Preparation of a Solvent-Resistant Nanofiltration Membrane of Liquefied Walnut Shell Modified by Ethylenediamine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
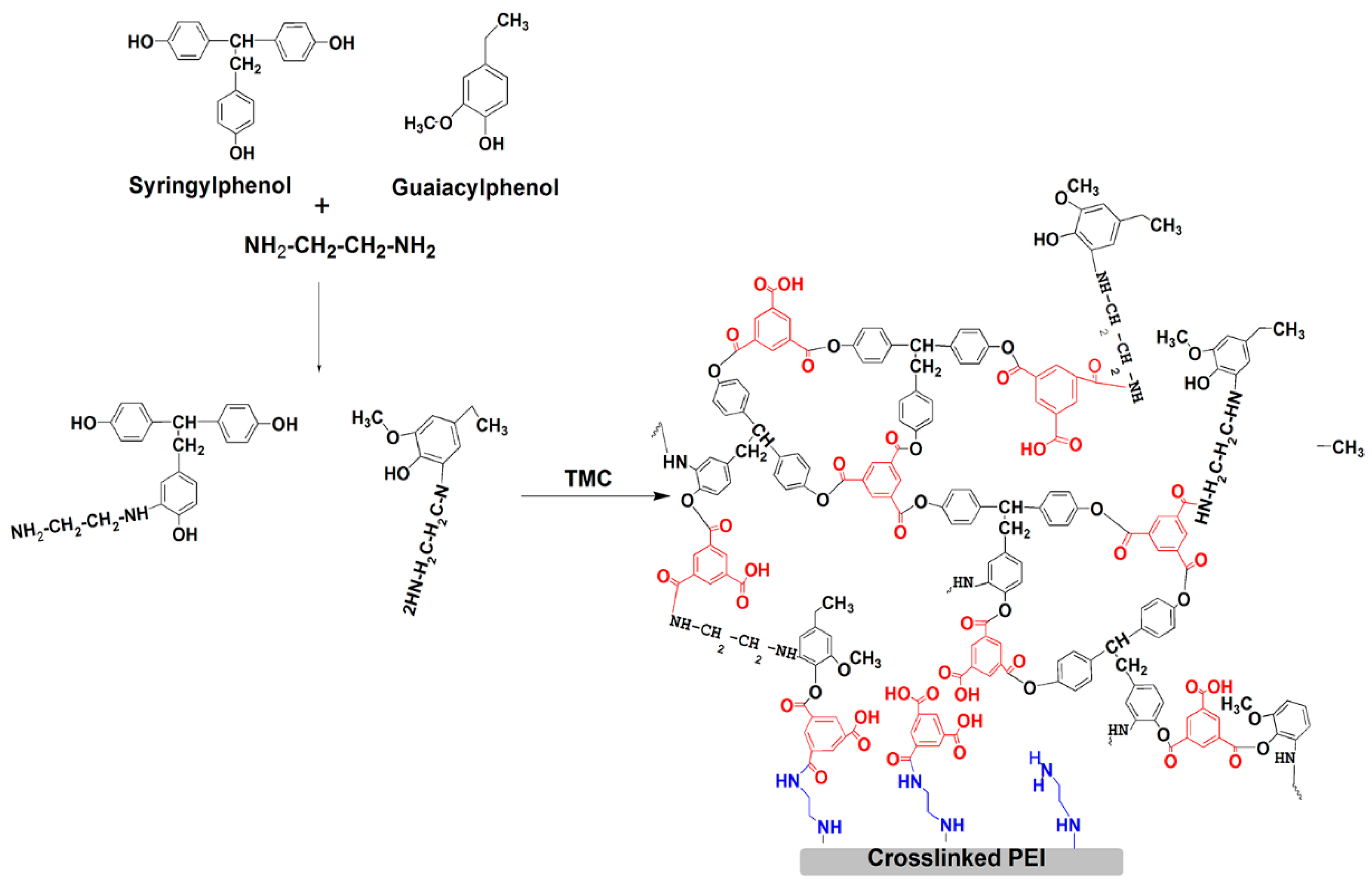
2.2.1. Preparation of Polyether-Imide Support Membrane and Crosslinked PEI Membrane by EDA
2.2.2. Preparation of EDA-Modified TFC Membranes
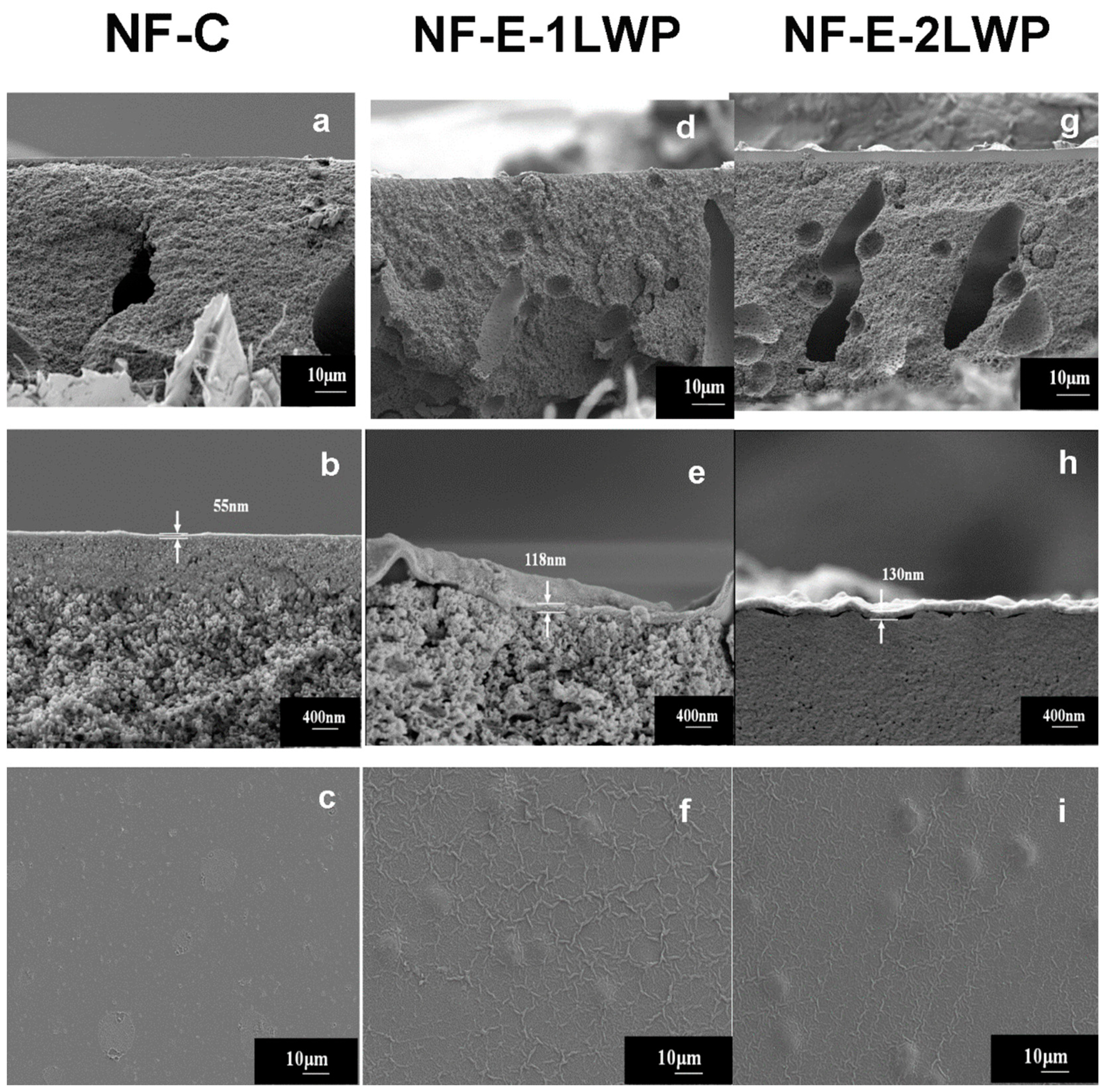
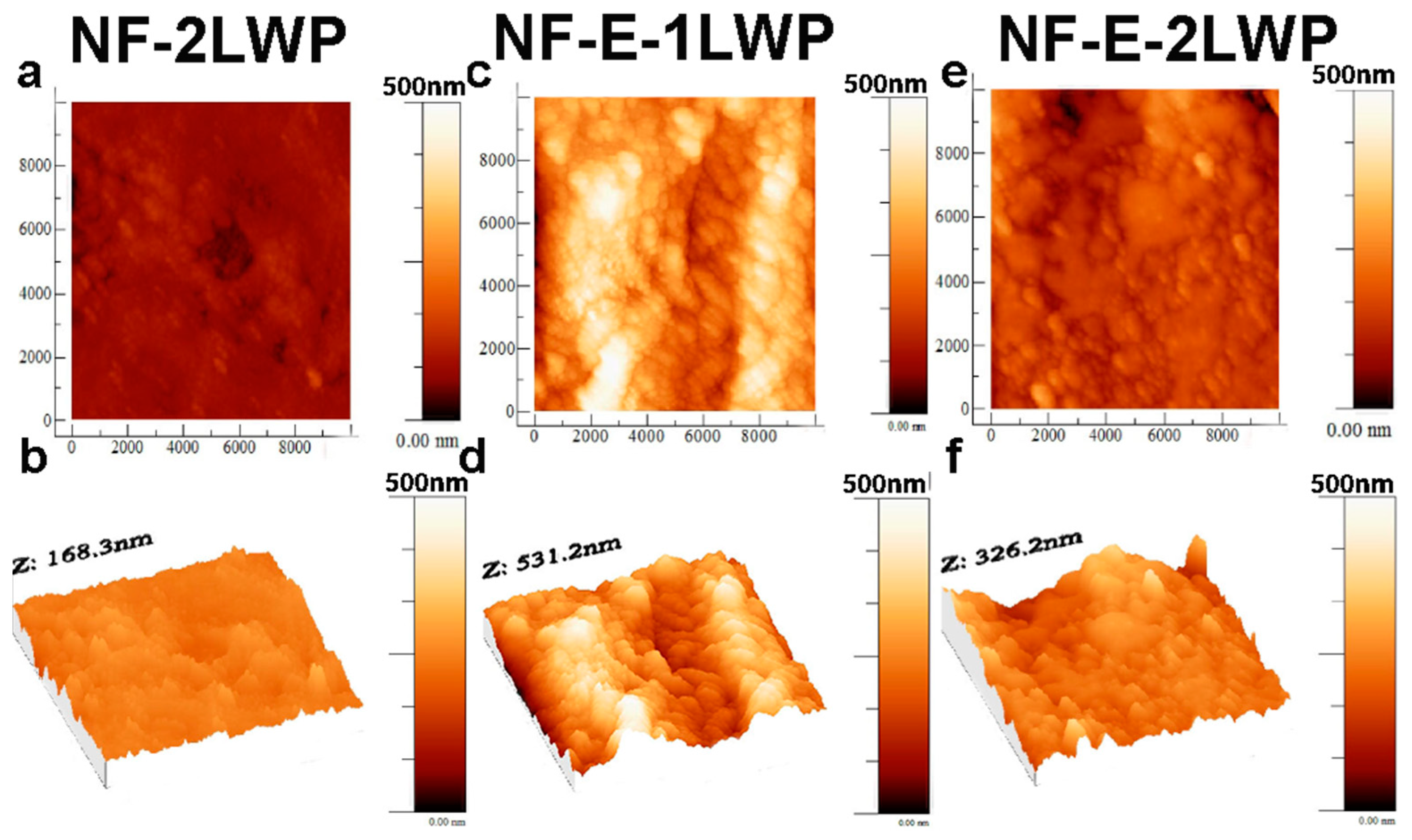
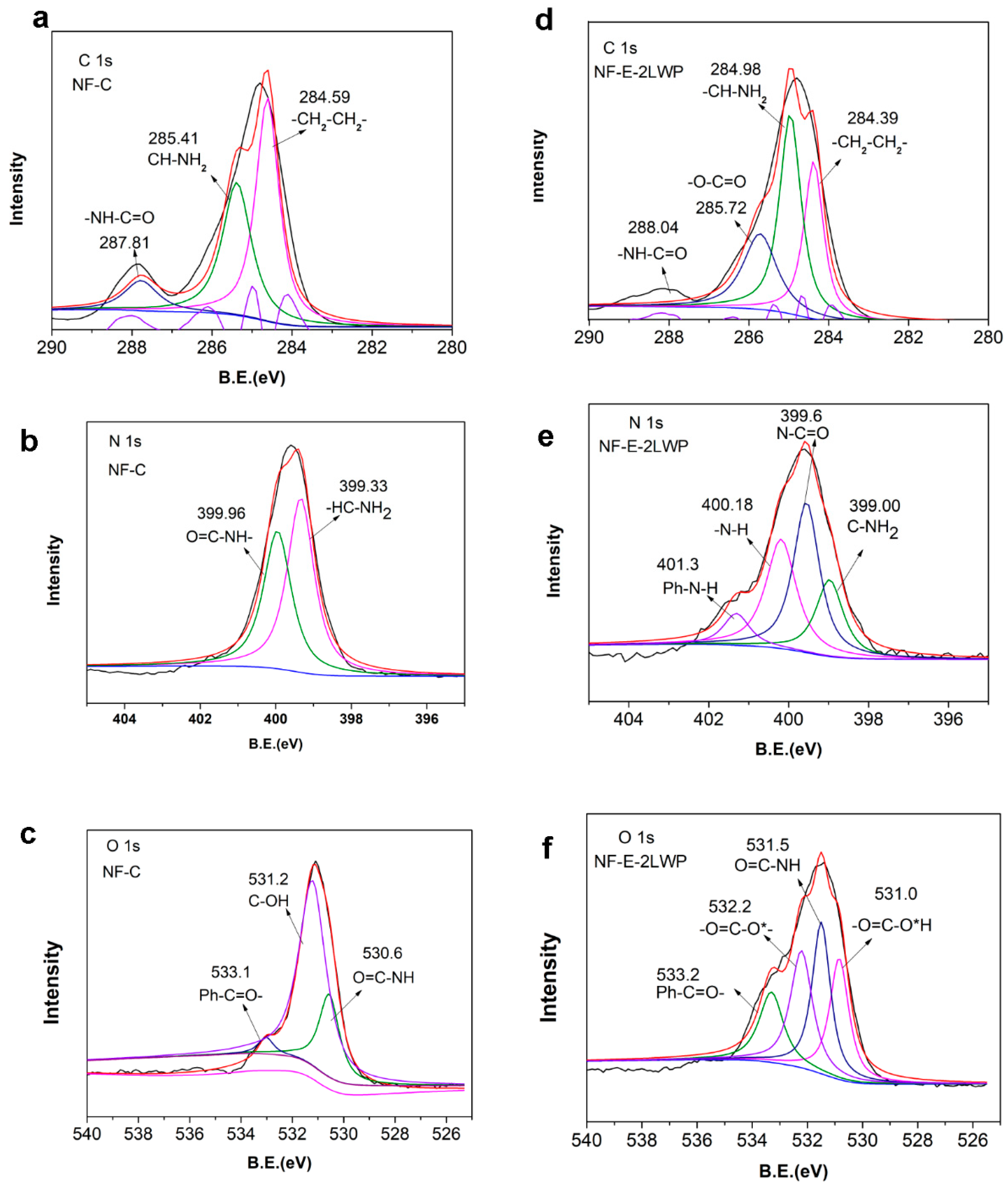
2.3. Membrane Structural Characterization
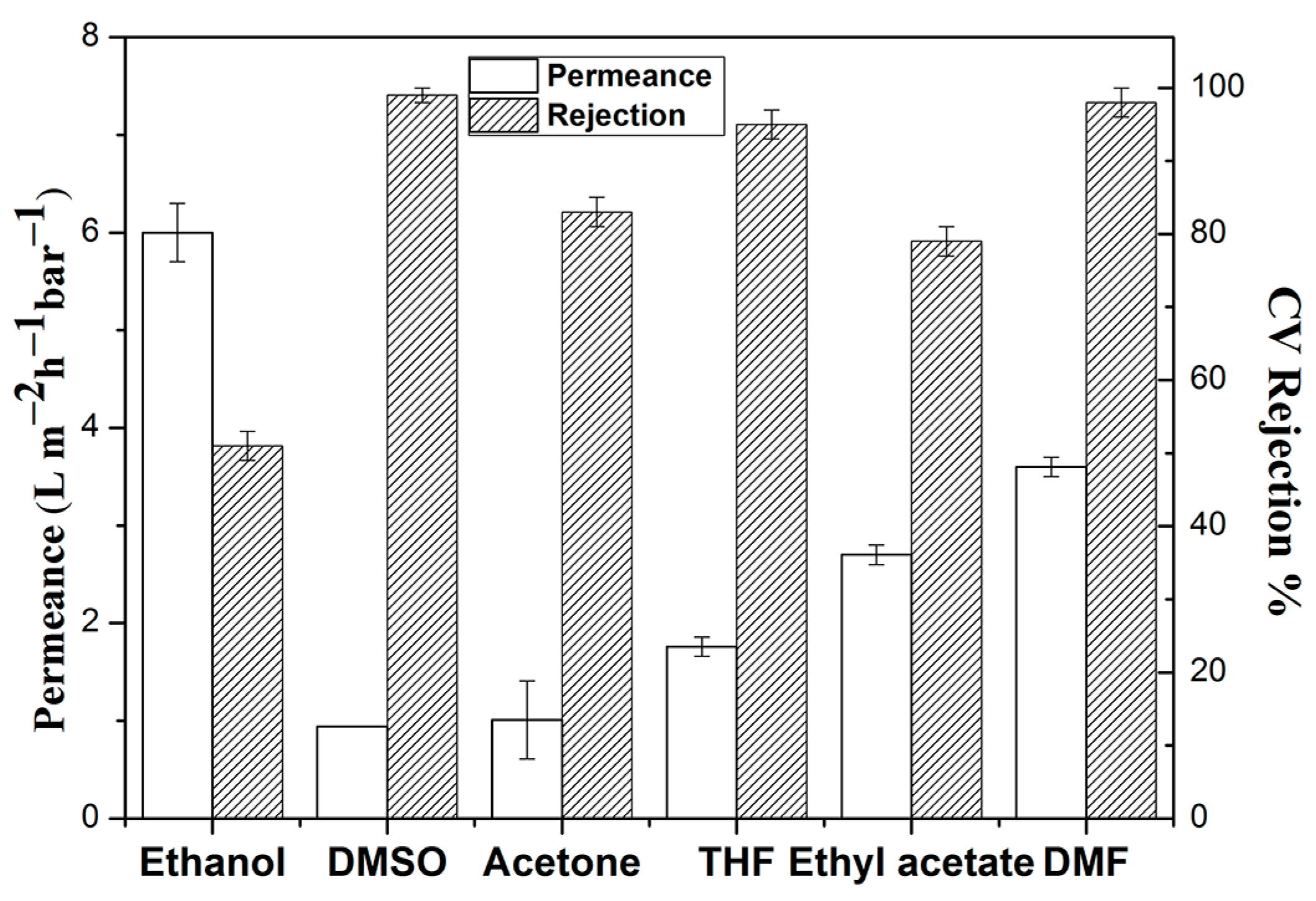
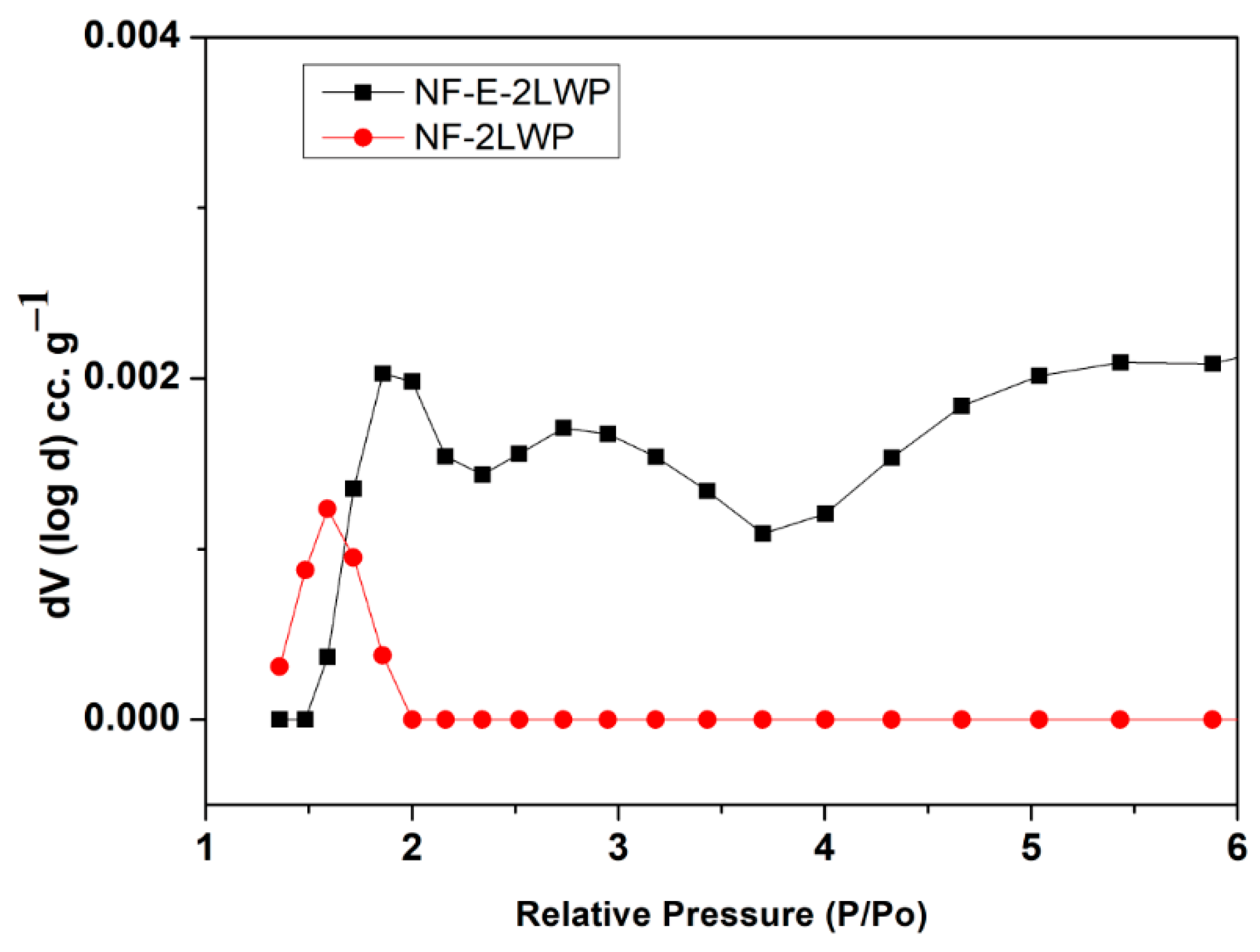
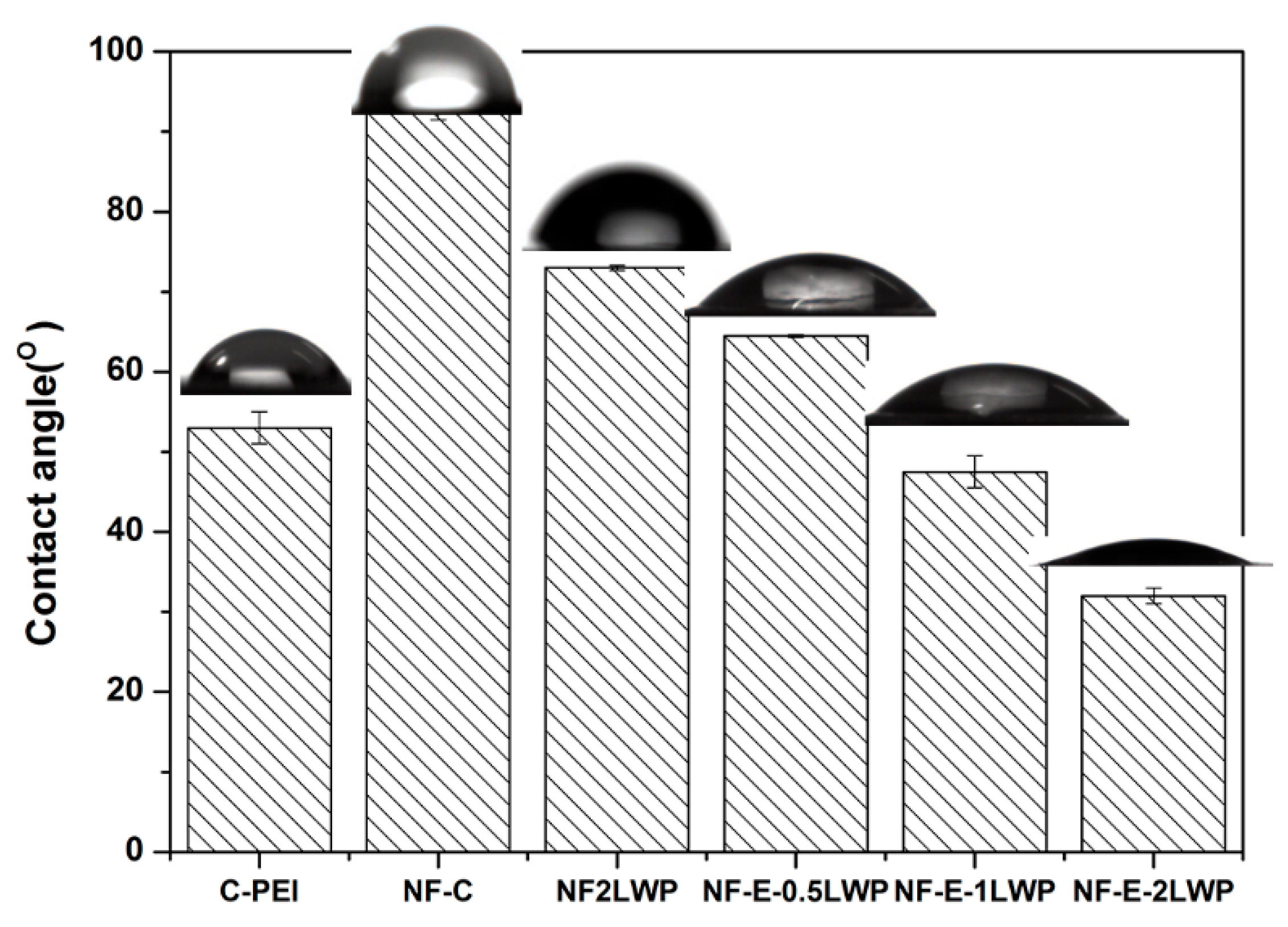
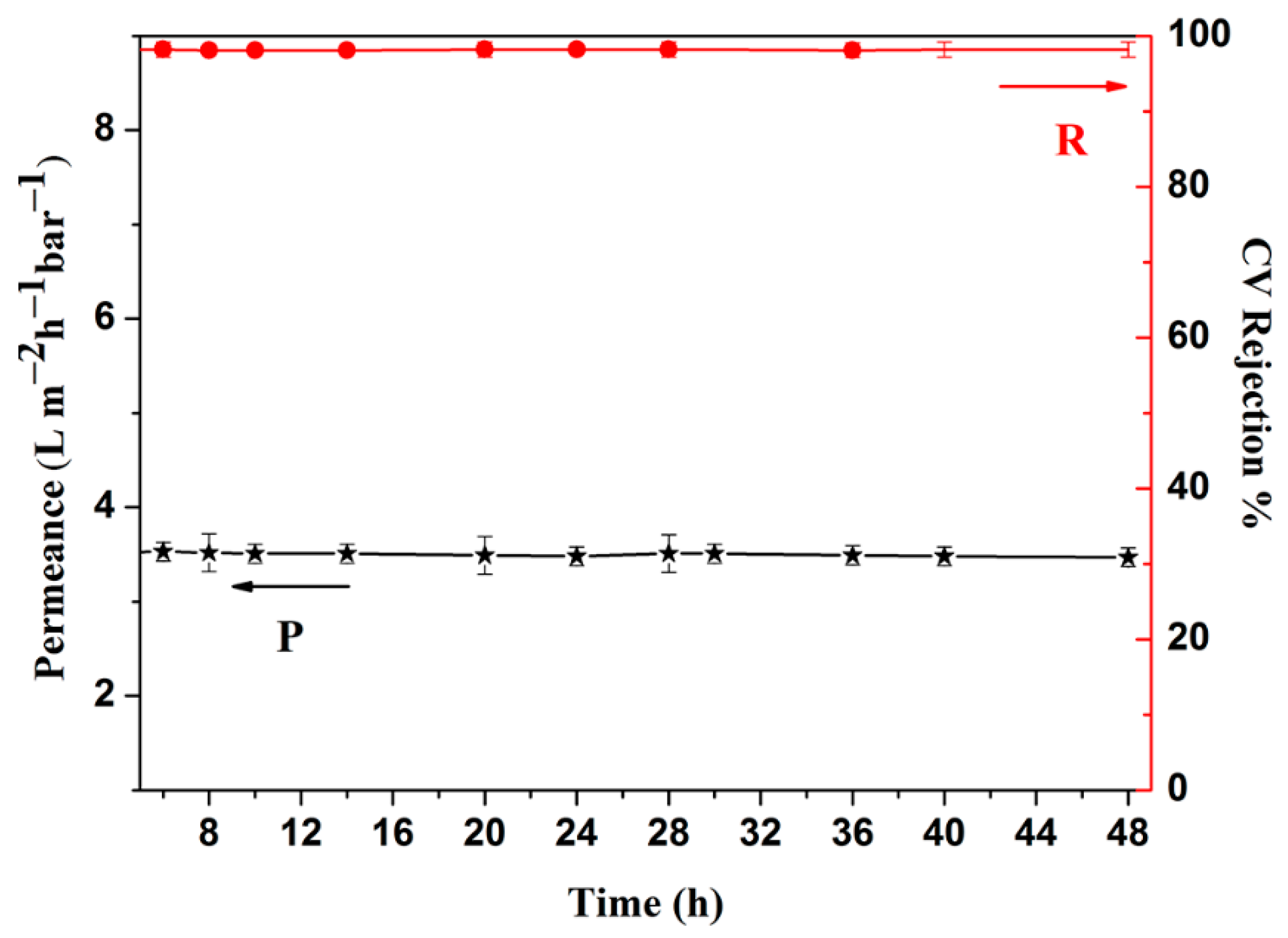
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Glarborg, P.; Miller, J.A.; Ruscic, B.; Klippenstein, S.J. Modeling nitrogen chemistry in combustion. Prog. Energy Combust. Sci. 2018, 67, 31–68. [Google Scholar] [CrossRef]
- Glarborg, P.; Jensen, A.D.; Johnsson, J.E. Fuel nitrogen conversion in solid fuel fired systems. Prog. Energy Combust. Sci. 2003, 29, 89–113. [Google Scholar] [CrossRef]
- Fan, Z.-G.; Tian, Z.-Y.; Li, W.; Zheng, Z.-H.; Yang, J.-Z. Pyrolysis study of N, N-dimethylformamide at low pressure. J. Anal. Appl. Pyrolysis 2022, 162, 105426. [Google Scholar] [CrossRef]
- Solak, E.K.; Şanlı, O. Separation Characteristics of Dimethylformamide/Water Mixtures through Alginate Membranes by Pervaporation, Vapor Permeation and Vapor Permeation with Temperature Difference Methods. Sep. Sci. Technol. 2006, 41, 627–646. [Google Scholar] [CrossRef]
- Abdur, R.; Singh, S.; Kuddus Sheikh, M.A.; Shaikh, M.A.A.; Jamal, M.S.; Lee, J. Modified post-annealing process with N, N-dimethylformamide vapor to control the growth of hybrid perovskite microstructure. Results Mater. 2022, 16, 100330. [Google Scholar] [CrossRef]
- Shah, D.; Kissick, K.; Ghorpade, A.; Hannah, R.; Bhattacharyya, D. Pervaporation of alcohol–water and dimethylformamide–water mixtures using hydrophilic zeolite NaA membranes: Mechanisms and experimental results. J. Membr. Sci. 2000, 179, 185–205. [Google Scholar] [CrossRef]
- Cao, L.; Su, C.; Lu, Y.; Wu, J.; Wei, L.; Liao, J.; Xian, Y.; Gao, S. Long-term performance study applying a tandem AnSBR-ASBR to treat wastewater containing N, N-dimethylformamide: Sludge physicochemical properties, microecology, and functional genes. J. Environ. Chem. Eng. 2023, 11, 109447. [Google Scholar] [CrossRef]
- Hai, Y.; Zhang, J.; Shi, C.; Zhou, A.; Bian, C.; Li, W. Thin film composite nanofiltration membrane prepared by the interfacial polymerization of 1,2,4,5-benzene tetracarbonyl chloride on the mixed amines cross-linked poly(ether imide) support. J. Membr. Sci. 2016, 520, 19–28. [Google Scholar] [CrossRef]
- Li, W.; Shi, C.; Zhou, A.; He, X.; Sun, Y.; Zhang, J. A positively charged composite nanofiltration membrane modified by EDTA for LiCl/MgCl2 separation. Sep. Purif. Technol. 2017, 186, 233–242. [Google Scholar] [CrossRef]
- Wang, X.; Wang, N.; Li, X.; An, Q.-F. A review of nano-confined composite membranes fabricated inside the porous support. Adv. Membr. 2021, 1, 100005. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, K.; Liu, Y.; Xia, S. A review on polyester and polyester-amide thin film composite nanofiltration membranes: Synthesis, characteristics and applications. Sci. Total Environ. 2023, 858, 159922. [Google Scholar] [CrossRef] [PubMed]
- Jimenez Solomon, M.F.; Bhole, Y.; Livingston, A.G. High flux hydrophobic membranes for organic solvent nanofiltration (OSN)—Interfacial polymerization, surface modification and solvent activation. J. Membr. Sci. 2013, 434, 193–203. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, X.; Zhang, J.; Zhang, F.; Fang, W.; Jin, J. Polymer membranes for organic solvent nanofiltration: Recent progress, challenges and perspectives. Adv. Membr. 2023, 3, 100063. [Google Scholar] [CrossRef]
- Aburabie, J.; Neelakanda, P.; Karunakaran, M.; Peinemann, K.-V. Thin-film composite crosslinked polythiosemicarbazide membranes for organic solvent nanofiltration (OSN). React. Funct. Polym. 2015, 86, 225–232. [Google Scholar] [CrossRef]
- Thiermeyer, Y.; Blumenschein, S.; Skiborowski, M. Fundamental insights into the rejection behavior of polyimide-based OSN membranes. Sep. Purif. Technol. 2021, 265, 118492. [Google Scholar] [CrossRef]
- Xu, S.-J.; Luo, L.-H.; Tong, Y.-H.; Shen, Q.; Xu, Z.-L.; Wu, Y.-Z.; Yang, H. Organic solvent nanofiltration (OSN) membrane with polyamantadinamide active layer for reducing separation performance inconformity. Sep. Purif. Technol. 2022, 278, 119582. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Feng, R.; Wang, W.-J.; Sun, Y.-X.; Tao, S.-N.; Wang, Y.-M.; Chen, Y.-F.; Fu, Z.-J.; Cao, X.-L.; Sun, S.-P.; et al. Robust braid reinforced hollow fiber membranes for organic solvent nanofiltration (OSN). Adv. Membr. 2021, 1, 100007. [Google Scholar] [CrossRef]
- Jimenez-Solomon, M.F.; Song, Q.; Jelfs, K.E.; Munoz-Ibanez, M.; Livingston, A.G. Polymer nanofilms with enhanced microporosity by interfacial polymerization. Nat. Mater. 2016, 15, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Jimenez Solomon, M.F.; Bhole, Y.; Livingston, A.G. High flux membranes for organic solvent nanofiltration (OSN)—Interfacial polymerization with solvent activation. J. Membr. Sci. 2012, 423–424, 371–382. [Google Scholar] [CrossRef]
- Kim, S.D.; Won, G.Y.; Shah, A.A.; Park, A.; Park, Y.-I.; Nam, S.-E.; Cho, Y.H.; Park, H. Reinforcing the polybenzimidazole membrane surface by an ultrathin co-crosslinked polydopamine layer for organic solvent nanofiltration applications. J. Membr. Sci. 2021, 636, 119587. [Google Scholar] [CrossRef]
- Nie, L.; Chuah, C.Y.; Bae, T.-H.; Lee, J.-M. Graphene-Based Advanced Membrane Applications in Organic Solvent Nanofiltration. Adv. Funct. Mater. 2021, 31, 2006949. [Google Scholar] [CrossRef]
- Huang, T.; Moosa, B.A.; Hoang, P.; Liu, J.; Chisca, S.; Zhang, G.; AlYami, M.; Khashab, N.M.; Nunes, S.P. Molecularly-porous ultrathin membranes for highly selective organic solvent nanofiltration. Nat. Commun. 2020, 11, 5882. [Google Scholar] [CrossRef] [PubMed]
- Li, S.L.; Chang, G.; Huang, Y.; Kinooka, K.; Chen, Y.; Fu, W.; Gong, G.; Yoshioka, T.; McKeown, N.B.; Hu, Y. 2,2′-Biphenol-based Ultrathin Microporous Nanofilms for Highly Efficient Molecular Sieving Separation. Angew. Chem. Int. Ed. Engl. 2022, 61, e202212816. [Google Scholar] [PubMed]
- Li, W.; Yang, Z.; Yang, W.; Guo, H.; Tang, C.Y. Vapor-phase polymerization of high-performance thin-film composite membranes for nanofiltration. AIChE J. 2021, 68, e17517. [Google Scholar] [CrossRef]
- Behboodi-Sadabad, F.; Zhang, H.; Trouillet, V.; Welle, A.; Plumeré, N.; Levkin, P.A. UV-Triggered Polymerization, Deposition, and Patterning of Plant Phenolic Compounds. Adv. Funct. Mater. 2017, 27, 1700127. [Google Scholar] [CrossRef]
- Zheng, Z.F.; Zou, J.C.; Zhang, H.J.; Ji-You, G.U.; Tian-Qi, M.A. Study on Liquefaction of Walnut Shell and its Application to Phenol-Formaldehyde Resin Adhesives. Acta Sci. Nat. Univ. Sunyatseni 2007, 46, 139–140. [Google Scholar]
- You, F.; Xu, Y.; Yang, X.; Zhang, Y.; Shao, L. Bio-inspired Ni2+-polyphenol hydrophilic network to achieve unconventional high-flux nanofiltration membranes for environmental remediation. Chem. Commun. 2017, 53, 6128–6131. [Google Scholar] [CrossRef]
- Huang, Y.; Li, S.-L.; Fu, Z.; Gong, G.; Hu, Y. Preparation of microporous organic solvent nanofiltration (OSN) composite membrane from a novel tris-phenol monomer. Sep. Purif. Technol. 2022, 301, 121985. [Google Scholar] [CrossRef]
- Shah, A.A.; Park, A.; Yoo, Y.; Nam, S.-E.; Park, Y.-I.; Cho, Y.H.; Park, H. Preparation of highly permeable nanofiltration membranes with interfacially polymerized biomonomers. J. Membr. Sci. 2021, 627, 119209. [Google Scholar] [CrossRef]
- Zhou, A.; Wang, Y.; Cheng, D.; Li, M.; Wang, L. Effective interfacially polymerized polyarylester solvent resistant nanofiltration membrane from liquefied walnut shell. Korean J. Chem. Eng. 2022, 39, 1566–1575. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Geens, J.; Vandecasteele, C. Fluxes and rejections for nanofiltration with solvent stable polymeric membranes in water, ethanol and n-hexane. Chem. Eng. Sci. 2002, 57, 2511–2518. [Google Scholar] [CrossRef]
- Bhanushali, D.; Kloos, S.; Kurth, C.; Bhattacharyya, D. Performance of solvent-resistant membranes for non-aqueous systems: Solvent permeation results and modeling. J. Membr. Sci. 2001, 189, 1–21. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, J.; Zhou, A.; Kong, A.; Alwan Almijbilee, M.M.; Zheng, X.; Zhang, J.; Li, W. Poly[acrylate-co-amide] network composite via photopolymerization for organic solvent nanofiltration separation. Sep. Purif. Technol. 2020, 246, 116855. [Google Scholar] [CrossRef]
- Zheng, X.; Zhou, A.; Wang, Y.; He, X.; Zhao, S.; Zhang, J.; Li, W. Modulating hydrophobicity of composite polyamide membranes to enhance the organic solvent nanofiltration. Sep. Purif. Technol. 2019, 223, 211–223. [Google Scholar] [CrossRef]
- He, X.; Zhou, A.; Shi, C.; Zhang, J.; Li, W. Solvent resistant nanofiltration membranes using EDA-XDA co-crosslinked poly(ether imide). Sep. Purif. Technol. 2018, 206, 247–255. [Google Scholar] [CrossRef]
- Scott, G. Properties of polymers: Their correlation with chemical structure; their numerical estimation and prediction from additive group contributions. Endeavour 2010, 16, 97–98. [Google Scholar] [CrossRef]
- Matthias, M.; Cédric, V.; Marloes, T.; Guy, K.; Vankelecom, I. Crosslinked PVDF-membranes for Solvent Resistant Nanofiltration. J. Membr. Sci. 2018, 566, 223–230. [Google Scholar]
- Li, Y.; Xue, J.; Zhang, X.; Cao, B.; Li, P. Formation of Macrovoid-Free PMDA-MDA Polyimide Membranes Using a Gelation/Non-Solvent-Induced Phase Separation Method for Organic Solvent Nanofiltration. Ind. Eng. Chem. Res. 2019, 58, 6712–6720. [Google Scholar] [CrossRef]
- See Toh, Y.H.; Lim, F.W.; Livingston, A.G. Polymeric membranes for nanofiltration in polar aprotic solvents. J. Membr. Sci. 2007, 301, 3–10. [Google Scholar] [CrossRef]
- Li, Y.; Cao, B.; Li, P. Effects of dope compositions on morphologies and separation performances of PMDA-ODA polyimide hollow fiber membranes in aqueous and organic solvent systems. Appl. Surf. Sci. 2019, 473, 1038–1048. [Google Scholar] [CrossRef]




| Membrane | Test Conditions | |||
|---|---|---|---|---|
| Width (mm) | Thickness (mm) | Velocity (mm/min) | Poisson’s Ratio | |
| C-PEI | 11.08 | 0.24 | 1 | 0.38121 |
| NF-2LWP | 10.58 | 0.21 | 1 | 0.46513 |
| NF-E-2LWP | 10.72 | 0.25 | 1 | 0.458808 |
| OSN Membrane | Solvent Permeance (L m−2 h−1 bar−1) | Solute | Mw of the Solute (g mol−1) | Rejection (%) | Ref. | |
|---|---|---|---|---|---|---|
| Crosslinked PVDF | DMF | 2.16 | Rose Bengal | 1017 | 75 | [37] |
| Lenzing P84 | DMF | 1.67 | Styrene oligomer | 200 | 93 | [39] |
| PMDA-ODA Polyimide | DMF | 2.5 | Rose Bengal | 1017 | 96 | [40] |
| PMDA-MDA Polyimide | DMF | 6.1 | Rose Bengal | 1017 | 92 | [38] |
| NF-E-1LWP | DMF | 3.70 | Crystal Violet | 407 | 96 | This work |
| NF-E-2LWP | DMF | 3.53 | Crystal Violet | 407 | 98 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, A.; Cao, M.; Qian, D.; Zhang, J.; Sun, Y. Preparation of a Solvent-Resistant Nanofiltration Membrane of Liquefied Walnut Shell Modified by Ethylenediamine. Membranes 2023, 13, 719. https://doi.org/10.3390/membranes13080719
Zhou A, Cao M, Qian D, Zhang J, Sun Y. Preparation of a Solvent-Resistant Nanofiltration Membrane of Liquefied Walnut Shell Modified by Ethylenediamine. Membranes. 2023; 13(8):719. https://doi.org/10.3390/membranes13080719
Chicago/Turabian StyleZhou, Ayang, Mingxue Cao, Demeng Qian, Jingyao Zhang, and Yaping Sun. 2023. "Preparation of a Solvent-Resistant Nanofiltration Membrane of Liquefied Walnut Shell Modified by Ethylenediamine" Membranes 13, no. 8: 719. https://doi.org/10.3390/membranes13080719
APA StyleZhou, A., Cao, M., Qian, D., Zhang, J., & Sun, Y. (2023). Preparation of a Solvent-Resistant Nanofiltration Membrane of Liquefied Walnut Shell Modified by Ethylenediamine. Membranes, 13(8), 719. https://doi.org/10.3390/membranes13080719







