raguz, M. Electroformation of Giant Unilamellar Vesicles from Damp Lipid Films with a Focus on Vesicles with High Cholesterol Content. Membranes 2024, 14, 79. [Google Scholar] [CrossRef] [PubMed]
- Politano, T.J.; Froude, V.E.; Jing, B.; Zhu, Y. AC-Electric Field Dependent Electroformation of Giant Lipid Vesicles. Colloids Surf. B Biointerfaces 2010, 79, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Billerit, C.; Jeffries, G.D.M.; Orwar, O.; Jesorka, A. Formation of Giant Unilamellar Vesicles from Spin-Coated Lipid Films by Localized IR Heating. Soft Matter 2012, 8, 10823–10826. [Google Scholar] [CrossRef]
- Baykal-Caglar, E.; Hassan-Zadeh, E.; Saremi, B.; Huang, J. Preparation of Giant Unilamellar Vesicles from Damp Lipid Film for Better Lipid Compositional Uniformity. Biochim. Biophys. Acta—Biomembr. 2012, 1818, 2598–2604. [Google Scholar] [CrossRef]
- Raguz, M.; Kumar, S.N.; Zareba, M.; Ilic, N.; Mainali, L.; Subczynski, W.K. Confocal Microscopy Confirmed That in Phosphatidylcholine Giant Unilamellar Vesicles with Very High Cholesterol Content Pure Cholesterol Bilayer Domains Form. Cell Biochem. Biophys. 2019, 77, 309–317. [Google Scholar] [CrossRef]
- Mainali, L.; Raguz, M.; Subczynski, W.K. Formation of Cholesterol Bilayer Domains Precedes Formation of Cholesterol Crystals in Cholesterol/Dimyristoylphosphatidylcholine Membranes: EPR and DSC Studies. J. Phys. Chem. B 2013, 117, 8994–9003. [Google Scholar] [CrossRef]
- Buboltz, J.T.; Feigenson, G.W. A Novel Strategy for the Preparation of Liposomes: Rapid Solvent Exchange. Biochim. Biophys. Acta—Biomembr. 1999, 1417, 232–245. [Google Scholar] [CrossRef]
- Buboltz, J.T. A More Efficient Device for Preparing Model-Membrane Liposomes by the Rapid Solvent Exchange Method. Rev. Sci. Instrum. 2009, 80, 124301. [Google Scholar] [CrossRef]
- Mainali, L.; Raguz, M.; O’Brien, W.J.; Subczynski, W.K. Properties of Membranes Derived from the Total Lipids Extracted from the Human Lens Cortex and Nucleus. Biochim. Biophys. Acta—Biomembr. 2013, 1828, 1432–1440. [Google Scholar] [CrossRef]
- Park, S.; Sut, T.N.; Ma, G.J.; Parikh, A.N.; Cho, N.J. Crystallization of Cholesterol in Phospholipid Membranes Follows Ostwald’s Rule of Stages. J. Am. Chem. Soc. 2020, 142, 21872–21882. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Ma, S.; Zhang, Y.; Han, X. Electroformation of Giant Unilamellar Vesicles in Saline Solution. Colloids Surf. B Biointerfaces 2016, 147, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Ghellab, S.E.; Mu, W.; Li, Q.; Han, X. Prediction of the Size of Electroformed Giant Unilamellar Vesicle Using Response Surface Methodology. Biophys. Chem. 2019, 253, 106217. [Google Scholar] [CrossRef] [PubMed]
- Preston Mason, R.; Tulenko, T.N.; Jacob, R.F. Direct Evidence for Cholesterol Crystalline Domains in Biological Membranes: Role in Human Pathobiology. Biochim. Biophys. Acta—Biomembr. 2003, 1610, 198–207. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mainali, L.; O’Brien, W.J.; Subczynski, W.K. Detection of Cholesterol Bilayer Domains in Intact Biological Membranes: Methodology Development and Its Application to Studies of Eye Lens Fiber Cell Plasma Membranes. Exp. Eye Res. 2019, 178, 72–81. [Google Scholar] [CrossRef]
- Herold, C.; Chwastek, G.; Schwille, P.; Petrov, E.P. Efficient Electroformation of Supergiant Unilamellar Vesicles Containing Cationic Lipids on ITO-Coated Electrodes. Langmuir 2012, 28, 5518–5521. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Development Core Team: Vienna, Austria, 2008. [Google Scholar]
- Richter, R.P.; Bérat, R.; Brisson, A.R. Formation of Solid-Supported Lipid Bilayers: An Integrated View. Langmuir 2006, 22, 3497–3505. [Google Scholar] [CrossRef]
- Brian, A.A.; McConnell, H.M. Allogeneic Stimulation of Cytotoxic T Cells by Supported Planar Membranes. Proc. Natl. Acad. Sci. USA 1984, 81, 6159–6163. [Google Scholar] [CrossRef]
- Lind, T.K.; Cárdenas, M.; Wacklin, H.P. Formation of Supported Lipid Bilayers by Vesicle Fusion: Effect of Deposition Temperature. Langmuir 2014, 30, 7259–7263. [Google Scholar] [CrossRef]
- Jackman, J.A.; Cho, N.-J. Supported Lipid Bilayer Formation: Beyond Vesicle Fusion. Langmuir 2020, 36, 1387–1400. [Google Scholar] [CrossRef]
- Tero, R. Substrate Effects on the Formation Process, Structure and Physicochemical Properties of Supported Lipid Bilayers. Materials 2012, 5, 2658–2680. [Google Scholar] [CrossRef]
- Reeves, J.P.; Dowben, R.M. Formation and Properties of Thin-Walled Phospholipid Vesicles. J. Cell. Physiol. 1969, 73, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Darszon, A.; Vandenberg, C.A.; Schönfeld, M.; Ellisman, M.H.; Spitzer, N.C.; Montal, M. Reassembly of Protein-Lipid Complexes into Large Bilayer Vesicles: Perspectives for Membrane Reconstitution. Proc. Natl. Acad. Sci. USA 1980, 77, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, N.; Pincet, F.; Cribier, S. Giant Vesicles Formed by Gentle Hydration and Electroformation: A Comparison by Fluorescence Microscopy. Colloids Surf. B Biointerfaces 2005, 42, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Cho, N.J.; Hwang, L.Y.; Solandt, J.J.R.; Frank, C.W. Comparison of Extruded and Sonicated Vesicles for Planar Bilayer Self-Assembly. Materials 2013, 6, 3294–3308. [Google Scholar] [CrossRef]
- Lapinski, M.M.; Castro-Forero, A.; Greiner, A.J.; Ofoli, R.Y.; Blanchard, G.J. Comparison of Liposomes Formed by Sonication and Extrusion: Rotational and Translational Diffusion of an Embedded Chromophore. Langmuir 2007, 23, 11677–11683. [Google Scholar] [CrossRef]
- Woodbury, D.J.; Richardson, E.S.; Grigg, A.W.; Welling, R.D.; Knudson, B.H. Reducing Liposome Size with Ultrasound: Bimodal Size Distributions. J. Liposome Res. 2006, 16, 57–80. [Google Scholar] [CrossRef]
- Maulucci, G.; De Spirito, M.; Arcovito, G.; Boffi, F.; Castellano, A.C.; Briganti, G. Particle Size Distribution in DMPC Vesicles Solutions Undergoing Different Sonication Times. Biophys. J. 2005, 88, 3545–3550. [Google Scholar] [CrossRef]
- Huang, J.; Feigenson, G.W. A Microscopic Interaction Model of Maximum Solubility of Cholesterol in Lipid Bilayers. Biophys. J. 1999, 76, 2142–2157. [Google Scholar] [CrossRef]
- Lira, R.B.; Leomil, F.S.C.; Melo, R.J.; Riske, K.A.; Dimova, R. To Close or to Collapse: The Role of Charges on Membrane Stability upon Pore Formation. Adv. Sci. 2021, 8, e2004068. [Google Scholar] [CrossRef]
- Uzun, H.D.; Tiris, Z.; Czarnetzki, M.; López-Marqués, R.L.; Günther Pomorski, T. Electroformation of Giant Unilamellar Vesicles from Large Liposomes. Eur. Phys. J. Spec. Top. 2024, 123. [Google Scholar] [CrossRef]
- Pott, T.; Bouvrais, H.; Méléard, P. Giant Unilamellar Vesicle Formation under Physiologically Relevant Conditions. Chem. Phys. Lipids 2008, 154, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Girard, P.; Pécréaux, J.; Lenoir, G.; Falson, P.; Rigaud, J.L.; Bassereau, P. A New Method for the Reconstitution of Membrane Proteins into Giant Unilamellar Vesicles. Biophys. J. 2004, 87, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Witkowska, A.; Jablonski, L.; Jahn, R. A Convenient Protocol for Generating Giant Unilamellar Vesicles Containing SNARE Proteins Using Electroformation. Sci. Rep. 2018, 8, 9422. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mardešić, I.; Boban, Z.; Raguz, M. Electroformation of Giant Unilamellar Vesicles from Damp Films in Conditions Involving High Cholesterol Contents, Charged Lipids, and Saline Solutions. Membranes 2024, 14, 215. https://doi.org/10.3390/membranes14100215
Mardešić I, Boban Z, Raguz M. Electroformation of Giant Unilamellar Vesicles from Damp Films in Conditions Involving High Cholesterol Contents, Charged Lipids, and Saline Solutions. Membranes. 2024; 14(10):215. https://doi.org/10.3390/membranes14100215
Chicago/Turabian StyleMardešić, Ivan, Zvonimir Boban, and Marija Raguz. 2024. "Electroformation of Giant Unilamellar Vesicles from Damp Films in Conditions Involving High Cholesterol Contents, Charged Lipids, and Saline Solutions" Membranes 14, no. 10: 215. https://doi.org/10.3390/membranes14100215
APA StyleMardešić, I., Boban, Z., & Raguz, M. (2024). Electroformation of Giant Unilamellar Vesicles from Damp Films in Conditions Involving High Cholesterol Contents, Charged Lipids, and Saline Solutions. Membranes, 14(10), 215. https://doi.org/10.3390/membranes14100215






