Amidoxime Modified UiO-66@PIM-1 Mixed-Matrix Membranes to Enhance CO2 Separation and Anti-Aging Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
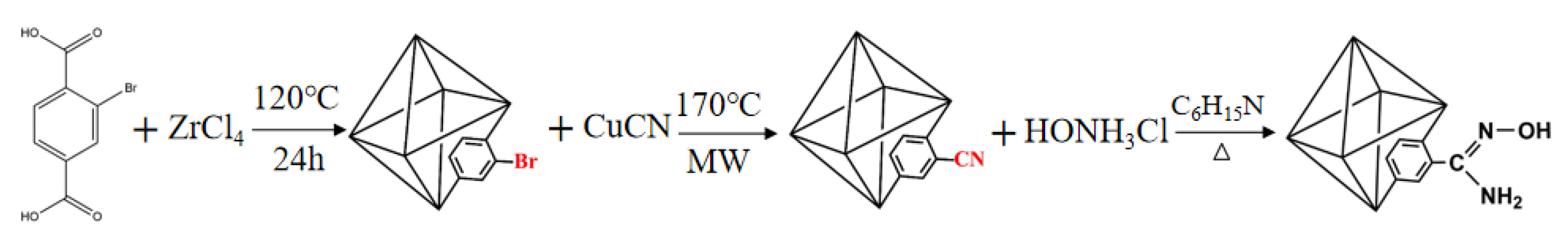
2.2. Synthesis of UiO-66-CN Nanoparticles
2.3. Synthesis of UiO-66-AO Nanoparticles
2.4. Synthesis of PIM-1
2.5. Membrane Preparation
2.6. Characterization
2.7. Gas Separation Experiment
3. Results
3.1. Characterization of UiO-66-CN and UiO-66-AO
3.2. Membrane Characterization
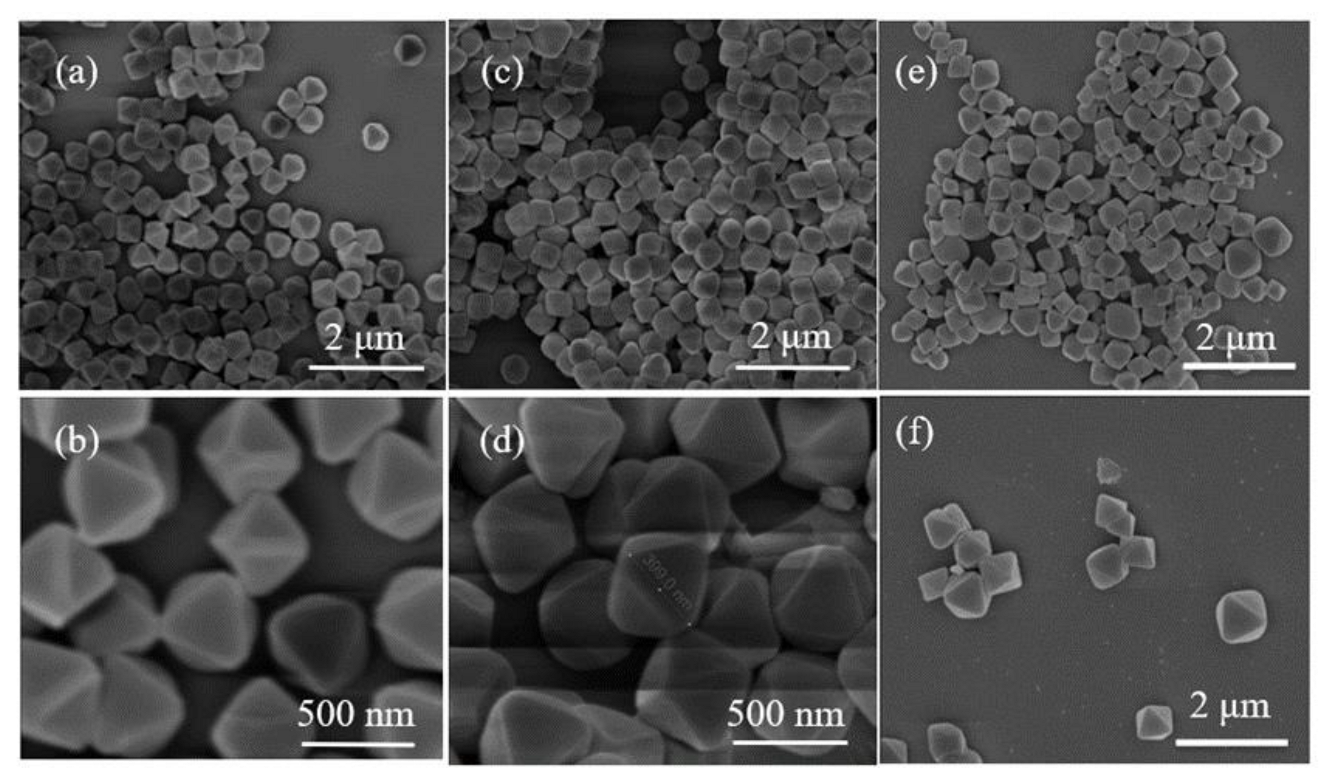
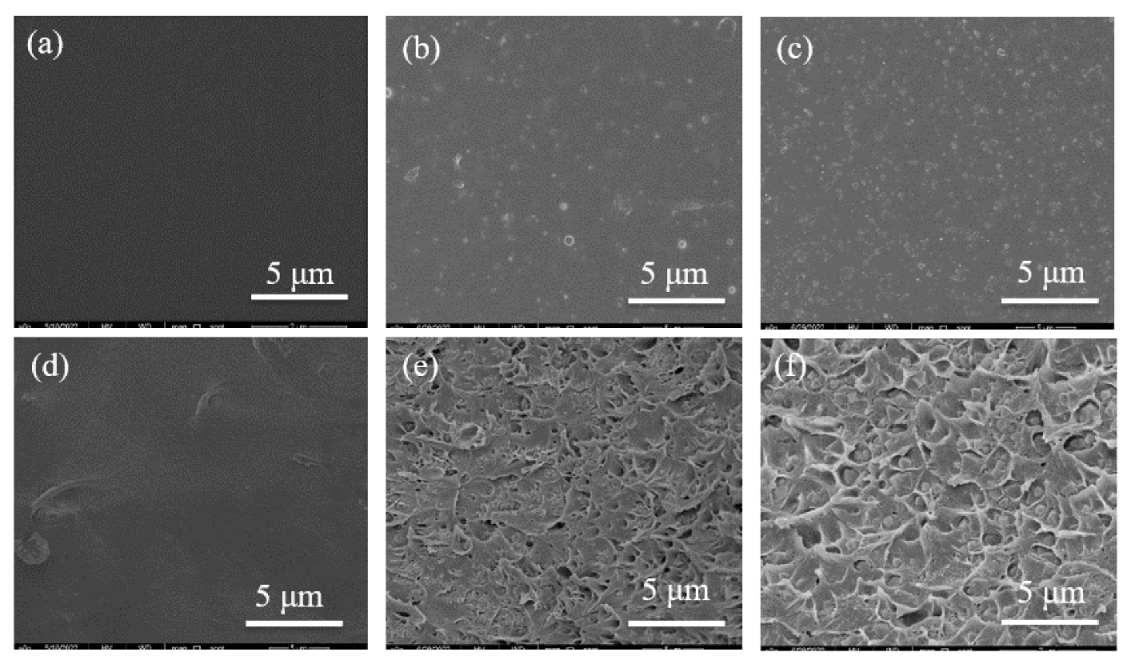
3.2.1. Scanning Electron Microscope (SEM)
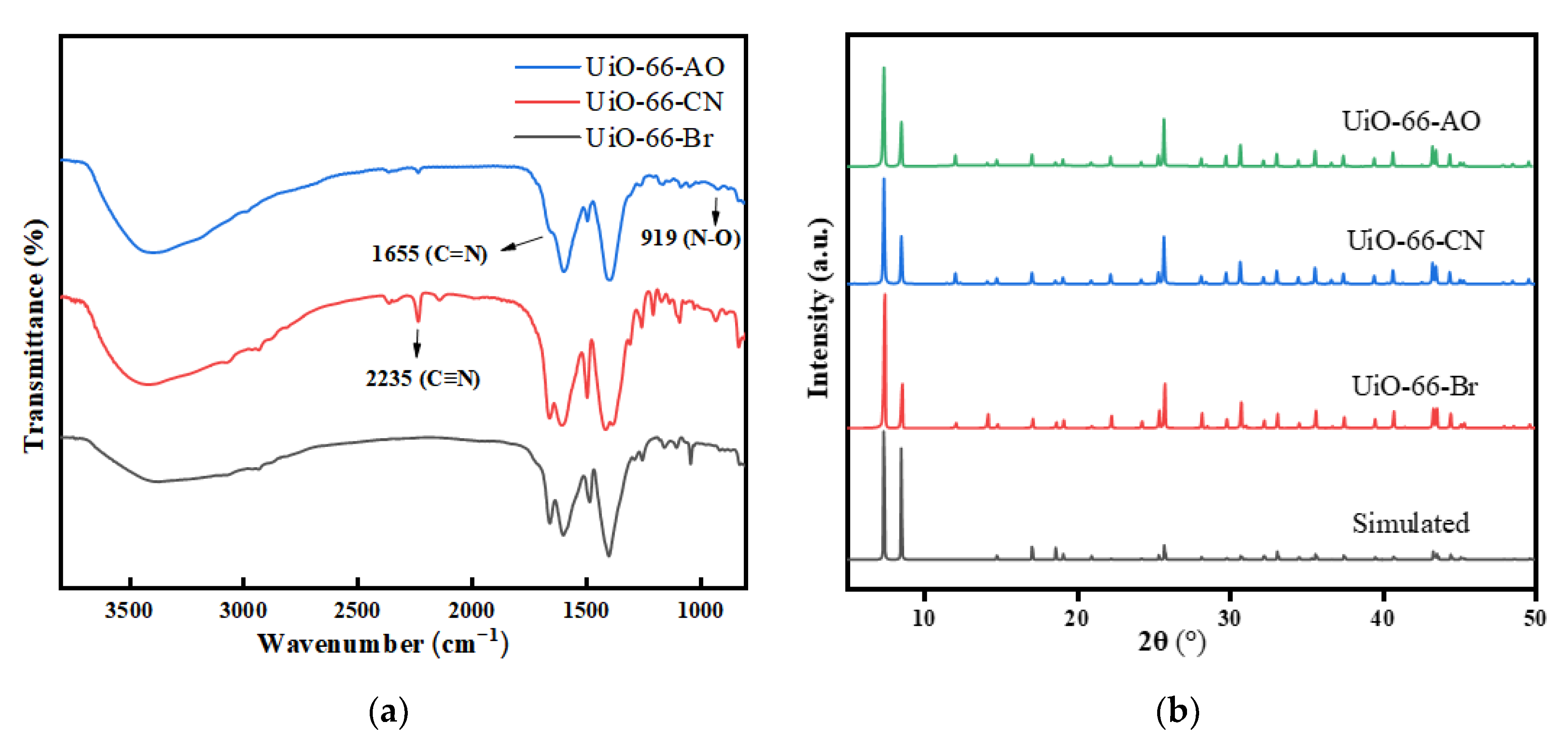
3.2.2. X-ray Powder Diffraction (XRD)
3.2.3. Fourier Transform Infrared Spectrometer (FT-IR)
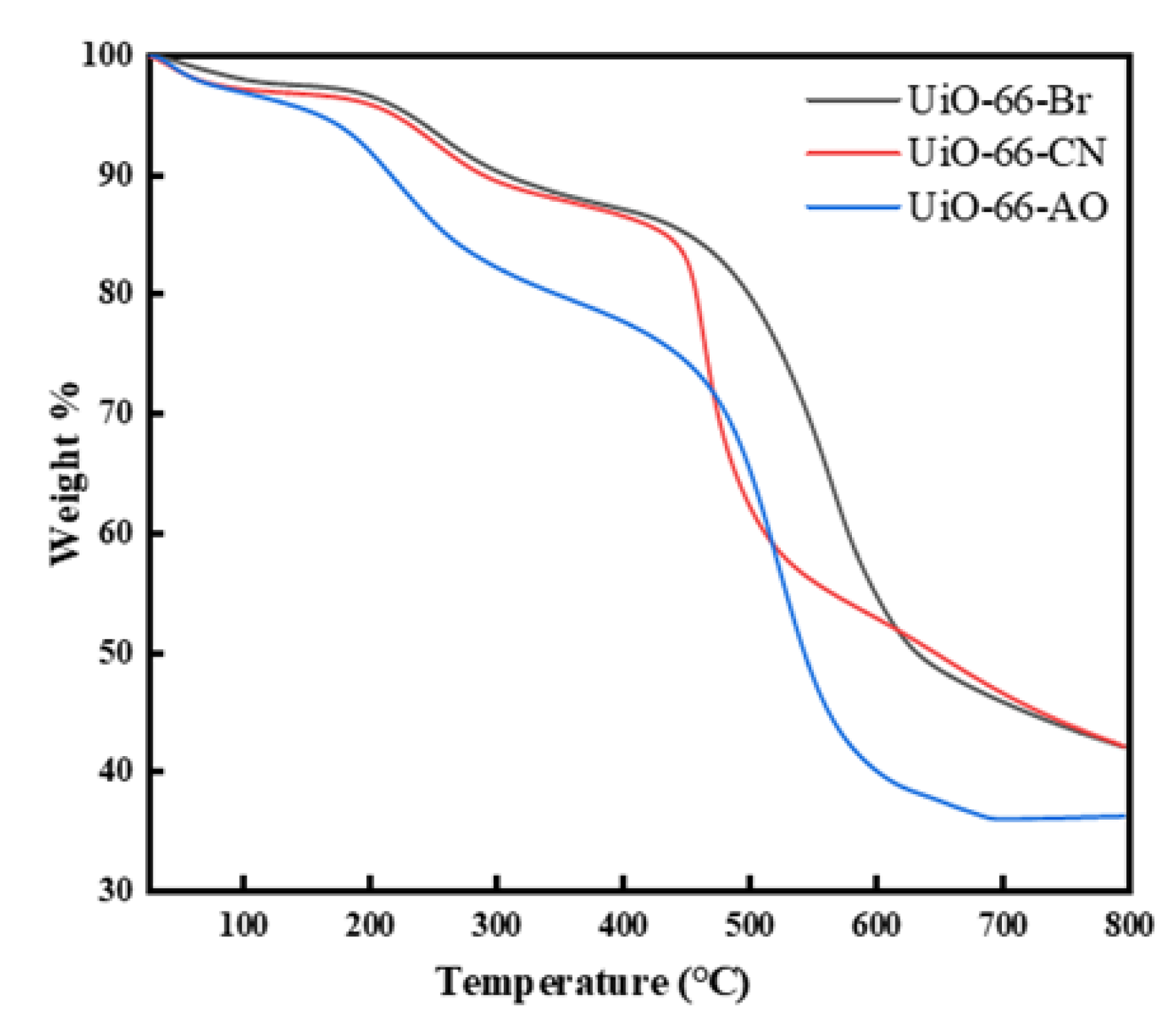
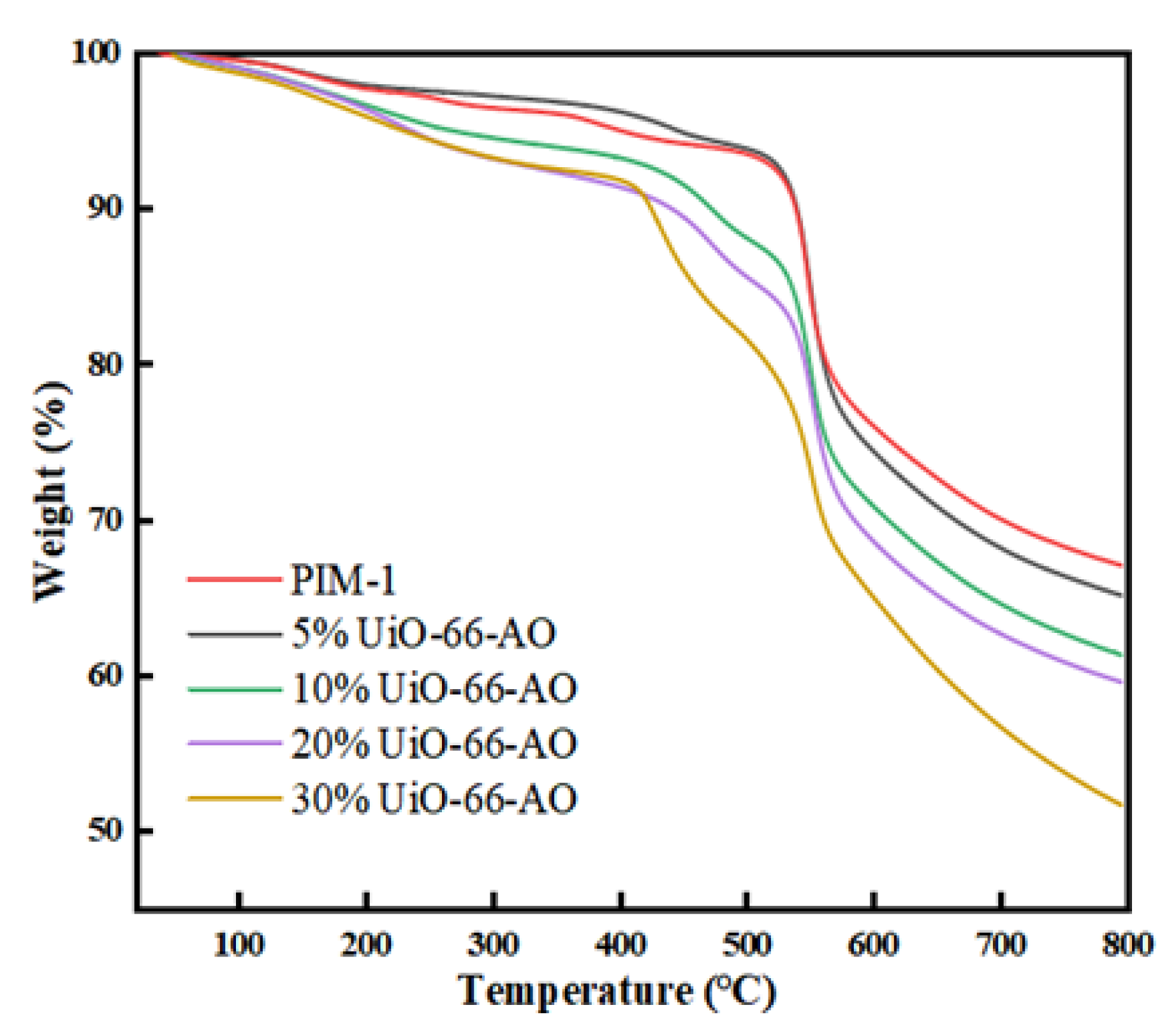
3.2.4. Thermogravimetric Analysis (TGA)
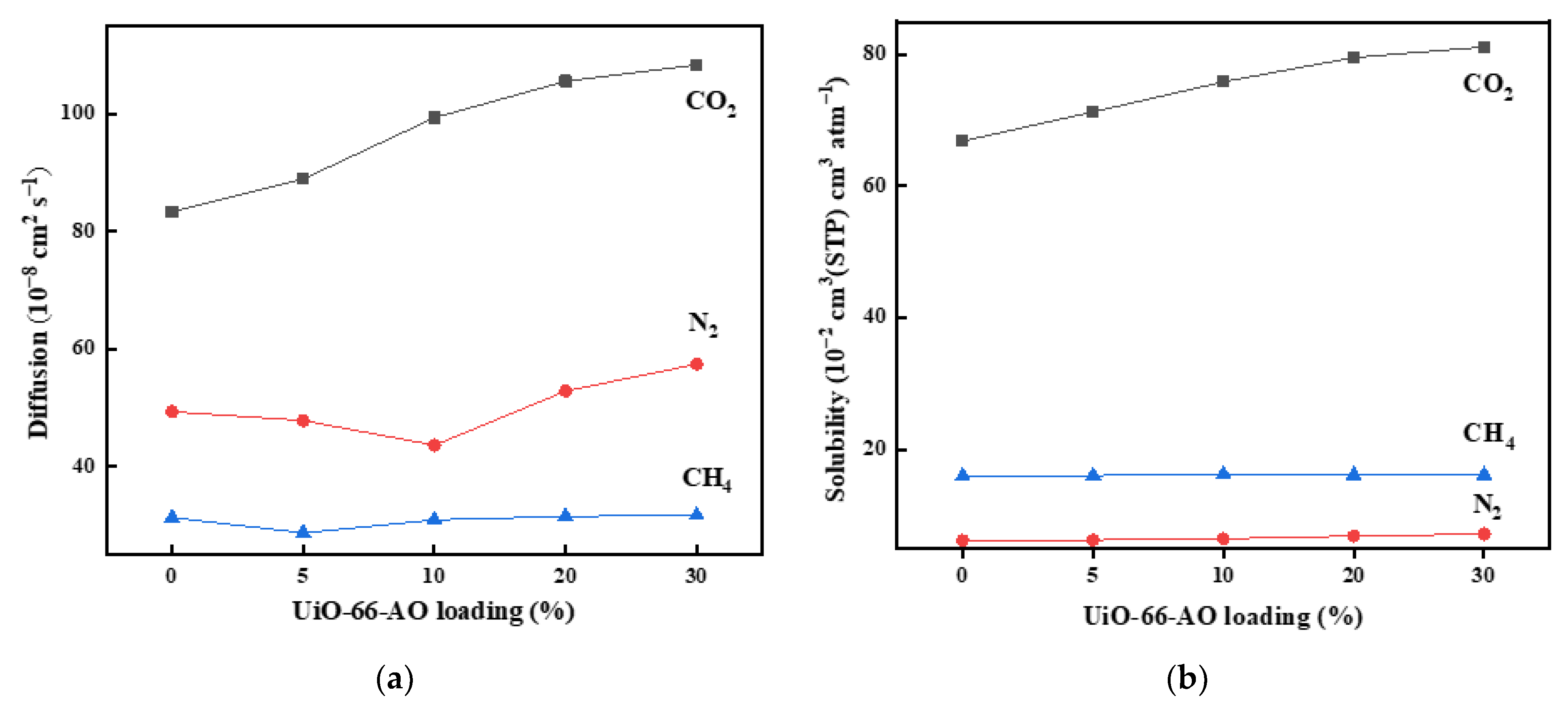
3.3. Gas Permeation Experiment
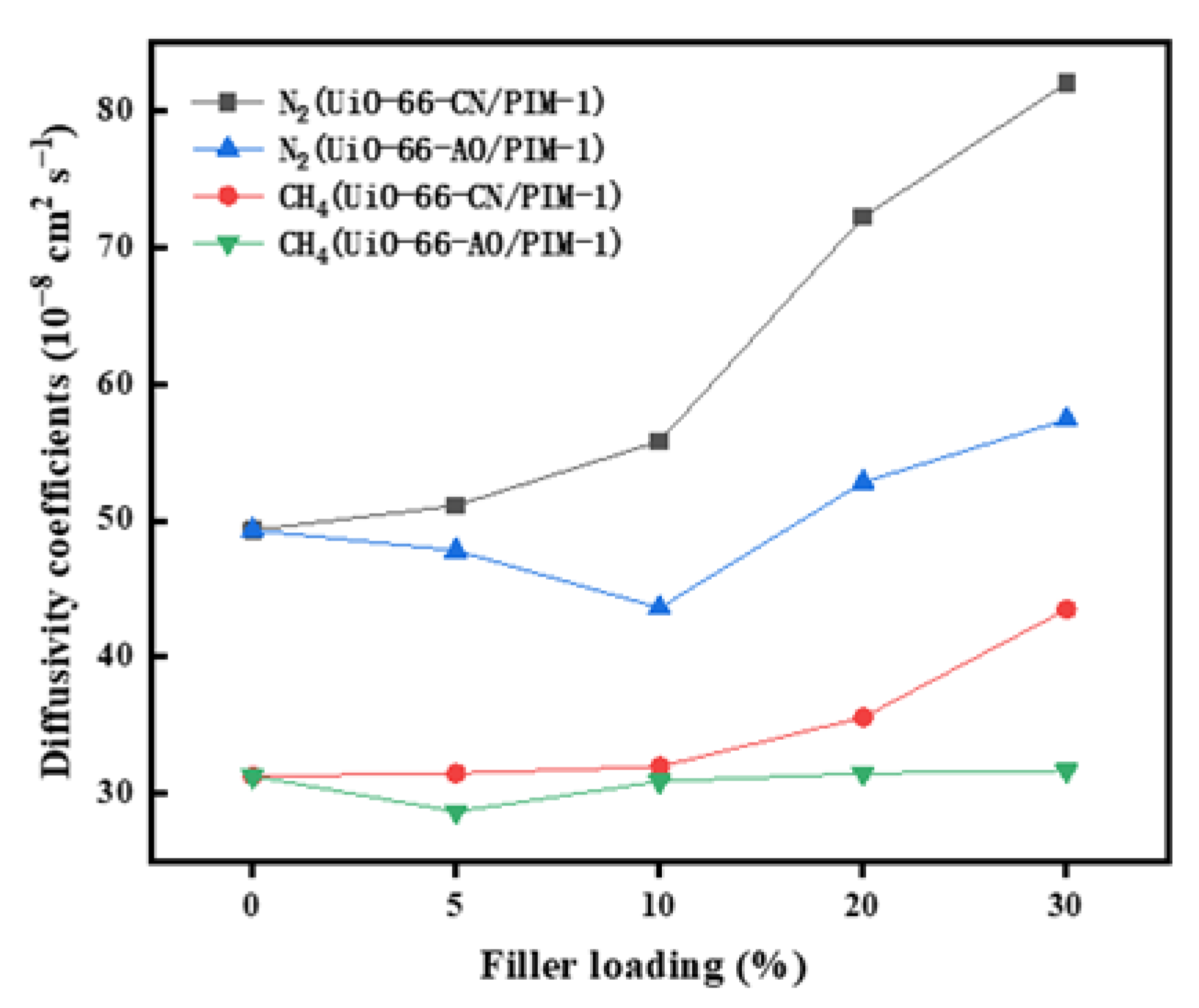
3.4. Anti-Aging Properties
3.5. Comparison with Literature
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Tollefson, J. IPCC climate report: Earth is warmer than it’s been in 125,000 years. Nature 2021, 596, 171–172. [Google Scholar] [CrossRef] [PubMed]
- Scott, V.; Gilfillan, S.; Markusson, N.; Chalmers, H.; Haszeldine, R.S. Last chance for carbon capture and storage. Nat. Clim. Chang. 2012, 3, 105–111. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, S.; Zhang, Y.; Chen, S.; Hou, Y.; Chen, L. Experimental evaluation of the performance of a cryogenic distillation system under offshore conditions. Chem. Eng. Sci. 2022, 263, 118084. [Google Scholar] [CrossRef]
- TRufford, E.; Smart, S.; Watson, G.C.Y.; Graham, B.F.; Boxall, J.; da Costa, J.C.D.; May, E.F. The removal of CO2 and N2 from natural gas: A review of conventional and emerging process technologies. J. Pet. Sci. Eng. 2012, 94–95, 123–154. [Google Scholar] [CrossRef]
- Li, J.-R.; Ma, Y.; McCarthy, M.C.; Sculley, J.; Yu, J.; Jeong, H.-K.; Balbuena, P.B.; Zhou, H.-C. Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coord. Chem. Rev. 2011, 255, 1791–1823. [Google Scholar] [CrossRef]
- Yuan, S.; Li, X.; Zhu, J.; Zhang, G.; Van Puyvelde, P.; Van der Bruggen, B. Covalent organic frameworks for membrane separation. Chem. Soc. Rev. 2019, 48, 2665–2681. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y. Perspective on Gas Separation Membrane Materials from Process Economics Point of View. Ind. Eng. Chem. Res. 2019, 59, 556–568. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Wu, H.; Tian, Z.; Xin, Q.; He, G.; Peng, D.; Chen, S.; Yin, Y.; Jiang, Z.; et al. Advances in high permeability polymer-based membrane materials for CO2 separations. Energy Environ. Sci. 2016, 9, 1863–1890. [Google Scholar] [CrossRef]
- Venna, S.R.; Carreon, M.A. Metal organic framework membranes for carbon dioxide separation. Chem. Eng. Sci. 2015, 124, 3–19. [Google Scholar] [CrossRef]
- Jusoh, N.; Yeong, Y.F.; Chew, T.L.; Lau, K.K.; Shariff, A.M. Current Development and Challenges of Mixed Matrix Membranes for CO2/CH4 Separation. Sep. Purif. Rev. 2016, 45, 321–344. [Google Scholar] [CrossRef]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Kosinov, N.; Gascon, J.; Kapteijn, F.; Hensen, E.J.M. Recent developments in zeolite membranes for gas separation. J. Membr. Sci. 2016, 499, 65–79. [Google Scholar] [CrossRef]
- Dechnik, J.; Gascon, J.; Doonan, C.J.; Janiak, C.; Sumby, C.J. Mixed-Matrix-Membranen. Angew. Chem. 2017, 129, 9420–9439. [Google Scholar] [CrossRef]
- Budd, P.M.; Ghanem, B.S.; Makhseed, S.; McKeown, N.B.; Msayib, K.J.; Tattershall, C.E. Polymers of intrinsic microporosity (PIMs): Robust, solution-processable, organic nanoporous materials. Chem. Commun. 2004, 2, 230–231. [Google Scholar] [CrossRef] [PubMed]
- Shamsabadi, A.A.; Rezakazemi, M.; Seidi, F.; Riazi, H.; Aminabhavi, T.; Soroush, M. Next generation polymers of intrinsic microporosity with tunable moieties for ultrahigh permeation and precise molecular CO2 separation. Prog. Energy Combust. Sci. 2021, 84, 100903. [Google Scholar] [CrossRef]
- Daglar, H.; Aydin, S.; Keskin, S. MOF-based MMMs breaking the upper bounds of polymers for a large variety of gas separations. Sep. Purif. Technol. 2022, 281, 119811. [Google Scholar] [CrossRef]
- Anbealagan, L.D.; Ng, T.Y.S.; Chew, T.L.; Yeong, Y.F.; Low, S.C.; Ong, Y.T.; Ho, C.D.; Jawad, Z.A. Modified Zeolite/Polysulfone Mixed Matrix Membrane for Enhanced CO2/CH4 Separation. Membranes 2021, 11, 630. [Google Scholar] [CrossRef]
- Vu, D.Q.; Koros, W.J.; Miller, S.J. Mixed matrix membranes using carbon molecular sieves. J. Membr. Sci. 2003, 211, 311–334. [Google Scholar] [CrossRef]
- Zhao, Y.; Yao, K.X.; Teng, B.; Zhang, T.; Han, Y. A perfluorinated covalent triazine-based framework for highly selective and water–tolerant CO2 capture. Energy Environ. Sci. 2013, 6, 3684–3692. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, S.; Wang, C.; Chang, H.-C.; Guo, R. Carbon nanotube-based mixed-matrix membranes with supramolecularly engineered interface for enhanced gas separation performance. J. Membr. Sci. 2020, 598, 1117794. [Google Scholar] [CrossRef]
- Sakaguchi, N.; Tanaka, M.; Yamato, M.; Kawakami, H. Superhigh CO2-Permeable Mixed Matrix Membranes Composed of a Polymer of Intrinsic Microporosity (PIM-1) and Surface-Modified Silica Nanoparticles. ACS Appl. Polym. Mater. 2019, 1, 2516–2524. [Google Scholar] [CrossRef]
- Wu, X.; Tian, Z.; Wang, S.; Peng, D.; Yang, L.; Wu, Y.; Xin, Q.; Wu, H.; Jiang, Z. Mixed matrix membranes comprising polymers of intrinsic microporosity and covalent organic framework for gas separation. J. Membr. Sci. 2017, 528, 273–283. [Google Scholar] [CrossRef]
- Sahoo, R.; Mondal, S.; Mukherjee, D.; Das, M.C. Metal–Organic Frameworks for CO2 Separation from Flue and Biogas Mixtures. Adv. Funct. Mater. 2022, 32, 2207197. [Google Scholar] [CrossRef]
- Bushell, A.F.; Attfield, M.P.; Mason, C.R.; Budd, P.M.; Yampolskii, Y.; Starannikova, L.; Rebrov, A.; Bazzarelli, F.; Bernardo, P.; Jansen, J.C.; et al. Gas permeation parameters of mixed matrix membranes based on the polymer of intrinsic microporosity PIM-1 and the zeolitic imidazolate framework ZIF-8. J. Membr. Sci. 2013, 427, 48–62. [Google Scholar] [CrossRef]
- Khdhayyer, M.; Bushell, A.F.; Budd, P.M.; Attfield, M.P.; Jiang, D.; Burrows, A.D.; Esposito, E.; Bernardo, P.; Monteleone, M.; Fuoco, A.; et al. Mixed matrix membranes based on MIL-101 metal–organic frameworks in polymer of intrinsic microporosity PIM-1. Sep. Purif. Technol. 2019, 212, 545–554. [Google Scholar] [CrossRef]
- Thomas, A.M.; de Grooth, J.; Wood, J.A. Synthetic guidelines for highly selective mixed matrix membranes. J. Membr. Sci. 2022, 649, 120311. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, K.; Wang, H.; Fan, Y.; Zhang, S.; He, S.; Wang, F.; Tao, Y.; Zhao, X.; Zhang, Y.B.; et al. Enhancing the Gas Separation Selectivity of Mixed-Matrix Membranes Using a Dual-Interfacial Engineering Approach. J. Am. Chem. Soc. 2020, 142, 18503–18512. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Luo, J.; Liu, X.; Liu, S.; Wang, Y.; Zong, X.; Xue, S. Fabrication of high-performance mixed-matrix membranes via constructing an in-situ crosslinked polymer matrix for gas separations. Sep. Purif. Technol. 2021, 271, 118859. [Google Scholar] [CrossRef]
- Ma, C.; Urban, J.J. Hydrogen-Bonded Polyimide/Metal-Organic Framework Hybrid Membranes for Ultrafast Separations of Multiple Gas Pairs. Adv. Funct. Mater. 2019, 29, 1903243. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, H.; Zhang, S.; Zhang, F.; Jin, J. Polymers of intrinsic microporosity/metal–organic framework hybrid membranes with improved interfacial interaction for high-performance CO2 separation. J. Mater. Chem. A 2017, 5, 10968–10977. [Google Scholar] [CrossRef]
- Tien-Binh, N.; Vinh-Thang, H.; Chen, X.Y.; Rodrigue, D.; Kaliaguine, S. Crosslinked MOF-polymer to enhance gas separation of mixed matrix membranes. J. Membr. Sci. 2016, 520, 941–950. [Google Scholar] [CrossRef]
- Li, F.Y.; Xiao, Y.; Chung, T.-S.; Kawi, S. High-Performance Thermally Self-Cross-Linked Polymer of Intrinsic Microporosity (PIM-1) Membranes for Energy Development. Macromolecules 2012, 45, 1427–1437. [Google Scholar] [CrossRef]
- Yu, G.; Zou, X.; Sun, L.; Liu, B.; Wang, Z.; Zhang, P.; Zhu, G. Constructing Connected Paths between UiO-66 and PIM-1 to Improve Membrane CO2 Separation with Crystal-Like Gas Selectivity. Adv. Mater. 2019, 31, e1806853. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Garibay, S.J.; Cohen, S.M. Microwave-assisted cyanation of an aryl bromide directly on a metal-organic framework. Inorg. Chem. 2011, 50, 729–731. [Google Scholar] [CrossRef]
- Chen, L.; Bai, Z.; Zhu, L.; Zhang, L.; Cai, Y.; Li, Y.; Liu, W.; Wang, Y.; Chen, L.; Diwu, J.; et al. Ultrafast and Efficient Extraction of Uranium from Seawater Using an Amidoxime Appended Metal-Organic Framework. ACS Appl. Mater. Interfaces 2017, 9, 32446–32451. [Google Scholar] [CrossRef]
- Alberto, M.; Luque-Alled, J.M.; Gao, L.; Iliut, M.; Prestat, E.; Newman, L.; Haigh, S.J.; Vijayaraghavan, A.; Budd, P.M.; Gorgojo, P. Enhanced organophilic separations with mixed matrix membranes of polymers of intrinsic microporosity and graphene-like fillers. J. Membr. Sci. 2017, 526, 437–449. [Google Scholar] [CrossRef]
- Du, N.; Song, J.; Robertson, G.P.; Pinnau, I.; Guiver, M.D. Linear High Molecular Weight Ladder Polymer via Fast Polycondensation of 5,5′,6,6′-Tetrahydroxy-3,3,3′,3′-tetramethylspirobisindane with 1,4-Dicyanotetrafluorobenzene. Macromol. Rapid Commun. 2008, 29, 783–788. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, J.; Li, H.; Fan, F.; Zhao, Q.; He, G.; Ma, C. Ester-crosslinked polymers of intrinsic microporosity membranes with enhanced plasticization resistance for CO2 separation. Sep. Purif. Technol. 2023, 314, 123623. [Google Scholar] [CrossRef]
- Tahir, Z.; Aslam, M.; Gilani, M.A.; Bilad, M.R.; Anjum, M.W.; Zhu, L.-P.; Khan, A.L. SO3H functionalized UiO-66 nanocrystals in Polysulfone based mixed matrix membranes: Synthesis and application for efficient CO2 capture. Sep. Purif. Technol. 2019, 224, 524–533. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, Y.; Liu, L.; Ma, F.; Zhang, C.; Dong, H. MOF modified with copolymers containing carboxyl and amidoxime groups and high efficiency U (VI) extraction from seawater. Sep. Purif. Technol. 2022, 291, 120946. [Google Scholar] [CrossRef]
- Garibay, S.J.; Cohen, S.M. Isoreticular synthesis and modification of frameworks with the UiO-66 topology. Chem. Commun. 2010, 46, 7700–7702. [Google Scholar] [CrossRef]
- Ghanem, B.S.; Swaidan, R.; Litwiller, E.; Pinnau, I. Ultra-microporous triptycene-based polyimide membranes for high-performance gas separation. Adv. Mater. 2014, 26, 3688–3692. [Google Scholar] [CrossRef]
- He, S.; Zhu, B.; Li, S.; Zhang, Y.; Jiang, X.; Lau, C.H.; Shao, L. Recent progress in PIM-1 based membranes for sustainable CO2 separations: Polymer structure manipulation and mixed matrix membrane design. Sep. Purif. Technol. 2022, 284, 120277. [Google Scholar] [CrossRef]
- Tien-Binh, N.; Rodrigue, D.; Kaliaguine, S. In-situ cross interface linking of PIM-1 polymer and UiO-66-NH2 for outstanding gas separation and physical aging control. J. Membr. Sci. 2018, 548, 429–438. [Google Scholar] [CrossRef]
- Mason, C.R.; Buonomenna, M.G.; Golemme, G.; Budd, P.M.; Galiano, F.; Figoli, A.; Friess, K.; Hynek, V. New organophilic mixed matrix membranes derived from a polymer of intrinsic microporosity and silicalite-1. Polymer 2013, 54, 2222–2230. [Google Scholar] [CrossRef]
- Han, J.; Bai, L.; Jiang, H.; Zeng, S.; Yang, B.; Bai, Y.; Zhang, X. Task-Specific Ionic Liquids Tuning ZIF-67/PIM-1 Mixed Matrix Membranes for Efficient CO2 Separation. Ind. Eng. Chem. Res. 2020, 60, 593–603. [Google Scholar] [CrossRef]
- Zhao, H.; Feng, L.; Ding, X.; Zhao, Y.; Tan, X.; Zhang, Y. The nitrogen-doped porous carbons/PIM mixed-matrix membranes for CO2 separation. J. Membr. Sci. 2018, 564, 800–805. [Google Scholar] [CrossRef]





| UiO-66-AO | Permeability (P) (Barrer) | Ideal Selectivity (α) | |||
|---|---|---|---|---|---|
| CO2 | N2 | CH4 | CO2/N2 | CO2/CH4 | |
| 0 | 5573.24 | 300.97 | 496.18 | 18.52 | 11.23 |
| 5% | 6335.77 | 296.06 | 458.45 | 21.44 | 13.82 |
| 10% | 7535.54 | 279.24 | 500.29 | 26.90 | 15.06 |
| 20% | 8398.75 | 358.77 | 506.75 | 23.41 | 16.57 |
| 30% | 8792.33 | 407.86 | 509.57 | 21.56 | 15.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, J.; Sun, Y.; Kang, F.; Guo, F.; He, G.; Wang, H.; Yang, Z.; Ma, C.; Jiang, X.; Xiao, W. Amidoxime Modified UiO-66@PIM-1 Mixed-Matrix Membranes to Enhance CO2 Separation and Anti-Aging Performance. Membranes 2023, 13, 781. https://doi.org/10.3390/membranes13090781
Gao J, Sun Y, Kang F, Guo F, He G, Wang H, Yang Z, Ma C, Jiang X, Xiao W. Amidoxime Modified UiO-66@PIM-1 Mixed-Matrix Membranes to Enhance CO2 Separation and Anti-Aging Performance. Membranes. 2023; 13(9):781. https://doi.org/10.3390/membranes13090781
Chicago/Turabian StyleGao, Jiaming, Yongchao Sun, Feifei Kang, Fei Guo, Gaohong He, Hanli Wang, Zhendong Yang, Canghai Ma, Xiaobin Jiang, and Wu Xiao. 2023. "Amidoxime Modified UiO-66@PIM-1 Mixed-Matrix Membranes to Enhance CO2 Separation and Anti-Aging Performance" Membranes 13, no. 9: 781. https://doi.org/10.3390/membranes13090781
APA StyleGao, J., Sun, Y., Kang, F., Guo, F., He, G., Wang, H., Yang, Z., Ma, C., Jiang, X., & Xiao, W. (2023). Amidoxime Modified UiO-66@PIM-1 Mixed-Matrix Membranes to Enhance CO2 Separation and Anti-Aging Performance. Membranes, 13(9), 781. https://doi.org/10.3390/membranes13090781








