1. Introduction
Interfacial protons at biological membranes play a crucial role in many bioenergetic processes and activity of membrane proteins [
1,
2]. These surface protons exchange with the bulk, overcoming a high energy barrier formed by water molecules oriented at the membrane–water interface [
3,
4,
5,
6]. Therefore, transfer of protons between donor and acceptor molecules in the membrane can proceed along the surface of the membrane instead of bulk water [
7,
8,
9]. This mechanism is essential for the functioning of membrane transporters, where the membrane can serve as a proton-collecting antenna [
1,
2]. A key question in testing this mechanism is how to evaluate the height of the energy barrier for proton surface to bulk release. This height has been estimated theoretically either by electrostatic models [
10] or by molecular dynamics simulations [
11]. For experimental values, the kinetics of the exchange of protons between the membrane surface and water is investigated using pH-sensitive fluorescent probes [
7,
8,
9]. The height of the barrier varies depending on experimental conditions and spans from 6 k
BT for the octanol–water interface [
6] to 30 k
BT in the case of bilayer lipid membranes (BLMs) [
7,
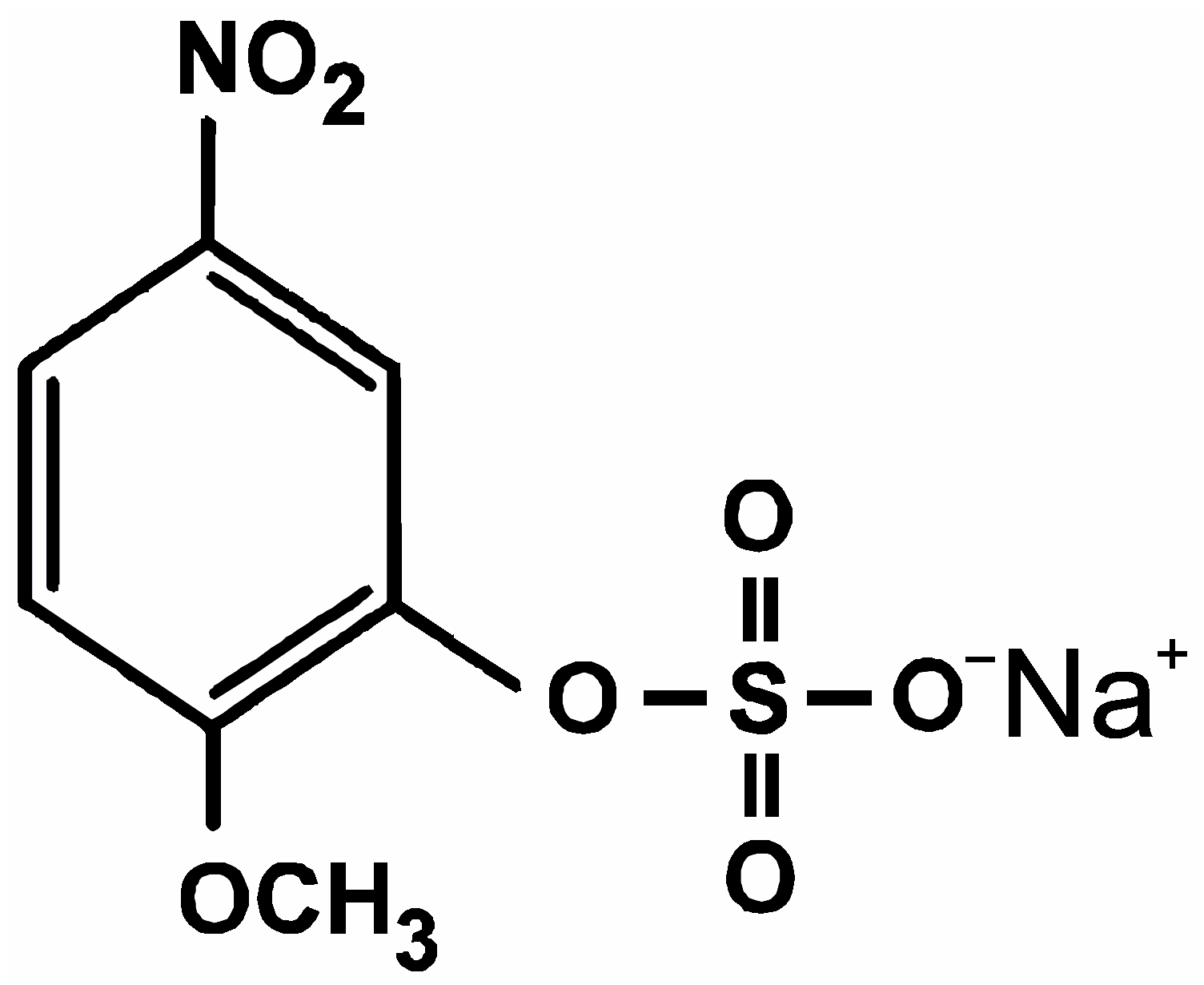
12]. More easy and rapid estimations of interfacial protons can be achieved by direct electric measurements of the membrane capacitance and electrostatic potentials. Recently, we have observed changes in these values caused by a fast light-driven release of protons from sodium 2-methoxy-5-nitrophenyl sulfate (MNPS) (
Figure 1) at the membrane surface that has relaxed in a timescale of about one minute [
13]. Study of the kinetics of such slow relaxation can be informative for better understanding of processes coupled with light-driven release of protons from photosensitive compounds. For such investigations, we used the intramembrane field compensation (IFC) method, which allows one to detect the adsorption of charged or dipole molecules at the membrane, as well as their damage by the chemical reactions [
14,
15,
16]. Here, we studied changes in the boundary potential at the membrane interface resulting from the adsorption and photolysis of MNPS molecules. We determined a contribution of the proton binding and the influence of the light intensity, buffer concentration, and pH of the solution.
2. Materials and Methods
Bilayer lipid membranes were formed by the Mueller–Rudin technique [
17] at a 0.8 mm aperture in a septum separating two 2 mL compartments of a Teflon cell. For BLM formation, 1,2-diphytanoyl-sn-glycero-3-phosphocholine (Avanti Polar Lipids, Alabaster, AL, USA) was dissolved in n-decane (Sigma-Aldrich, St. Louis, MO, USA) to a final concentration of 15 mg/mL. This lipid is chemically stable, in contrast to natural lipids with unsaturated hydrocarbon tails, thus allowing long-lasting experiments with UV illumination without membrane damage. The cell had two windows: one was for the visual control of the BLM and the other was for illuminating. Compartments were filled with 20 mM KCl, 0.2 mM HEPES, 0.2 mM TRIS, and 0.2 mM citric acid (all from Sigma-Aldrich, St. Louis, MO, USA) in double-distilled water and continuously stirred during experiments. Sodium 2-methoxy-5-nitrophenyl sulfate was synthesized in the Zelinsky Institute of Organic Chemistry, Russian Academy of Sciences. Solution of MNPS was added to the distant (relative to the light source) compartment of the cell. To illuminate the BLM, a UV LED (wavelength 375 nm, maximum electric power 3 W) was used. The intensity of the illumination was varied in the range 0.1–0.8 W by changing the voltage applied to the LED.
For electrical measurements at the BLM, we used a pair of Ag/AgCl electrodes. Electrodes contacted the working buffer solution in the cell through plastic tips, which bottom part was filled with agar, prepared in the solution of 0.1 M KCl in double-distilled water. Electric current was converted to voltage by a Keithley-427 current amplifier (Cleveland, OH, USA) and further analyzed with self-made software. To measure the boundary potential difference (∆ϕ
b) across the BLM, we used the IFC method ([
13], see also reviews [
14,
15]). This method allowed measuring the difference in boundary potentials between two sides of the BLM after the addition of charged substances or the appearance of charge due to chemical reactions at the one side of the membrane. To measure the open circuit potential (OCP), we added to the lipid solution 10
−5 M of ionophore pentachlorophenol (PCP) (Sigma-Aldrich, St. Louis, MO, USA).
3. Results
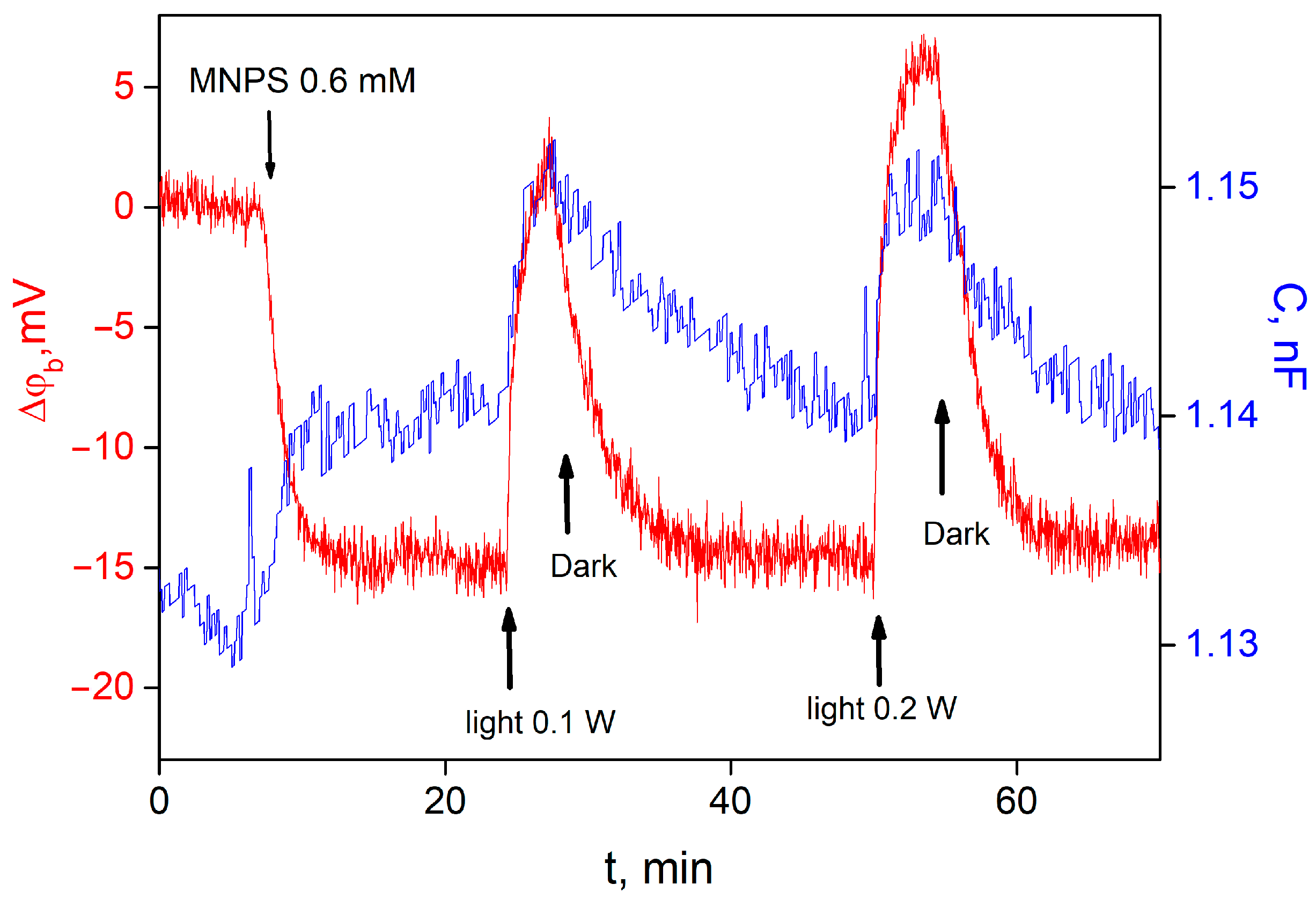
MNPS is a well-known source of protons that are released after a flash of UV light. Recently, we have demonstrated that MNPS can bond to a lipid membrane and change its boundary potential as well as electrical capacitance under illumination [
13]. Addition of MNPS into the water solution at one side of the BLM led to a boundary potential difference across the membrane, the sign of which corresponded to the adsorption of MNPS anions on the membrane. Illumination of the BLM with adsorbed MNPS molecules led to a reversible change in the ∆ϕ
b and the membrane electrical capacitance with the magnitude depending on the light intensity (
Figure 2). An increase in the intensity of the illumination raised the magnitude and the rate of ∆ϕ
b changes.
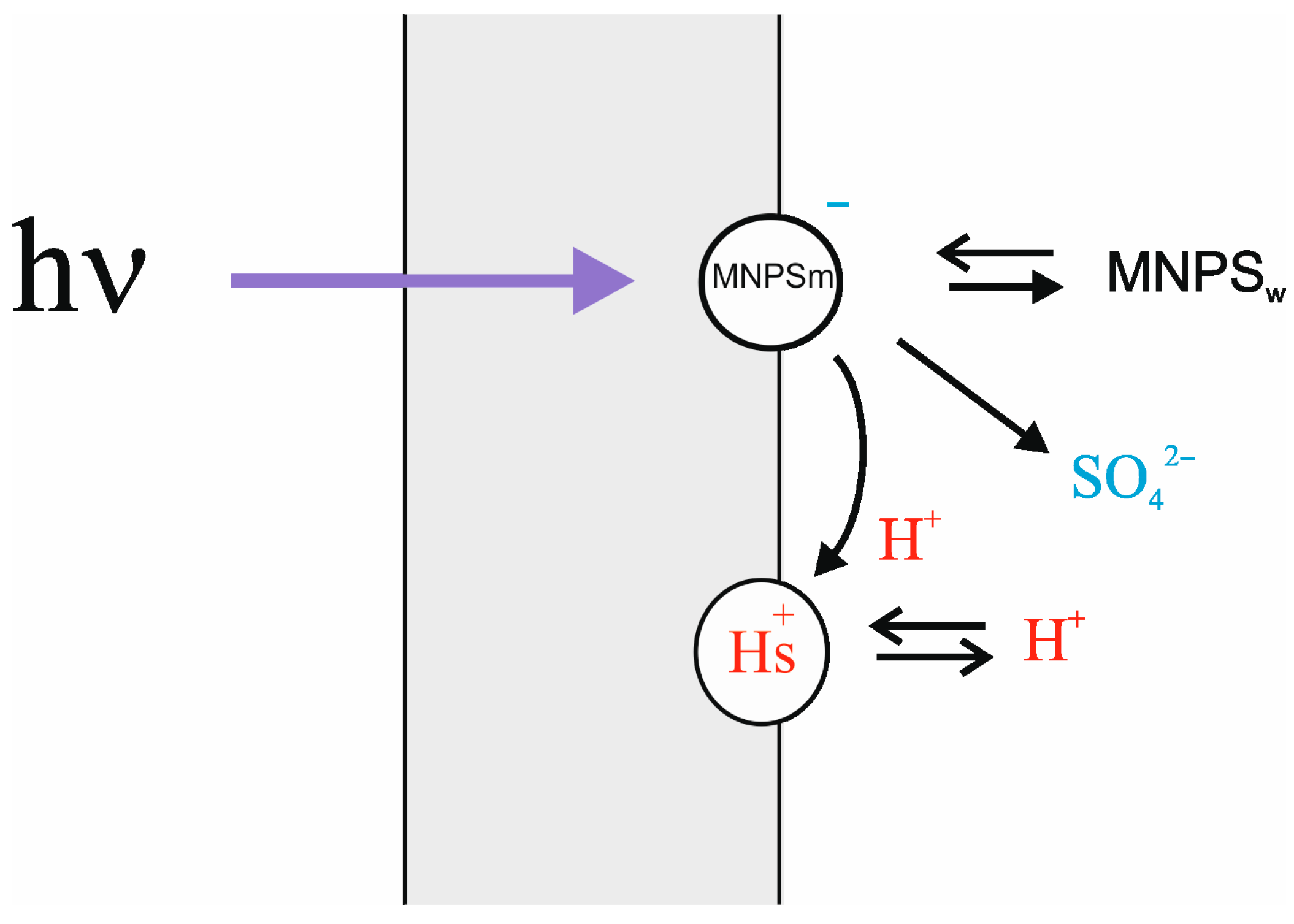
There are two processes causing the change in ∆ϕ
b due to illumination: damage of the MNPS anions at the membrane surface and binding of released protons [
13]. To extract the effect of protons from the total change in ∆ϕ
b, a simple theoretical model was applied (see
Appendix A). According to the model, the change in ∆ϕ
b under illumination is a result of a decrease in the number of MNPS anions at the membrane surface with a simultaneous increase in the amount of released protons. The model also accounts for the exchange of protons and MNPS anions between the BLM and the bulk water solution. The scheme of processes is shown in
Figure 3.
Based on the model, kinetics of the ∆ϕ
b change can be fitted by two exponential functions using four independent parameters: rate constants
kd and
kdh of the desorption from the BLM of MNPS anions and protons, respectively, the rate
k of the damage of MNPS at the BLM, and the coefficient
B, which determines the ratio of contributions to the change in ∆ϕ
b from the proton binding and MNPS damage. The ratio of these steady-state changes is equal to
(see
Appendix A).
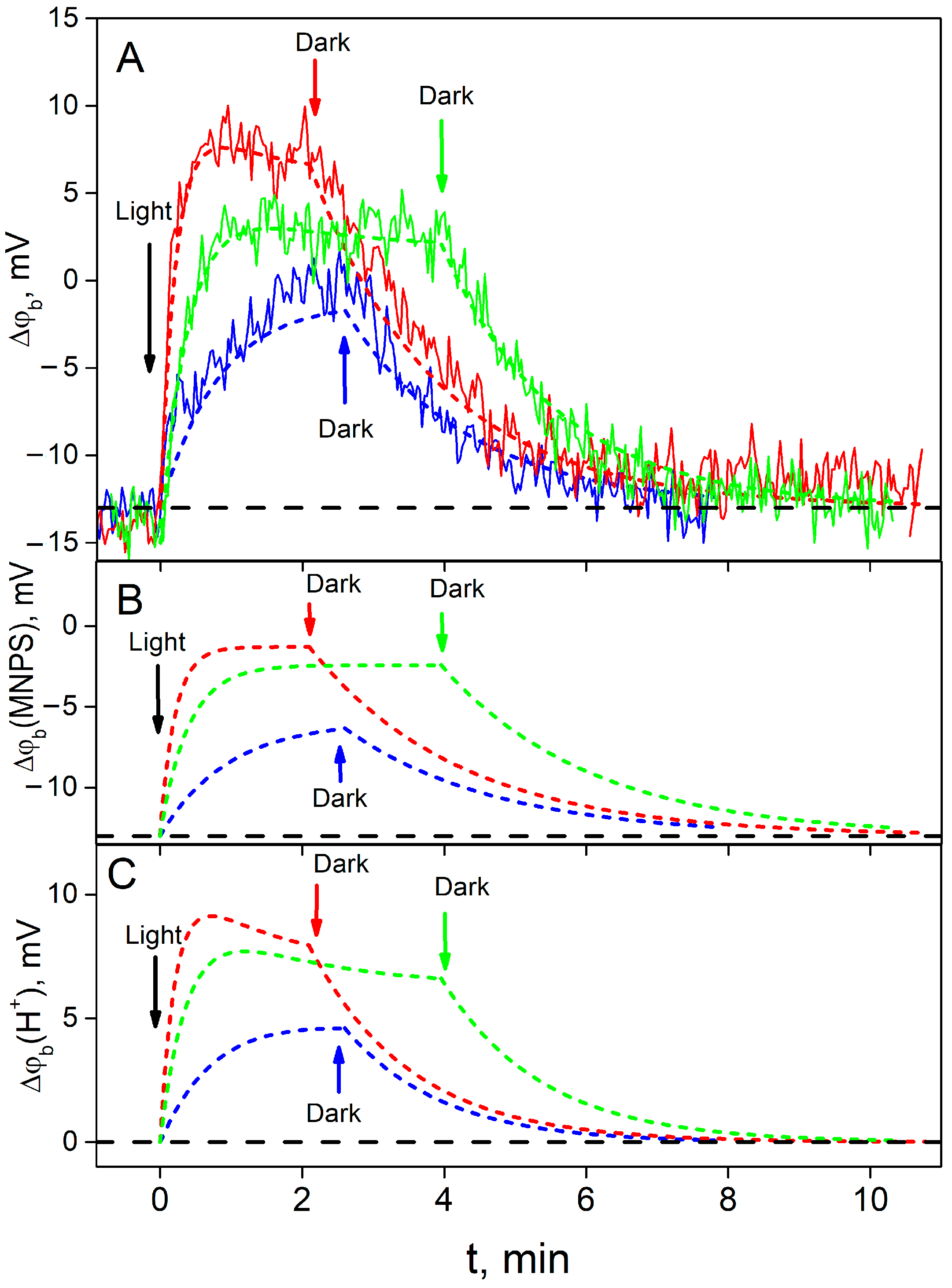
Typical experimental kinetics of the ∆ϕ
b change for various light intensities and their fit by the model are shown in
Figure 4A.
Figure 4B,C demonstrate calculated contributions to the total value of ∆ϕ
b from the MNPS and proton binding at the BLM, respectively. As one can see, at the beginning (t = 0), ∆ϕ
b was equal to the part produced by the binding of MNPS anions (compare
Figure 4A and
Figure 4B). After the illumination was switched on, the ∆ϕ
b value reflected the sum of a decrease in the number of MNPS anions (
Figure 4B) and an increase in the amount of released protons (
Figure 4C). The steady-state amount of MNPS anions at the BLM is considerably less than the initial one. The contribution of protons to the ∆ϕ
b (
Figure 4C) for the high light intensity is non-monotonic and could be described by two exponential functions.
Contribution of released protons to ∆ϕ
b should depend on the concentration of the buffer and pH of the solution. An increase in the buffer concentration reduces the amount of non-equilibrium protons bound to the BLM and the respective part of the ∆ϕ
b change [
7,
9].
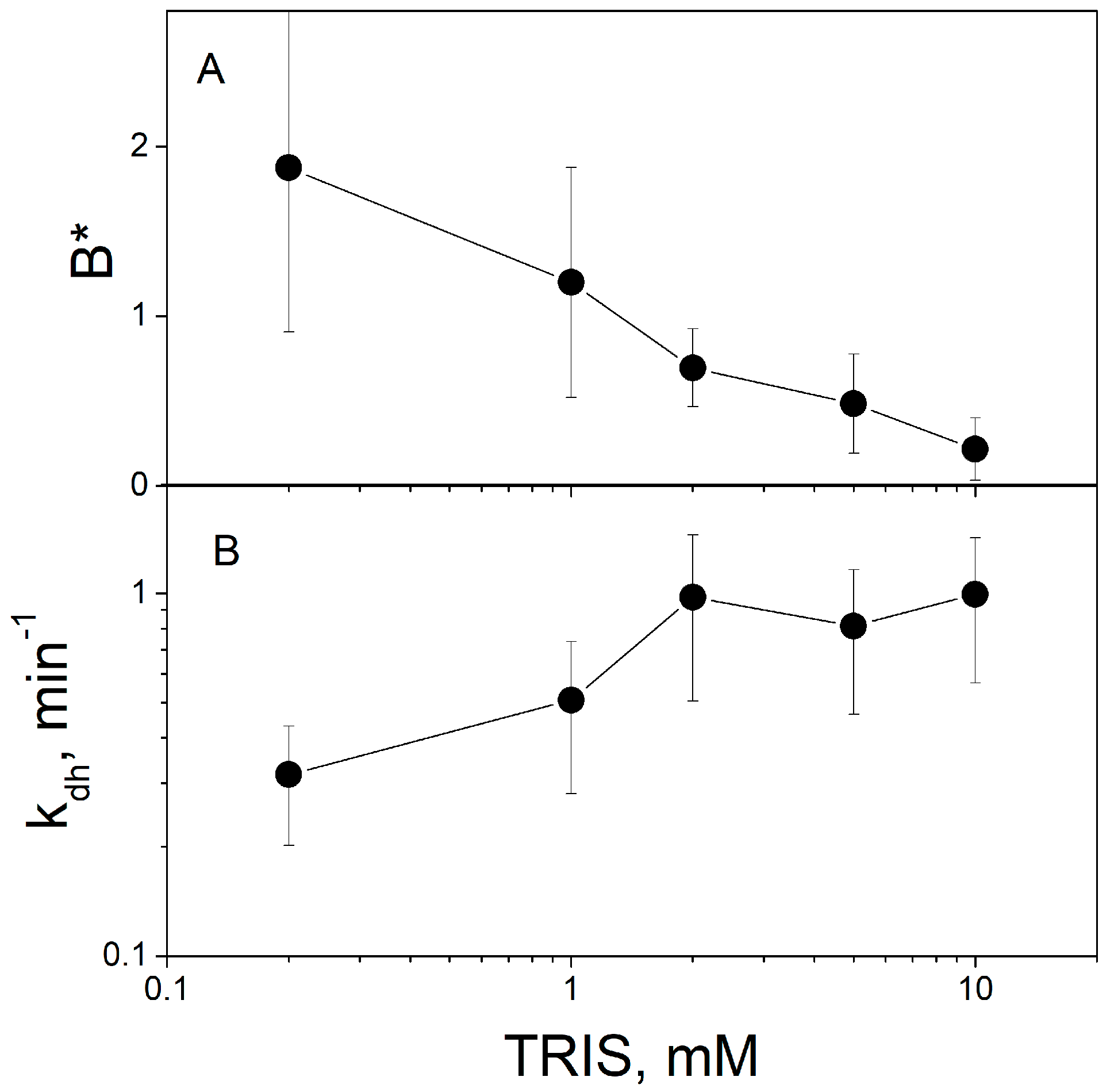
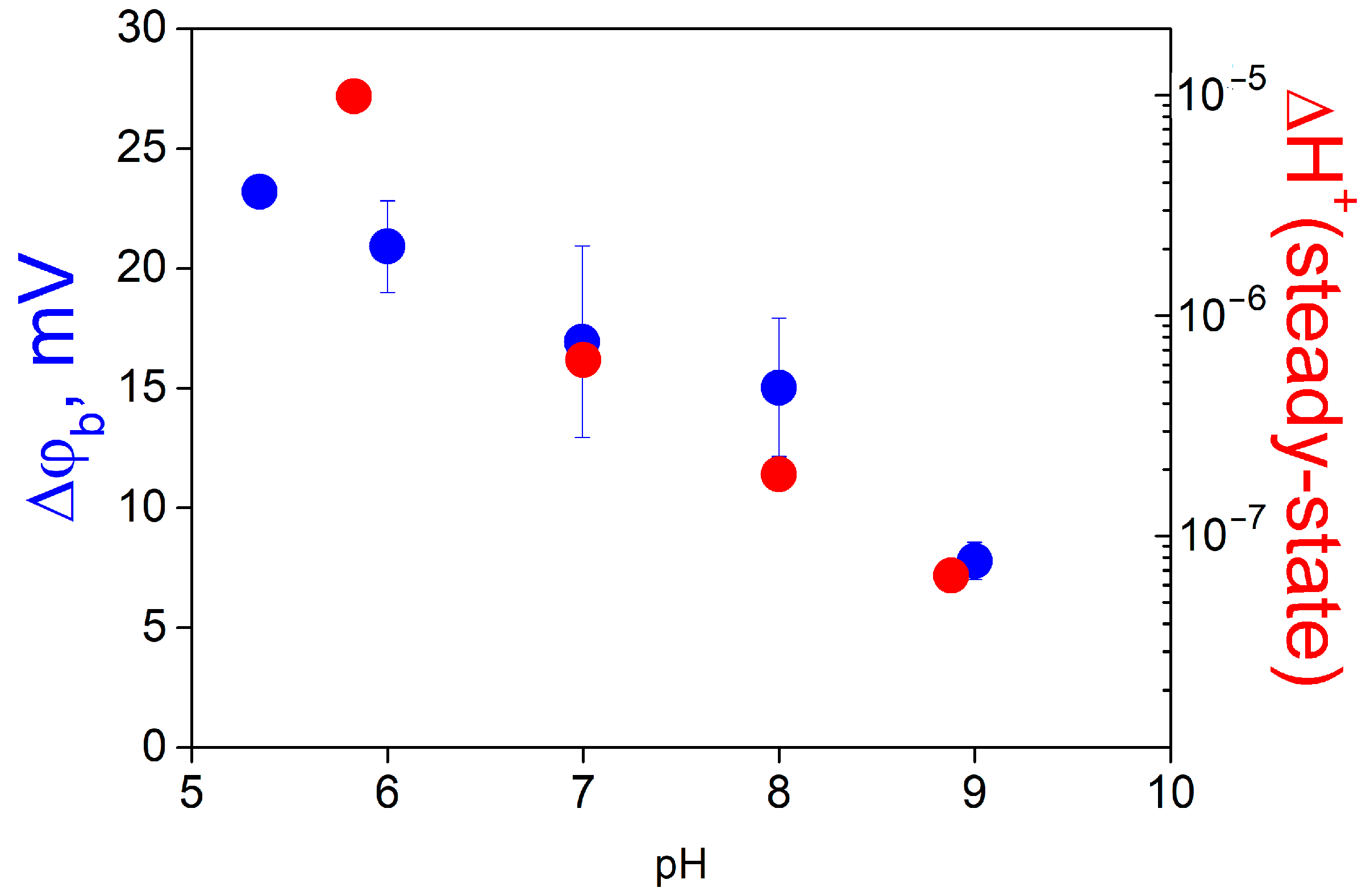
Figure 5 demonstrates that the increase in the TRIS concentration decreases the parameter
B* (ratio of steady-state ∆ϕ
b values caused by protons and MNPS anions) determined by the fitting of the experimental data (
Figure 5A) and increases the rate constant
kdh (
Figure 5B).
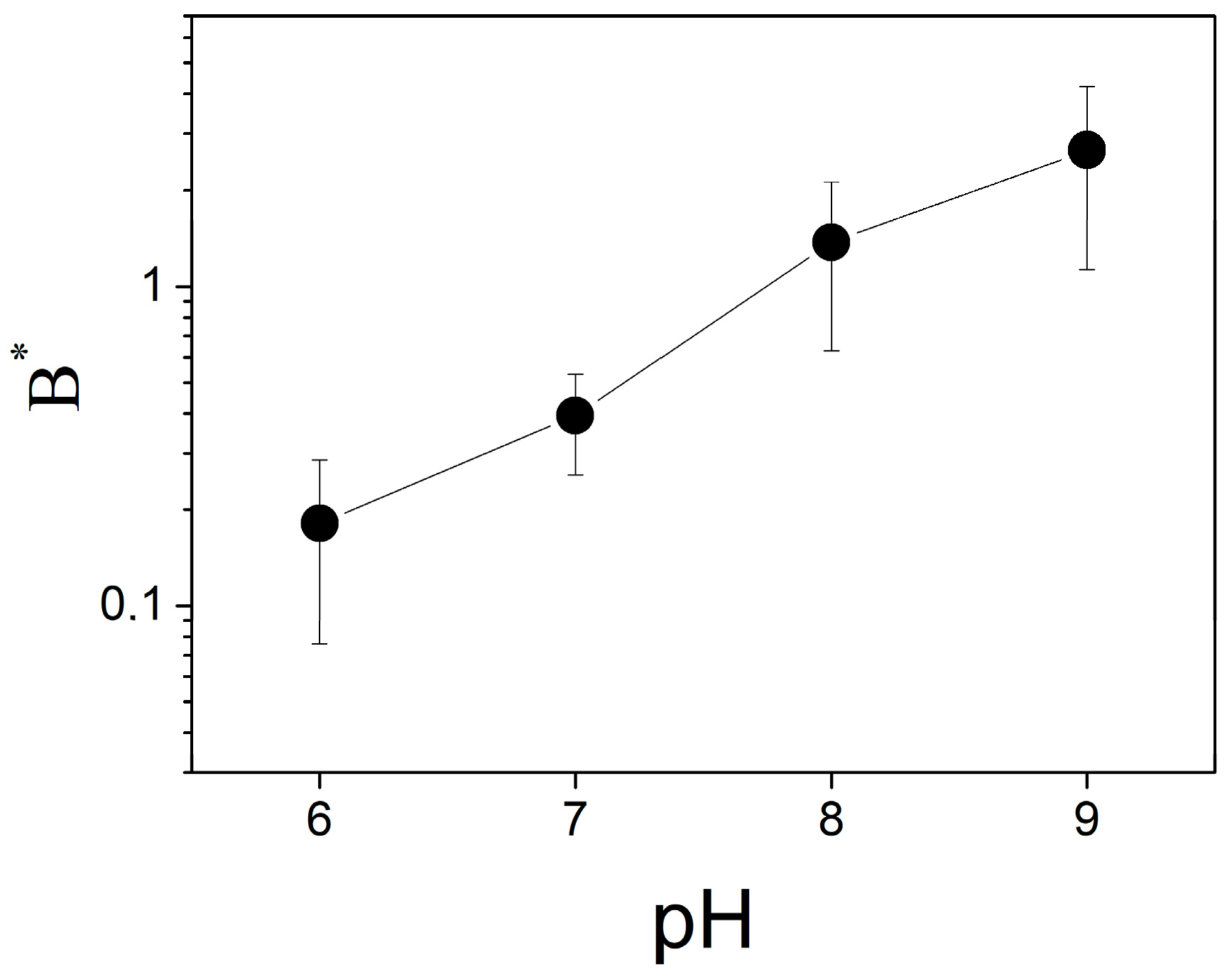
The change in ∆ϕ
b due to binding of protons depends on the pH of the solution (
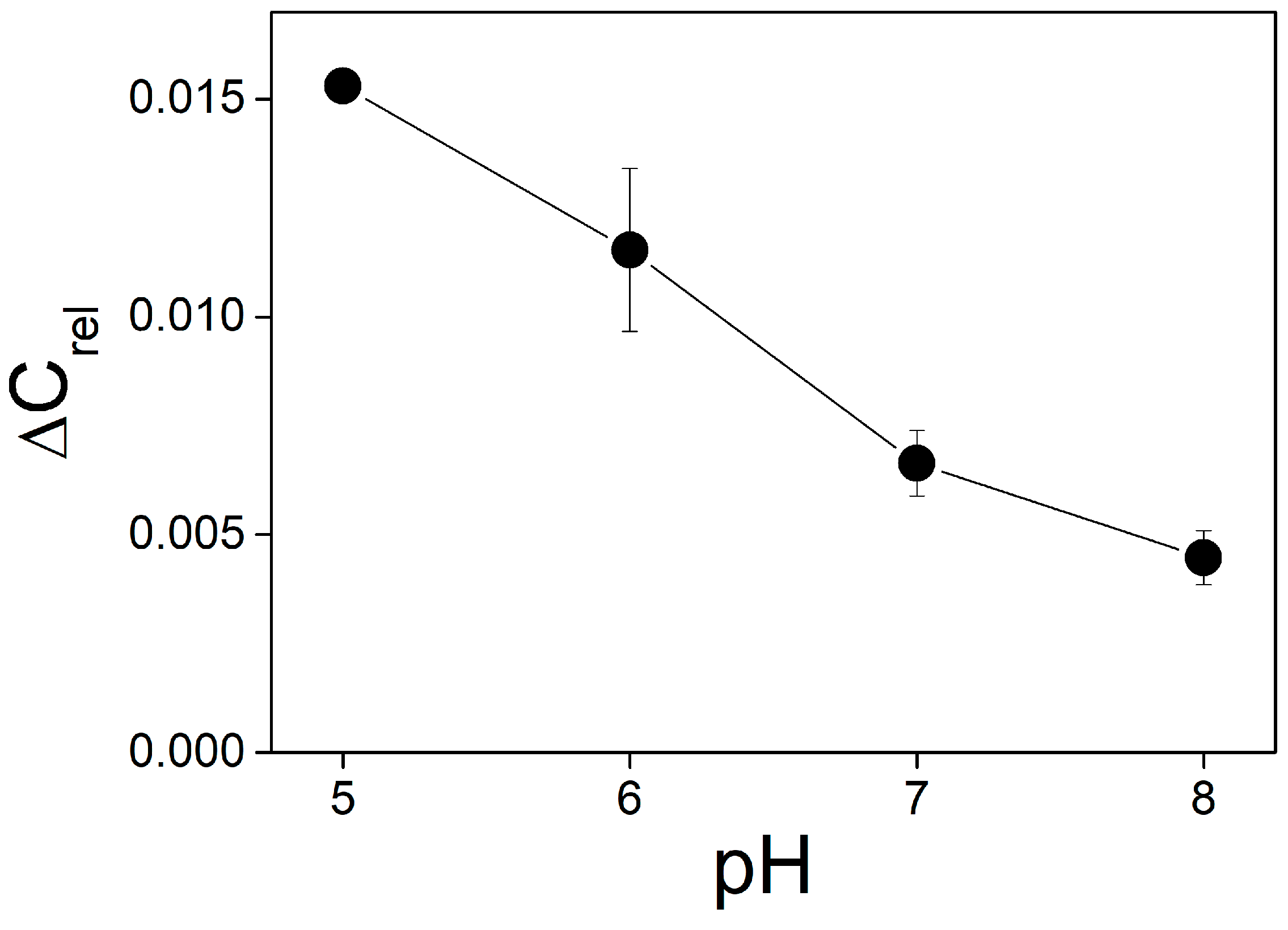
Figure 6). The parameter
B* increases with the pH in the entire range of the measurements, from 6.0 to 9.0. It means that the decrease in pH attenuates the ∆ϕ
b change due to bound protons similarly to the effect of the buffer.
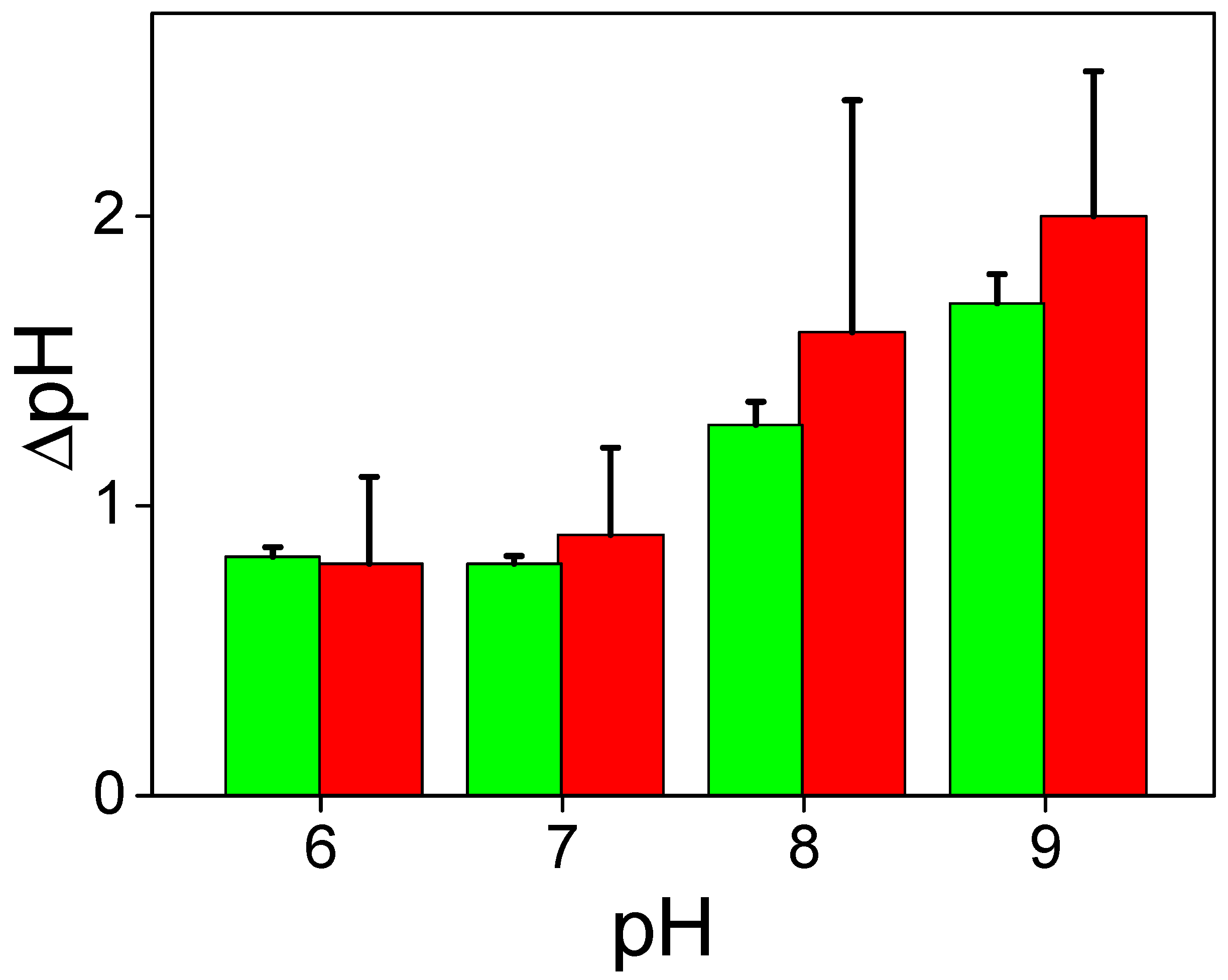
The pH dependence of the ∆ϕ
b part caused by protons is determined by the change in the surface charge of the BLM, resulting from the shift in the acid–base dissociation of titratable groups in the polar part of lipids. To study this dependence, we measured the equilibrium change in ∆ϕ
b by adding a small amount of HCl or KOH in the solution at one side of the BLM. This dependence is shown in
Figure 7A. We observed a similar dependence earlier [
18]. Like in [
18], the absolute value of ∆ϕ
b was taken to be zero at pH 5, because ζ-potential measurements indicate zero surface charge of the BLM at this pH value. We observed changes in ∆ϕ
b under illumination at high pH values, where the surface charge of the BLM was negative and release of protons increased the ∆ϕ
b value (
Figure 7A). The pH dependence of the equilibrium value of ∆ϕ
b can be used for evaluating the relative change in the concentration of protons released at the BLM surface. In addition to this curve, we determined the dependence of the ∆ϕ
b change due to adsorption of the MNPS on the BLM. As follows from
Figure 7B, this part of ∆ϕ
b decreased with pH, which could be explained by electrostatic interactions of MNPS anions with the BLM, the negative charge of which increased with pH.
Release of protons from MNPS decreased pH at the membrane surface that resulted in the ∆ϕ
b increase. The total change in ∆ϕ
b is a product of boundary potential difference resulting from the MNPS adsorption (
Figure 7B, blue symbols) and the coefficient
B*, which determines the relative contribution of protons to ∆ϕ
b (
Figure 6). The proton-related part of ∆ϕ
b is plotted in
Figure 7B by red symbols. If we add this value to the equilibrium ∆ϕ
b (
Figure 7A, red symbols) and plot a horizontal line up to the intersection with the pH dependence of equilibrium ∆ϕ
b (
Figure 7A, black curve), we should obtain the correspondence between equilibrium pH at the membrane surface and change in pH resulting from the illumination.
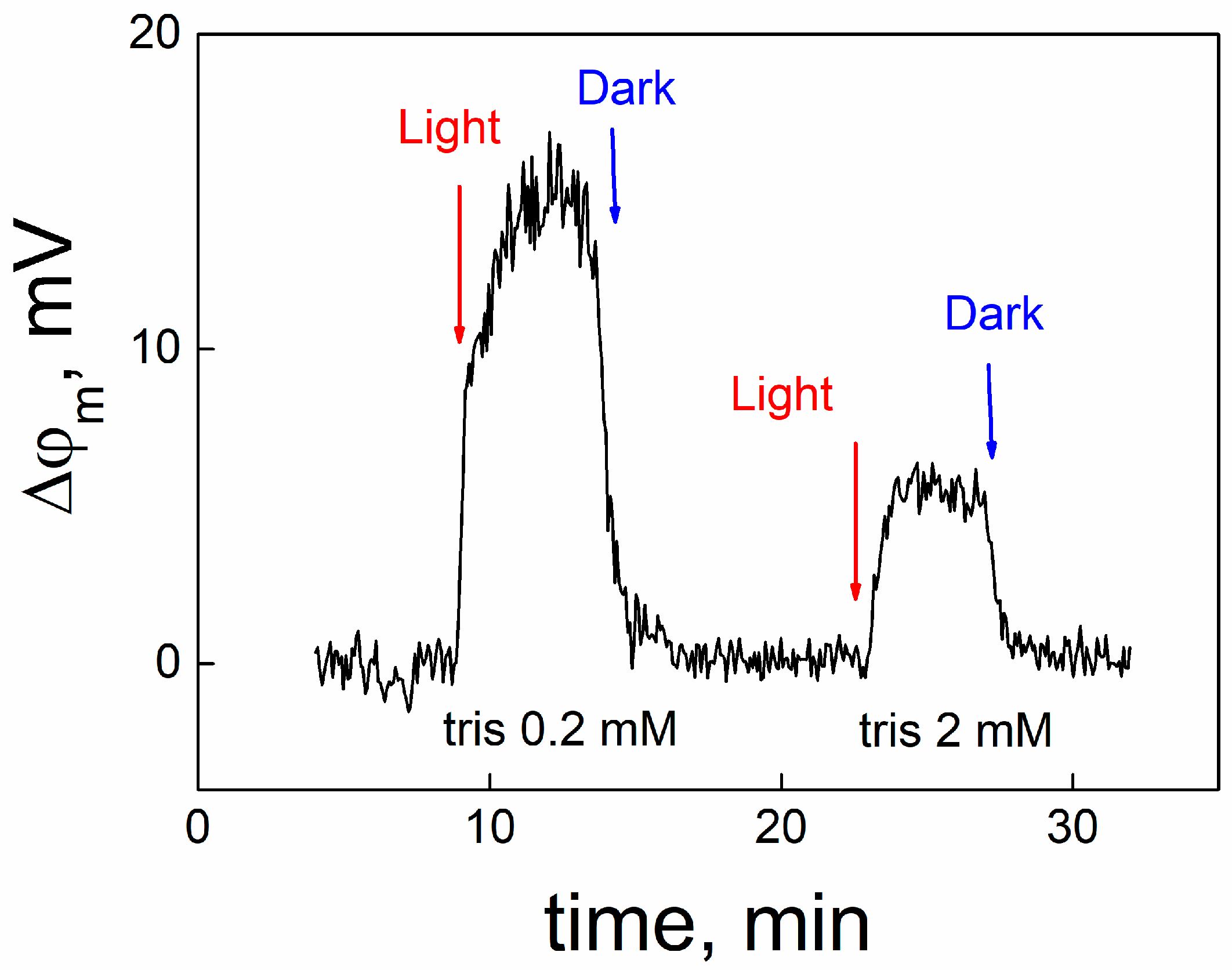
Release of protons from MNPS into the unstirred layer of the solution adjacent to the membrane can be measured by another method, described in [
19,
20]. We adsorbed MNPS at one side of the BLM, which conducted protons due to the presence of protonophore pentachlorophenol, and measured the open circuit potential with an electrometer. Typical kinetics of the change in OCP under illumination and its following restoration in the dark are presented in
Figure 8.
After the beginning of the illumination, the OCP changed due to release of protons from MNPS into the unstirred layer near the BLM. The termination of the illumination led to restoration of the OCP to its initial value. The increase in the concentration of buffer in the solution suppressed the change in OCP caused by illumination. Similar experiments have been performed earlier, when a BLM with adsorbed MNPS was illuminated by a UV light flash [
21]. To determine the shift in pH in the unstirred layer, we performed calibration measurements, when the pH value was changed in the bulk water solution by adding HCl into one compartment of the cell. The pH shift in the unstirred layer of the water solution was compared with the change in pH at the membrane surface determined by the ∆ϕ
b measurements (
Figure 9). This comparison showed that pH shifts determined by these two methods almost coincided in the whole range of pH.
Illumination of the BLM with adsorbed molecules of MNPS led not only to a change in ∆ϕ
b and pH in the unstirred layer but also to an increase of the membrane capacitance. Like changes in ∆ϕ
b, the light-induced change in the capacitance depended on the concentration of MNPS, intensity of the illumination, and pH of the solution. In contrast to the changes in ∆ϕ
b, the change in the capacitance decreased with pH (
Figure 10).
4. Discussion
Measurements of the kinetics of ∆ϕ
b and OCP showed that the total change in ∆ϕ
b consisted of two parts connected with the amount of MNPS anions and protons at the surface of the BLM. The contribution corresponding to protons was determined using the simple model accounting for MNPS photolysis and the exchange of MNPS anions and protons between the BLM surface and the bulk solution (see
Appendix A). The part of ∆ϕ
b caused by protons increases with the MNPS concentration and light intensity, decreases with the concentration of buffer, and depends on pH. Changes in the relative concentration of protons released by the photolysis of MNPS at the surface of the BLM and in the unstirred layer near the membrane were very close. This allows us to conclude that protons released from MNPS molecules remain in an equilibrium between the BLM surface and the adjacent water layer. Therefore, the energy barrier for proton transfer between the lipid membrane and water has a negligible effect on the distribution of protons between the BLM and water. This can be explained by a fast transfer over the barrier at the membrane–water interface and a slow diffusion in the unstirred layer. The coincidence of proton concentration shifts at the membrane surface and in water (
Figure 9) indicates that the proton transfer is much faster than diffusion.
The conclusion that the diffusion of protons in water is the rate-limiting step of the ∆ϕ
b change is supported by the evaluation of the rate of the process. Values of
kdh determined by fitting the experimental data by our theoretical model varied in the range 0.4–2.0 min
−1 that yielded the lowest time constant of the proton surface to bulk release of about 30 s. OCP measurements characterizing the rate of change in pH in the unstirred layer near the membrane gave a time constant of about 10 s. Nevertheless, all these rates were 1–2 orders of magnitude slower than the corresponding rates found from experiments with fluorescent probes [
6,
7,
9]. The slower rate in our experiments indicates that the rate-limiting process is the change in the proton concentration in the unstirred layer.
The time of equilibration of the concentration of molecules in the unstirred layer can be roughly evaluated as:
where
L is the thickness of the unstirred layer (which is about 10
−2 cm),
D is the diffusion coefficient (protons diffuse in the water together with buffer molecules, and the value of
D for most buffer molecules can be taken approximately as 10
−5 cm
2 s
−1). This yields a time of about 10 s, similar to that determined from our experiment. Perhaps experiments with fluorescent probes give faster rates of the proton surface to bulk release because of the better time resolution of the method or usage of other compounds, such as “Caged-H+”. Values of the rate constant
kdh of the proton desorption from the BLM in our experiments were close, by an order of magnitude, to that of rate constant
kd of the MNPS desorption. This could be explained if we assume that both these values correspond to the diffusion of molecules of buffer or MNPS in the water solution.
Our results suggest that the main contribution to the change in the ∆ϕ
b is determined by the change in pH in the unstirred layer adjacent to the membrane. Surface charge of the BLM should influence the distribution of protons between the membrane and water. Recently, the effect of surface charge on the lateral transfer of protons along the membrane and their release in the bulk solution has been found in experiments with fluorescent probes [
12]. The dependence of photoinduced ∆ϕ
b change on pH does not reflect the influence of pH on the rate of the reaction of release of protons from MNPS. To find the actual dependence of the reaction on pH, the absolute value of the pH shift caused by the illumination was determined by subtracting the initial concentration of protons from the total values (
Figure 7A). These values are plotted in
Figure 11. Absolute shifts in the concentration of protons released from MNPS indicate that the reaction of photo release of protons from MNPS does not depend on pH and is determined only by the pH dependence of the adsorption of MNPS anions at the membrane. Note that the pH dependence of the absolute shift in the concentration of protons released from MNPS (
Figure 11) is similar to the pH dependence of the membrane capacitance (
Figure 10).
The mechanism of the membrane capacitance change under illumination is unknown. Earlier we observed similar effects, when the BLM was illuminated by a flash of light [
13]. There are several factors which could lead to a total change in the capacitance: (i) change in the capacitance of the double layer; (ii) change in the geometric size (thickness or area) of the membrane; or (iii) change in the dielectric permittivity of the water layer near the membrane surface. The first reason can be excluded: the evaluation of the change in the capacitance due to the change in the surface charge using Gouy–Chapman theory gives a value two orders of magnitude lower than the measured one [
13]. The most probable mechanism of the capacitance change could be a change in the surface tension at the membrane–water interface due to the damage of MNPS molecules and binding of the protons at the membrane surface. This can influence the membrane geometric size. The most interesting mechanism is the third one. The dielectric permittivity of the water layer at the membrane surface could vary either by the change in the concentration of MNPS molecules and protons at the membrane–water interface or by the reorientation of water molecules near the membrane surface.
The equilibrium dependence of ∆ϕ
b on pH (
Figure 7) contradicts simple theoretical expectations, if we assume that the surface charge of the BLM formed from PC lipids should be determined only by two charged groups of the phospholipid molecule: negatively charged phosphate (pK
a about 2.2) and positively charged choline, which should not dissociate. Therefore, an expected dependence of the surface charge of the BLM on pH should include only the region of the positive charge at low pH, where the phosphate group of the PC molecule becomes neutral. At a pH of around 7, the surface charge of the lipid membrane should be zero. However, the negative surface charge of PC membranes at neutral pH has been observed by various methods [
18,
22,
23] and explained as a result of spontaneous degradation of PC molecules [
24], adsorption of OH
– or anions of buffer [
25], or other inorganic salts [
26,
27]. The hypothesis about the possible adsorption of OH
– on lipid membranes is supported by ζ-potential measurements of gas or oil bubbles that show a negative surface charge at high pH values [
28,
29,
30].
An alternative assumption is that the pH of the solution influences the dipole potential of the membrane resulting from the change in the projection of the PC headgroup dipole to the membrane surface [
31]. This hypothesis is supported by the corresponding changes in PC molecule shape influencing membrane fusion [
32]. The change in dipole potential also reflects the change in the orientation of water molecules bound to polar lipid parts. It is established that the change in entropy of water molecules at the membrane interface relative to the bulk solution gives the main contribution to the energy barrier of proton surface to bulk release [
7]. Thus, proton transport in the unstirred layer of the bound water can determine the lateral proton diffusion and its release from the membrane surface to water.