Facile Preparation of Durable and Eco-Friendly Superhydrophobic Filter with Self-Healing Ability for Efficient Oil/Water Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Silica Sol
2.3. Fabrication of MA/SiO2@cotton
2.4. Characterisation
2.5. Oil/Water Separation (OWS)
2.6. Mechanical and Chemical Durability Test
3. Results and Discussion
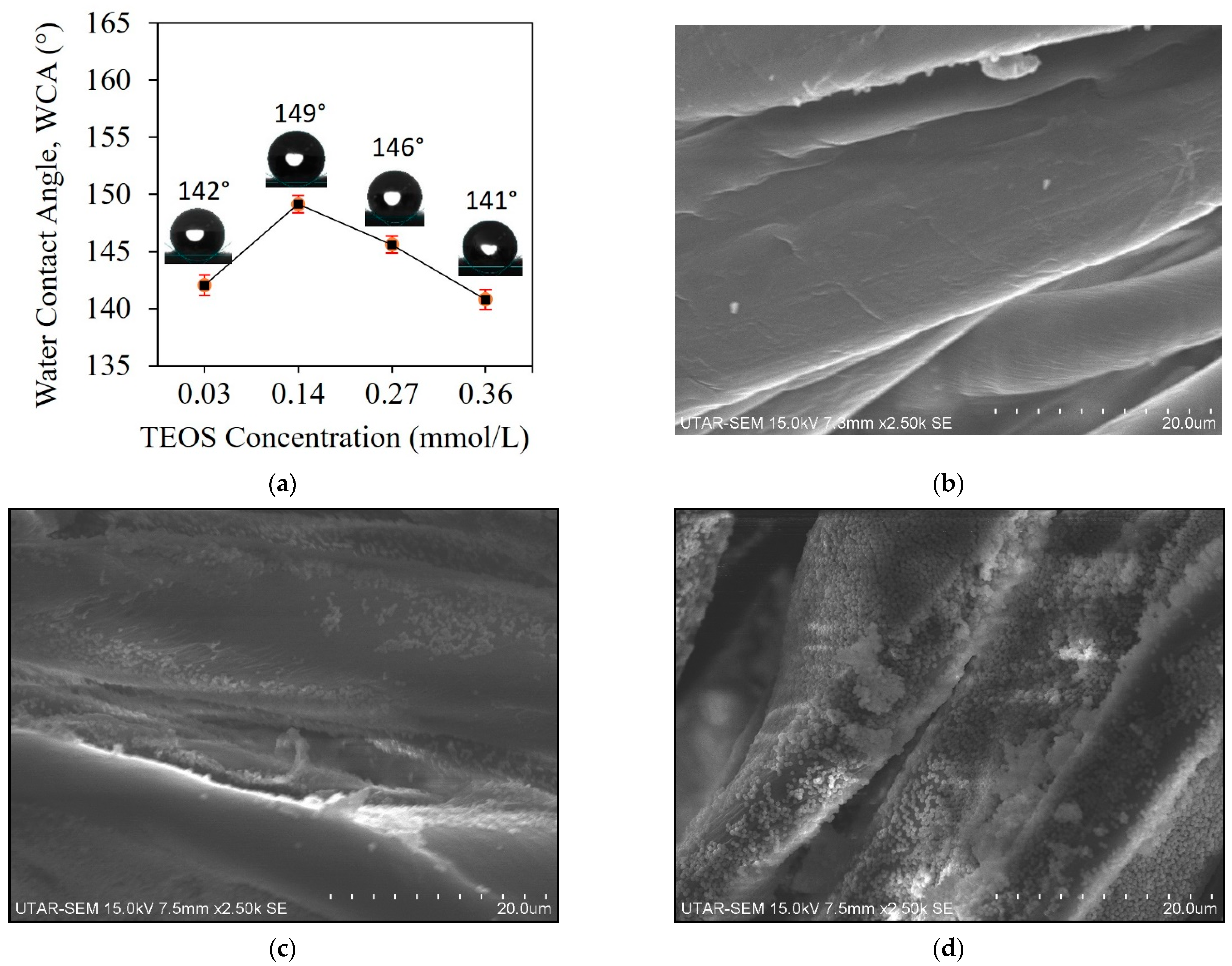
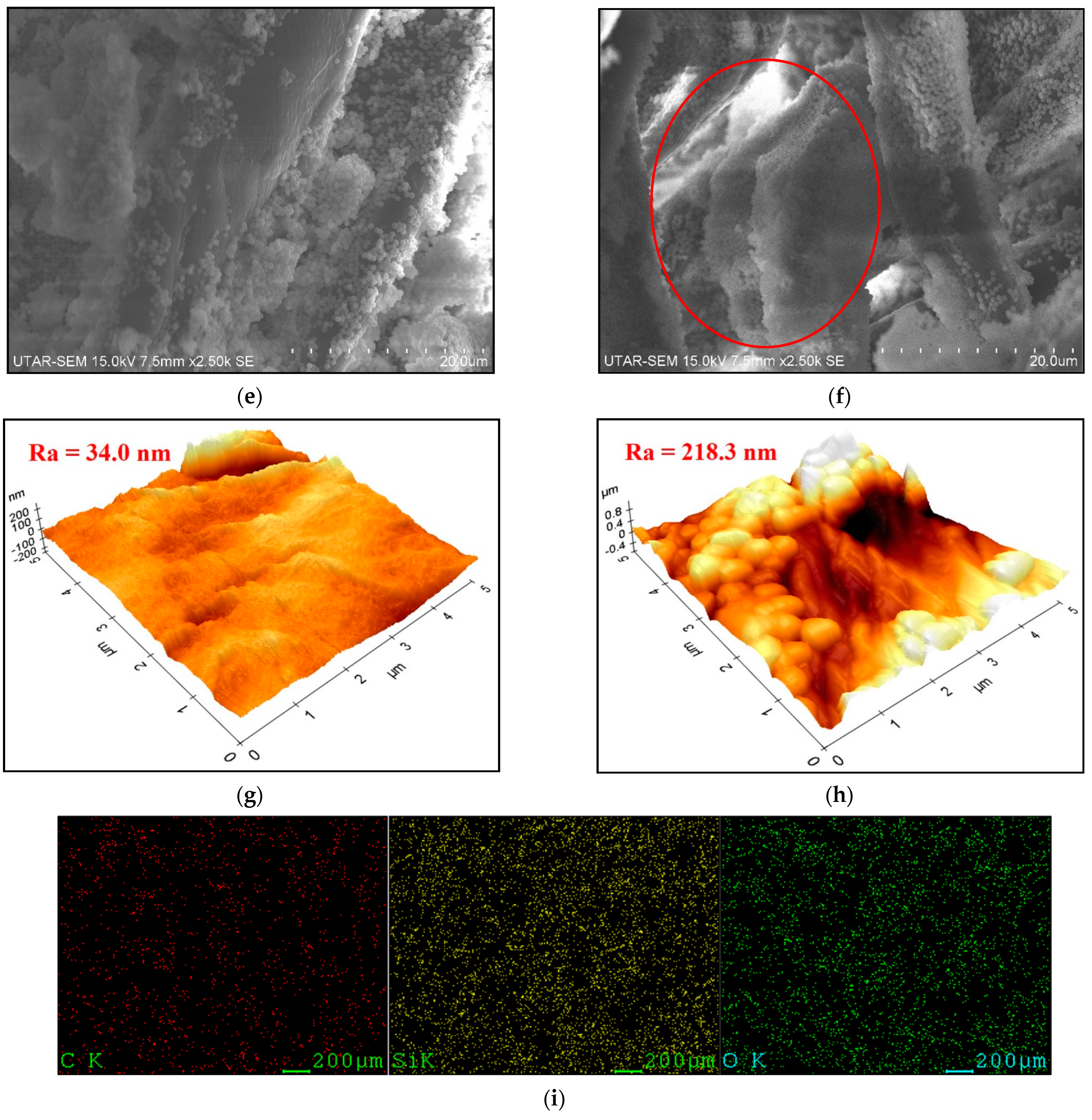
3.1. Effect of Silica Particles Loading
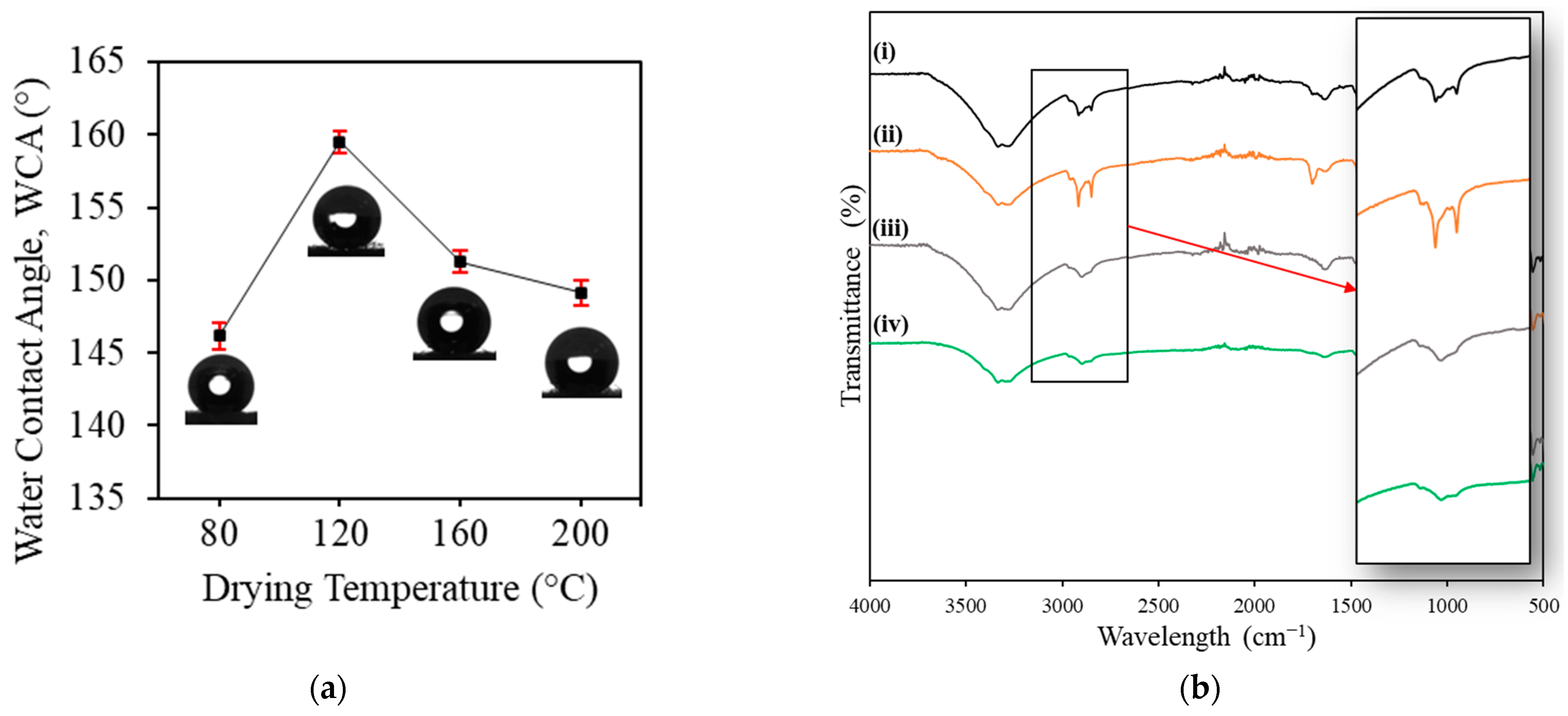
3.2. Effect of Drying Temperature
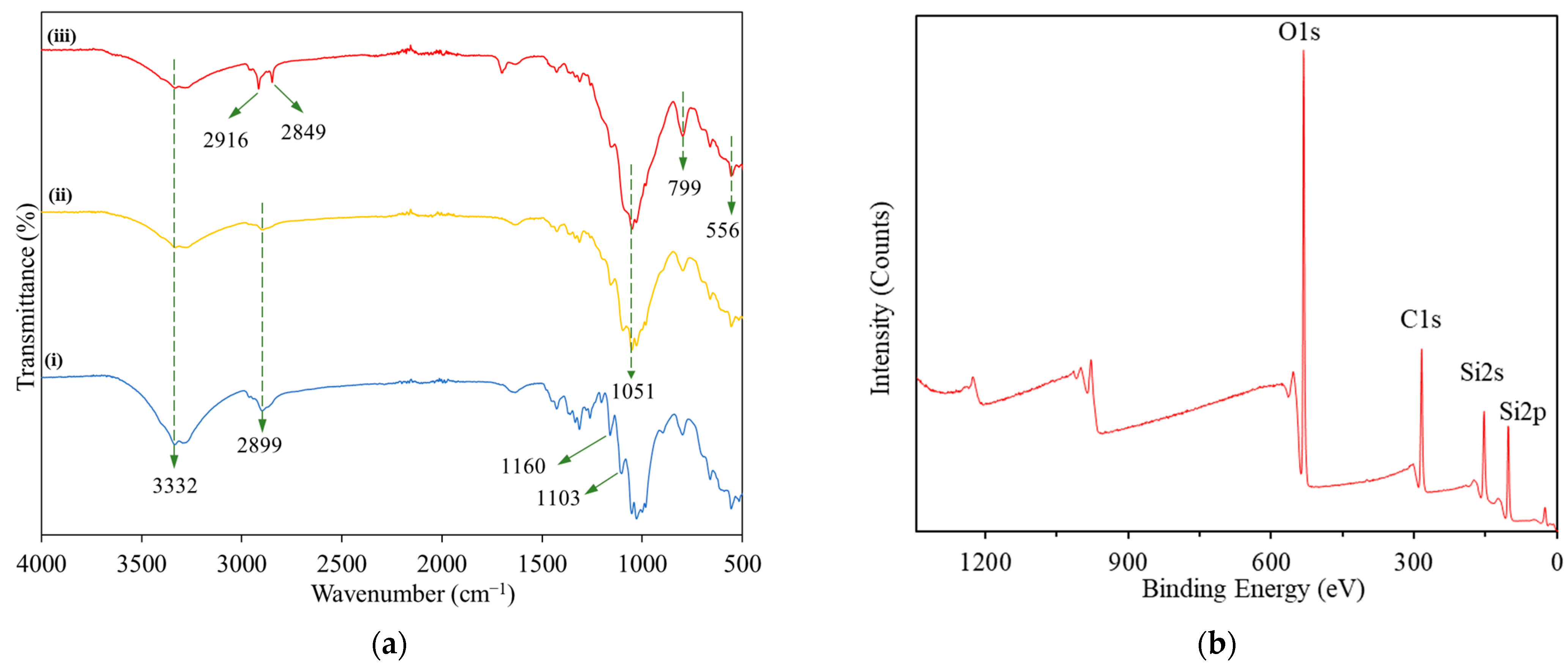
3.3. FTIR and XPS Analysis
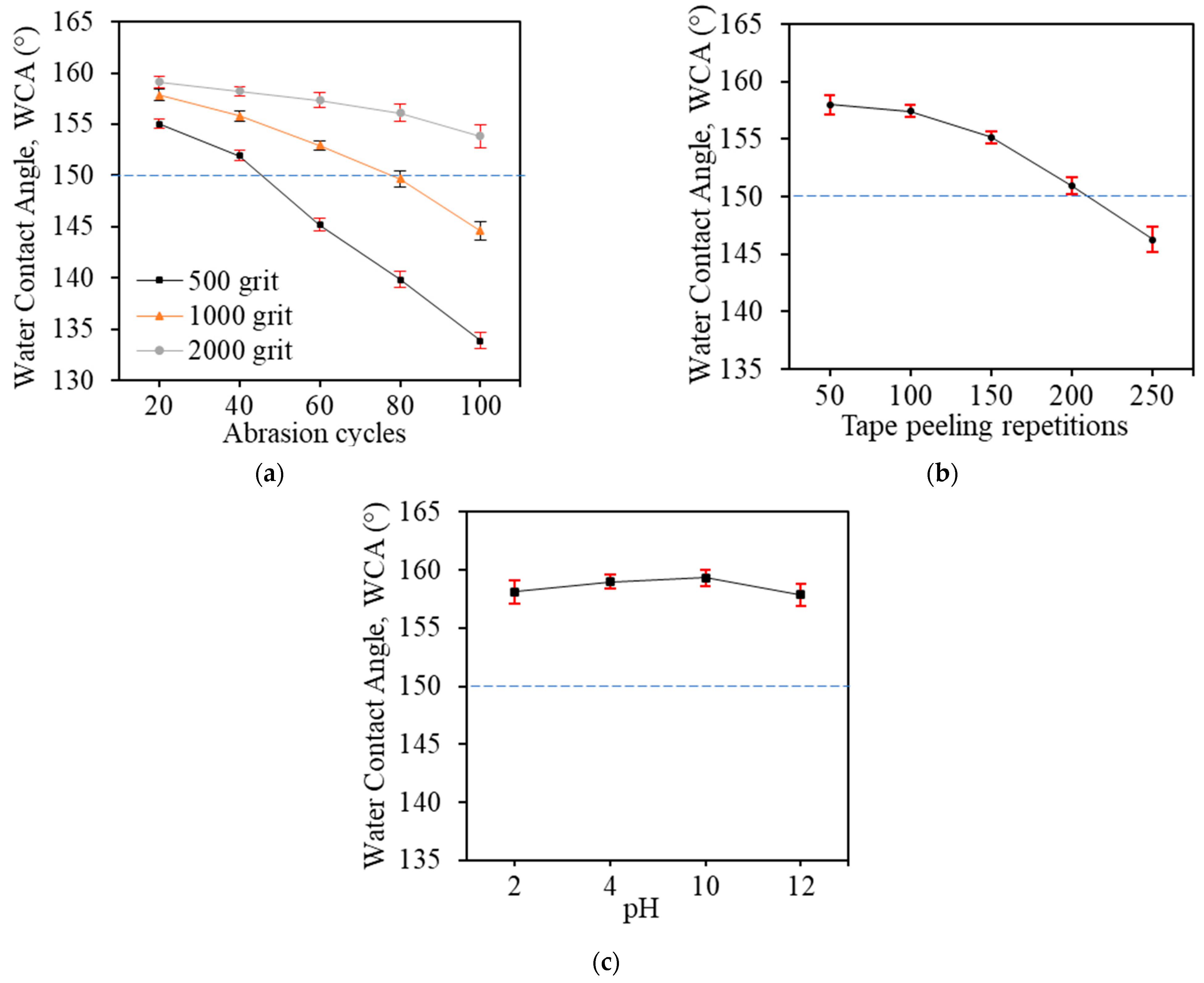
3.4. Mechanical and Chemical Durability
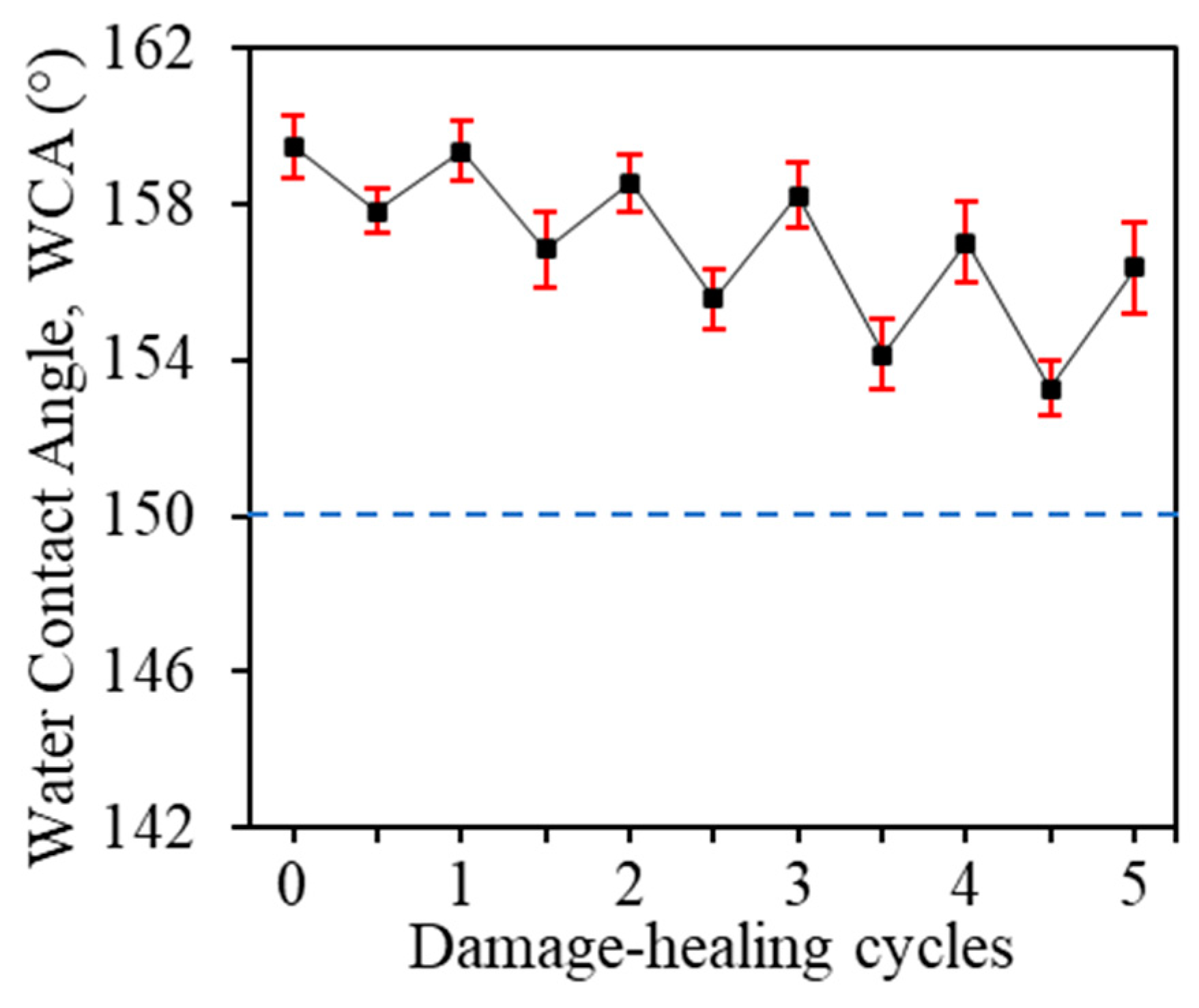
3.5. Self-Healing Property
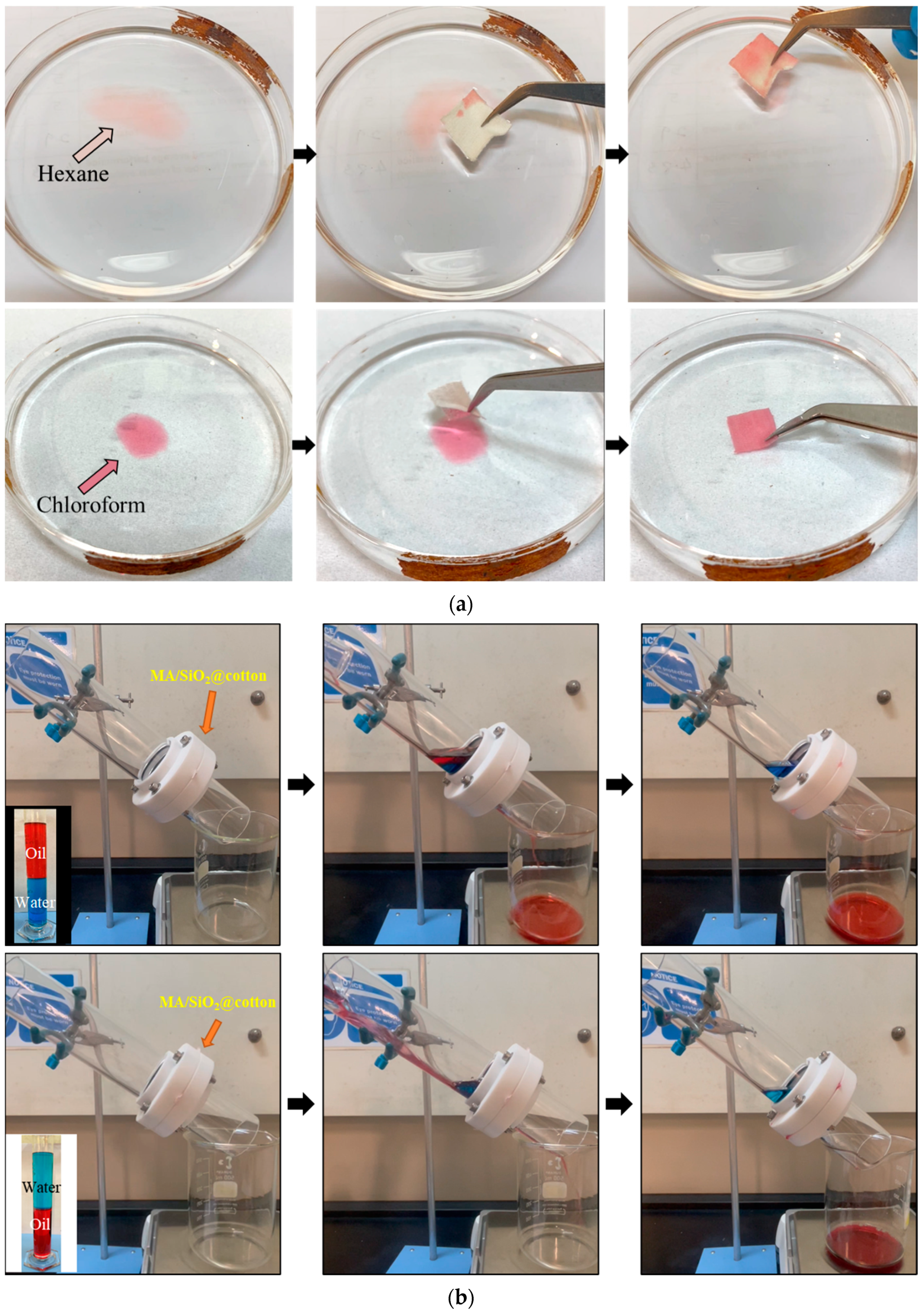
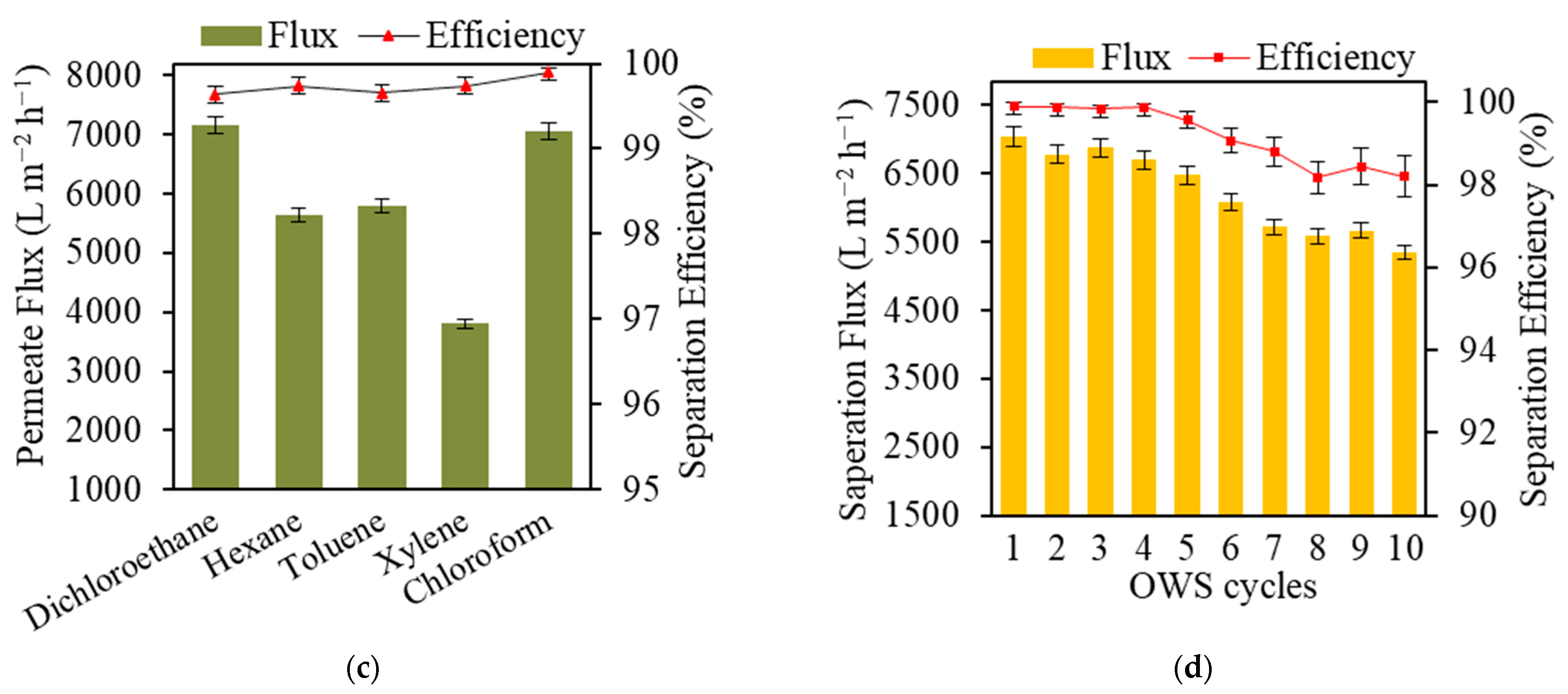
3.6. Performance in Oil/Water Separation and Reusability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bao, Y.; Chang, J.; Zhang, Y.; Chen, L. Robust Superhydrophobic Coating with Hollow SiO2/PAA-b-PS Janus Microspheres for Self-Cleaning and Oil–Water Separation. Chem. Eng. J. 2022, 446, 136959. [Google Scholar] [CrossRef]
- Fan, S.; Wang, Z.; Liang, P.; Li, H.; Zhang, Y.; Fan, W.; Xu, G. Fabrication of Polypyrrole Coated Superhydrophobic Surfaces for Effective Oil/Water Separation. J. Mater. Res. Technol. 2022, 19, 4337–4349. [Google Scholar] [CrossRef]
- Shang, Q.; Cheng, J.; Liu, C.; Hu, L.; Bo, C.; Hu, Y.; Yang, X.; Ren, X.; Zhou, Y.; Lei, W. Fabrication of Sustainable and Durable Bio-Polybenzoxazine Based Superhydrophobic Cotton Fabric for Efficient Oil/Water Separation. Prog. Org. Coat. 2021, 158, 106343. [Google Scholar] [CrossRef]
- Dhaka, A.; Chattopadhyay, P. A Review on Physical Remediation Techniques for Treatment of Marine Oil Spills. J. Environ. Manag. 2021, 288, 112428. [Google Scholar] [CrossRef]
- Gupta, R.K.; Dunderdale, G.J.; England, M.W.; Hozumi, A. Oil/Water Separation Techniques: A Review of Recent Progresses and Future Directions. J. Mater. Chem. A R. Soc. Chem. 2017, 5, 16025–16058. [Google Scholar] [CrossRef]
- Yu, L.; Han, M.; He, F. A Review of Treating Oily Wastewater. Arab. J. Chem. 2017, 10, S1913–S1922. [Google Scholar] [CrossRef]
- Rasouli, S.; Rezaei, N.; Hamedi, H.; Zendehboudi, S.; Duan, X. Superhydrophobic and Superoleophilic Membranes for Oil-Water Separation Application: A Comprehensive Review. Mater. Des. 2021, 204, 109599. [Google Scholar] [CrossRef]
- Motta, F.L.; Stoyanov, S.R.; Soares, J.B.P. Application of Solidifiers for Oil Spill Containment: A Review. Chemosphere 2018, 194, 837–846. [Google Scholar] [CrossRef]
- Abidli, A.; Huang, Y.; Park, C.B. In Situ Oils/Organic Solvents Cleanup and Recovery Using Advanced Oil-Water Separation System. Chemosphere 2020, 260, 127586. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.N.; Maity, J.P.; Bundschuh, J.; Li, C.F.; Lee, C.R.; Hsu, C.M.; Lee, W.C.; Huang, C.H.; Chen, C.Y. Green Technological Approach to Synthesis Hydrophobic Stable Crystalline Calcite Particles with One-Pot Synthesis for Oil–Water Separation during Oil Spill Cleanup. Water Res. 2017, 123, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Chen, M.; Dong, Y.; Tang, C.Y.; Huang, A.; Li, L. A Low-Cost Mullite-Titania Composite Ceramic Hollow Fiber Microfiltration Membrane for Highly Efficient Separation of Oil-in-Water Emulsion. Water Res. 2016, 90, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Goyat, M.S.; Hooda, A.; Pandey, J.K.; Kumar, A.; Gupta, R.; Upadhyay, A.K.; Prakash, R.; Kirabira, J.B.; Mandal, P.; et al. Recent Progress in Nano-Oxides and CNTs Based Corrosion Resistant Superhydrophobic Coatings: A Critical Review. Prog. Org. Coat. 2020, 140, 105512. [Google Scholar] [CrossRef]
- Peng, F.; Zhang, D.; Liu, X.; Zhang, Y. Recent Progress in Superhydrophobic Coating on Mg Alloys: A General Review. J. Magnes. Alloy. 2020, 9, 1471–1486. [Google Scholar] [CrossRef]
- Li, D.; Guo, Z. Stable and Self-Healing Superhydrophobic MnO2@fabrics: Applications in Self-Cleaning, Oil/Water Separation and Wear Resistance. J. Colloid Interface Sci. 2017, 503, 124–130. [Google Scholar] [CrossRef]
- Xiang, S.; Liu, W. Self-Healing Superhydrophobic Surfaces: Healing Principles and Applications. Adv. Mater. Interfaces 2021, 8, 2100247. [Google Scholar] [CrossRef]
- Yin, X.; Yu, S.; Wang, K.; Cheng, R.; Lv, Z. Fluorine-Free Preparation of Self-Healing and Anti-Fouling Superhydrophobic Ni3S2 Coating on 304 Stainless Steel. Chem. Eng. J. 2020, 394, 124925. [Google Scholar] [CrossRef]
- Yuan, D.; Zhang, T.; Guo, Q.; Qiu, F.; Yang, D.; Ou, Z. Recyclable Biomass Carbon@SiO2@MnO2 Aerogel with Hierarchical Structures for Fast and Selective Oil-Water Separation. Chem. Eng. J. 2018, 351, 622–630. [Google Scholar] [CrossRef]
- Perween, S.; Khan, Z.; Singh, S.; Ranjan, A. PVA-PDMS-Stearic Acid Composite Nanofibrous Mats with Improved Mechanical Behavior for Selective Filtering Applications. Sci. Rep. 2018, 8, 16038. [Google Scholar] [CrossRef]
- Qing, Y.; Yang, C.; Yu, Z.; Zhang, Z.; Hu, Q.; Liu, C. Large-Area Fabrication of Superhydrophobic Zinc Surface with Reversible Wettability Switching and Anticorrosion. J. Electrochem. Soc. 2016, 163, D385–D391. [Google Scholar] [CrossRef]
- Ma, W.; Ding, Y.; Li, Y.; Gao, S.; Jiang, Z.; Cui, J.; Huang, C.; Fu, G. Durable, Self-Healing Superhydrophobic Nanofibrous Membrane with Self-Cleaning Ability for Highly-Efficient Oily Wastewater Purification. J. Memb. Sci. 2021, 634, 119402. [Google Scholar] [CrossRef]
- Jiang, C.; Liu, W.; Yang, M.; Liu, C.; He, S.; Xie, Y.; Wang, Z. Facile Fabrication of Robust Fluorine-Free Self-Cleaning Cotton Textiles with Superhydrophobicity, Photocatalytic Activity, and UV Durability. Colloids Surf. A Physicochem. Eng. Asp. 2018, 559, 235–242. [Google Scholar] [CrossRef]
- Peng, S.; Meng, W.; Guo, J.; Wang, B.; Wang, Z.; Xu, N.; Li, X.; Wang, J.; Xu, J. Photocatalytically Stable Superhydrophobic and Translucent Coatings Generated from PDMS-Grafted-SiO2/TiO2@PDMS with Multiple Applications. Langmuir 2019, 35, 2760–2771. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Li, Z.; Cheng, F.; Wu, C.; Cai, D.; Zhang, Q.; Zhang, H. Preparation of Durable Superhydrophobic Composite Coatings with Photothermal Conversion Precisely Targeted Configuration Self-Healability and Great Degradability. Compos. Sci. Technol. 2021, 213, 108926. [Google Scholar] [CrossRef]
- Shen, S.; Wang, C.; Sun, M.; Jia, M.; Tang, Z.; Yang, J. Free-Standing Sodium Titanate Ultralong Nanotube Membrane with Oil-Water Separation, Self-Cleaning, and Photocatalysis Properties. Nanoscale Res. Lett. 2020, 15, 22. [Google Scholar] [CrossRef]
- Sam, E.K.; Ge, Y.; Liu, J.; Lv, X. Robust, Self-Healing, Superhydrophobic Fabric for Efficient Oil/Water Emulsion Separation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126860. [Google Scholar] [CrossRef]
- Li, X.; Li, B.; Li, Y.; Sun, J. Nonfluorinated, Transparent, and Spontaneous Self-Healing Superhydrophobic Coatings Enabled by Supramolecular Polymers. Chem. Eng. J. 2021, 404, 126504. [Google Scholar] [CrossRef]
- Talbot, G. Tropical Exotic Oils: Properties and Processing for Use in Food; Elsevier Ltd.: Amsterdam, The Netherlands, 2015. [Google Scholar] [CrossRef]
- Chowdhury, R.; Steur, M.; Patel, P.S.; Franco, O.H. Individual Fatty Acids in Cardiometabolic Disease; Elsevier Inc.: Amsterdam, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Voo, W.; Chong, W.; Teoh, H.; Mohammad, A. Fluorine-Free Superhydrophobic Cotton Fabric for Highly-Efficient Gravity-Driven Oil/Water Separation. IOP Conf. Ser. Mater. Sci. Eng. 2022, 1257, 012048. [Google Scholar] [CrossRef]
- Wang, X.; Ding, H.; Sun, S.; Zhang, H.; Zhou, R.; Li, Y.; Liang, Y.; Wang, J. Preparation of a Temperature-Sensitive Superhydrophobic Self-Cleaning SiO2-TiO2@PDMS Coating with Photocatalytic Activity. Surf. Coat. Technol. 2021, 408, 126853. [Google Scholar] [CrossRef]
- Zhao, L.; Du, Z.; Tai, X.; Ma, Y. One-Step Facile Fabrication of Hydrophobic SiO2 Coated Super-Hydrophobic/Super-Oleophilic Mesh via an Improved Stöber Method to Efficient Oil/Water Separation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 623, 126404. [Google Scholar] [CrossRef]
- Jannatun, N.; Taraqqi-A-Kamal, A.; Rehman, R.; Kuker, J.; Lahiri, S.K. A Facile Cross-Linking Approach to Fabricate Durable and Self-Healing Superhydrophobic Coatings of SiO2-PVA@PDMS on Cotton Textile. Eur. Polym. J. 2020, 134, 109836. [Google Scholar] [CrossRef]
- Chen, C.; Li, Z.; Hu, Y.; Huang, Q.; Li, X.; Qing, Y.; Wu, Y. Rosin Acid and SiO2 Modified Cotton Fabric to Prepare Fluorine-Free Durable Superhydrophobic Coating for Oil-Water Separation. J. Hazard. Mater. 2022, 440, 129797. [Google Scholar] [CrossRef] [PubMed]
- Vafaei, S.; Borca-Tasciuc, T.; Podowski, M.Z.; Purkayastha, A.; Ramanath, G.; Ajayan, P.M. Effect of Nanoparticles on Sessile Droplet Contact Angle. Nanotechnology 2006, 17, 2523–2527. [Google Scholar] [CrossRef] [PubMed]
- Ogihara, H.; Xie, J.; Okagaki, J.; Saji, T. Simple Method for Preparing Superhydrophobic Paper: Spray-Deposited Hydrophobic Silica Nanoparticle Coatings Exhibit High Water-Repellency and Transparency. Langmuir 2012, 28, 4605–4608. [Google Scholar] [CrossRef] [PubMed]
- Silvia, L.; Hasanah, I.; Zainuri, M. Hydrophobic of Polyethylene/SiO2 Modified Coating for Self Cleaning Material. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2019; Volume 2202. [Google Scholar] [CrossRef]
- Drelich, J.; Chibowski, E.; Meng, D.D.; Terpilowski, K. Hydrophilic and Superhydrophilic Surfaces and Materials. Soft Matter 2011, 7, 9804–9828. [Google Scholar] [CrossRef]
- Kuo, S.W.; Wu, Y.C.; Wang, C.F.; Jeong, K.U. Preparing Low-Surface-Energy Polymer Materials by Minimizing Intermolecular Hydrogen-Bonding Interactions. J. Phys. Chem. C 2009, 113, 20666–20673. [Google Scholar] [CrossRef]
- Jiang, B.; Chen, Z.; Sun, Y.; Yang, H.; Zhang, H.; Dou, H.; Zhang, L. Fabrication of Superhydrophobic Cotton Fabrics Using Crosslinking Polymerization Method. Appl. Surf. Sci. 2018, 441, 554–563. [Google Scholar] [CrossRef]
- Chung, C.; Lee, M.; Choe, E.K. Characterization of Cotton Fabric Scouring by FT-IR ATR Spectroscopy. Carbohydr. Polym. 2004, 58, 417–420. [Google Scholar] [CrossRef]
- Yu, N.; Xiao, X.; Pan, G. A Stearic Acidified-ZnO/Methyl Polysiloxane/PDMS Superhydrophobic Coating with Good Mechanical Durability and Physical Repairability. J. Dispers. Sci. Technol. 2019, 40, 1548–1558. [Google Scholar] [CrossRef]
- Gao, S.; Dong, X.; Huang, J.; Li, S.; Li, Y.; Chen, Z.; Lai, Y. Rational Construction of Highly Transparent Superhydrophobic Coatings Based on a Non-Particle, Fluorine-Free and Water-Rich System for Versatile Oil-Water Separation. Chem. Eng. J. 2018, 333, 621–629. [Google Scholar] [CrossRef]
- Wang, X.; Ding, H.; Wang, C.; Zhou, R.; Li, Y.; Li, W.; Ao, W. Self-Healing Superhydrophobic A-SiO2/N-TiO2@HDTMS Coating with Self-Cleaning Property. Appl. Surf. Sci. 2021, 567, 150808. [Google Scholar] [CrossRef]
- Xue, C.H.; Bai, X.; Jia, S.T. Robust, Self-Healing Superhydrophobic Fabrics Prepared by One-Step Coating of PDMS and Octadecylamine. Sci. Rep. 2016, 6, 27262. [Google Scholar] [CrossRef]
- Ray, S.C.; Mishra, D.K.; Strydom, A.M.; Papakonstantinou, P. Magnetic Behavioural Change of Silane Exposed Graphene Nanoflakes. J. Appl. Phys. 2015, 118, 115302. [Google Scholar] [CrossRef]
- Yang, X.; Li, H.; Li, L.; Li, M.; Xing, T.; Chen, G. Fluorine-Free, Short-Process and Robust Superhydrophobic Cotton Fabric and Its Oil-Water Separation Ability. Prog. Org. Coat. 2022, 172, 107122. [Google Scholar] [CrossRef]
- Zeng, S.; Feng, W.; Peng, S.; Teng, Z.; Chen, C.; Zhang, H.; Peng, S. Dual-Functional SiOC Ceramics Coating Modified Carbon Fibers with Enhanced Microwave Absorption Performance. RSC Adv. 2019, 9, 30685–30692. [Google Scholar] [CrossRef]
- Kaur, A.; Chahal, P.; Hogan, T. Selective Fabrication of SiC/Si Diodes by Excimer Laser under Ambient Conditions. IEEE Electron Device Lett. 2016, 37, 142–145. [Google Scholar] [CrossRef]
- Zhang, X.; Geng, T.; Guo, Y.; Zhang, Z.; Zhang, P. Facile Fabrication of Stable Superhydrophobic SiO2/Polystyrene Coating and Separation of Liquids with Different Surface Tension. Chem. Eng. J. 2013, 231, 414–419. [Google Scholar] [CrossRef]
- Cheng, J.; Shang, Q.; Liu, C.; Hu, L.; Bo, C.; Hu, Y.; Yang, X.; Zhou, Y.; Lei, W. Fabrication of Cardanol-Based Superhydrophobic Cotton Fabric for Highly Effective Oil-Water Separation. Mater. Today Commun. 2021, 29, 102820. [Google Scholar] [CrossRef]
- Pal, S.; Mondal, S.; Pal, P.; Das, A.; Pramanik, S.; Maity, J. Fabrication of Durable, Fluorine-Free Superhydrophobic Cotton Fabric for Efficient Self-Cleaning and Heavy/Light Oil-Water Separation. Colloids Interface Sci. Commun. 2021, 44, 100469. [Google Scholar] [CrossRef]
- Lin, H.; Chen, K.; Zheng, S.; Zeng, R.; Lin, Y.; Jian, R.; Bai, W.; Xu, Y. Facile Fabrication of Natural Superhydrophobic Eleostearic Acid-SiO2@cotton Fabric for Efficient Separation of Oil/Water Mixtures and Emulsions. Sustain. Mater. Technol. 2022, 32, e00418. [Google Scholar] [CrossRef]
- Zhang, Z.; Dong, H.; Liao, Y. Facile Fabrication of SWCNT Film-Based Superhydrophobic Cotton Fabrics for Oil/Water Separation and Self-Cleaning. J. Environ. Chem. Eng. 2023, 11, 109570. [Google Scholar] [CrossRef]
- Ren, H.-T.; Cai, C.-C.; Cao, W.-B.; Li, D.-S.; Li, T.-T.; Lou, C.-W.; Lin, J.-H. Superhydrophobic TiN-Coated Cotton Fabrics with Nanoscale Roughness and Photothermal Self-Healing Properties for Effective Oil–Water Separation. ACS Appl. Nano Mater. 2023. [Google Scholar] [CrossRef]

| Material(s) | Efficiency η (%) | Flux, J (L m−2 h−1) | OWS Cycles, m | ηm (%) | Self-Healing | References |
|---|---|---|---|---|---|---|
| PDMS/ZIF-90/FAS@linen fabric | 99.5 | 1213 | 10 | 98 | Yes | [25] |
| Eleostearic acid/SiO2@cotton | 99.5 | 3600 | 15 | 99.5 | No | [52] |
| SiO2/PAA-b-PS Janus microspheres coated on fabric | 99.36 | 3851 | 40 | 98 | No | [1] |
| Rosin acid, SiO2 on cotton fabric | 98.8 | 7810 | 10 | 96 | No | [33] |
| Single-walled carbon nanotubes (SWCNT) on cotton fabric treated with 1 H,1 H,2 H,2 H-perfluorooctyltriethoxysilane (POTS) | 99.2 | 28,710 | 20 | 98.8 | No | [53] |
| Nano-titaniumnitride(TiN), PDMS-SiO2 on cotton fabric | 98.16 | >5500 | 10 | 98 | Yes | [54] |
| Myristic acid, SiO2, on cotton fabric | 99.9 | 7050 | 10 | 98 | Yes | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voo, W.X.; Chong, W.C.; Teoh, H.C.; Lau, W.J.; Chan, Y.J.; Chung, Y.T. Facile Preparation of Durable and Eco-Friendly Superhydrophobic Filter with Self-Healing Ability for Efficient Oil/Water Separation. Membranes 2023, 13, 793. https://doi.org/10.3390/membranes13090793
Voo WX, Chong WC, Teoh HC, Lau WJ, Chan YJ, Chung YT. Facile Preparation of Durable and Eco-Friendly Superhydrophobic Filter with Self-Healing Ability for Efficient Oil/Water Separation. Membranes. 2023; 13(9):793. https://doi.org/10.3390/membranes13090793
Chicago/Turabian StyleVoo, Wei Xin, Woon Chan Chong, Hui Chieh Teoh, Woei Jye Lau, Yi Jing Chan, and Ying Tao Chung. 2023. "Facile Preparation of Durable and Eco-Friendly Superhydrophobic Filter with Self-Healing Ability for Efficient Oil/Water Separation" Membranes 13, no. 9: 793. https://doi.org/10.3390/membranes13090793
APA StyleVoo, W. X., Chong, W. C., Teoh, H. C., Lau, W. J., Chan, Y. J., & Chung, Y. T. (2023). Facile Preparation of Durable and Eco-Friendly Superhydrophobic Filter with Self-Healing Ability for Efficient Oil/Water Separation. Membranes, 13(9), 793. https://doi.org/10.3390/membranes13090793






