Copolyimide Brushes as a Component of a Hybrid Poly(phenylene Oxide) Membrane for Controlling Gas Separation: Effect of Water, Methanol, and Hexane Vapors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
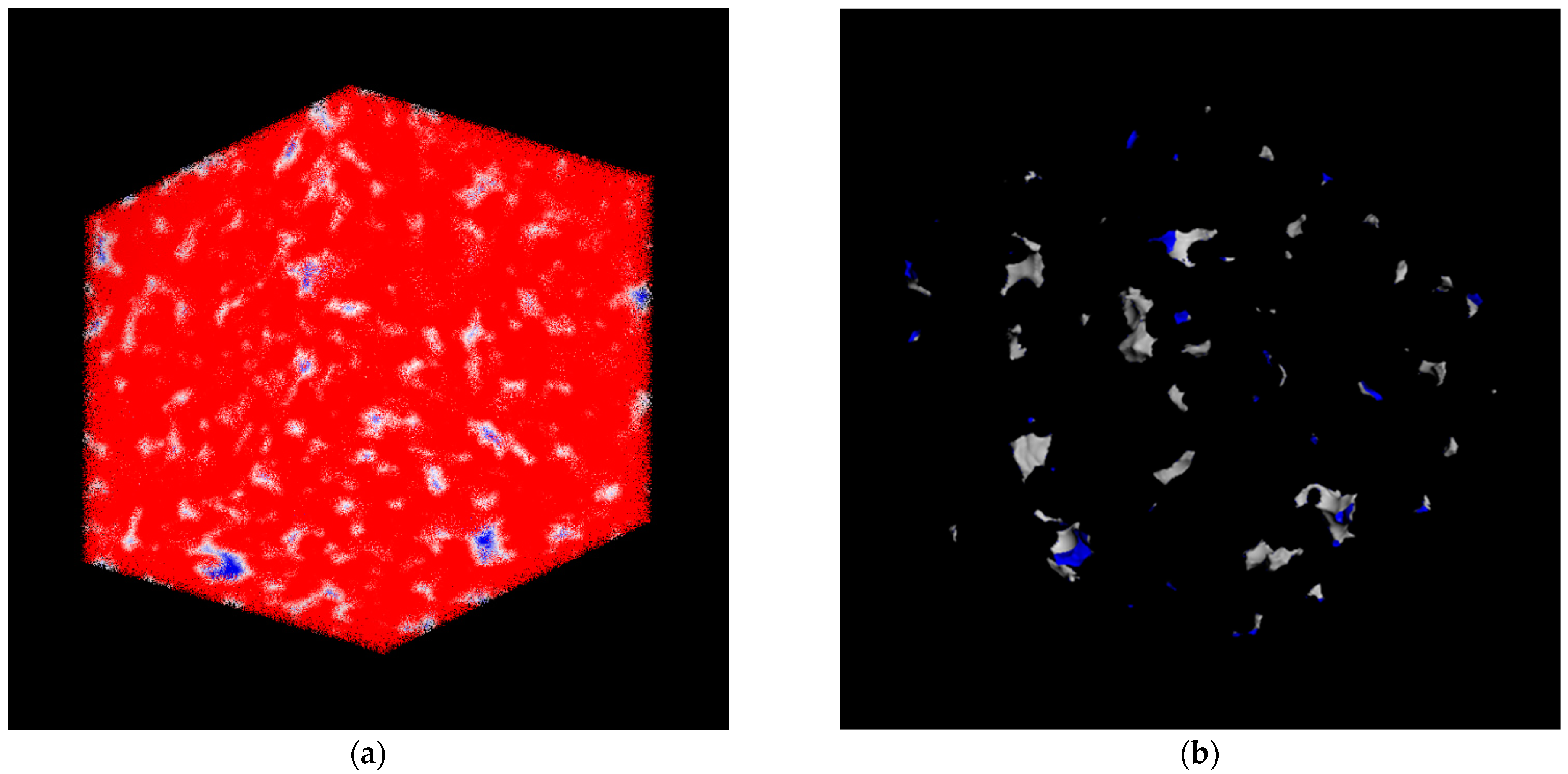
2.3. Computer Simulation
2.4. Membrane Characterization
2.5. Gas Transport
3. Results and Discussions
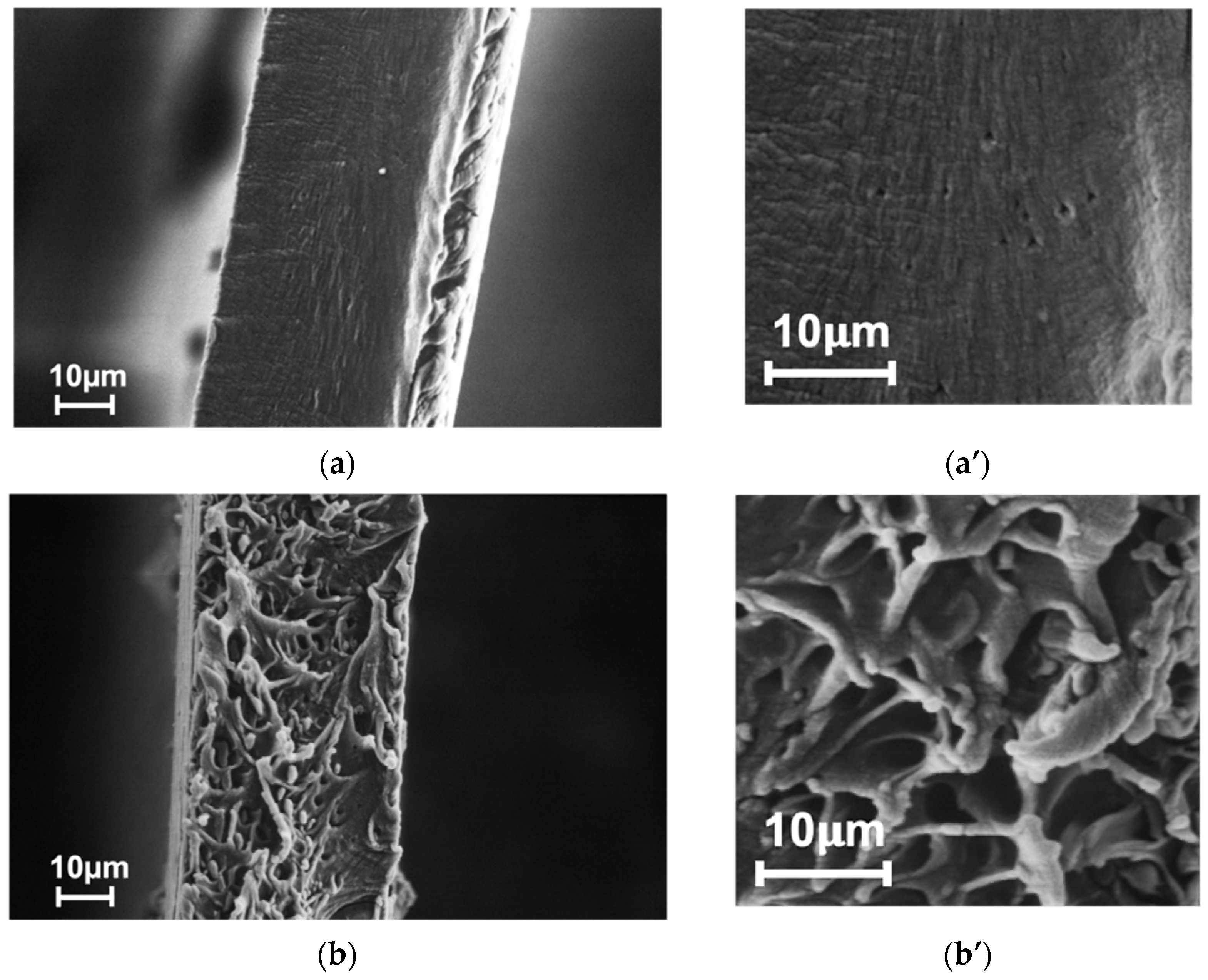
3.1. Membrane Structure
3.2. Mechanical Properties
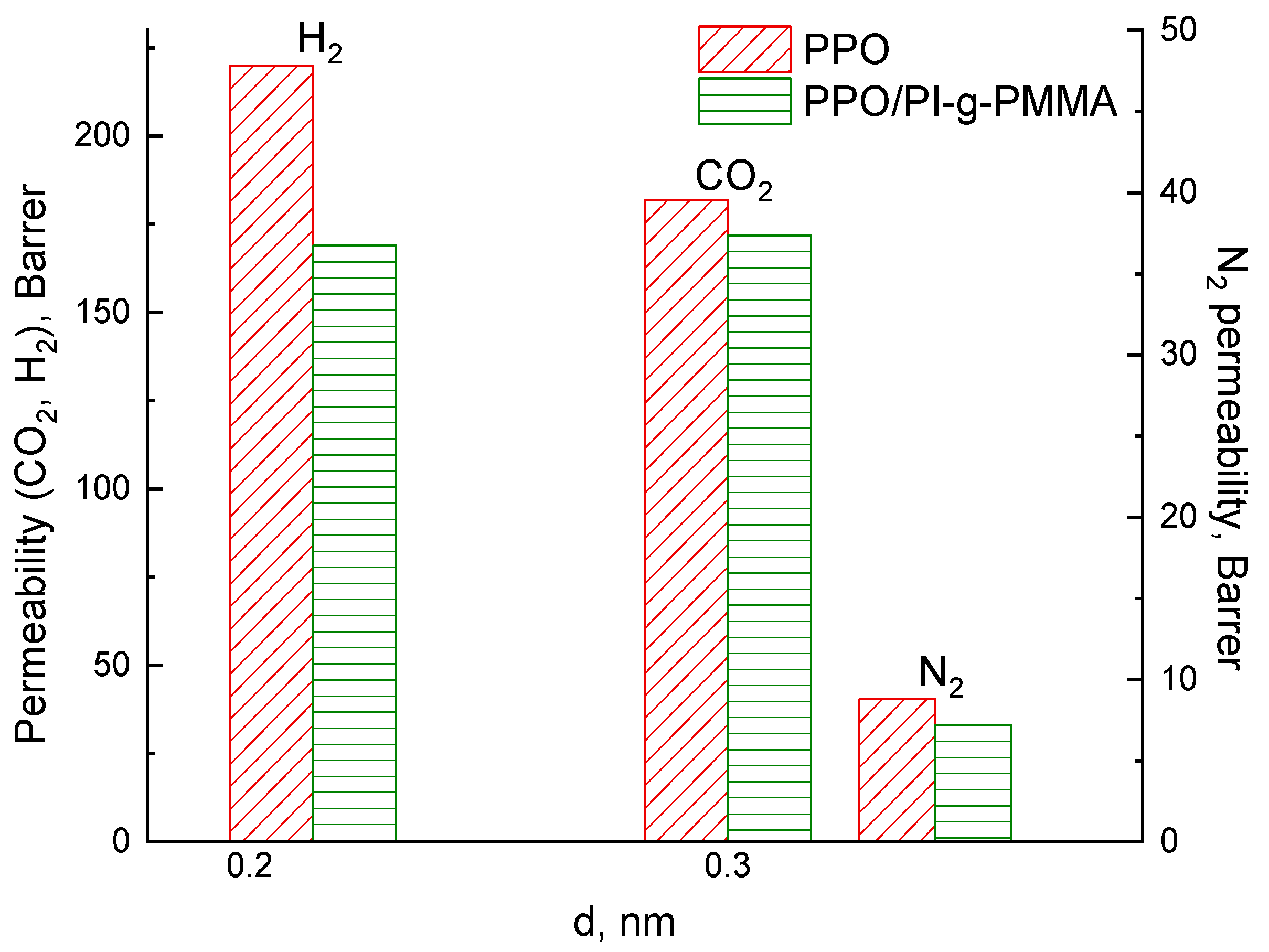
3.3. Transport Properties
- -
- The channels and cavities of the polymer saturated with water vapor become more polar, which leads to a local increase in the dielectric constant of the membrane and a decrease in the nitrogen solubility.
- -
- Filling the cavities with H2O molecules reduces the available volume required for the relay nature of gas diffusion in the polymer; therefore, the standard deviation of the adsorbate molecules from the local equilibrium positions decreases. This change especially affects the CO2 molecules due to their size and strong electrostatic effects with water.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | Atomic force microscopy |
| GCMC | Grand Canonical Monte-Carlo |
| MD | Molecular dynamics |
| MMA | Methyl methacrylate |
| MS | Materials Studio |
| MW | Molecular weight |
| PI | Polyimide |
| PMMA | Poly(methyl methacrylate) |
| PI-g-PMMA | Copolyimide brush with poly(methyl methacrylate) side chains |
| PPO | Poly(2,6-dimethyl-1,4-phenylene oxide) |
| SEM | Scanning electron microscopy |
| E | Young’s modulus |
| αi/j | ideal selectivity |
| σb | break stress |
| Σb | ultimate deformation |
| ρ | density |
| δ | Hildebrand solubility parameter |
| D | diffusion coefficient |
| Ecoh | cohesion energy |
| Vm | molar volume |
| P | permeability |
| Vp | calibrated volume of the product part of the cell |
| t | time |
| l | membrane thickness |
| S | membrane area/solubility coefficient |
| T | temperature |
| R | gas constant |
References
- Ananenkov, A.G.; Mastepanov, A.M. Gas Industry in Russia at the Turn of XX and XXI Centuries: Some Results and Prospects; Gazoil Press: Moscow, Russia, 2010. [Google Scholar]
- Bazhenova, O.K.; Burlin, Y.K.; Sokolov, B.A.; Khain, V.E. Geology and Geochemistry of Oil and Gas; MSU Press: Moscow, Russia, 2012. [Google Scholar]
- Fink, J.K. Hydraulic Fracturing Chemicals and Fluids Technology; Elsevier: Waltham, MA, USA, 2013. [Google Scholar]
- Miroshnichenko, D.; Teplyakov, V.; Shalygin, M. Recovery of Methanol during Natural Gas Dehydration Using Polymeric Membranes: Modeling of the Process. Membranes 2022, 12, 1176. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.W.; Lokhandwala, K. Natural Gas Processing with Membranes: An Overview. Ind. Eng. Chem. Res. 2008, 47, 2109–2121. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Kraftschik, B.E.; Babu, V.P.; Bhuwania, N.; Chinn, D.; Koros, W.J. Natural gas sweetening using TEGMC polyimide hollow fiber membranes. J. Membr. Sci. 2021, 632, 119361. [Google Scholar] [CrossRef]
- Yampolksii, Y.; Pinnau, I.; Freeman, B.D. (Eds.) Materials Science of Membranes for Gas and Vapor Separation; John Wiley and Sons, Ltd.: Chichester, UK, 2006. [Google Scholar]
- Vu, D.Q.; Koros, W.J.; Miller, S.J. Mixed matrix membranes using carbon molecular sieves: I. Preparation and experimental results. J. Membr. Sci. 2003, 211, 311–334. [Google Scholar] [CrossRef]
- Sunder, N.; Fong, Y.Y.; Bustam, M.A.; Suhaimi, N.H. Development of Amine-Functionalized Metal-Organic Frameworks Hollow Fiber Mixed Matrix Membranes for CO2 and CH4 Separation: A Review. Polymers 2022, 14, 1408. [Google Scholar] [CrossRef] [PubMed]
- Araújo, T.; Bernardo, G.; Mendes, A. Cellulose-Based Carbon Molecular Sieve Membranes for Gas Separation: A Review. Molecules 2020, 25, 3532. [Google Scholar] [CrossRef] [PubMed]
- Markova, S.Y.; Dukhov, A.V.; Pelzer, M.; Shalygin, M.G.; Vad, T.; Gries, T.; Teplyakov, V.V. Designing 3D membrane modules for gas separation based on hollow fibers from poly(4-methyl-1-pentene). Membranes 2022, 12, 36. [Google Scholar] [CrossRef]
- Liu, L.; Chakma, A.; Feng, X. Gas permeation through water-swollen hydrogel membranes. J. Membr. Sci. 2008, 310, 66–75. [Google Scholar] [CrossRef]
- Žitková, A.; Kárászová, M.; Stanovský, P.; Vejražka, J.; Izák, P. Application of Water-Swollen Thin-Film Composite Membrane in Flue Gas Purification. Chem. Eng. Technol. 2019, 42, 1304–1309. [Google Scholar] [CrossRef]
- Suleman, M.S.; Lau, K.K.; Yeong, Y.F. Development, characterization and performance evaluation of a swelling resistant membrane for CO2/CH4 separation. J. Nat. Gas Eng. 2018, 52, 390–400. [Google Scholar] [CrossRef]
- Dai, Z.; Ansaloni, L.; Ryan, J.J.; Spontak, R.J.; Deng, L. Nafion/IL hybrid membranes with tuned nanostructure for enhanced CO2 separation: Effects of ionic liquid and water vapor. Green Chem. 2018, 20, 1391–1404. [Google Scholar] [CrossRef]
- Otvagina, K.V.; Atlaskin, A.A.; Trubyanov, M.M.; Kryuchkov, S.S.; Smorodin, K.A.; Mochalova, A.E.; Vorotyntsev, I.V. Effect of Moisture Presence on Gas Permeability through Gas Separation Membranes Based on Poly(Vinyltrimethylsilane) and Quaternized Chitosan. Membr. Membr. Technol. 2020, 2, 125–131. [Google Scholar] [CrossRef]
- Tsvigu, C.; Pavesi, E.; De Angelis, M.G.; Giacinti Baschetti, M. Effect of relative humidity and temperature on the gas transport properties of 6FDA–6FpDA polyimide: Experimental study and modelling. J. Membr. Sci. 2015, 485, 60–68. [Google Scholar] [CrossRef]
- Olivieri, L.; Tena, A.; De Angelis, M.G.; Hernández Giménez, A.; Lozano, A.E.; Sarti, G.C. The effect of humidity on the CO2/N2 separation performance of copolymers based on hard polyimide segments and soft polyether chains: Experimental and modeling. Green Energy Environ. 2016, 1, 201–210. [Google Scholar] [CrossRef]
- Kida, K.; Maeta, Y.; Yogo, K. Pure silica CHA-type zeolite membranes for dry and humidified CO2/CH4 mixtures separation. Sep. Purif. Technol. 2018, 197, 116–121. [Google Scholar] [CrossRef]
- Ansaloni, L.; Minelli, M.; Giacinti Baschetti, M.; Sarti, G.C. Effect of relative humidity and temperature on gas transport in Matrimid®: Experimental study and modeling. J. Membr. Sci. 2014, 471, 392–401. [Google Scholar] [CrossRef]
- Scholes, C.A.; Freeman, B.D.; Kentish, S.E. Water vapor permeability and competitive sorption in thermally rearranged (TR) membranes. J. Membr. Sci. 2014, 470, 132–137. [Google Scholar] [CrossRef]
- Galizia, M.; Chi, W.S.; Smith, Z.P.; Merkel, T.C.; Baker, R.W.; Freeman, B.D. 50th anniversary perspective: Polymers and mixed matrix membranes for gas and vapor separation: A review and prospective opportunities. Macromolecules 2017, 50, 7809–7843. [Google Scholar] [CrossRef]
- Lin, H.; Freeman, B.D. Materials selection guidelines for membranes that remove CO2 from gas mixtures. J. Mol. Struct. 2005, 739, 57. [Google Scholar] [CrossRef]
- Nunes, S.P.; Culfaz-Emecen, P.Z.; Ramon, G.Z.; Visser, T.; Koops, G.H.; Jin, W.; Ulbricht, M. Thinking the future of membranes: Perspectives for advanced and new membrane materials and manufacturing processes. J. Membr. Sci. 2020, 598, 117761. [Google Scholar] [CrossRef]
- González-Revuelta, D.; Fallanza, M.; Ortiz, A.; Gorri, D. Thin-Film Composite Matrimid-Based Hollow Fiber Membranes for Oxygen/Nitrogen Separation by Gas Permeation. Membranes 2023, 13, 218. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, M.; Mobili, R.; Milanese, C.; Esposito, E.; Fuoco, A.; La Cognata, S.; Amendola, V.; Jansen, J.C. PEEK–WC-Based Mixed Matrix Membranes Containing Polyimine Cages for Gas Separation. Molecules 2021, 26, 5557. [Google Scholar] [CrossRef] [PubMed]
- Nikolaeva, D.; Luis, P. Top-Down Polyelectrolytes for Membrane-Based Post-Combustion CO2 Capture. Molecules 2020, 25, 323. [Google Scholar] [CrossRef] [PubMed]
- Berned-Samatán, V.; Téllez, C.; Coronas, J. Double-Layered Pebax®3533/ZIF-8 Membranes with Single-Walled Carbon Nanotube Buckypapers as Support for Gas Separation. Membranes 2023, 13, 71. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Fíla, V. Progress on Incorporating Zeolites in Matrimid®5218 Mixed Matrix Membranes towards Gas Separation. Membranes 2018, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Elhenawy, S.; Khraisheh, M.; AlMomani, F.; Hassan, M. Key Applications and Potential Limitations of Ionic Liquid Membranes in the Gas Separation Process of CO2, CH4, N2, H2 or Mixtures of These Gases from Various Gas Streams. Molecules 2020, 25, 4274. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.; Asinger, P.A.; Lee, M.J.; Han, G.; Rodriguez, K.M.; Lin, S.; Benedetti, F.M.; Wu, A.X.; Chi, W.S.; Smith, Z.P. MOF-based membranes for gas separations. Chem. Rev. 2020, 120, 8161–8266. [Google Scholar] [CrossRef]
- Kulak, H.; Thür, R.; Vankelecom, I.F.J. MOF/Polymer mixed-matrix membranes preparation: Effect of main synthesis parameters on CO2/CH4 separation performance. Membranes 2022, 12, 425. [Google Scholar] [CrossRef]
- Tian, N.; Meleshko, T.; Polotskaya, G.; Gofman, I.; Kashina, A.; Kukarkina, N.; Vlasova, E.; Zoolshoev, Z.; Yakimansky, A. Influence of macromolecular brushes with polyimide backbones and poly(methyl methacrylate) side chains on structure, physical and transport properties of polyphthalamide. Polym. Eng. Sci. 2020, 60, 481–490. [Google Scholar] [CrossRef]
- Deng, L.; Kim, T.-J.; Hägg, M.-B. Facilitated transport of CO2 in novel PVAm/PVA blend membrane. J. Membr. Sci. 2009, 340, 154–163. [Google Scholar] [CrossRef]
- Chowdhury, G.; Kruczek, B.; Matsuura, T. (Eds.) Polyphenylene Oxide and Modified Polyphenylene Oxide Membranes: Gas, Vapor and Liquid Separation; Kluwer Academic Publishers: Boston, MA, USA, 2001. [Google Scholar]
- Polotskaya, G.A.; Penkova, A.V.; Toikka, A.M.; Pientka, Z.; Brozova, L.; Bleha, M. Transport of small molecules through polyphenylene oxide membranes modified by fullerene. Sep. Sci. Technol. 2007, 42, 333–347. [Google Scholar] [CrossRef]
- Giel, V.; Galajdová, B.; Popelková, D.; Kredatusová, J.; Trchová, M.; Pavlova, E.; Beneš, H.; Válek, R.; Peter, J. Gas transport properties of novel mixed matrix membranes made of titanate nanotubes and PBI or PPO. Desalin. Water Treat. 2015, 56, 3285–3293. [Google Scholar] [CrossRef]
- Zhuang, G.-L.; Wey, M.-Y.; Tseng, H.-H. The density and crystallinity properties of PPO-silica mixed-matrix membranes produced via the in situ sol-gel method for H2/CO2 separation. II: Effect of thermal annealing treatment. Chem. Eng. Res. Des. 2015, 104, 319–332. [Google Scholar] [CrossRef]
- Benedetti, F.M.; De Angelis, M.G.; Degli Esposti, M.; Fabbri, P.; Masili, A.; Orsini, A.; Pettinau, A. Enhancing the Separation Performance of Glassy PPO with the Addition of a Molecular Sieve (ZIF-8): Gas Transport at Various Temperatures. Membranes 2020, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Tyan, N.S.; Polotskaya, G.A.; Meleshko, T.K.; Yakimansky, A.V.; Pientka, Z. Influence of the Molecular Polyimide Brush on the Gas Separation Properties of Polyphenylene Oxide. Russ. J. Appl. Chem. 2019, 92, 360–366. [Google Scholar] [CrossRef]
- Tian, N.S.; Meleshko, T.K.; Polotskaya, G.A.; Kashina, A.V.; Gofman, I.V.; Zoolshoev, Z.F.; Lavrentyev, V.K.; Pientka, Z.; Yakimansky, A.V. Dual-phase polyphenylene oxide membranes with copolyimide branched modifiers. J. Appl. Polym. Sci. 2020, 137, 49543. [Google Scholar] [CrossRef]
- Meleshko, T.K.; Il’gach, D.M.; Bogorad, N.N.; Kukarkina, N.V.; Yakimansky, A.V. Synthesis of graft copolyimides via controlled radical polymerization of methacrylates with a polyimide macroinitiator. Polym. Sci. Ser. B 2014, 56, 118–126. [Google Scholar] [CrossRef]
- Sun, H.; Jin, Z.; Yang, C.; Akkermans, R.L.C.; Robertson, S.H.; Spenley, N.A.; Miller, S.; Todd, S.M. COMPASS II: Extended coverage for polymer and drug-like molecule databases. J. Mol. Mod. 2016, 22, 47. [Google Scholar] [CrossRef]
- Metropolis, N.; Rosenbluth, A.W.; Rosenbluth, M.N.; Teller, A.H.; Teller, E. Equation of State Calculations by Fast Computing Machines. J. Chem. Phys. 1953, 21, 1087–1092. [Google Scholar] [CrossRef]
- Karasawa, N.; Goddard, W.A., III. Acceleration of convergence for lattice sums. J. Phys. Chem. 1989, 93, 7320–7327. [Google Scholar] [CrossRef]
- Allen, M.P.; Tildesley, D.J.; Banavar, J.R. Computer Simulation of Liquids. Phys. Today 1989, 42, 105–106. [Google Scholar] [CrossRef]
- Belmares, M.; Blanco, M.; Goddard, W.A.; Ross, R.B.; Caldwell, G.; Chou, S.H.; Pham, J.; Olofson, P.M.; Thomas, C. Hildebrand and Hansen solubility parameters from molecular dynamics with applications to electronic nose polymer sensors. J. Comput. Chem. 2004, 25, 1814–1826. [Google Scholar] [CrossRef] [PubMed]
- Freeman, B.D.; Pinnau, I. Polymeric materials for gas separations. In Polymer Membranes for Gas and Vapor Separation; American Chemical Society: Washington, DC, USA, 1999. [Google Scholar]
- Meleshko, T.K.; Pulyalina, A.Y.; Tyan, N.S.; Polotskaya, G.A.; Ivanov, I.V.; Kukarkina, N.V.; Toikka, A.M.; Yakimansky, A.V. Molecular polyimide brushes as a novel membrane material for pervaporation processes. Polym. Sci. Ser. B 2017, 59, 183–193. [Google Scholar] [CrossRef]
- Habib, N.; Durak, O.; Zeeshan, M.; Uzun, A.; Keskin, S. A novel IL/MOF/polymer mixed matrix membrane having superior CO2/N2 selectivity. J. Membr. Sci. 2022, 658, 120712. [Google Scholar] [CrossRef]
- Huang, J.; Zou, J.; Ho, W.S.W. Carbon dioxide capture using a CO2-selective facilitated transport membrane. Ind. Eng. Chem. Res. 2008, 47, 1261–1267. [Google Scholar] [CrossRef]
- Barton, A.F.M. CRC Handbook of Solubility Parameters and Other Cohesion Parameters; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Comesaña-Gandara, B.; Ansaloni, L.; Lee, Y.M.; Lozano, A.E.; De Angelis, M.G. Sorption, diffusion, and permeability of humid gases and aging of thermally rearranged (TR) polymer membranes from a novel ortho-hydroxypolyimide. J. Membr. Sci. 2017, 542, 439–455. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications, 3rd ed.; Wiley: Chichester, UK, 2012. [Google Scholar]





| Membrane | E, GPa | σb, MPa | εb, % | Density, g/cm3 |
|---|---|---|---|---|
| PPO | 1.90 ± 0.16 | 44 ± 1 | 14 ± 2 | 1.060 ± 0.060 |
| PPO/PI-g-PMMA | 1.87 ± 0.20 | 48 ± 2 | 5.7 ± 0.3 | 1.065 ± 0.060 |
| Liquid | Mol. Weight, g/mol | Density, g/cm3 | Mol. Volume, cm3/mol | Dynamic Viscosity, mPa∙s | Solubility Parameter, δ, MPa1/2 |
|---|---|---|---|---|---|
| Water | 18.0 | 0.997 | 18.0 | 1.0 | 49.6 |
| Methanol | 32.0 | 0.792 | 40.4 | 0.55 | 29.7 |
| Hexane | 86.2 | 0.655 | 131.6 | 0.30 | 14.9 |
| Gas | Diffusion Coefficient, D298, 10−6 cm2·s−1 | Solubility Coefficient, S298, 10−4 mol·dm−3·bar−1 | Simulation Permeability Coefficient, P, Barrer | Experimental Permeability Coefficient, P, Barrer |
|---|---|---|---|---|
| N2 | 7.15 ± 0.10 | 0.40 ± 0.10 | 9.6 | 8.8 |
| N2 (for membrane saturated with water vapor) | 6.10 ± 0.16 | 0.23 ± 0.05 | 4.7 | 6.9 |
| CO2 | 3.93 ± 0.12 | 14.90 ± 0.16 | 196 | 182 |
| CO2 (for membrane saturated with water vapor) | 2.92 ± 0.11 | 13.6 ± 0.13 | 133 | 117 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, N.; Pulyalina, A.; Faykov, I.; Gofman, I.; Zolotovsky, K.; Polotskaya, G. Copolyimide Brushes as a Component of a Hybrid Poly(phenylene Oxide) Membrane for Controlling Gas Separation: Effect of Water, Methanol, and Hexane Vapors. Membranes 2023, 13, 805. https://doi.org/10.3390/membranes13090805
Tian N, Pulyalina A, Faykov I, Gofman I, Zolotovsky K, Polotskaya G. Copolyimide Brushes as a Component of a Hybrid Poly(phenylene Oxide) Membrane for Controlling Gas Separation: Effect of Water, Methanol, and Hexane Vapors. Membranes. 2023; 13(9):805. https://doi.org/10.3390/membranes13090805
Chicago/Turabian StyleTian, Nadezhda, Alexandra Pulyalina, Ilya Faykov, Iosif Gofman, Konstantin Zolotovsky, and Galina Polotskaya. 2023. "Copolyimide Brushes as a Component of a Hybrid Poly(phenylene Oxide) Membrane for Controlling Gas Separation: Effect of Water, Methanol, and Hexane Vapors" Membranes 13, no. 9: 805. https://doi.org/10.3390/membranes13090805
APA StyleTian, N., Pulyalina, A., Faykov, I., Gofman, I., Zolotovsky, K., & Polotskaya, G. (2023). Copolyimide Brushes as a Component of a Hybrid Poly(phenylene Oxide) Membrane for Controlling Gas Separation: Effect of Water, Methanol, and Hexane Vapors. Membranes, 13(9), 805. https://doi.org/10.3390/membranes13090805









