Acrylonitrile–Acrylic Acid Copolymer Ultrafiltration Membranes for Selective Asphaltene Removal from Crude Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polymer Synthesis
2.3. Polymer Characterization
2.4. Solution Preparation and Characterization
2.5. Membrane Preparation
2.6. Membrane Characterization
3. Results and Discussion
3.1. Copolymer Characterization
3.2. Membrane Preparation and Characterization
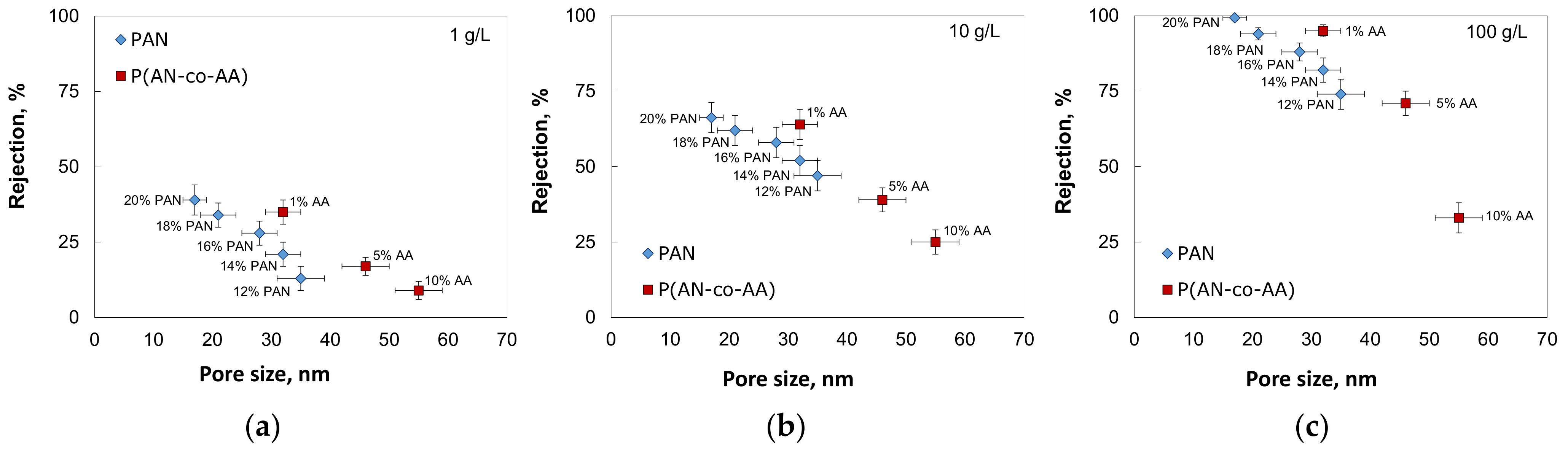
3.3. Oil/Toluene Solution Filtration
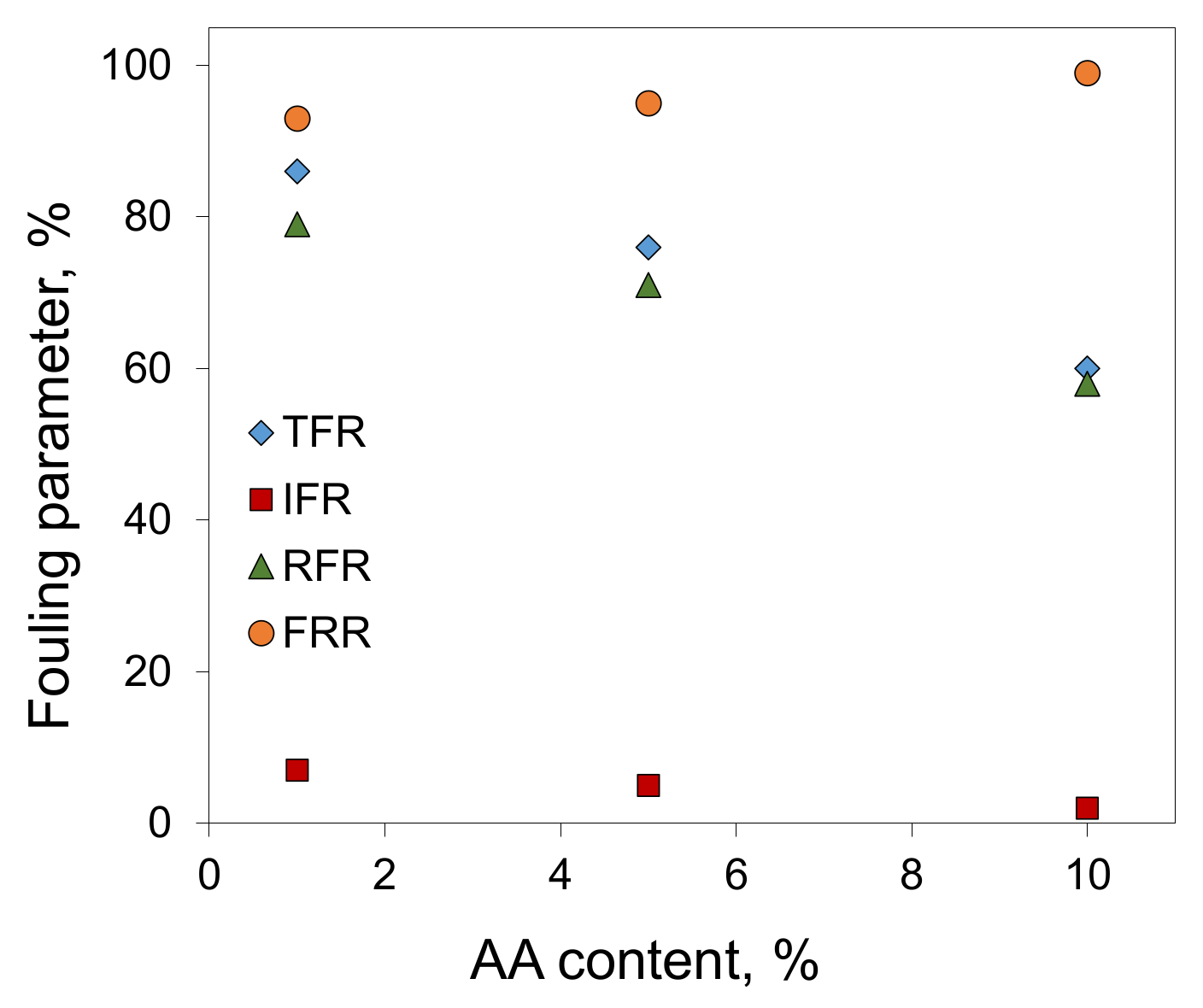
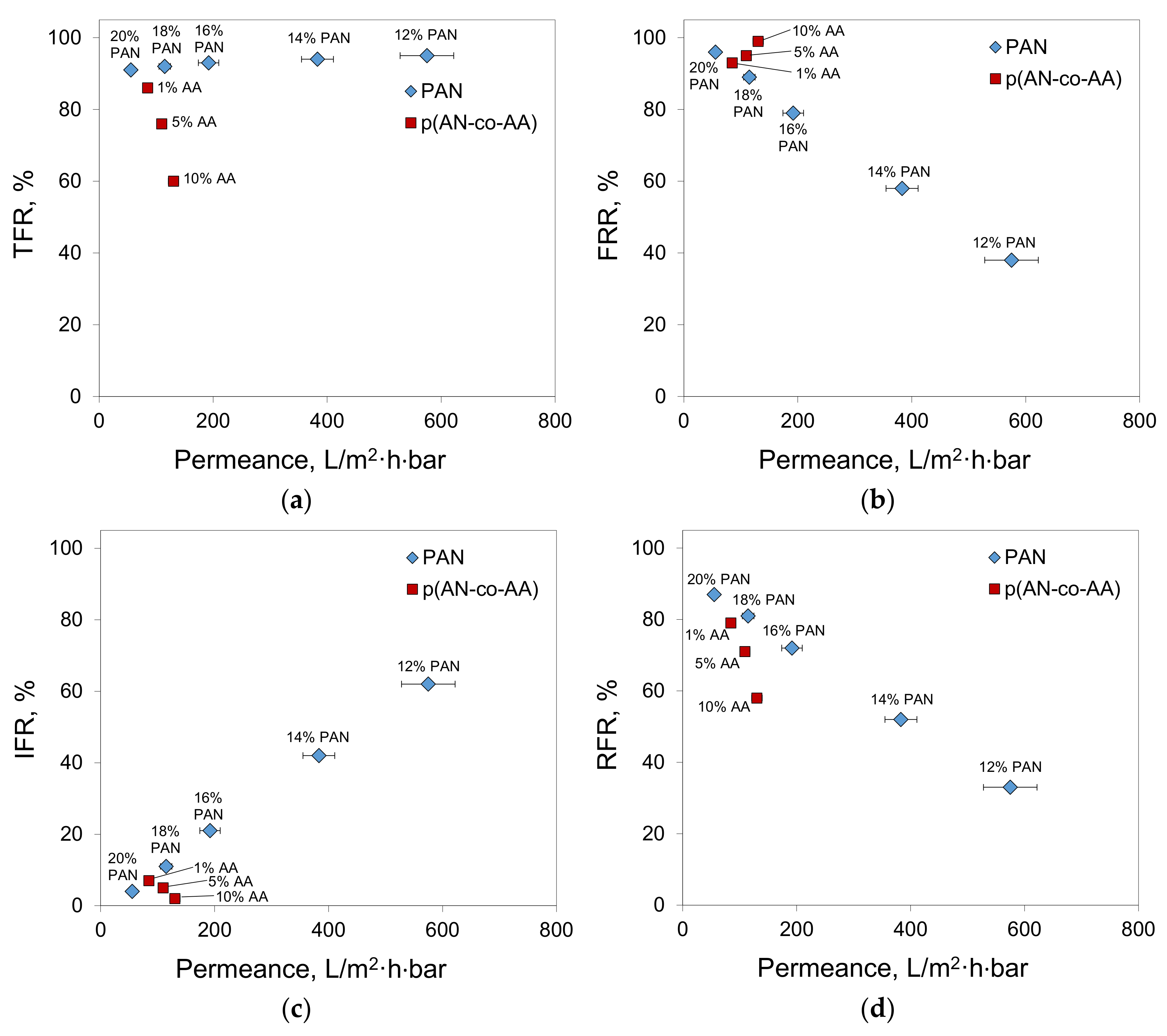
3.4. Antifouling Properties of Obtained Membranes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ganeeva, Y.M.; Yusupova, T.Y.N.; Romanov, G.V.E. Asphaltene nano-aggregates: Structure, phase transitions and effect on petroleum systems. Russ. Chem. Rev. 2011, 80, 993–1008. [Google Scholar] [CrossRef]
- Farooq, U.; Patil, A.; Panjwani, B.; Simonsen, G. Review on application of nanotechnology for asphaltene adsorption, crude oil demulsification, and produced water treatment. Energy Fuels 2021, 35, 19191–19210. [Google Scholar] [CrossRef]
- Kashefi, S.; Lotfollahi, M.N.; Shahrabadi, A. Asphaltene adsorption using nanoparticles with different surface chemistry: Equilibrium and thermodynamics studies. Pet. Chem. 2019, 59, 1201–1206. [Google Scholar] [CrossRef]
- Rogel, E.; Moir, M.; Witt, M. Atmospheric Pressure Photoionization and Laser Desorption Ionization Coupled to Fourier Transform Ion Cyclotron Resonance Mass Spectrometry to Characterize Asphaltene Solubility Fractions: Studying the Link between Molecular Composition and Physical Behavior. Energy Fuels 2015, 29, 4201–4209. [Google Scholar] [CrossRef]
- Hurt, M.R.; Borton, D.J.; Choi, H.J.; Kenttämaa, H.I. Comparison of the Structures of Molecules in Coal and Petroleum Asphaltenes by Using Mass Spectrometry. Energy Fuels 2013, 27, 3653–3658. [Google Scholar] [CrossRef]
- Eyssautier, J.; Frot, D.; Barré, L. Structure and Dynamic Properties of Colloidal Asphaltene Aggregates. Langmuir 2012, 28, 11997–12004. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Nguyen, D.L.T.; Xia, C.; Nguyen, T.B.; Shokouhimehr, M.; Sana, S.S.; Grace, A.N.; Aghbashlo, M.; Tabatabaei, M.; Sonne, C.; et al. Recent advances in asphaltene transformation in heavy oil hydroprocessing: Progress, challenges, and future perspectives. Fuel Process. Technol. 2021, 213, 106681. [Google Scholar] [CrossRef]
- Zuo, P.; Qu, S.; Shen, W. Asphaltenes: Separations, structural analysis and applications. J. Energy Chem. 2019, 34, 186–207. [Google Scholar] [CrossRef]
- Rueda-Velasquez, R.I.; Freund, H.; Qian, K.; Olmstead, W.N.; Gray, M.R. Characterization of asphaltene building blocks by cracking under favorable hydrogenation conditions. Energy Fuels 2013, 27, 1817–1829. [Google Scholar] [CrossRef]
- Karambeigi, M.A.; Kharrat, R. An investigation of inhibitors performance on asphaltene precipitation due to associated gas injection. Pet. Sci. Technol. 2014, 32, 1213–1218. [Google Scholar] [CrossRef]
- Jafari Behbahani, T.; Miranbeigi, A.A.; Sharifi, K. A new experimental investigation on upgrading of waxy crude oils by methacrylate polymers. Pet. Chem. 2017, 57, 874–880. [Google Scholar] [CrossRef]
- Marczewski, A.W.; Szymula, M. Adsorption of asphaltenes from toluene on mineral surface. Colloids Surf. A Physicochem. Eng. Asp. 2002, 208, 259–266. [Google Scholar] [CrossRef]
- Abdallah, W.A.; Taylor, S.D. Surface characterization of adsorbed asphaltene on a stainless steel surface. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2007, 258, 213–217. [Google Scholar] [CrossRef]
- Franco, C.; Patiño, E.; Benjumea, P.; Ruiz, M.A.; Cortés, F.B. Kinetic and thermodynamic equilibrium of asphaltenes sorption onto nanoparticles of nickel oxide supported on nanoparticulated alumina. Fuel 2013, 105, 408–414. [Google Scholar] [CrossRef]
- Ramirez-Corredores, M.M. The Science and Technology of Unconventional Oils: Finding Refining Opportunities; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Ashtari, M.; Ashrafizadeh, S.N.; Bayat, M. Asphaltene removal from crude oil by means of ceramic membranes. J. Pet. Sci. Eng. 2012, 82, 44–49. [Google Scholar] [CrossRef]
- Ashtari, M.; Bayat, M.; Sattarin, M. Investigation on asphaltene and heavy metal removal from crude oil using a thermal effect. Energy Fuels 2011, 25, 300–306. [Google Scholar] [CrossRef]
- Barbier, J.; Marques, J.; Caumette, G.; Merdrignac, I.; Bouyssiere, B.; Lobinski, R.; Lienemann, C.P. Monitoring the behaviour and fate of nickel and vanadium complexes during vacuum residue hydrotreatment and fraction separation. Fuel Process. Technol. 2014, 119, 185–189. [Google Scholar] [CrossRef]
- Marques, J.; Merdrignac, I.; Baudot, A.; Barré, L.; Guillaume, D.; Espinat, D.; Brunet, S. Asphaltenes size polydispersity reduction by nano-and ultrafiltration separation methods–comparison with the flocculation method. Oil Gas Sci. Technol. Rev. IFP 2008, 63, 139–149. [Google Scholar] [CrossRef]
- Ching, M.J.T.M.; Pomerantz, A.E.; Andrews, A.B.; Dryden, P.; Schroeder, R.; Mullins, O.C.; Harrison, C. On the nanofiltration of asphaltene solutions, crude oils, and emulsions. Energy Fuels 2010, 24, 5028–5037. [Google Scholar] [CrossRef]
- Chisca, S.; Musteata, V.E.; Zhang, W.; Vasylevskyi, S.; Falca, G.; Abou-Hamad, E.; Emwas, A.-H.; Altunkaya, M.; Nunes, S.P. Polytriazole membranes with ultrathin tunable selective layer for crude oil fractionation. Science 2022, 376, 1105–1110. [Google Scholar] [CrossRef]
- Yushkin, A.A.; Balynin, A.V.; Nekhaev, A.I.; Volkov, A.V. Separation of Archipelago and Continent-Type Asphaltenes on Ultrafiltration Membranes. Membr. Membr. Technol. 2021, 3, 139–145. [Google Scholar] [CrossRef]
- Tsai, H.A.; Chen, Y.L.; Lee, K.R.; Lai, J.Y. Preparation of heat-treated PAN hollow fiber membranes for pervaporation of NMP/H2O mixtures. Sep. Purif. Technol. 2012, 100, 97–105. [Google Scholar] [CrossRef]
- Mirjalili, M.; Zohoori, S. Review for application of electrospinning and electrospun nanofibers technology in textile industry. J. Nanostruct. Chem. 2016, 6, 207–213. [Google Scholar] [CrossRef]
- Yushkin, A.A.; Balynin, A.V.; Efimov, M.N.; Muratov, D.G.; Karpacheva, G.P.; Volkov, A.V. Formation of Multilayer Membranes from One Polymer Using IR Treatment. Membr. Membr. Technol. 2022, 4, 251–257. [Google Scholar] [CrossRef]
- Pérez-Manríquez, L.; Aburabi’e, J.; Neelakanda, P.; Peinemann, K.V. Cross-linked PAN-based thin-film composite membranes for non-aqueous nanofiltration. React. Funct. Polym. 2015, 86, 243–247. [Google Scholar] [CrossRef]
- Saufi, S.M.; Ismail, A.F. Fabrication of carbon membranes for gas separation—A review. Carbon 2004, 42, 241–259. [Google Scholar] [CrossRef]
- Tran, T.D.; Mori, S.; Suzuki, M. Plasma modification of polyacrylonitrile ultrafiltration membrane. Thin Solid Film. 2007, 515, 4148–4152. [Google Scholar] [CrossRef]
- Marbelia, L.; Mulier, M.; Vandamme, D.; Muylaert, K.; Szymczyk, A.; Vankelecom, I.F. Polyacrylonitrile membranes for microalgae filtration: Influence of porosity, surface charge and microalgae species on membrane fouling. Algal Res. 2016, 19, 128–137. [Google Scholar] [CrossRef]
- Yushkin, A.; Basko, A.; Balynin, A.; Efimov, M.; Lebedeva, T.; Ilyasova, A.; Pochivalov, K.; Volkov, A. Effect of Acetone as Co-Solvent on Fabrication of Polyacrylonitrile Ultrafiltration Membranes by Non-Solvent Induced Phase Separation. Polymers 2022, 14, 4603. [Google Scholar] [CrossRef]
- Miller, D.J.; Dreyer, D.R.; Bielawski, C.W.; Paul, D.R.; Freeman, B.D. Surface modification of water purification membranes. Angew. Chem. Int. Ed. 2017, 56, 4662–4711. [Google Scholar] [CrossRef]
- Ismail, N.H.; Salleh, W.N.W.; Ismail, A.F.; Hasbullah, H.; Yusof, N.; Aziz, F.; Jaafar, J. Hydrophilic polymer-based membrane for oily wastewater treatment: A review. Sep. Purif. Technol. 2020, 233, 116007. [Google Scholar] [CrossRef]
- Grushevenko, E.; Balynin, A.; Ashimov, R.; Sokolov, S.; Legkov, S.; Bondarenko, G.; Borisov, I.; Sadeghi, M.; Bazhenov, S.; Volkov, A. Hydrophobic Ag-Containing Polyoctylmethylsiloxane-Based Membranes for Ethylene/Ethane Separation in Gas-Liquid Membrane Contactor. Polymers 2022, 14, 1625. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, Y.; Tian, E.; Li, J.; Ren, Y. Graphene oxide-based polymeric membranes for water treatment. Adv. Mater. Interfaces 2018, 5, 1701427. [Google Scholar] [CrossRef]
- Eremin, Y.; Grekhov, A.; Belogorlov, A. Percolation Effects in Mixed Matrix Membranes with Embedded Carbon Nanotubes. Membranes 2022, 12, 1100. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Huang, S.; Zhang, Y.; Zhao, S. Comparing the antifouling effects of activated carbon and TiO2 in ultrafiltration membrane development. J. Colloid Interface Sci. 2018, 515, 109–118. [Google Scholar] [CrossRef]
- Pulyalina, A.Y.; Tyan, N.S.; Faykov, I.I.; Polotskaya, G.A.; Rostovtseva, V.A. Transport Properties of Ultrafiltration Membranes Based on Copolyimide/Nanodiamonds Composites. Membr. Membr. Technol. 2022, 4, 328–335. [Google Scholar] [CrossRef]
- Qiu, J.H.; Zhang, Y.W.; Zhang, Y.T.; Zhang, H.Q.; Liu, J.D. Synthesis and antibacterial activity of copper-immobilized membrane comprising grafted poly (4-vinylpyridine) chains. J. Colloid Interface Sci. 2011, 354, 152–159. [Google Scholar] [CrossRef]
- Younas, H.; Bai, H.; Shao, J.; Han, Q.; Ling, Y.; He, Y. Super-hydrophilic and fouling resistant PVDF ultrafiltration membranes based on a facile prefabricated surface. J. Membr. Sci. 2017, 541, 529–540. [Google Scholar] [CrossRef]
- Arthanareeswaran, G.; Devi, T.S.; Raajenthiren, M. Effect of silica particles on cellulose acetate blend ultrafiltration membranes: Part I. Sep. Purif. Technol. 2008, 64, 38–47. [Google Scholar] [CrossRef]
- Feng, Y.; Shamsaei, E.; Davies, C.H.; Wang, H. Inorganic particle enhanced polymer hollow fiber membranes with high mechanical properties. Mater. Chem. Phys. 2015, 167, 209–218. [Google Scholar] [CrossRef]
- Zhang, X.; Fang, X.; Li, J.; Pan, S.; Sun, X.; Shen, J.; Han, W.; Wang, L.; Zhao, S. Developing new adsorptive membrane by modification of support layer with iron oxide microspheres for arsenic removal. J. Colloid Interface Sci. 2018, 514, 760–768. [Google Scholar] [CrossRef]
- Amirilargani, M.; Sabetghadam, A.; Mohammadi, T. Polyethersulfone/polyacrylonitrile blend ultrafiltration membranes with different molecular weight of polyethylene glycol: Preparation, morphology and antifouling properties. Polym. Adv. Technol. 2012, 23, 398–407. [Google Scholar] [CrossRef]
- Rekha Panda, S.; De, S. Effects of polymer molecular weight, concentration, and role of polyethylene glycol as additive on polyacrylonitrile homopolymer membranes. Polym. Eng. Sci. 2014, 54, 2375–2391. [Google Scholar] [CrossRef]
- Matveev, D.N.; Plisko, T.V.; Volkov, V.V.; Vasilevskii, V.P.; Bazhenov, S.D.; Shustikov, A.A.; Chernikova, E.V.; Bildyukevich, A.V. Ultrafiltration membranes based on various acrylonitrile copolymers. Membr. Membr. Technol. 2019, 1, 386–393. [Google Scholar] [CrossRef]
- Oh, D.; Ouchi, M. Synthesis of methacrylic acid-acrylonitrile alternating copolymer using an extremely bulky methacrylate and the sequence-dependent thermal reaction behaviors. Eur. Polym. J. 2023, 193, 112063. [Google Scholar] [CrossRef]
- Goosen, M.F.; Sablani, S.S.; Roque-Malherbe, R. Membrane fouling: Recent strategies and methodologies for its minimization. In Handbook of Membrane Separations: Chemical, Pharmaceuticals, Food and Biotechnological Applications; CRC Press: Boca Raton, FL, USA, 2008; pp. 325–341. [Google Scholar]
- Mohammad, A.W.; Ng, C.Y.; Lim, Y.P.; Ng, G.H. Ultrafiltration in food processing industry: Review on application, membrane fouling, and fouling control. Food Bioprocess Technol. 2012, 5, 1143–1156. [Google Scholar] [CrossRef]
- Su, Y.L.; Cheng, W.; Li, C.; Jiang, Z. Preparation of antifouling ultrafiltration membranes with poly (ethylene glycol)-graft-polyacrylonitrile copolymers. J. Membr. Sci. 2009, 329, 246–252. [Google Scholar] [CrossRef]
- Zhao, Z.P.; Li, J.; Zhang, D.X.; Chen, C.X. Nanofiltration membrane prepared from polyacrylonitrile ultrafiltration membrane by low-temperature plasma: I. Graft of acrylic acid in gas. J. Membr. Sci. 2004, 232, 9. [Google Scholar] [CrossRef]
- Yushkin, A.; Balynin, A.; Efimov, M.; Pochivalov, K.; Petrova, I.; Volkov, A. Fabrication of Polyacrylonitrile UF Membranes by VIPS Method with Acetone as Co-Solvent. Membranes 2022, 12, 523. [Google Scholar] [CrossRef]
- Zeman, L.J.; Zydney, A. Microfiltration and Ultrafiltration: Principles and Applications; Marcel Dekker: New York, NY, USA, 1996; pp. 197–201. [Google Scholar] [CrossRef]
- Yushkin, A.A.; Efimov, M.N.; Malakhov, A.O.; Karpacheva, G.P.; Bondarenko, G.; Marbelia, L.; Vankelecom, I.F.J.; Volkov, A.V. Creation of highly stable porous polyacrylonitrile membranes using infrared heating. React. Funct. Polym. 2021, 158, 104793. [Google Scholar] [CrossRef]
- Toms, R.V.; Balashov, M.S.; Gervald, A.Y.; Prokopov, N.I.; Plutalova, A.V.; Berkovich, A.K.; Chernikova, E.V. Influence of synthesis method on the properties of carbon fiber precursors based on acrylonitrile and acrylic acid copolymers. Polym. Sci. Ser. B 2020, 62, 660–670. [Google Scholar] [CrossRef]
- Zhang, H.; Quan, L.; Gao, A.; Tong, Y.; Shi, F.; Xu, L. Thermal analysis and crystal structure of poly (acrylonitrile-co-itaconic acid) copolymers synthesized in water. Polymers 2020, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, P.; Paliwal, D.K.; Gupta, A.K. Acrylonitrile—Acrylic acids copolymers. I. Synthesis and characterization. J. Appl. Polym. Sci. 1993, 49, 823–833. [Google Scholar] [CrossRef]
- Parlayıcı, Ş.; Yar, A.; Pehlivan, E.; Avcı, A. ZnO-TiO2 doped polyacrylonitrile nano fiber-Mat for elimination of Cr (VI) from polluted water. J. Anal. Sci. Technol. 2019, 10, 24. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C.; Wang, Y.; Zhu, B. Aqueous deposited copolymerization of acrylonitrile and itaconic acid. J. Appl. Polym. Sci. 2009, 111, 3163–3169. [Google Scholar] [CrossRef]
- Ouyang, Q.; Wang, X.; Wang, X.; Huang, J.; Huang, X.; Chen, Y. Simultaneous DSC/TG analysis on the thermal behavior of PAN polymers prepared by aqueous free-radical polymerization. Polym. Degrad. Stab. 2016, 130, 320–327. [Google Scholar] [CrossRef]
- Adegbola, T.A.; Agboola, O.; Fayomi, O.S.I. Review of polyacrylonitrile blends and application in manufacturing technology: Recycling and environmental impact. Results Eng. 2020, 7, 100144. [Google Scholar] [CrossRef]
- Toms, R.V.; Balashov, M.S.; Shaova, A.A.; Gerval’d, A.Y.; Prokopov, N.I.; Plutalova, A.V.; Grebenkina, N.A.; Chernikova, E.V. Copolymers of Acrylonitrile and Acrylic Acid: Effect of Composition and Distribution of Chain Units on the Thermal Behavior of Copolymers. Polym. Sci. Ser. B 2020, 62, 102–115. [Google Scholar] [CrossRef]
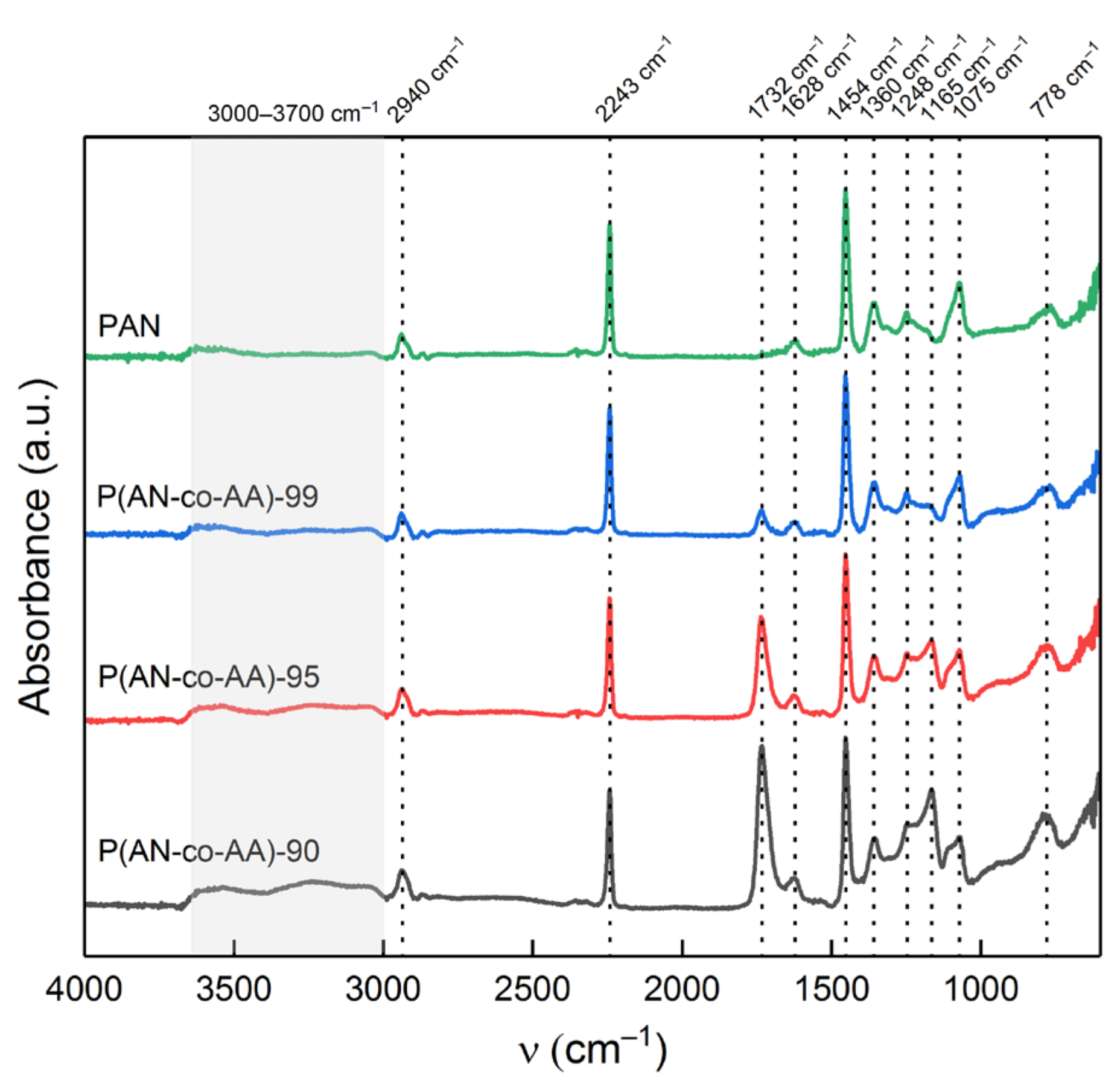

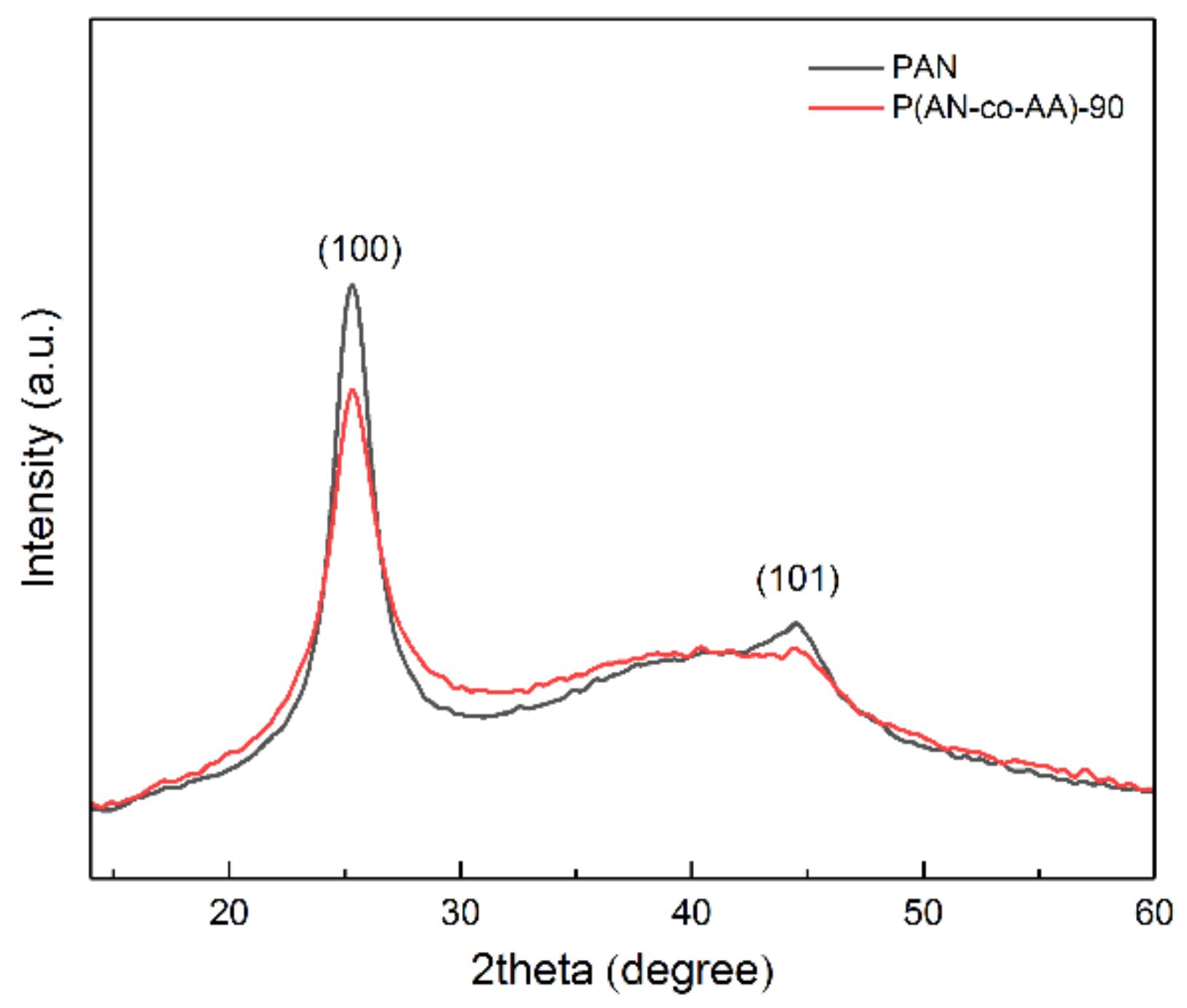

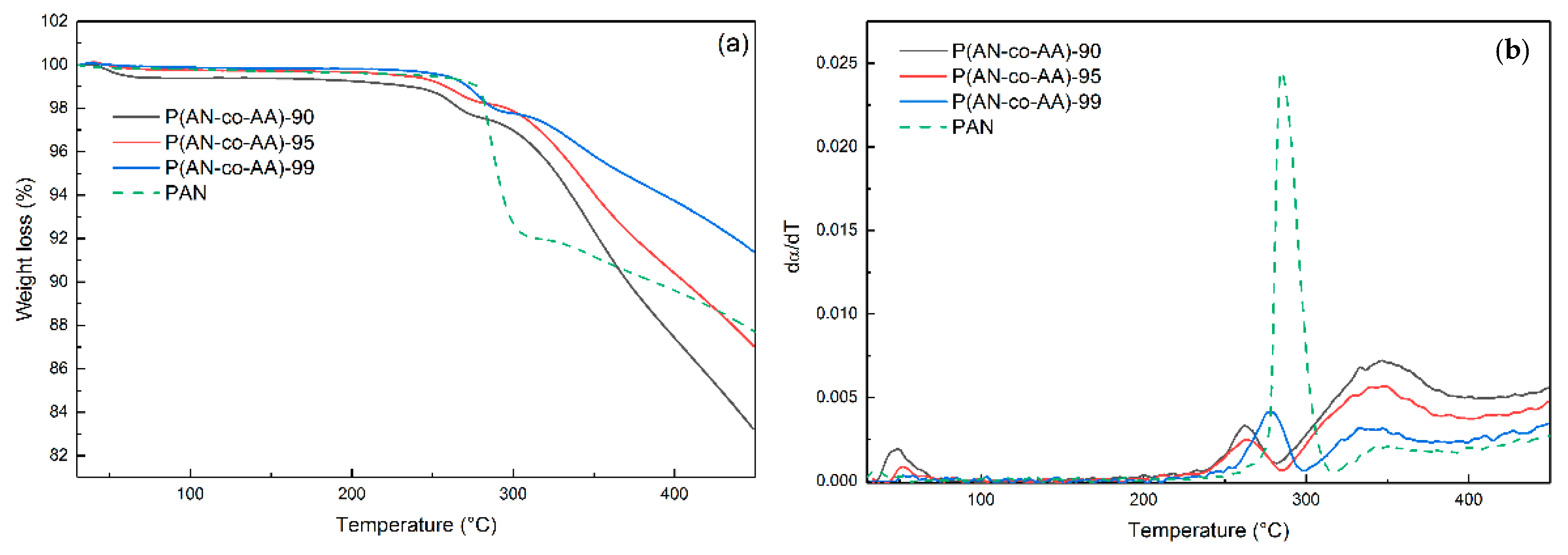


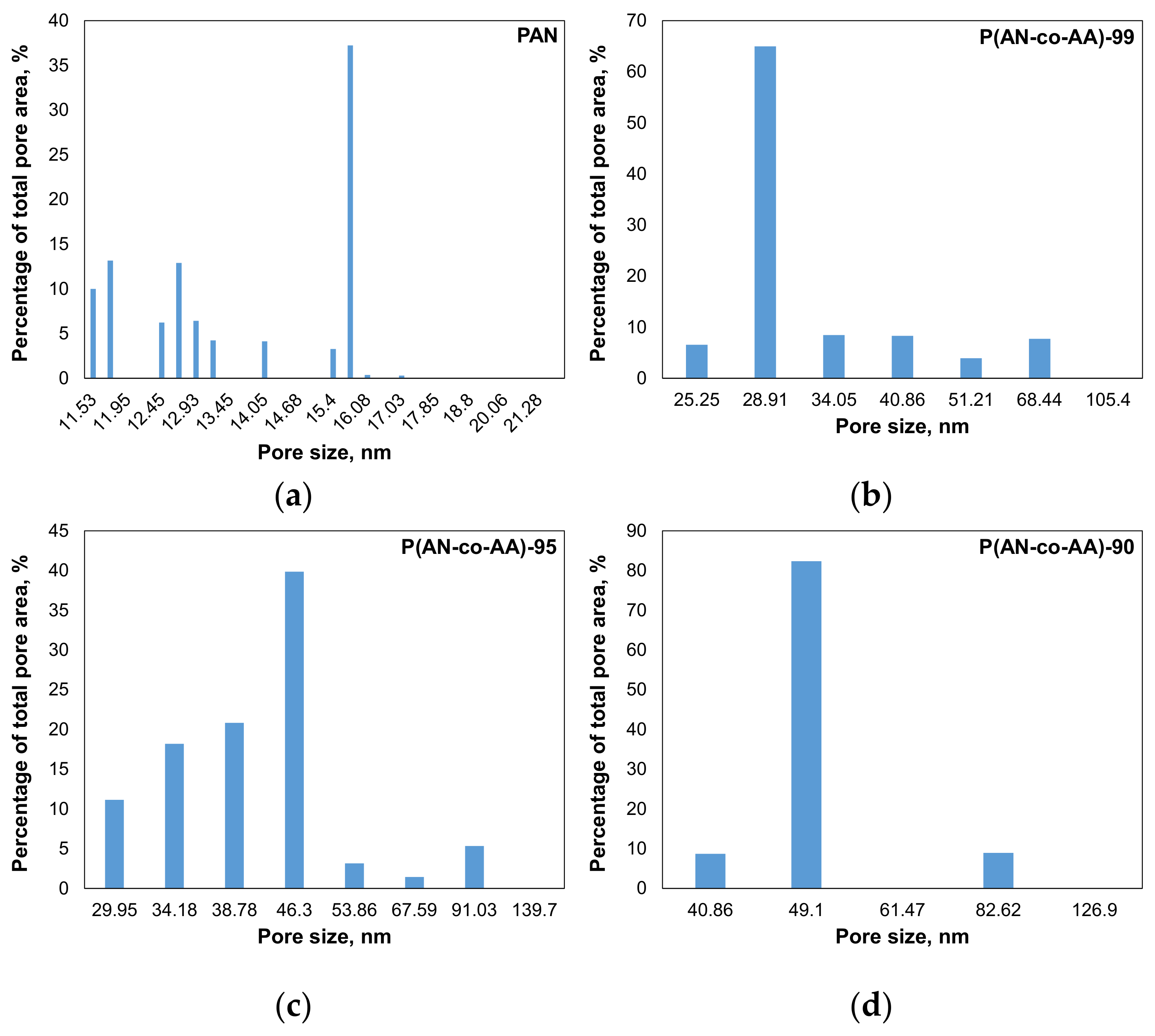



| Sample | AN:AA Ratio | Mn | Mw | Mw/Mn | Yield, % |
|---|---|---|---|---|---|
| P(AN-co-AA)-90 | 90:10 | 59,300 | 165,100 | 2.8 | 81.4 |
| P(AN-co-AA)-95 | 95:5 | 73,900 | 257,200 | 3.5 | 82.4 |
| P(AN-co-AA)-99 | 99:1 | 96,400 | 313,000 | 3.2 | 82.3 |
| PAN | 100:0 | 36,900 | 118,800 | 3.2 | 92.4 |
| Sample | A1732/A2243 | Calculated AA Content, mol.% |
|---|---|---|
| P(AN-co-AA)-90 | 1.3827 | 8.02 |
| P(AN-co-AA)-99 | 0.8361 | 4.37 |
| P(AN-co-AA)-99 | 0.1962 | 0.11 |
| Sample | Tmax, °C | −ΔH, J/g | Heat Flow, W/g | ΔT, °C | ΔH/ΔT |
|---|---|---|---|---|---|
| P(AN-co-AA)-90 | 272.3 | −444.7 | 1.6 | 105 | 4.2 |
| P(AN-co-AA)-95 | 283.4 | −479.5 | 2.3 | 104 | 4.6 |
| P(AN-co-AA)-99 | 282 | −589.4 | 8.8 | 100 | 6.0 |
| PAN | 284 | −441 | 15.6 | 73 | 5.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yushkin, A.A.; Balynin, A.V.; Nebesskaya, A.P.; Chernikova, E.V.; Muratov, D.G.; Efimov, M.N.; Karpacheva, G.P. Acrylonitrile–Acrylic Acid Copolymer Ultrafiltration Membranes for Selective Asphaltene Removal from Crude Oil. Membranes 2023, 13, 775. https://doi.org/10.3390/membranes13090775
Yushkin AA, Balynin AV, Nebesskaya AP, Chernikova EV, Muratov DG, Efimov MN, Karpacheva GP. Acrylonitrile–Acrylic Acid Copolymer Ultrafiltration Membranes for Selective Asphaltene Removal from Crude Oil. Membranes. 2023; 13(9):775. https://doi.org/10.3390/membranes13090775
Chicago/Turabian StyleYushkin, Alexey A., Alexey V. Balynin, Alexandra P. Nebesskaya, Elena V. Chernikova, Dmitriy G. Muratov, Mikhail N. Efimov, and Galina P. Karpacheva. 2023. "Acrylonitrile–Acrylic Acid Copolymer Ultrafiltration Membranes for Selective Asphaltene Removal from Crude Oil" Membranes 13, no. 9: 775. https://doi.org/10.3390/membranes13090775
APA StyleYushkin, A. A., Balynin, A. V., Nebesskaya, A. P., Chernikova, E. V., Muratov, D. G., Efimov, M. N., & Karpacheva, G. P. (2023). Acrylonitrile–Acrylic Acid Copolymer Ultrafiltration Membranes for Selective Asphaltene Removal from Crude Oil. Membranes, 13(9), 775. https://doi.org/10.3390/membranes13090775








