Improving PFSA Membranes Using Sulfonated Nanodiamonds
Abstract
1. Introduction
2. Experimental Procedure
2.1. Sample Preparation
2.1.1. Sulfonation of Nanodiamonds
2.1.2. Preparation of Compositional Membranes
2.2. Methods of Investigation
2.2.1. Energy-Dispersive X-ray Analysis
2.2.2. Fourier-Transform Infrared (FTIR) Spectroscopy
2.2.3. ζ-Metric Titrations
2.2.4. Proton Conductivity Measurements
2.2.5. Stress-Strain Mechanical Tests
2.2.6. Small-Angle Neutron Scattering
2.2.7. Scanning Electron Microscopy
3. Results and Discussions
3.1. Identification of Sulfonated Nanodiamonds
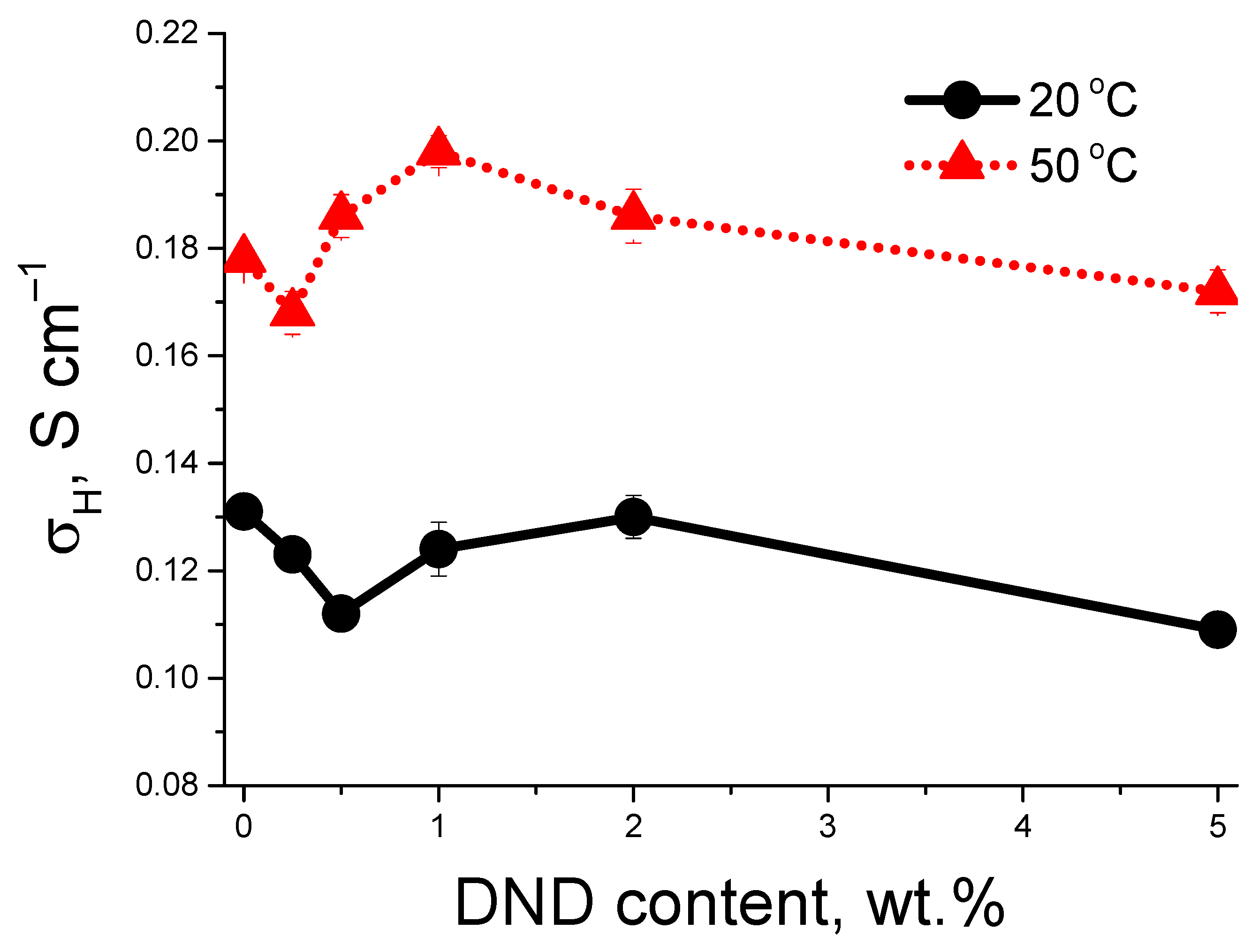
3.2. Proton Conductivity of the Membranes
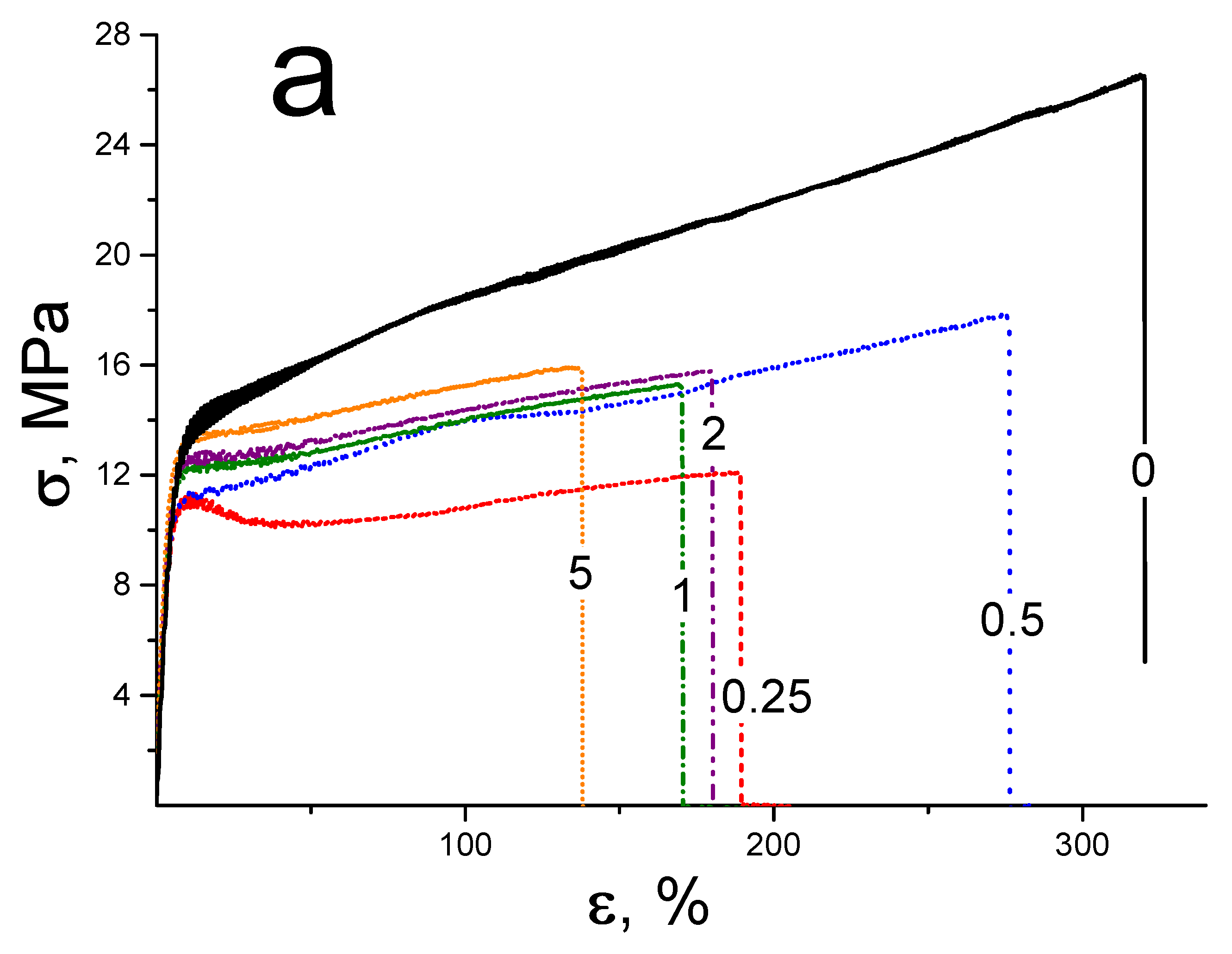
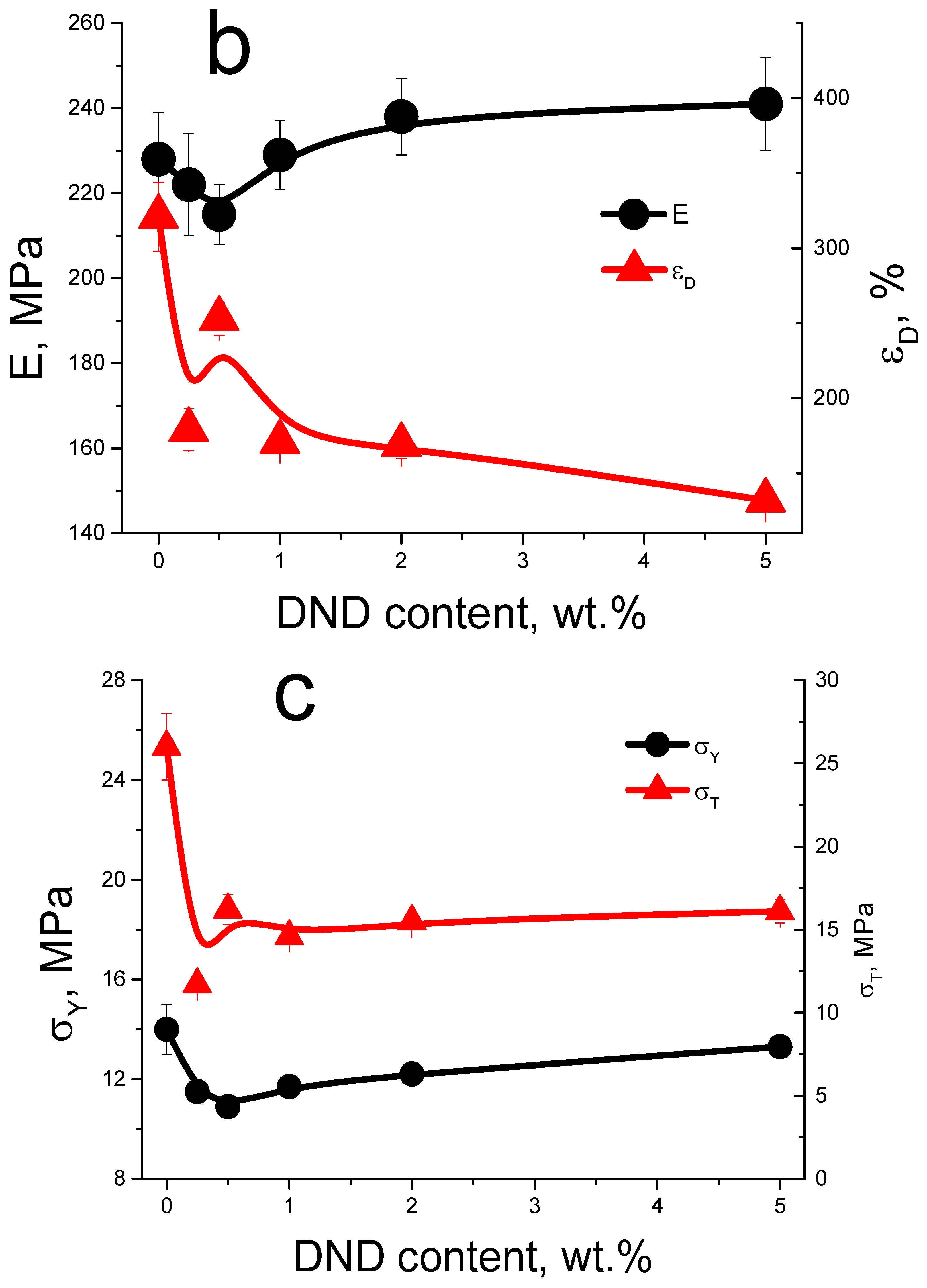
3.3. Mechanical Tests of the Membranes
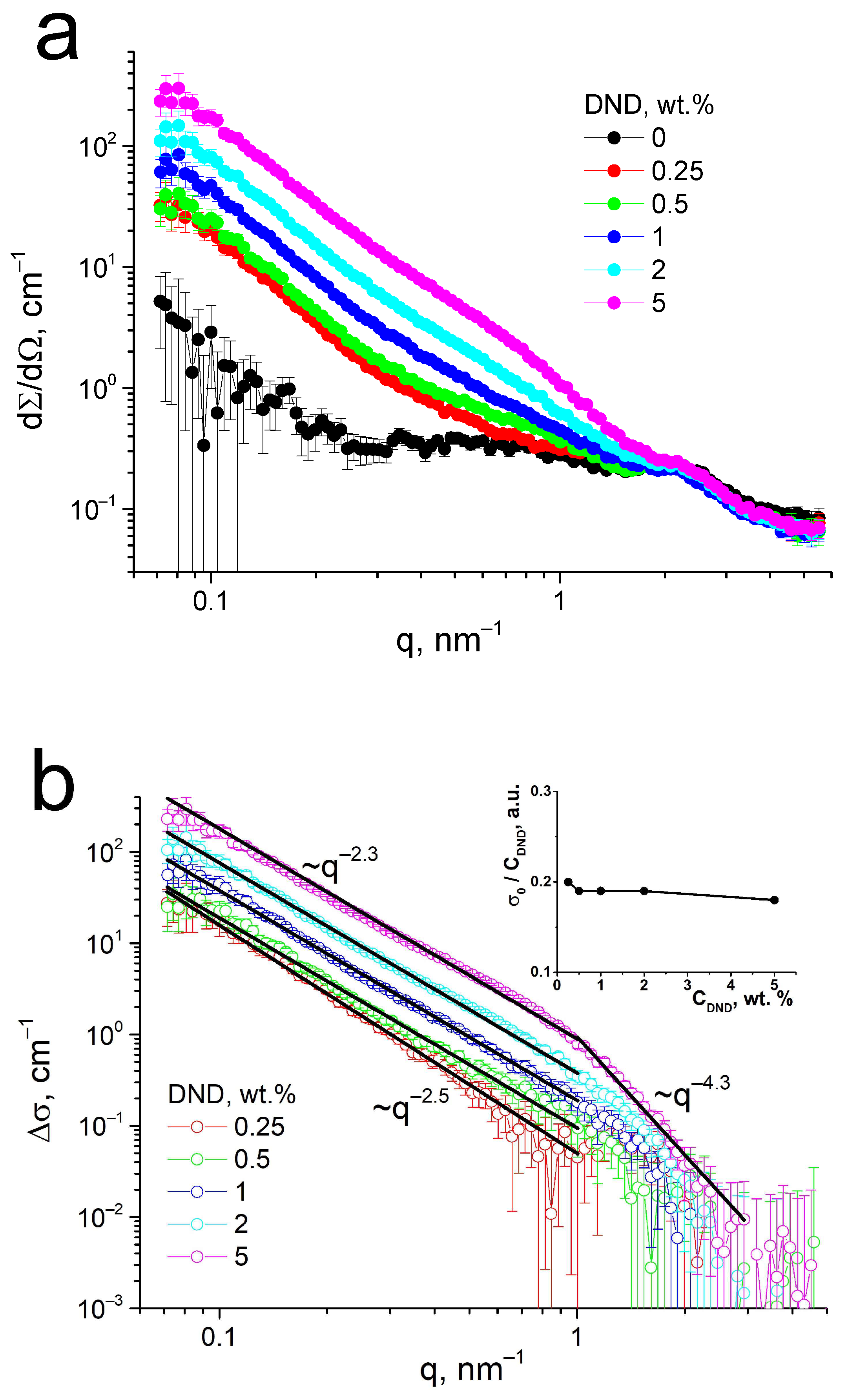
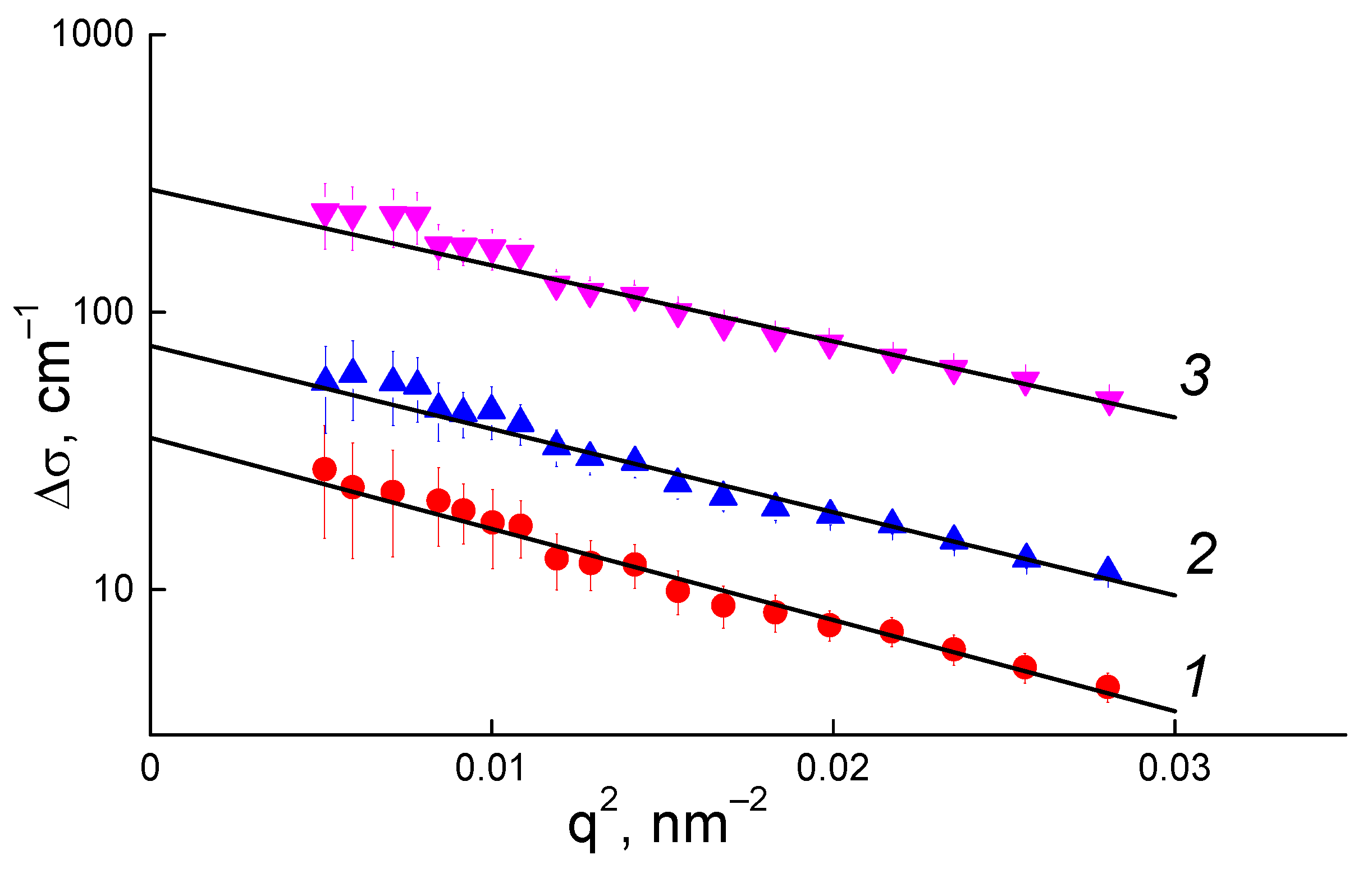
3.4. Structural Studies of the Membranes by Small-Angle Neutron Scattering
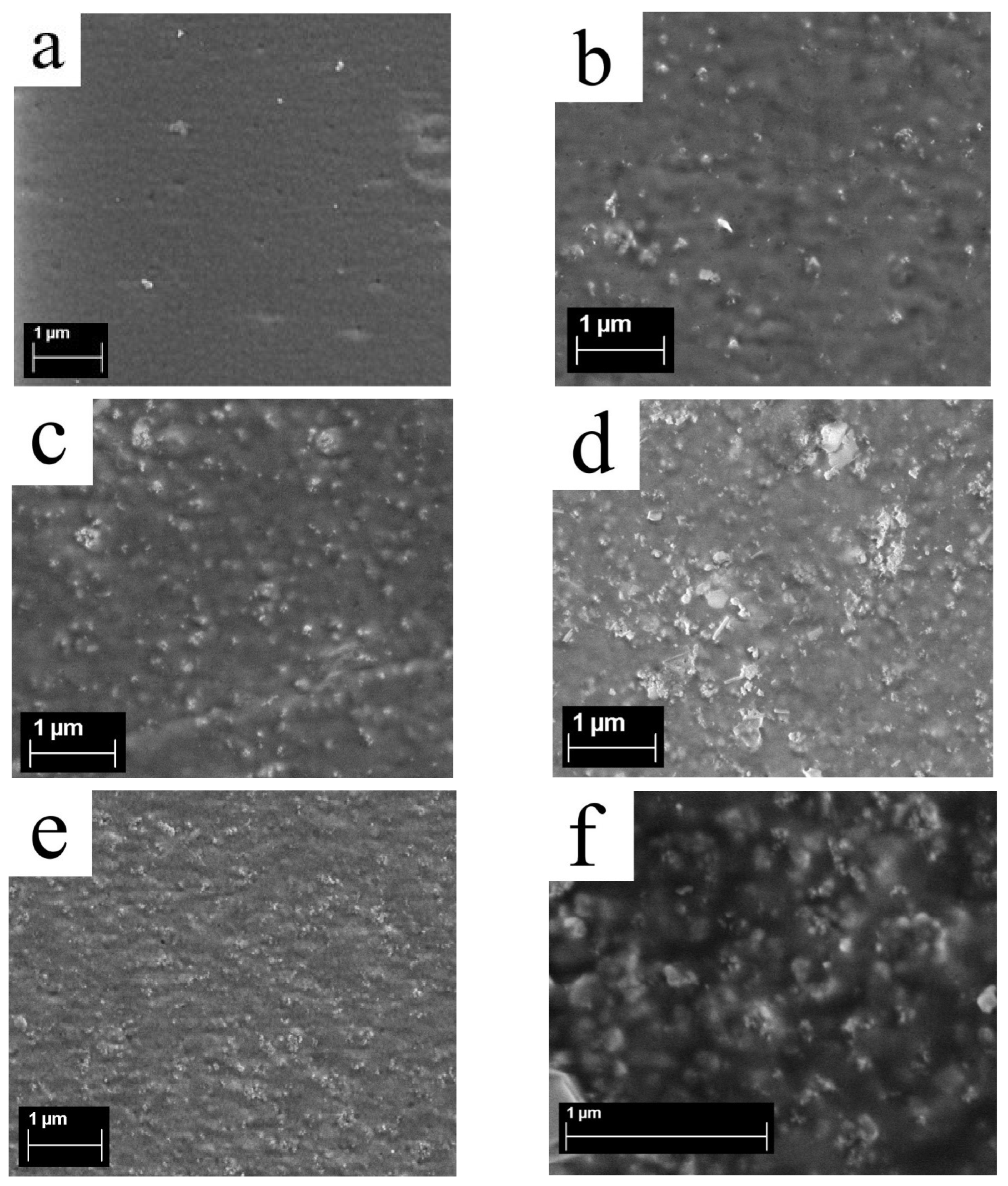
3.5. Surface Structure of Membranes from Scanning Electron Microscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Filippov, S.P.; Yaroslavtsev, A.B. Hydrogen energy: Development prospects and materials. Russ. Chem. Rev. 2021, 90, 627–643. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, P.; Niu, M.; Maddy, J. The survey of key technologies in hydrogen energy storage. Int. J. Hydrogen Energy 2016, 41, 14535–14552. [Google Scholar] [CrossRef]
- Kusoglu, A.; Weber, A.Z. New insights into perfluorinated sulfonic-acid ionomers. Chem. Rev. 2017, 117, 987–1104. [Google Scholar] [CrossRef]
- Alent’ev, A.Y.; Volkov, A.V.; Vorotyntsev, I.V.; Maksimov, A.L.; Yaroslavtsev, A.B. Membrane technologies for decarbonization. Membr. Membr. Technol. 2021, 3, 255–273. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Vezzù, K.; Pagot, G.; Cavinato, G.; Nale, A.; Bang, Y.H.; Di Noto, V. Hybrid inorganic-organic proton-conducting membranes based on SPEEK doped with WO3 nanoparticles for application in vanadium redox flow batteries. Electrochim. Acta 2019, 309, 311–325. [Google Scholar] [CrossRef]
- Fathabadi, H. Utilizing solar and wind energy in plug-in hybrid electric vehicles. Energy Convers. Manag. 2018, 156, 317–328. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, H. Review the development of first-generation redox flow batteries: Iron-chromium system. ChemSusChem 2022, 15, e202101798. [Google Scholar] [CrossRef]
- Ivanchev, S.S.; Myakin, S.V. Polymer membranes for fuel cells: Manufacture, structure, modification, properties. Russ. Chem. Rev. 2010, 79, 101–117. [Google Scholar] [CrossRef]
- Danilczuck, M.; Lancucki, L.; Schlick, S.; Hamrock, S.J.; Haugen, G.M. In-depth profiling of degradationin processes in a fuel cell: 2D spectral-spatial FTIR spectra of Nafion membranes. ACS Macro Lett. 2012, 1, 280–285. [Google Scholar] [CrossRef]
- Banergjee, S.; Curtin, D.E. Nafion® perfluorinated membranes in fuel cell. J. Fluor. Chem. 2004, 125, 1211–1216. [Google Scholar] [CrossRef]
- Hiesgen, R.; Aleksandrova, E.; Meichsner, G.; Wehl, I.; Roduner, E.; Friedrich, K.A. High-resolution imaging of ion conductivity of Nafion® membranes with electrochemical atomic force microscopy. Electrochim. Acta 2009, 55, 423–429. [Google Scholar] [CrossRef]
- Xie, T. Tunable polymer multi-shape memory effect. Nature 2010, 464, 267–270. [Google Scholar] [CrossRef]
- Komarov, P.V.; Khalatur, P.G.; Khokhlov, A.R. Large-scale atomistic and quantum-mechanical simulations of a Nafion membrane: Morphology, proton solvation and charge transport. Beilstein J. Nanotechnol. 2013, 4, 567–587. [Google Scholar] [CrossRef]
- Xiao, P.; Li, J.; Cho, C. Experimental investigation and discussion on the mechanical endurance limit of Nafion membrane used in proton membrane fuel cell. Energies 2014, 7, 6401–6411. [Google Scholar] [CrossRef]
- Primachenko, O.N.; Marinenko, E.A.; Odinokov, A.S.; Kononova, S.V.; Kulvelis, Y.V.; Lebedev, V.T. State of the art and prospects in the development of proton conducting perfluorinated membranes with short side chains: A review. Polym. Adv. Technol. 2021, 32, 1386–1408. [Google Scholar] [CrossRef]
- Kulvelis, Y.V.; Ivanchev, S.S.; Lebedev, V.T.; Primachenko, O.N.; Likhomanov, V.S.; Török., G. Structure characterization of perfluorosulfonic short side chain polymer membranes. RSC Adv. 2015, 5, 73820–73826. [Google Scholar] [CrossRef]
- Primachenko, O.N.; Odinokov, A.S.; Barabanov, V.G.; Tyul’mankov, V.P.; Marinenko, E.A.; Gofman, I.V.; Ivanchev, S.S. Relationship between the morphology, nanostructure, and strength properties of Aquivion® type perfluorinated proton-conducting membranes prepared by casting from solution. Russ. J. Appl. Chem. 2018, 91, 101–104. [Google Scholar] [CrossRef]
- Safronova, E.Y.; Osipov, A.K.; Yaroslavtsev, A.B. Short Side Chain Aquivion Perfluorinated Sulfonated Proton-Conductive Membranes: Transport and Mechanical Properties. Pet. Chem. 2018, 58, 130–136. [Google Scholar] [CrossRef]
- Xu, F.; Mu, S. Nanoceramic Oxide Hybrid Electrolyte Membranes for Proton Exchange Membrane Fuel Cells. J. Nanosci. Nanotechnol. 2014, 14, 1169–1180. [Google Scholar] [CrossRef]
- Wong, C.Y.; Wong, W.Y.; Ramya, K.; Khali, M.; Loh, K.S.; Daud, K.L.; Lim, W.R.W.; Walvekar, R.; Kadhum, A.A.H. Additives in proton exchange membranes for low and high-temperature fuel cell applications: A review. Int. J. Hydrogen Energy 2019, 44, 6116–6135. [Google Scholar] [CrossRef]
- Bakangura, E.; Wu, L.; Ge, L.; Yang, Z.; Xu, T. Mixed matrix proton exchange membranes for fuel cells: State of the art and perspectives. Prog. Polym. Sci. 2016, 57, 103–152. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, Q.; Zheng, X.; Yin, Y.; Wu, H.; Jiang, Z. Incorporating phosphoric acid-functionalized polydopamine into Nafion polymer by in situ sol-gel method for enhanced proton conductivity. J. Membr. Sci. 2019, 570, 236–244. [Google Scholar] [CrossRef]
- Ying, Y.P.; Kamarudin, S.K.; Masdar, M.S. Silica-related membranes in fuel cell applications: An overview. Int. J. Hydrogen Energy 2018, 43, 16068–16084. [Google Scholar] [CrossRef]
- Park, J.-S.; Shin, M.-S.; Kim, C.-S. Proton exchange membranes for fuel cell operation at low relative humidity and intermediate temperature: An updated review. Curr. Opin. Electrochem. 2017, 5, 43–55. [Google Scholar] [CrossRef]
- Pethaiah, S.S.; Kalaignan, G.P.; Ulaganathan, M.; Arunkumar, J. Preparation of durable nanocatalyzed MEA for PEM fuel cell applications. Ionics 2011, 17, 361–366. [Google Scholar] [CrossRef]
- Kim, D.J.; Jo, M.J.; Nam, S.Y. A review of polymer-nanocomposite electrolyte membranes for fuel cell application. J. Ind. Eng. Chem. 2015, 21, 36–52. [Google Scholar] [CrossRef]
- Porozhnyy, M.V.; Shkirskaya, S.A.; Butylskii, D.Y.; Dotsenko, V.V.; Safronova, E.Y.; Yaroslavtsev, A.B.; Deabate, S.; Huguet, P.; Nikonenko, V.V. Physicochemical and electrochemical characterization of Nafion-type membranes with embedded silica nanoparticles: Effect of functionalization. Electrochim. Acta 2021, 370, 137689. [Google Scholar] [CrossRef]
- Yadav, R.; Subhash, A.; Chemmenchery, N.; Kandasubramanian, B. Graphene and Graphene Oxide for Fuel Cell Technology. Ind. Eng. Chem. Res. 2018, 57, 9333–9350. [Google Scholar] [CrossRef]
- Nunn, N.; Torelli, M.; McGuire, G.; Shenderova, O. Nanodiamond: A high impact nanomaterial. Curr. Opin. Solid State Mater. Sci. 2017, 21, 1–9. [Google Scholar] [CrossRef]
- Yang, N.; Foord, J.S.; Jiang, X. Diamond electrochemistry at the nanoscale: A review. Carbon 2016, 99, 90–110. [Google Scholar] [CrossRef]
- Postnov, V.N.; Mel’nikova, N.A.; Shul’meister, G.A.; Novikov, A.G.; Murin, I.V.; Zhukov, A.N. Nafion- and Aquivion-Based Nanocomposites Containing Detonation Nanodiamonds. Russ. J. Gen. Chem. 2017, 87, 2754–2755. [Google Scholar] [CrossRef]
- Aleksenskiy, A.E.; Eydelman, E.D.; Vul’, A.Y. Deagglomeration of detonation nanodiamonds. Nanosci. Nanotechnol. Lett. 2011, 3, 68–74. [Google Scholar] [CrossRef]
- Kulvelis, Y.V.; Primachenko, O.N.; Odinokov, A.S.; Shvidchenko, A.V.; Bairamukov, V.Y.; Gofman, I.V.; Lebedev, V.T.; Ivanchev, S.S.; Vul, A.Y.; Kuklin, A.I. Composite proton-conducting membranes with nanodiamonds. Fuller. Nanotub. Carbon Nanostruct. 2020, 28, 140–146. [Google Scholar] [CrossRef]
- Primachenko, O.N.; Kulvelis, Y.V.; Lebedev, V.T.; Odinokov, A.S.; Bayramukov, V.Y.; Marinenko, E.A.; Gofman, I.V.; Shvidchenko, A.V.; Vul, A.Y.; Ivanchev, S.S. Perfluorinated proton-conducting membrane composites with functionalized nanodiamonds. Membr. Membr. Technol. 2020, 2, 1–9. [Google Scholar] [CrossRef]
- Kulvelis, Y.V.; Primachenko, O.N.; Gofman, I.V.; Odinokov, A.S.; Shvidchenko, A.V.; Yudina, E.B.; Marinenko, E.A.; Lebedev, V.T.; Vul, A.Y. Modification of the mechanism of proton conductivity of the perfluorinated membrane copolymer by nanodiamonds. Russ. Chem. Bull. Int. Ed. 2021, 70, 1713–1717. [Google Scholar] [CrossRef]
- Williams, O.A.; Hees, J.; Dieker, C.; Jager, W.; Kirste, L.; Nebel, C.E. Size-Dependent Reactivity of Diamond Nanoparticles. ACS Nano 2010, 4, 4824–4830. [Google Scholar] [CrossRef] [PubMed]
- Primachenko, O.N.; Kulvelis, Y.V.; Odinokov, A.S.; Glebova, N.V.; Krasnova, A.O.; Antokolskiy, L.A.; Nechitailov, A.A.; Shvidchenko, A.V.; Gofman, I.V.; Marinenko, E.A.; et al. New Generation of Compositional Aquivion®-Type Membranes with Nanodiamonds for Hydrogen Fuel Cells: Design and Performance. Membranes 2022, 12, 827. [Google Scholar] [CrossRef] [PubMed]
- Simari, C.; Stallworth, P.; Peng, J.; Coppola, L.; Greenbaum, S.; Nicotera, I. Graphene oxide and sulfonated-derivative: Proton transport properties and electrochemical behavior of Nafion-based nanocomposites. Electrochim. Acta 2019, 297, 240–249. [Google Scholar] [CrossRef]
- Sgambetterra, M.; Brutti, S.; Allodi, V.; Mariotto, G.; Panero, S.; Navarra, M.A. Critical filler concentration in sulfated titania-added Nafion™ membranes for fuel cell applications. Energies 2016, 9, 272. [Google Scholar] [CrossRef]
- Vinothkannan, M.; Kim, A.R.; Ramakrishnan, S.; Yu, Y.-T.; Yoo, D.J. Advanced Nafion nanocomposite membrane embedded with unzipped and functionalized graphite nanofibers for high-temperature hydrogen-air fuel cell system: The impact of filler on power density, chemical durability and hydrogen permeability of membrane. Composites Part B. 2021, 215, 108828. [Google Scholar] [CrossRef]
- Rambabu, G.; Nagaraju, N.; Bhat, S.D. Functionalized fullerene embedded in Nafion matrix: A modified composite membrane electrolyte for direct methanol fuel cells. Chem. Eng. J. 2016, 306, 43–52. [Google Scholar] [CrossRef]
- Kuznetsov, O.; Sun, Y.; Thaner, R.; Bratt, A.; Shenoy, V.; Wong, M.S.; Jones, J.; Billups, W.E. Water-soluble nanodiamond. Langmuir 2012, 28, 5243–5248. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lei, Y.; Huang, Q.; Gan, D.; Huang, H.; Chen, J.; Deng, F.; Liu, M.; Li, X.; Zhang, X.; Wei, Y. A novel one-step method for preparation of sulfonate functionalized nanodiamonds and their utilization for ultrafast removal of organic dyes with high efficiency: Kinetic and isotherm studies. J. Environ. Chem. Eng. 2020, 8, 103780. [Google Scholar] [CrossRef]
- Petit, T.; Girard, H.A.; Trouvé, A.; Batonneau-Gener, I.; Bergonzo, P.; Arnault, J.-C. Surface transfer doping can mediate both colloidal stability and selfassembly of nanodiamonds. Nanoscale 2013, 5, 8958–8962. [Google Scholar] [CrossRef]
- Ginés, L.; Mandal, S.; Cheng, C.-L.; Sow, M.; Williams, O.A. Positive zeta potential of nanodiamonds. Nanoscale 2017, 9, 12549. [Google Scholar] [CrossRef]
- Vul, A.Y.; Eidelman, E.D.; Aleksenskiy, A.E.; Shvidchenko, A.V.; Dideikin, A.T.; Yuferev, V.S.; Lebedev, V.T.; Kul’velis, Y.V.; Avdeev, M.V. Transition sol-gel in nanodiamond hydrosols. Carbon 2017, 114, 242–249. [Google Scholar] [CrossRef]
- Primachenko, O.N.; Odinokov, A.S.; Marinenko, E.A.; Kulvelis, Y.V.; Barabanov, V.G.; Kononova, S.V. Influence of sulfonyl fluoride monomers on the mechanism of emulsion copolymerization with the preparation of proton-conducting membrane precursors. J. Fluor. Chem. 2021, 244, 109736. [Google Scholar] [CrossRef]
- Delgado, A.V.; González-Caballero, F.; Hunter, R.J.; Koopal, L.K.; Lyklema, J. Measurement and interpretation of electrokinetic phenomena. J. Coll. Interf. Sci. 2007, 309, 194–224. [Google Scholar] [CrossRef]
- Kuklin, A.I.; Ivankov, O.I.; Rogachev, A.V.; Soloviov, D.V.; Islamov, A.K.; Skoi, V.V.; Kovalev, Y.S.; Vlasov, A.V.; Ryzhykau, Y.L.; Soloviev, A.G.; et al. Small-Angle Neutron Scattering at the Pulsed Reactor IBR-2: Current Status and Prospects. Crystallogr. Rep. 2021, 66, 231–241. [Google Scholar] [CrossRef]
- Kuklin, A.I.; Ivankov, A.I.; Soloviov, D.V.; Rogachev, A.V.; Kovalev, Y.S.; Soloviev, A.G.; Islamov, A.K.; Balasoiu, M.; Vlasov, A.V.; Kutuzov, S.A. High-throughput SANS experiment on two-detector system of YuMO spectrometer. J. Phys. Conf. Ser. 2018, 994, 012016. [Google Scholar] [CrossRef]
- Soloviev, A.G.; Solovjeva, T.M.; Ivankov, O.I.; Soloviov, D.V.; Rogachev, A.V.; Kuklin, A.I. SAS program for two-detector system: Seamless curve from both detectors. J. Phys. Conf. Ser. 2017, 848, 012020. [Google Scholar] [CrossRef]
- Petit, T.; Puskar, L. FTIR spectroscopy of nanodiamonds: Methods and interpretation. Diam. Rel. Mat. 2018, 89, 52–66. [Google Scholar] [CrossRef]
- Danilczuk, M.; Lin, L.; Schlick, S.; Hamrock, S.J.; Schaberg, M.S. Understanding the fingerprint region in the infra-red spectra of perfluorinated ionomer membranes and corresponding model compounds: Experiments and theoretical calculations. J. Pow. Sourc. 2011, 196, 8216–8224. [Google Scholar] [CrossRef]
- Kulvelis, Y.V.; Ivanchev, S.S.; Primachenko, O.N.; Lebedev, V.T.; Marinenko, E.A.; Ivanova, I.N.; Kuklin, A.I.; Ivankov, O.I.; Soloviov, D.V. Structure and property optimization of perfluorinated short side chain membranes for hydrogen fuel cells using orientational stretching. RSC Adv. 2016, 6, 108864–108875. [Google Scholar] [CrossRef]
- Teixeira, J. Small-angle scattering by fractal systems. J. Appl. Cryst. 1988, 21, 781–785. [Google Scholar] [CrossRef]
- Grigoriev, S.V.; Iashina, E.G.; Bairamukov, V.Y.; Pipich, V.; Radulescu, A.; Filatov, M.V.; Pantina, R.A.; Varfolomeeva, E.Y. Switch of fractal properties of DNA in chicken erythrocytes nuclei by mechanical stress. Phys. Rev. E 2020, 102, 032415. [Google Scholar] [CrossRef]
- Iashina, E.G.; Varfolomeeva, E.Y.; Pantina, R.A.; Bairamukov, V.Y.; Kovalev, R.A.; Fedorova, N.D.; Pipich, V.; Radulescu, A.; Grigoriev, S.V. Bifractal structure of chromatin in rat lymphocyte nuclei. Phys. Rev. E 2021, 104, 064409. [Google Scholar] [CrossRef]
- Lebedev, V.; Kulvelis, Y.; Kuklin, A.; Vul, A. Neutron Study of Multilevel Structures of Diamond Gels. Cond. Matter. 2016, 1, 10. [Google Scholar] [CrossRef]
- Avdeev, M.V.; Tomchuk, O.V.; Ivankov, O.I.; Alexenskii, A.E.; Dideikin, A.T.; Vul, A.Y. On the structure of concentrated detonation nanodiamond hydrosols with a positive ζ potential: Analysis of small-angle neutron scattering. Chem. Phys. Lett. 2016, 658, 58–62. [Google Scholar] [CrossRef]
- Tomchuk, O.V.; Mchedlov-Petrossyan, N.O.; Kyzyma, O.A.; Kriklya, N.N.; Bulavin, L.A.; Zabulonov, Y.L.; Ivankov, O.I.; Garamus, V.M.; Ōsawa, E.; Avdeev, M.V. Cluster-cluster interaction in nanodiamond hydrosols by small-angle scattering. J. Mol. Liq. 2022, 354, 118816. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shvidchenko, A.V.; Odinokov, A.S.; Primachenko, O.N.; Gofman, I.V.; Yevlampieva, N.P.; Marinenko, E.A.; Lebedev, V.T.; Kuklin, A.I.; Kulvelis, Y.V. Improving PFSA Membranes Using Sulfonated Nanodiamonds. Membranes 2023, 13, 712. https://doi.org/10.3390/membranes13080712
Shvidchenko AV, Odinokov AS, Primachenko ON, Gofman IV, Yevlampieva NP, Marinenko EA, Lebedev VT, Kuklin AI, Kulvelis YV. Improving PFSA Membranes Using Sulfonated Nanodiamonds. Membranes. 2023; 13(8):712. https://doi.org/10.3390/membranes13080712
Chicago/Turabian StyleShvidchenko, Alexandr V., Alexei S. Odinokov, Oleg N. Primachenko, Iosif V. Gofman, Natalia P. Yevlampieva, Elena A. Marinenko, Vasily T. Lebedev, Alexander I. Kuklin, and Yuri V. Kulvelis. 2023. "Improving PFSA Membranes Using Sulfonated Nanodiamonds" Membranes 13, no. 8: 712. https://doi.org/10.3390/membranes13080712
APA StyleShvidchenko, A. V., Odinokov, A. S., Primachenko, O. N., Gofman, I. V., Yevlampieva, N. P., Marinenko, E. A., Lebedev, V. T., Kuklin, A. I., & Kulvelis, Y. V. (2023). Improving PFSA Membranes Using Sulfonated Nanodiamonds. Membranes, 13(8), 712. https://doi.org/10.3390/membranes13080712







