Heparin-Immobilized Polyethersulfone for Hemocompatibility Enhancement of Dialysis Membrane: In Situ Synchrotron Imaging, Experimental, and Ex Vivo Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
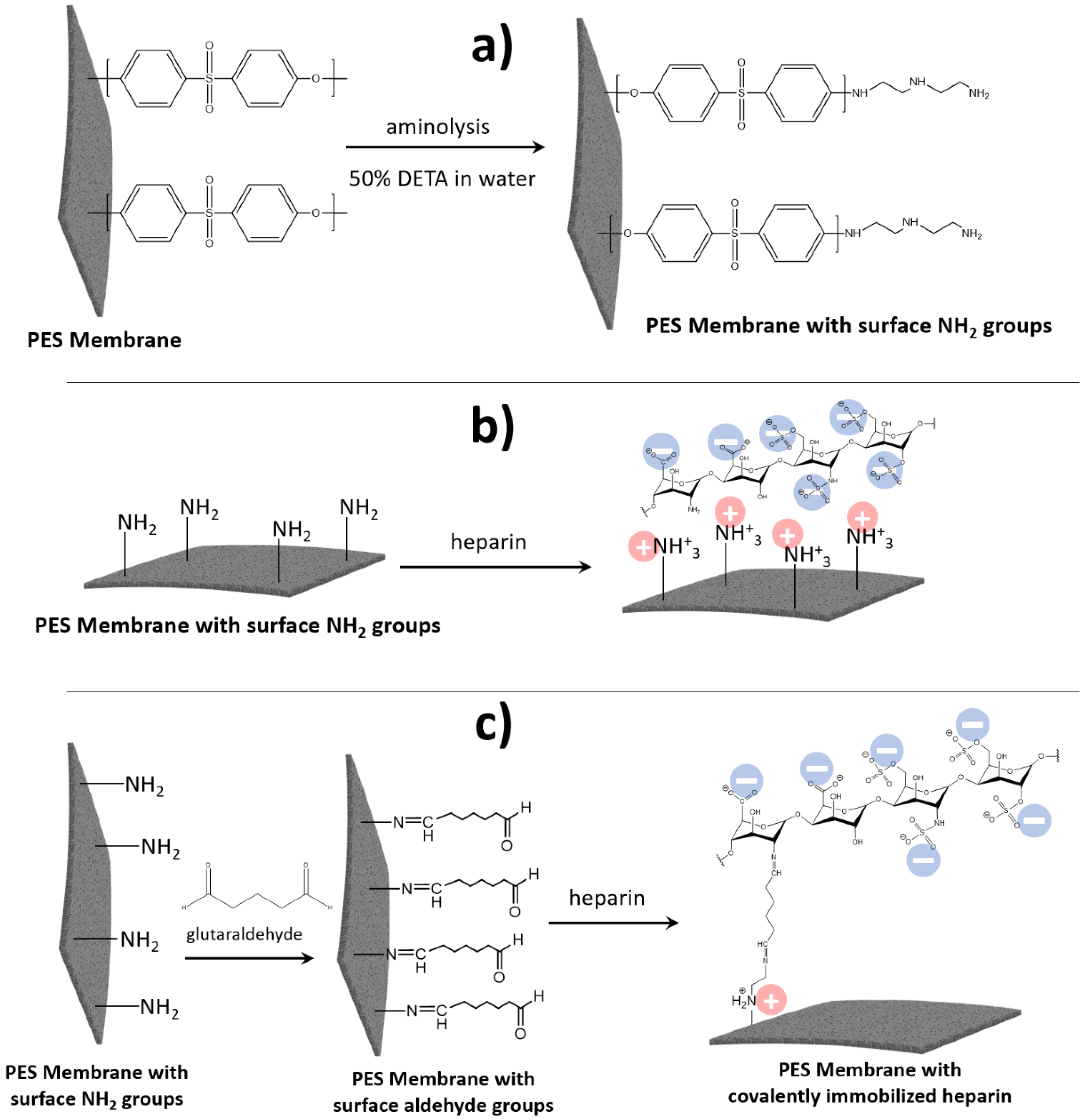
2.1.1. PES Membrane Surface Modifications
PES Membrane Surface Modification with NH2 Groups
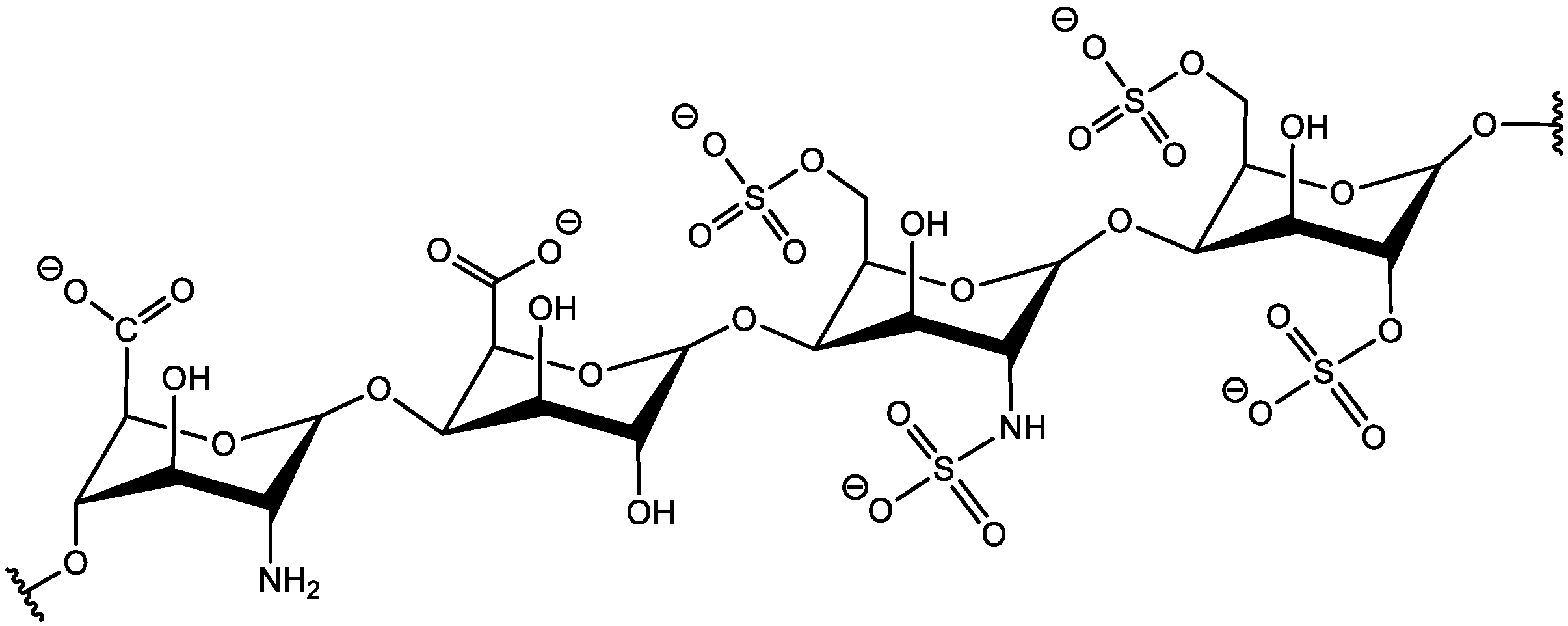
PES Membrane Modification with Heparin–Pseudo-ZW Complex
PES Membrane Modification with Covalently Attached Heparin
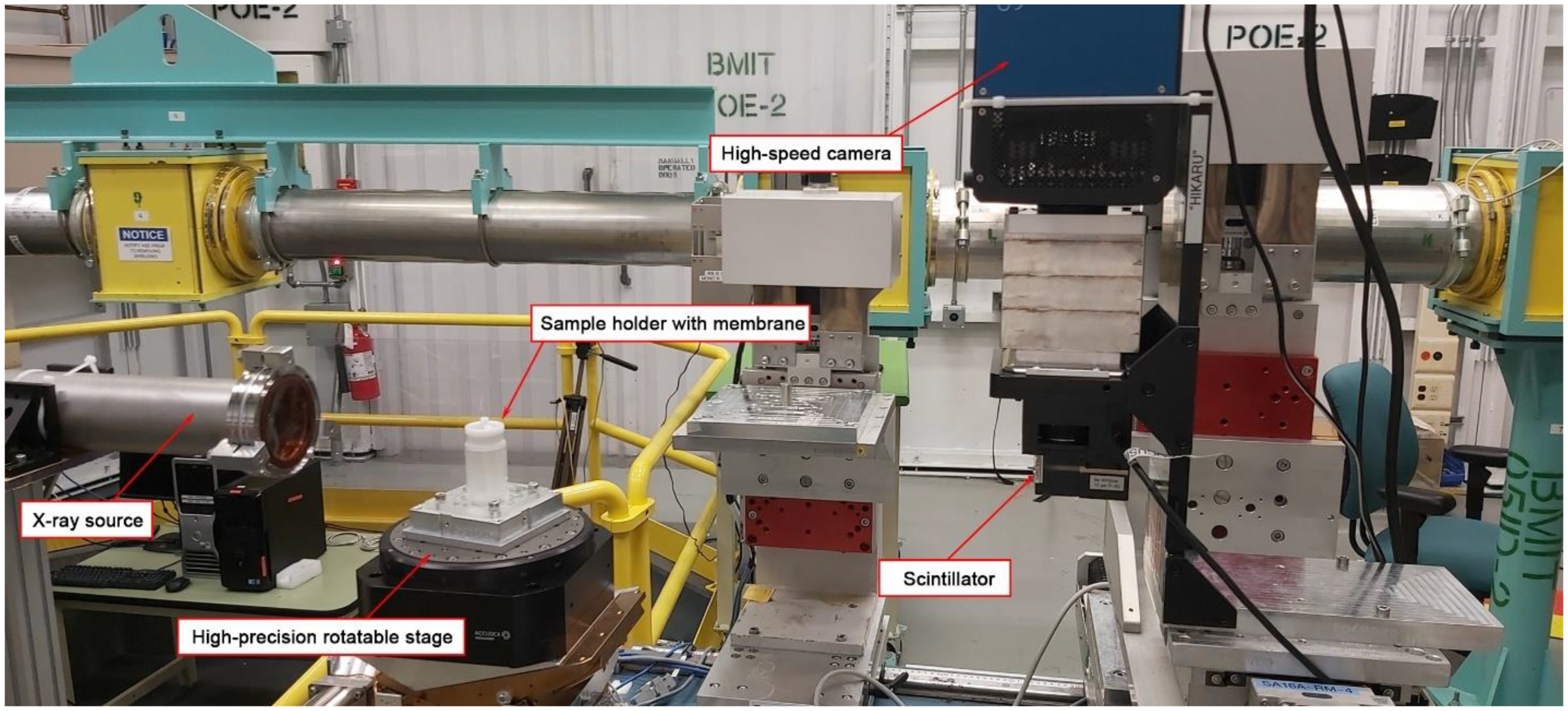
2.1.2. Synchrotron Imaging at Canadian Light Source (CLS)
2.1.3. In Vitro Investigation of FB Depletion and FB Adsorption
2.1.4. Analytical Techniques
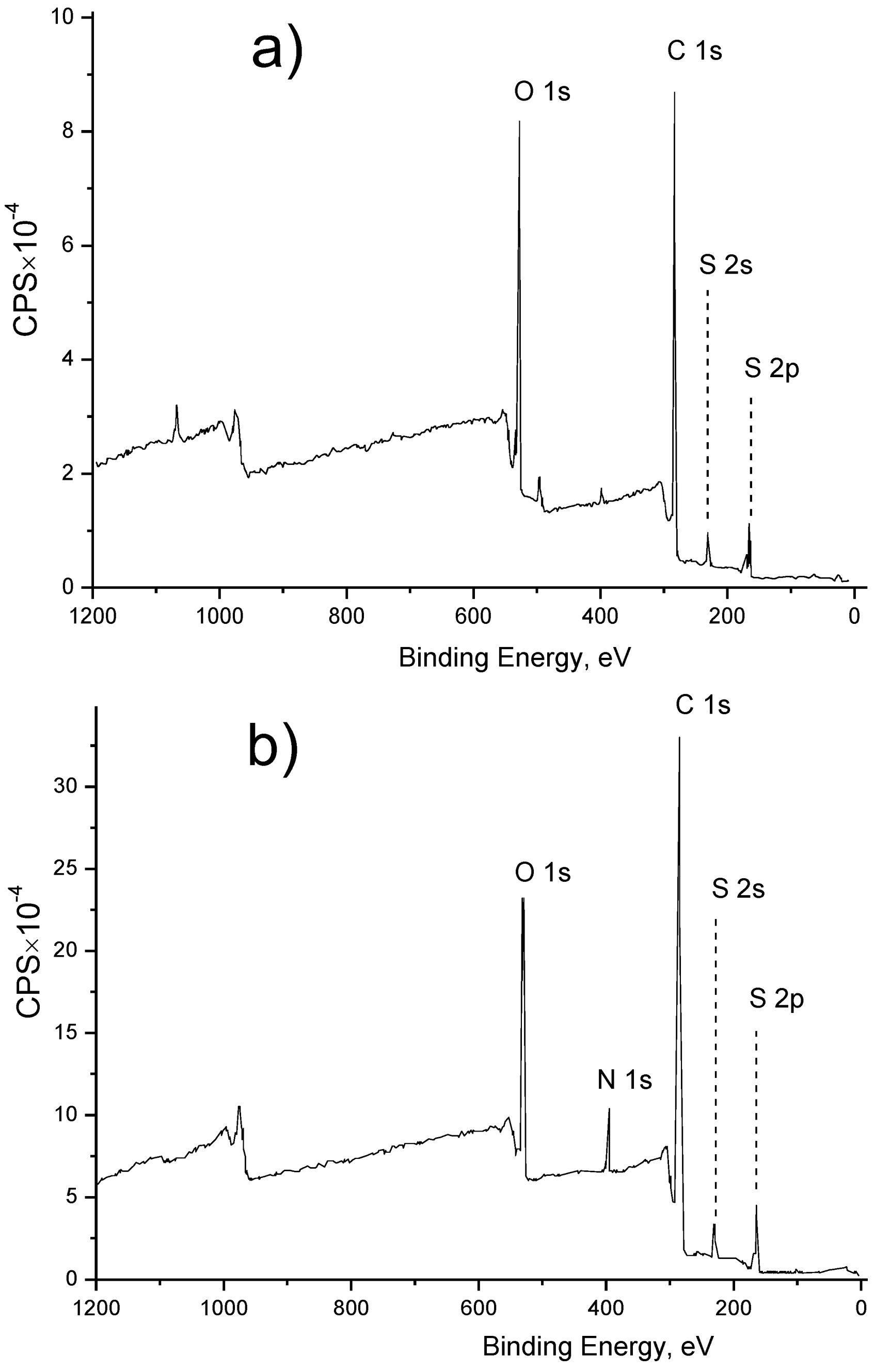
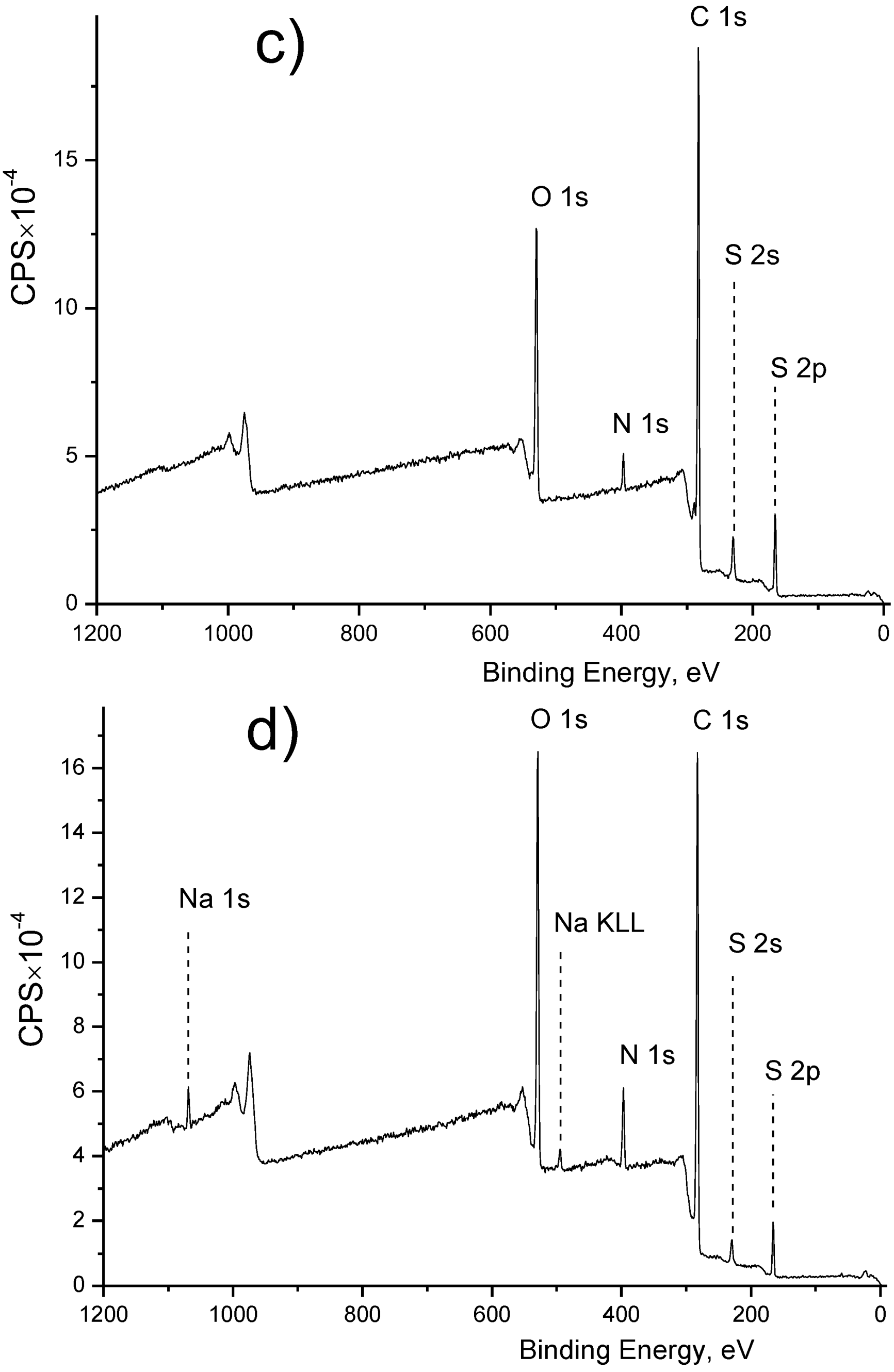
X-ray Photoelectron Spectroscopy (XPS) Analysis
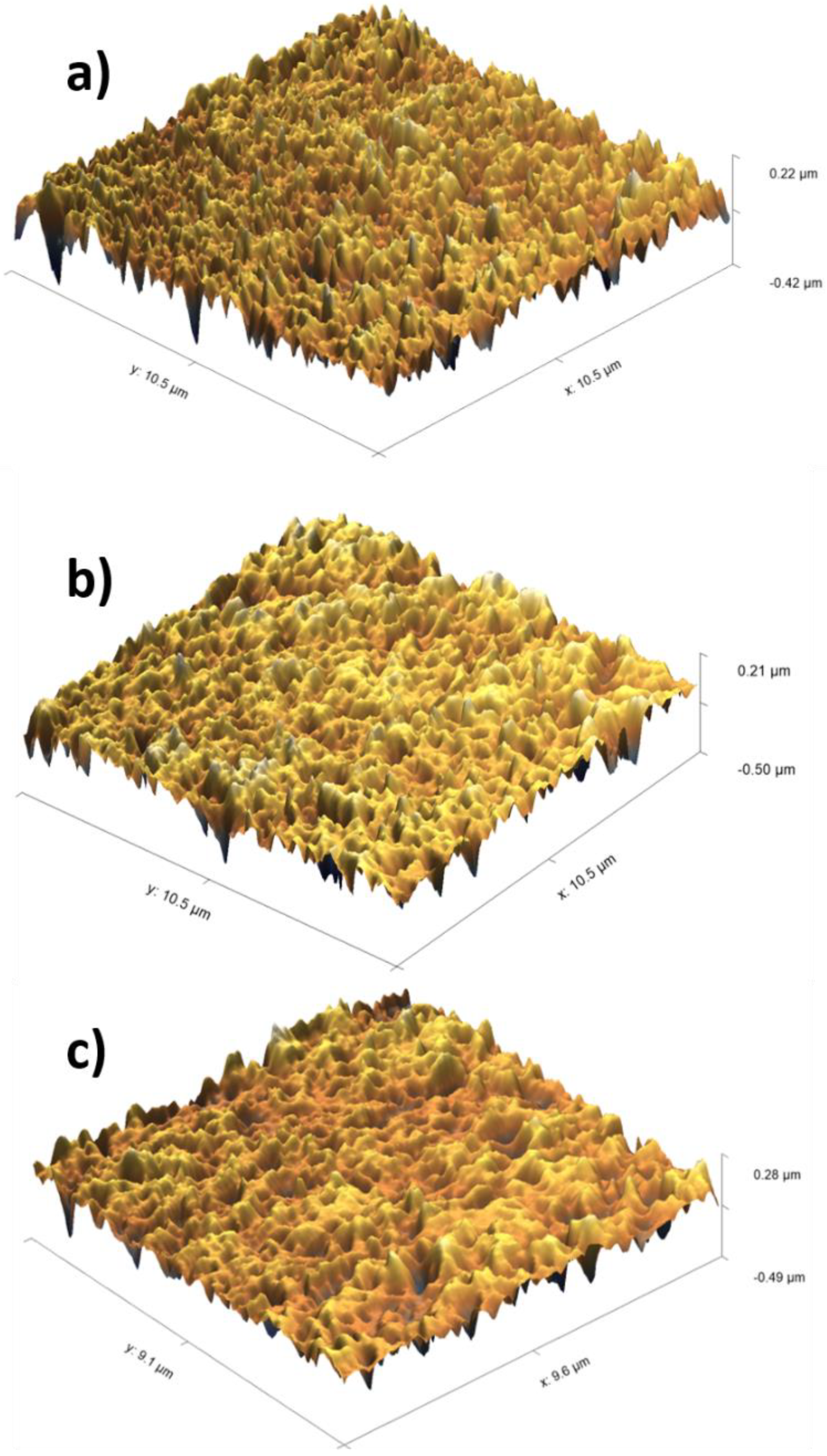
Atomic Force Microscopy (AFM) Analysis
UV–Visible Spectrometer Analysis
Surface Charge Measurement
Differential Scanning Calorimetry (DSC)
2.1.5. In Vitro Assessment of Inflammatory Biomarkers
2.1.6. Heparin Coating Stability Assessment
3. Results and Discussion
3.1. Membrane Surface Modification
3.2. Enhancement of Membrane Surface Roughness
3.3. Assessment of FB Depletion
3.4. Assessment of In Vitro FB Adsorption on Membrane Surface
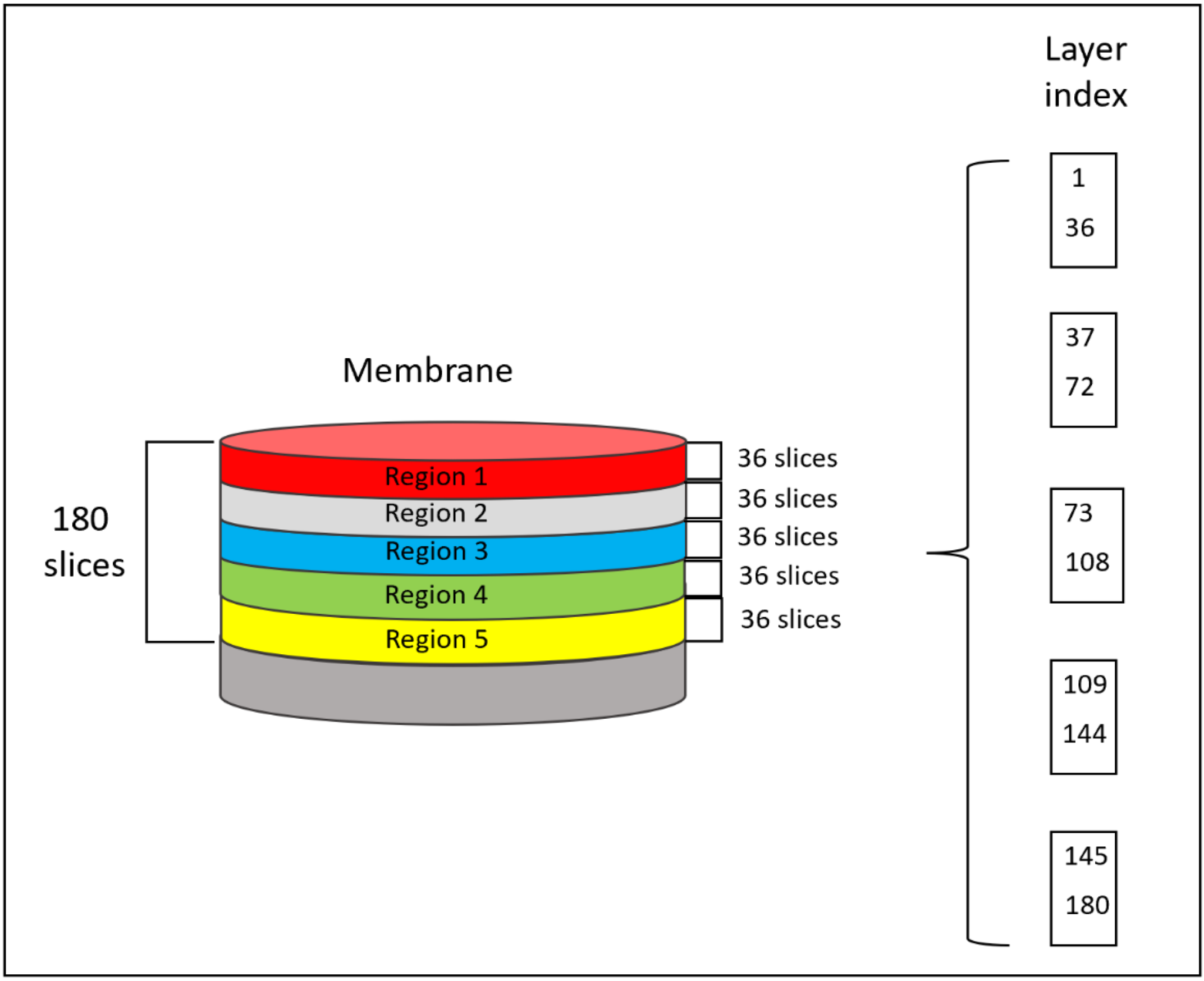
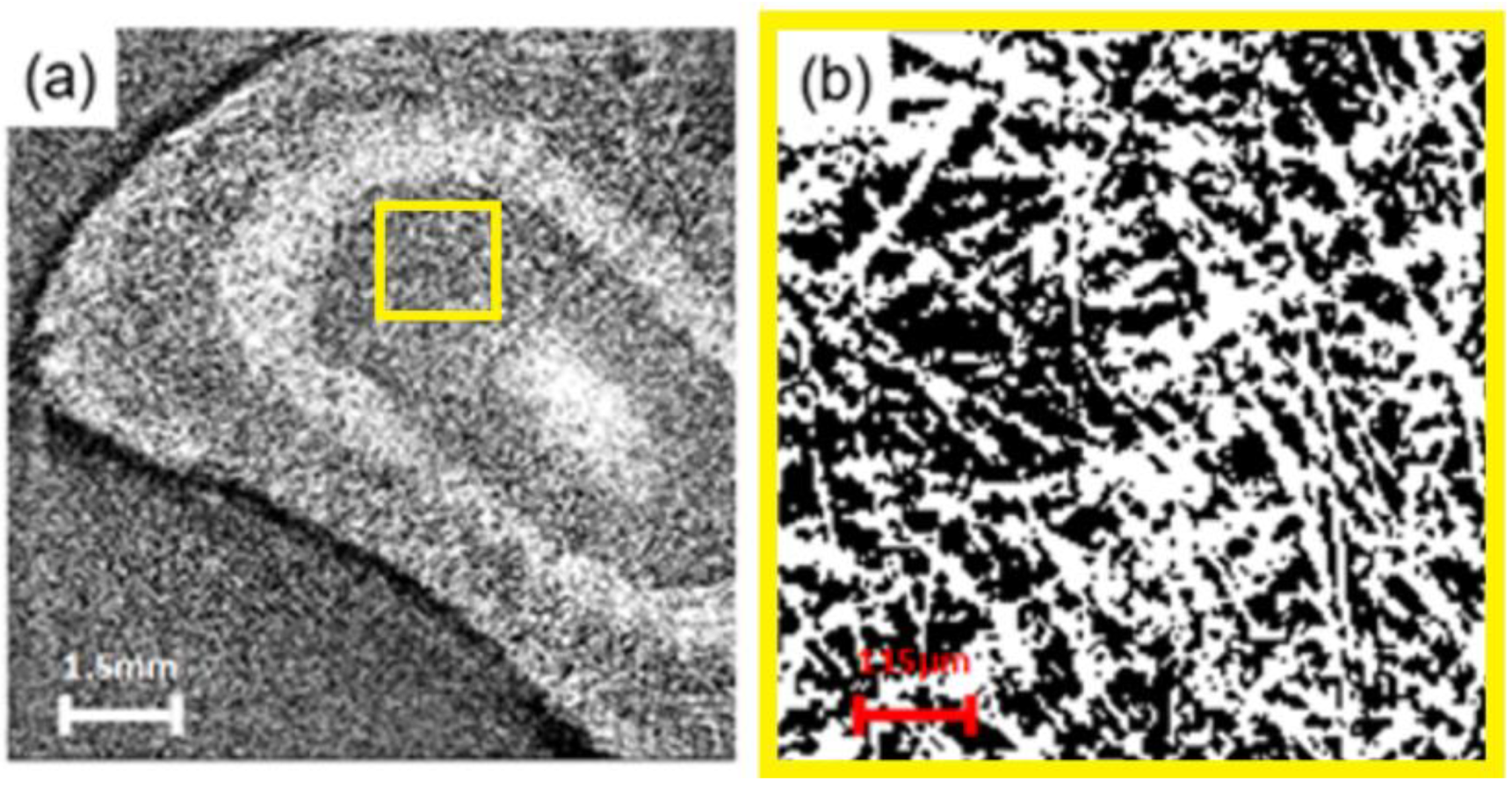
3.5. In Situ X-ray Synchrotron SR-μCT Imaging for Layer-by-Layer FB Adsorption Assessment
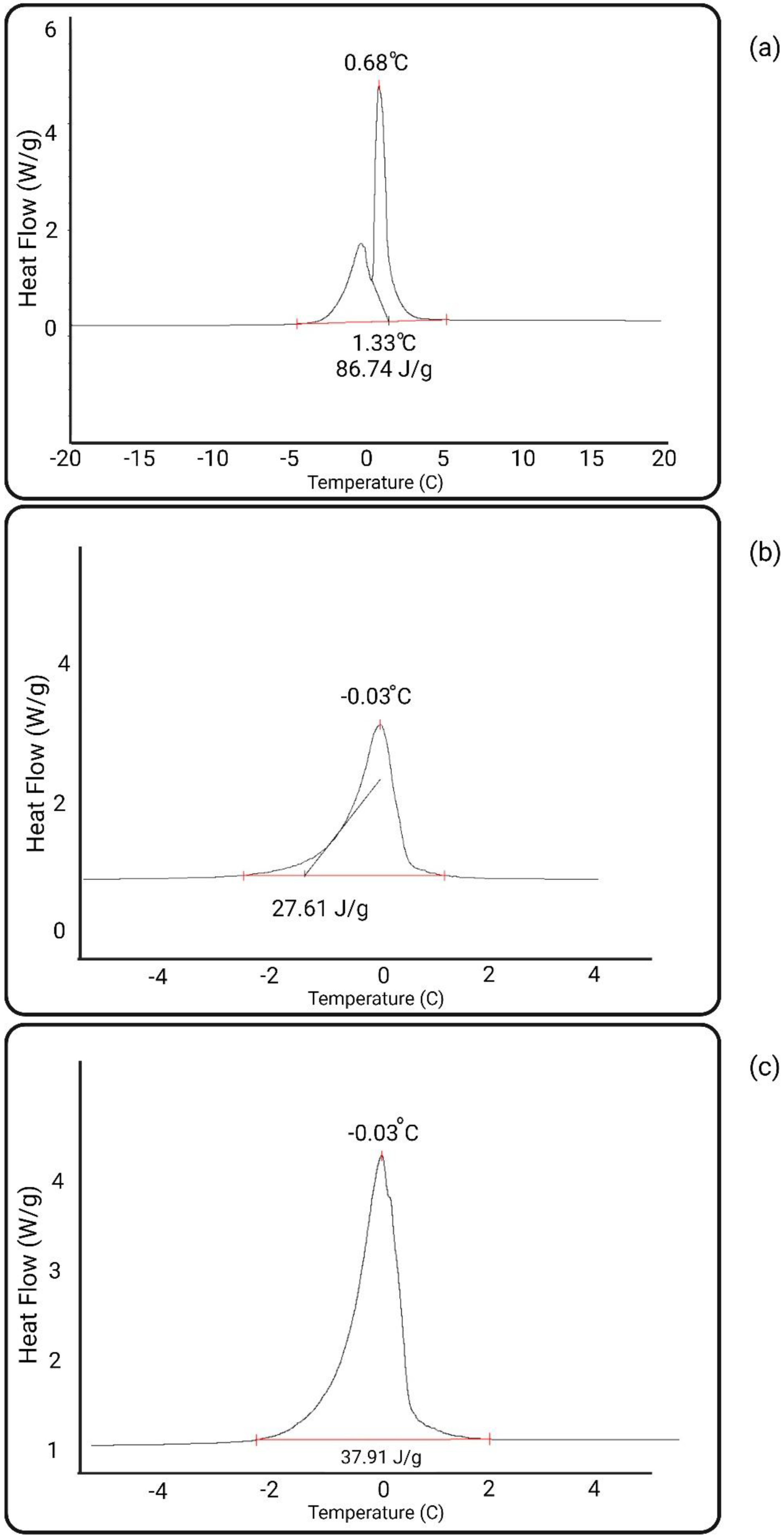
3.6. Assessment of Membranes’ Non-Freezable Water
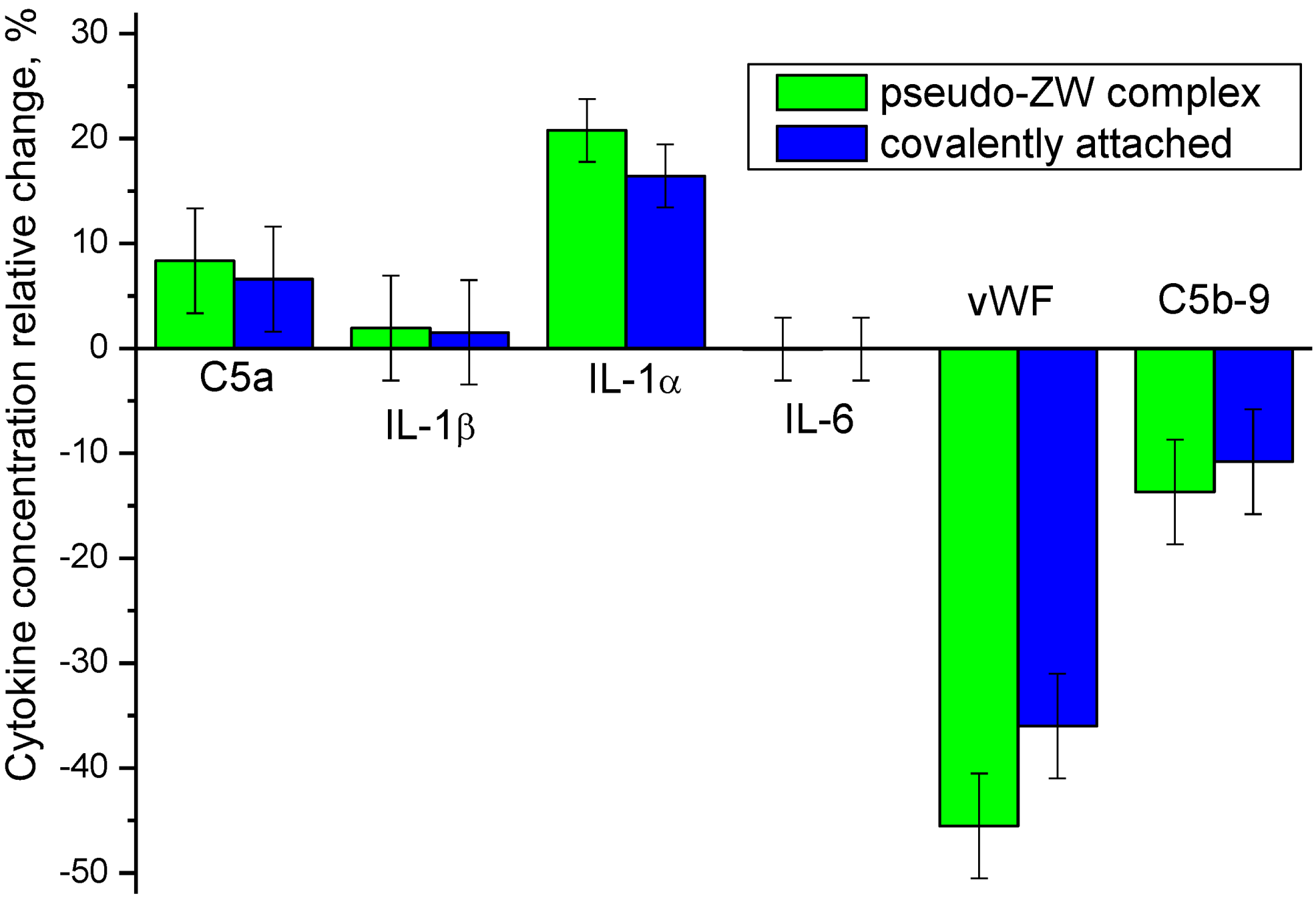
3.7. Ex Vivo Study of Inflammatory Biomarkers Released in Patients’ Blood
3.7.1. C5a
3.7.2. Interleukins
3.7.3. vWF
3.7.4. C5b9
3.8. Assessment of the Stability of Membrane Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Couser, W.G.; Remuzzi, G.; Mendis, S.; Tonelli, M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011, 80, 1258–1270. [Google Scholar] [PubMed]
- Yin, G.; Janson, J.C.; Liu, Z. Characterization of protein adsorption on membrane surface by enzyme linked immunoassay. J. Membr. Sci. 2000, 178, 99–105. [Google Scholar]
- Tong, J.; Liu, M.; Li, H.; Luo, Z.; Zhong, X.; Huang, J.; Liu, R.; He, F.; Fu, J. Mortality and associated risk factors in dialysis patients with cardiovascular disease. Kidney Blood Press. Res. 2016, 41, 479–487. [Google Scholar] [PubMed]
- Sarnak, M.J. Cardiovascular complications in chronic kidney disease. Am. J. Kidney Dis. 2003, 41, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Kanso, A.; Sedor, J.R. Sedor Chronic kidney disease and its complications. Prim. Care Clin. Off. Pract. 2008, 35, 329–344. [Google Scholar] [CrossRef]
- Saadati, S.; Eduok, U.; Westphalen, H.; Abdelrasoul, A.; Shoker, A.; Choi, P.; Doan, H.; Ein-Mozaffari, F.; Zhu, N. Assessment of polyethersulfone and polyacrylonitrile hemodialysis clinical membranes: In situ synchrotron-based imaging of human serum proteins adsorption, interaction analyses, molecular docking and clinical inflammatory biomarkers investigations. Mater. Today Commun. 2021, 29, 102928. [Google Scholar]
- Abdelrasoul, A.; Shoker, A. Induced hemocompatibility of polyethersulfone (PES) hemodialysis membrane using polyvinylpyrrolidone: Investigation on human serum fibrinogen adsorption and inflammatory biomarkers released. Chem. Eng. Res. Des. 2022, 177, 615–624. [Google Scholar]
- Westphalen, H.; Saadati, S.; Eduok, U.; Abdelrasoul, A.; Shoker, A.; Choi, P.; Doan, H.; Ein-Mozaffari, F. Case studies of clinical hemodialysis membranes: Influences of membrane morphology and biocompatibility on uremic blood-membrane interactions and inflammatory biomarkers. Sci. Rep. 2020, 10, 14808. [Google Scholar]
- Westphalen, H.; Abdelrasoul, A.; Shoker, A.; Zhu, N. Assessment of hemodialysis clinical practices using polyaryl ether sulfone-polyvinylpyrrolidone (PAES: PVP) clinical membrane: Modeling of in vitro fibrinogen adsorption, in situ synchrotron-based imaging, and clinical inflammatory biomarkers investigations. Sep. Purif. Technol. 2021, 259, 118136. [Google Scholar]
- Saadati, S.; Westphalen, H.; Eduok, U.; Abdelrasoul, A.; Shoker, A.; Choi, P.; Doan, H.; Ein-Mozaffari, F.; Zhu, N. Biocompatibility enhancement of hemodialysis membranes using a novel zwitterionic copolymer: Experimental, in situ synchrotron imaging, molecular docking, and clinical inflammatory biomarkers investigations. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 117, 111301. [Google Scholar]
- Borawski, J.; Naumnik, B.; Mysliwiec, M. Activation of hepatocyte growth factor/activin A/follistatin system during hemodialysis: Role of heparin. Kidney Int. 2003, 64, 2229–2237. [Google Scholar]
- Baird, C.W.; Zurakowski, D.; Robinson, B.; Gandhi, S.; Burdis-Koch, L.; Tamblyn, J.; Munoz, R.; Fortich, K.; Pigula, F.A. Anticoagulation and pediatric extracorporeal membrane oxygenation: Impact of activated clotting time and heparin dose on survival. Ann. Thorac. Surg. 2007, 83, 912–919; discussion 919–920. [Google Scholar]
- Lulev, C.; Ambrosch, S.W.; Neumann, K.H. Anticoagulation with heparin during hemodialysis causes pronounced LDL alterations. Atherosclerosis 1997, 134, 225. [Google Scholar]
- Evenepoel, P.; Dejagere, T.; Verhamme, P.; Claes, K.; Kuypers, D.; Bammens, B.; Vanrenterghem, Y. Heparin-coated polyacrylonitrile membrane versus regional citrate anticoagulation: A prospective randomized study of 2 anticoagulation strategies in patients at risk of bleeding. Am. J. Kidney Dis. 2007, 49, 642–649. [Google Scholar]
- Laville, M.; Dorval, M.; Fort Ros, J.; Fay, R.; Cridlig, J.; Nortier, J.L.; Juillard, L.; Debska-Slizien, A.; Fernandez Lorente, L.; Thibaudin, D.; et al. Results of the HepZero study comparing heparin-grafted membrane and standard care show that heparin-grafted dialyzer is safe and easy to use for heparin-free dialysis. Kidney Int. 2014, 86, 1260–1267. [Google Scholar]
- Zea, N.; Menard, G.; Le, L.; Luo, Q.; Bazan, H.A.; Sternbergh, W.C., 3rd; Smith, T.A. Heparin-Bonded Polytetrafluorethylene Does Not Improve Hemodialysis Arteriovenous Graft Function. Ann. Vasc. Surg. 2016, 30, 28–33. [Google Scholar]
- Dumont, G. C0281 Heparin-induced thrombocytopenia (HIT) type 2 caused by preventive anticoagulation of the circuit of hemodialysis (HD): A case report. Thromb. Res. 2012, 130, S185. [Google Scholar]
- Glick, D.; Dzierba, A.L.; Abrams, D.; Muir, J.; Eisenberger, A.; Diuguid, D.; Abel, E.; Agerstrand, C.; Bacchetta, M.; Brodie, D. Clinically suspected heparin-induced thrombocytopenia during extracorporeal membrane oxygenation. J. Crit. Care 2015, 30, 1190–1194. [Google Scholar]
- Pham, P.T.; Miller, J.M.; Demetrion, G.; Lew, S.Q. Clotting by Heparin of Hemoaccess for Hemodialysis in an End-Stage Renal Disease Patient. Am. J. Kidney Dis. 1995, 25, 642–647. [Google Scholar]
- Doi, Y.; Koga, K.; Sugioka, S.; Inoue, Y.; Arisato, T.; Nishioka, K.; Ishihara, T.; Sugawara, A. Heparin-induced thrombocytopenia among incident hemodialysis patients anticoagulated with low molecular weight heparin: A single-center retrospective study. Nefrología 2021, 41, 356–358. [Google Scholar]
- Welp, H.; Ellger, B.; Scherer, M.; Lanckohr, C.; Martens, S.; Gottschalk, A. Heparin-induced thrombocytopenia during extracorporeal membrane oxygenation. J. Cardiothorac. Vasc. Anesth. 2014, 28, 342–344. [Google Scholar] [PubMed]
- Yamamoto, S.; Koide, M.; Matsuo, M.; Suzuki, S.; Ohtaka, M.; Saika, S.; Matsuo, T. Heparin-Induced Thrombocytopenia in Hemodialysis Patients. Am. J. Kidney Dis. 1996, 28, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Nasstrom, B.; Olivecrona, G.; Olivecrona, T.; Stegmayr, B.G. Lipoprotein lipase during heparin infusion: Lower activity in hemodialysis patients. Scand. J. Clin. Lab. Investig. 2003, 63, 45–53. [Google Scholar]
- Blossom, D.B.; Kallen, A.J.; Patel, P.R.; Elward, A.; Robinson, L.; Gao, G.; Langer, R.; Perkins, K.M.; Jaeger, J.L.; Kurkjian, K.M.; et al. Outbreak of Adverse Reactions Associated with Contaminated Heparin. N. Engl. J. Med. 2008, 329, 2674–2684. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.N.; Ho, K.; Cheung, R.C.K.; Lit, L.C.W.; Lee, S.K.M.; Fung, K.S.; Tong, M.K.L.; Lam, C.W.K. Effect of low molecular weight heparin on bone metabolism and hyperlipidemia in patients on maintenance hemodialysis. Int. J. Artif. Organs 2001, 24, 447–455. [Google Scholar]
- Edes, T.E.; Sunderrajan, E.V. Heparin-Induced Hyperkalemia. Arch. Intern. Med. 1985, 145, 1070–1072. [Google Scholar] [CrossRef]
- Diskin, C.J.; Stokes, T.J.; Dansby, L.M.; Radcliff, L.; Carter, T.B. Is systemic heparin a risk factor for catheter-related sepsis in dialysis patients? An evaluation of various biofilm and traditional risk factors. Nephron Clin. Pract. 2007, 107, c128–c132. [Google Scholar]
- Humphries, J.E.; Kaplan, D.M.; Bolton, W.K. Heparin Skin Necrosis: Delayed Occurrence in a Patient on Hemodialysis. Am. J. Kidney Dis. 1991, 17, 233–236. [Google Scholar]
- Islam, M.S.; Hassan, Z.A.; Chalmin, F.; Vido, S.; Berrada, M.; Verhelst, D.; Donnadieu, P.; Moranne, O.; Esnault, V.L. Vitamin E-Coated and Heparin-Coated Dialyzer Membranes for Heparin-Free Hemodialysis: A Multicenter, Randomized, Crossover Trial. Am. J. Kidney Dis. 2016, 68, 752–762. [Google Scholar]
- Guo, J.; Li, K.; Ning, C.; Liu, X. Improved cellular bioactivity by heparin immobilization on polycarbonate film via an aminolysis modification for potential tendon repair. Int. J. Biol. Macromol. 2020, 142, 835–845. [Google Scholar]
- Warkentin, T.E. Hemodialysis-associated acute systemic reactions and heparin-induced thrombocytopenia. Thromb. Res. 2012, 129, 405–406. [Google Scholar] [CrossRef]
- Baudeau, C.; Fitt, H. Heparin and Urokinase in Situ for Partial Thrombosis of Indwelling Hemodialysis Catheters. Am. J. Kidney Dis. 1993, 22, 622. [Google Scholar] [CrossRef]
- Mureebe, L.; Coats, R.D.; Silliman, W.R. Heparin-associated antiplatelet antibodies increase morbidity and mortality in hemodialysis patients. J. Vasc. Surg. 2005, 41, 560. [Google Scholar] [CrossRef][Green Version]
- Carrier, M.; Rodger, M.A.; Fergusson, D.; Doucette, S.; Kovacs, M.J.; Moore, J.; Kelton, J.G.; Knoll, G.A. Increased mortality in hemodialysis patients having specific antibodies to the platelet factor 4-heparin complex. Kidney Int. 2008, 73, 213–219. [Google Scholar] [CrossRef]
- Novak, M.; Cvitkovic, M.; Galic, S.; Luetic, T.; Cavar, S.; Puretic, Z. The life-threatening hemodialysis catheter heparin lock caused bleeding in a child after peritoneal catheter removal. J. Pediatr. Surg. 2008, 43, E41–E44. [Google Scholar] [CrossRef]
- Sela, S.; Shurtz-Swirski, R.; Shapiro, G.; Nasser, L.; Hamzi, M.; Shasha, S.M.; Kristal, B. Oxidative stress during hemodialysis: Effect of heparin. Kidney Int. Suppl. 2001, 78, S159–S163. [Google Scholar]
- Carrier, M.; Knoll, G.A.; Kovacs, M.J.; Moore, J.C.; Fergusson, D.; Rodger, M.A. The prevalence of antibodies to the platelet factor 4 -heparin complex and association with access thrombosis in patients on chronic hemodialysis. Thromb. Res. 2007, 120, 215–220. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Amin, T.; Amin, A.; Nashawi, M.; Haloot, J.; Fichardt, H.; Prasad, A.; Almomani, A. Valve-in-Valve Transcatheter Aortic-Valve Replacement with Basilica in a Patient with History of Evans Syndrome, Heparin Induced Thrombocytopenia and Hemodialysis. Is It Even Possible? J. Am. Coll. Cardiol. 2021, 77, 2357. [Google Scholar]
- Liu, P.; Chen, Q.; Li, L.; Lin, S.; Shen, J. Anti-biofouling ability and cytocompatibility of the zwitterionic brushes-modified cellulose membrane. J. Mater. Chem. B 2014, 2, 7222–7231. [Google Scholar] [CrossRef]
- Choi, S.-Y.; Li, M.-X.; Kang, J.H.; Yang, D.H.; Joung, Y.K. Anti-thrombotic polymer surfaces modified with zwitterionic and fluorinated surface-migrating oligomers. Surf. Interfaces 2021, 25, 101280. [Google Scholar] [CrossRef]
- Venault, A.; Wei, T.-C.; Shih, H.-L.; Yeh, C.-C.; Chinnathambi, A.; Alharbi, S.A.; Carretier, S.; Aimar, P.; Lai, J.-Y.; Chang, Y. Antifouling pseudo-zwitterionic poly(vinylidene fluoride) membranes with efficient mixed-charge surface grafting via glow dielectric barrier discharge plasma-induced copolymerization. J. Membr. Sci. 2016, 516, 13–25. [Google Scholar]
- Cai, N.; Li, Q.; Zhang, J.; Xu, T.; Zhao, W.; Yang, J.; Zhang, L. Antifouling zwitterionic hydrogel coating improves hemocompatibility of activated carbon hemoadsorbent. J. Colloid. Interface Sci. 2017, 503, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Erathodiyil, N.; Chan, H.-M.; Wu, H.; Ying, J.Y. Zwitterionic polymers and hydrogels for antibiofouling applications in implantable devices. Mater. Today 2020, 38, 84–98. [Google Scholar] [CrossRef]
- Venault, A.; Ye, C.C.; Lin, Y.C.; Tsai, C.W.; Jhong, J.F.; Ruaan, R.C.; Higuchi, A.; Chinnathambi, A.; Ho, H.T.; Chang, Y. Zwitterionic fibrous polypropylene assembled with amphiphatic carboxybetaine copolymers for hemocompatible blood filtration. Acta Biomater. 2016, 40, 130–141. [Google Scholar]
- Hoseinpour, V.; Ghaee, A.; Vatanpour, V.; Ghaemi, N. Surface modification of PES membrane via aminolysis and immobilization of carboxymethylcellulose and sulphated carboxymethylcellulose for hemodialysis. Carbohydr. Polym. 2018, 188, 37–47. [Google Scholar]
- Remigy, J.-C.; Meireles, M. Assessment of pore geometry and 3-D architecture of filtration membranes by synchrotron radiation computed microtomography. Desalination 2006, 199, 501–503. [Google Scholar]
- Fairley, N. Systematic and Collaborative Approach to Problem Solving using X-ray Photoelectron Spectroscopy. Appl. Surf. Sci. Adv. 2021, 5, 100112. [Google Scholar]
- Abdelrasoul, A.; Westphalen, H.; Saadati, S.; Shoker, A. Hemodialysis biocompatibility mathematical models to predict the inflammatory biomarkers released in dialysis patients based on hemodialysis membrane characteristics and clinical practices. Sci. Rep. 2021, 11, 23080. [Google Scholar]
- Robbins, K.C. Hemolysis during hemodialysis: Etiology, identification, interventions, and acute and long-term sequalae. Nephrol. Nurs. J. 2017, 44, 546–550. [Google Scholar]
- Niwa, T.; Yazawa, T.; Kodama, T.; Uehara, Y.; Maeda, K.; Yamada, K. Efficient removal of albumin-bound furancarboxylic acid, an inhibitor of erythropoiesis, by continuous ambulatory peritoneal dialysis. Nephron 1990, 56, 241–245. [Google Scholar]
- Dou, L.; Bertrand, E.; Cerini, C.; Faure, V.; Sampol, J.; Vanholder, R.; Berland, Y.; Brunet, P. The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int. 2004, 65, 442–451. [Google Scholar]
- Owen, W.F., Jr.; Lew, N.L.; Liu, Y.; Lowrie, E.G.; Lazarus, J.M. The urea reduction ratio and serum albumin concentration as predictors of mortality in patients undergoing hemodialysis. N. Engl. J. Med. 1993, 329, 1001–1006. [Google Scholar]
- Foley, R.N.; Parfrey, P.S.; Harnett, J.D.; Kent, G.M.; Murray, D.C.; Barre, P.E. Hypoalbuminemia, cardiac morbidity, and mortality in end-stage renal disease. J. Am. Soc. Nephrol. 1996, 7, 728–736. [Google Scholar] [CrossRef]
- Osicka, T.M.; Houlihan, C.A.; Chan, J.G.; Jerums, G.; Comper, W.D. Albuminuria in patients with type 1 diabetes is directly linked to changes in the lysosome-mediated degradation of albumin during renal passage. Diabetes 2000, 49, 1579–1584. [Google Scholar]
- Kaysen, G.A. Biological basis of hypoalbuminemia in ESRD. J. Am. Soc. Nephrol. 1998, 9, 2368–2376. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.H.; Goodnough, L.T. How I use fibrinogen replacement therapy in acquired bleeding. Blood 2015, 125, 1387–1393. [Google Scholar] [PubMed]
- Rahmati, M.; Mozafari, M. Protein adsorption on polymers. Mater. Today Commun. 2018, 17, 527–540. [Google Scholar] [CrossRef]
- Whitford, D. Protein expression, purification and characterization. In Proteins Structure and Function; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2005; Volume 314. [Google Scholar]
- Chenoweth, D.E. The properties of human C5a anaphylatoxin. The significance of C5a formation during hemodialysis. Contrib. Nephrol. 1987, 59, 51–71. [Google Scholar]
- Kourtzelis, I.; Markiewski, M.M.; Doumas, M.; Rafail, S.; Kambas, K.; Mitroulis, I.; Panagoutsos, S.; Passadakis, P.; Vargemezis, V.; Magotti, P. Complement anaphylatoxin C5a contributes to hemodialysis-associated thrombosis. Blood J. Am. Soc. Hematol. 2010, 116, 631–639. [Google Scholar]
- Pertosa, G.; Grandaliano, G.; Gesualdo, L.; Schena, F.P. Clinical relevance of cytokine production in hemodialysis. Kidney Int. 2000, 58, S104–S111. [Google Scholar] [CrossRef]
- Pertosa, G.; Gesualdo, L.; Bottalico, D.; Schena, F. Endotoxins modulate chronically tumour necrosis factor α and interleukin 6 release by uraemic monocytes. Nephrol. Dial. Transplant. 1995, 10, 328–333. [Google Scholar] [PubMed]
- Sioulis, A.; Malindretos, P.; Makedou, A.; Makris, P.; Grekas, D. Coagulation factors as biological risk markers of endothelial dysfunction. Association with the thrombotic episodes of chronic hemodialysis patients. Hippokratia 2009, 13, 237. [Google Scholar] [PubMed]
- Abdelrasoul, A.; Shoker, A. POS-600 Investigations on the Impact of Hemodialysis Clinical Practices on Human Plasma Proteins Loss and von Willebrand factor. Kidney Int. Rep. 2022, 7, S258. [Google Scholar]
- Lenting, P.J.; Christophe, O.D.; Denis, C.V. von Willebrand factor biosynthesis, secretion, and clearance: Connecting the far ends. Blood J. Am. Soc. Hematol. 2015, 125, 2019–2028. [Google Scholar]
- Péquériaux, N.C.; Fijnheer, R.; Gemen, E.F.; Barendrecht, A.D.; Dekker, F.W.; Krediet, R.T.; Beutler, J.J.; Boeschoten, E.W.; Roest, M. Plasma concentration of von Willebrand factor predicts mortality in patients on chronic renal replacement therapy. Nephrol. Dial. Transplant. 2012, 27, 2452–2457. [Google Scholar]
- Kizhakkedathu, J.N.; Conway, E.M. Biomaterial and cellular implants: Foreign surfaces where immunity and coagulation meet. Blood J. Am. Soc. Hematol. 2022, 139, 1987–1998. [Google Scholar]
- Pertosa, G.; Tarantino, E.A.; Gesualdo, L.; Montinaro, V.; Schena, F.P. C5b-9 generation and cytokine production in hemodialyzed patients. Kidney Int. Suppl. 1993, 41, S221–S225. [Google Scholar]
- Shahidi, M. Thrombosis and von Willebrand Factor. Adv. Exp. Med. Biol. 2017, 906, 285–306. [Google Scholar]
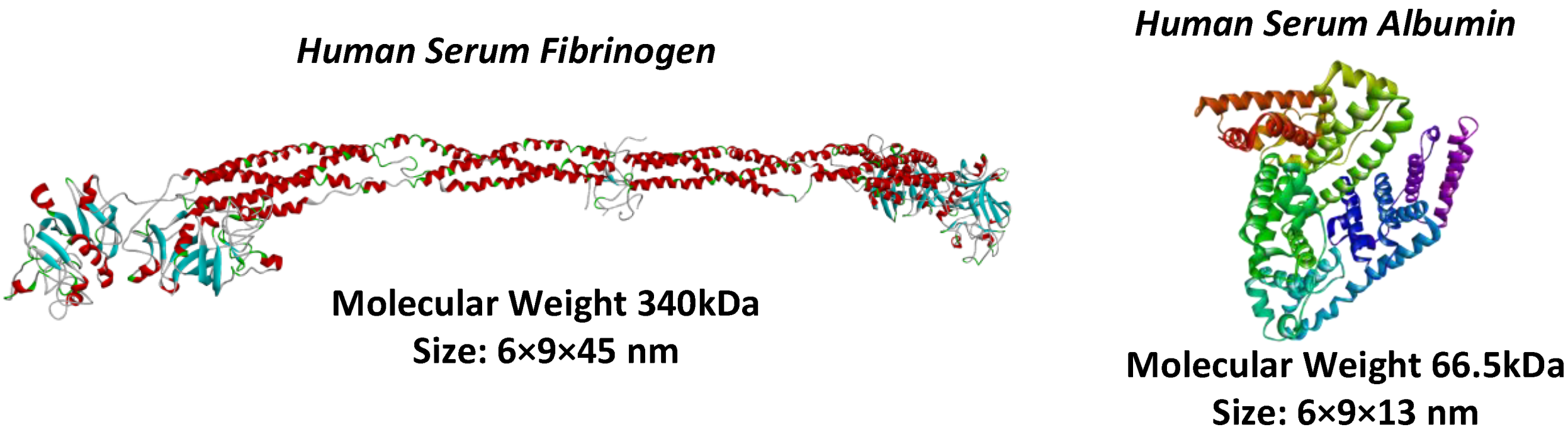
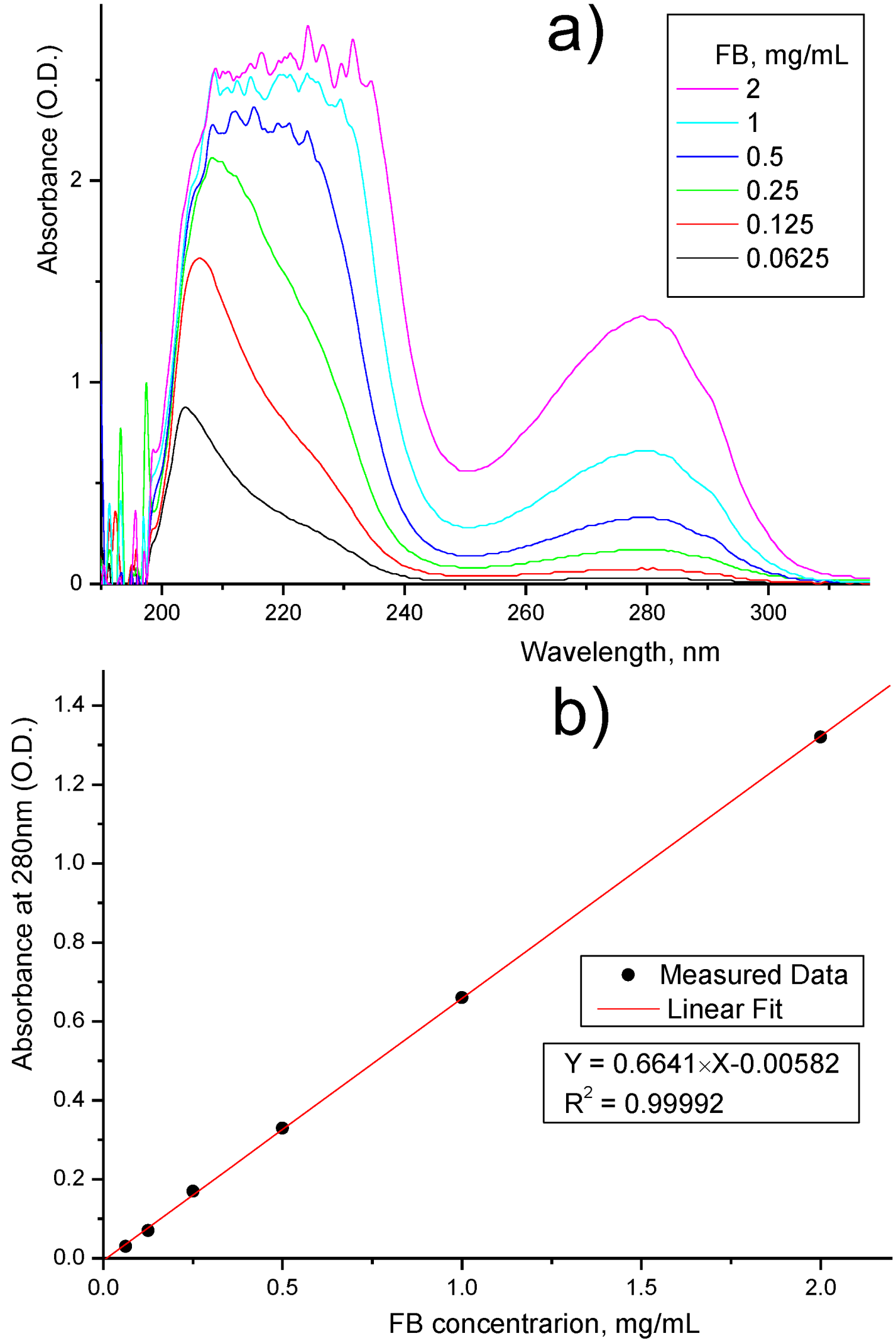
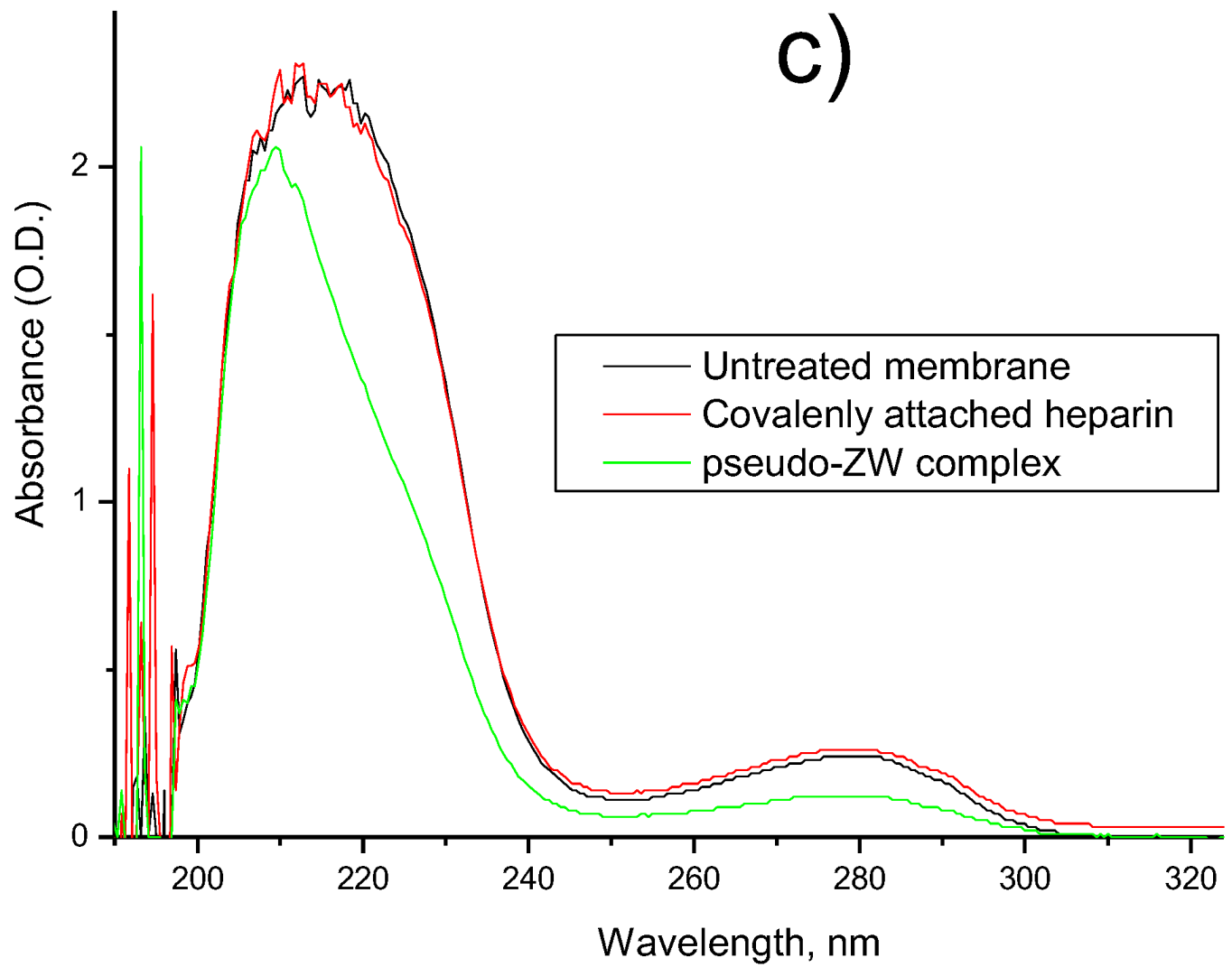
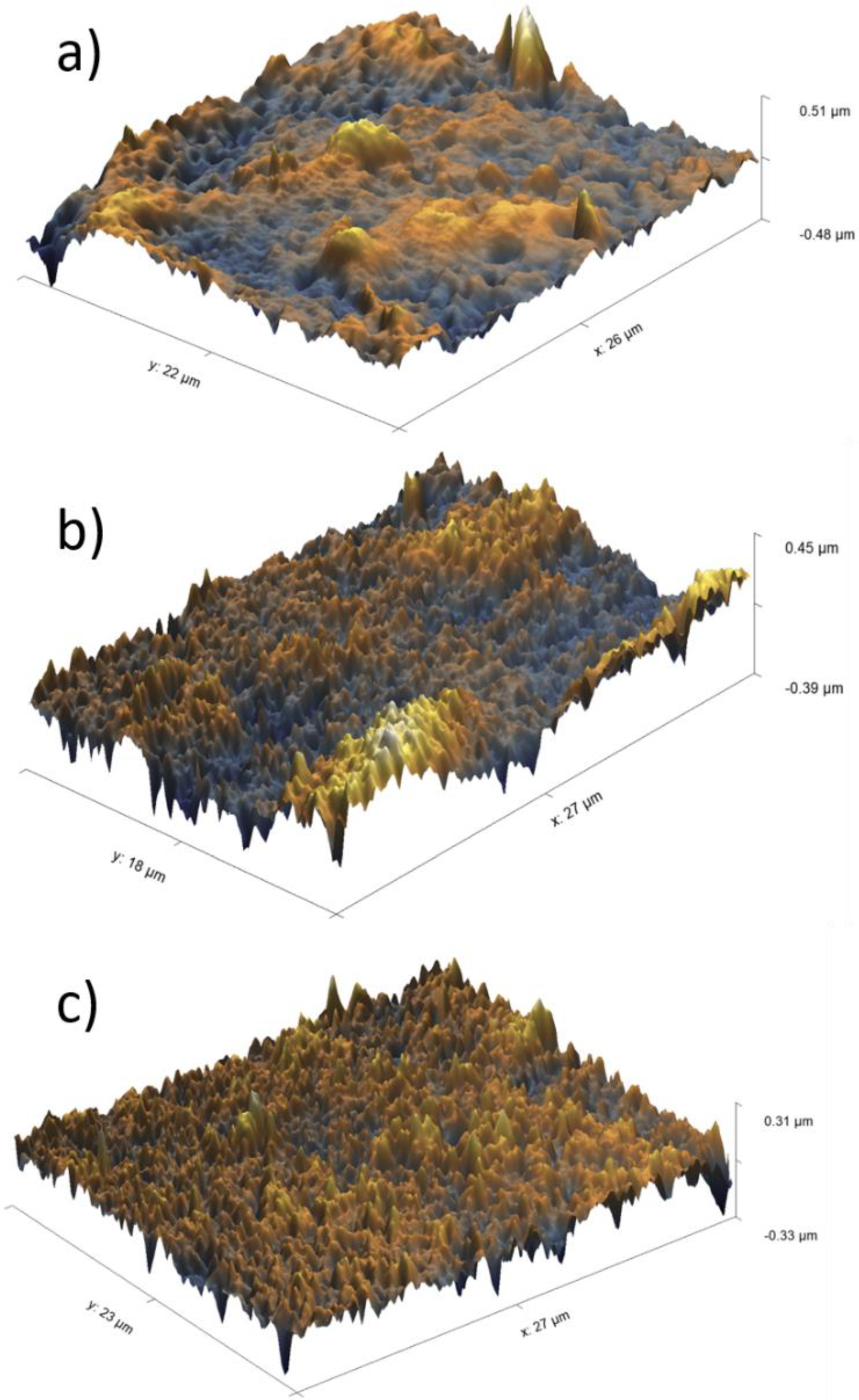
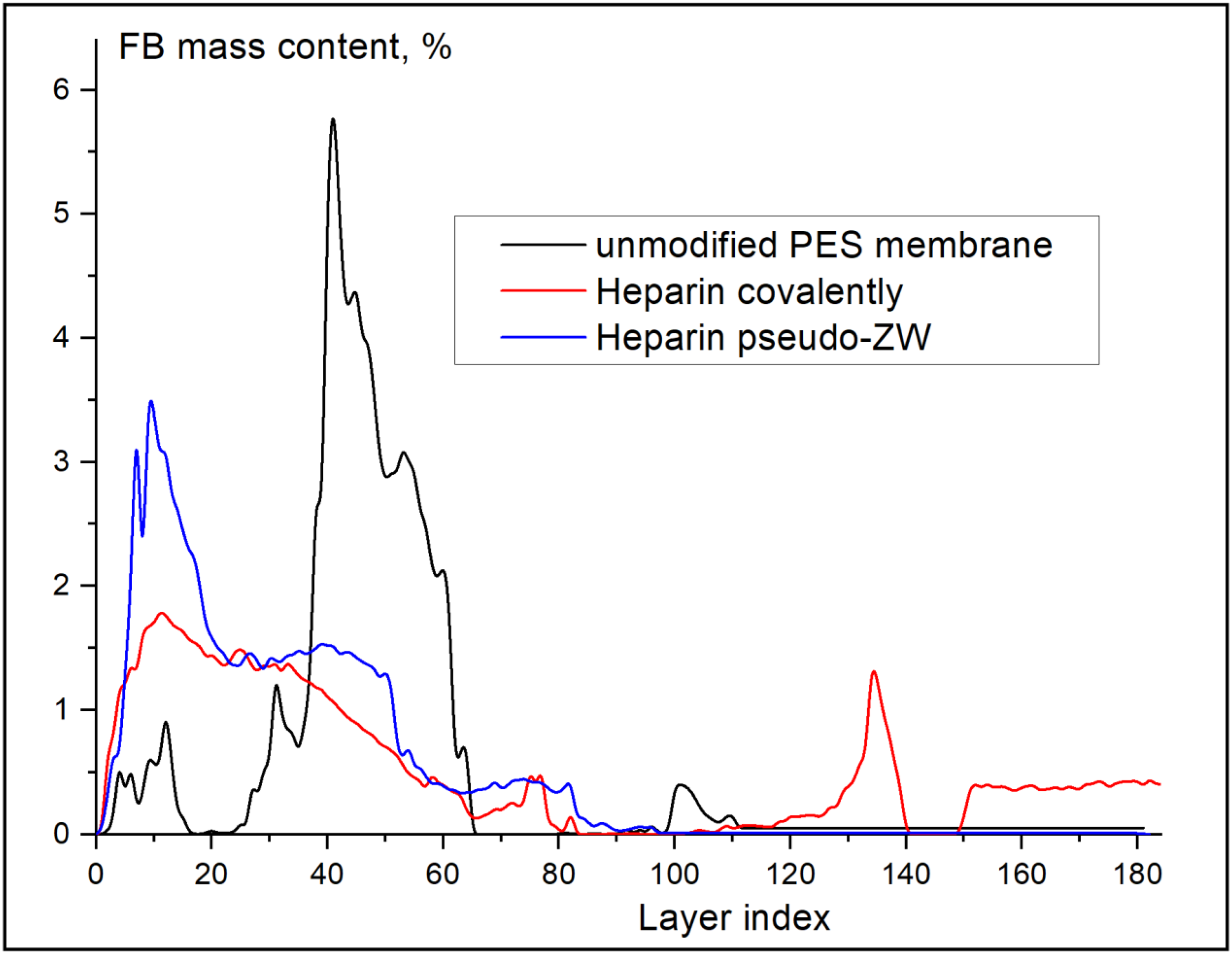
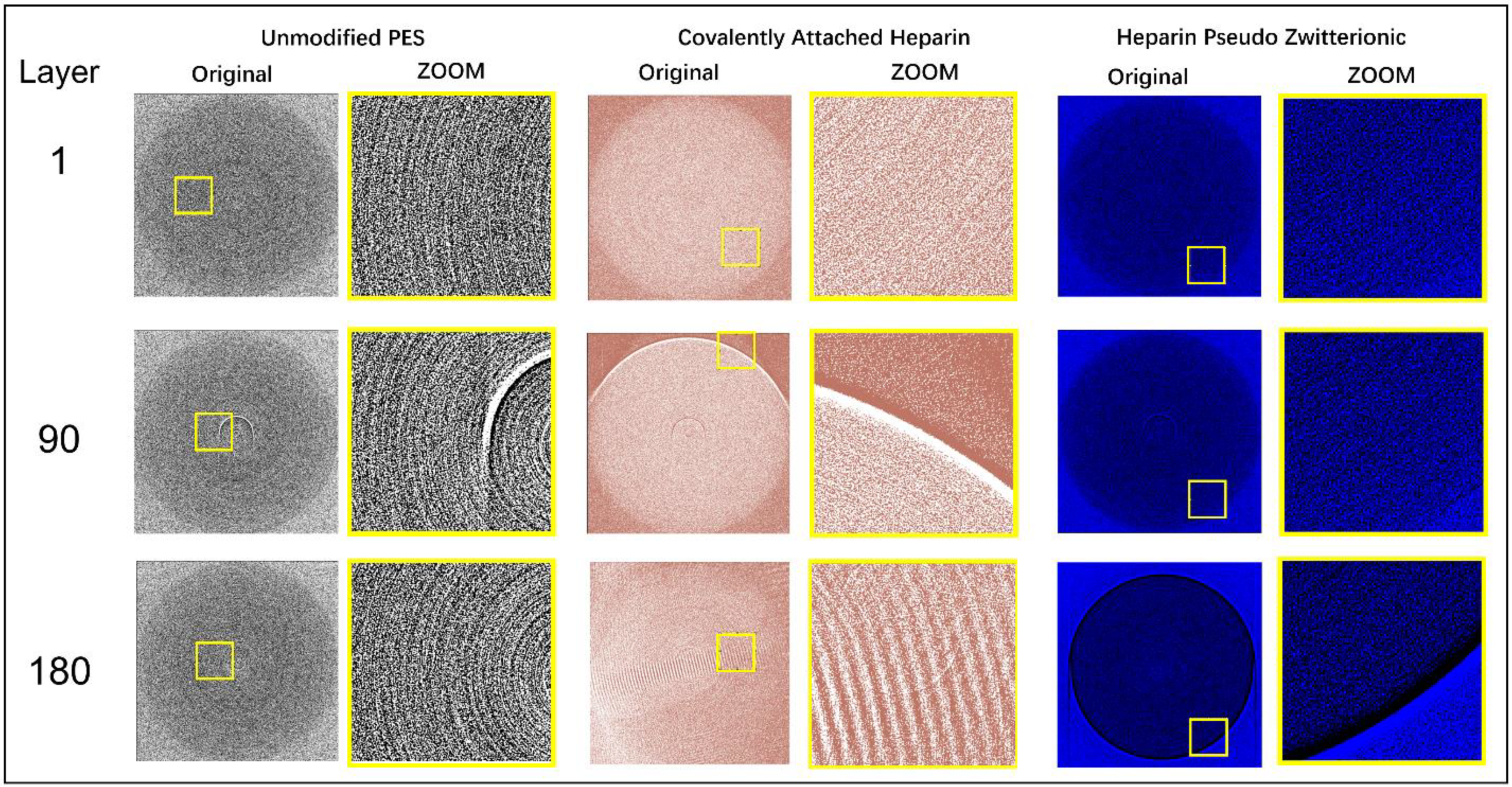
| Membrane | FB Concentration in Injected Solution, mg/mL | FB Concentration in Permeate (Based on UV Analysis), mg/mL | FB Retention, % |
|---|---|---|---|
| Unmodified PES | 2 | 0.374 | 81.3 |
| Covalently attached heparin | 0.405 | 79.8 | |
| Heparin–pseudo-ZW | 0.190 | 90.5 |
| Membrane | Non-Freezable Water (%) | Freezing Point (°C) |
|---|---|---|
| PES | 2.84 | 0.68 |
| PES Heparin–pZW | 32.22 | −0.03 |
| PES Covalent Heparin | 30.99 | −0.03 |
| Membrane | Cytokine Concentration, pg/mL | |||||
|---|---|---|---|---|---|---|
| C5a | IL-1β | IL-1α | IL-6 | vWF | C5b-9 | |
| Unmodified PES | 139,502 | 4.861 | 5.278 | 12.653 | 497 | 3806 |
| Heparin attached covalently | 148,726 | 4.936 | 6.146 | 12.645 | 318 | 3395 |
| Heparin–pseudo-ZW | 151,169 | 4.956 | 6.376 | 12.643 | 270 | 3286 |
| Filtration Time, Hours | PES Heparin–pZW | PES Heparin Covalently |
|---|---|---|
| 0 | −13 ± 2.66 | −9.16 ± 2.18 |
| 1 | −7.59 ± 0.448 | −5.69 ± 1.57 |
| 2 | −11.2 ± 0.647 | −8.84 ± 1.56 |
| 4 | 5.66 ± 1.30 | 5.09 ± 1.21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalugin, D.; Bahig, J.; Shoker, A.; Abdelrasoul, A. Heparin-Immobilized Polyethersulfone for Hemocompatibility Enhancement of Dialysis Membrane: In Situ Synchrotron Imaging, Experimental, and Ex Vivo Studies. Membranes 2023, 13, 718. https://doi.org/10.3390/membranes13080718
Kalugin D, Bahig J, Shoker A, Abdelrasoul A. Heparin-Immobilized Polyethersulfone for Hemocompatibility Enhancement of Dialysis Membrane: In Situ Synchrotron Imaging, Experimental, and Ex Vivo Studies. Membranes. 2023; 13(8):718. https://doi.org/10.3390/membranes13080718
Chicago/Turabian StyleKalugin, Denis, Jumanah Bahig, Ahmed Shoker, and Amira Abdelrasoul. 2023. "Heparin-Immobilized Polyethersulfone for Hemocompatibility Enhancement of Dialysis Membrane: In Situ Synchrotron Imaging, Experimental, and Ex Vivo Studies" Membranes 13, no. 8: 718. https://doi.org/10.3390/membranes13080718
APA StyleKalugin, D., Bahig, J., Shoker, A., & Abdelrasoul, A. (2023). Heparin-Immobilized Polyethersulfone for Hemocompatibility Enhancement of Dialysis Membrane: In Situ Synchrotron Imaging, Experimental, and Ex Vivo Studies. Membranes, 13(8), 718. https://doi.org/10.3390/membranes13080718






