Preparation of Cross-Sectional Membrane Samples for Scanning Electron Microscopy Characterizations Using a New Frozen Section Technique
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Liquid Nitrogen Cryogenic Fracture
2.3. BIB Polishing
2.4. Frozen Section Technique
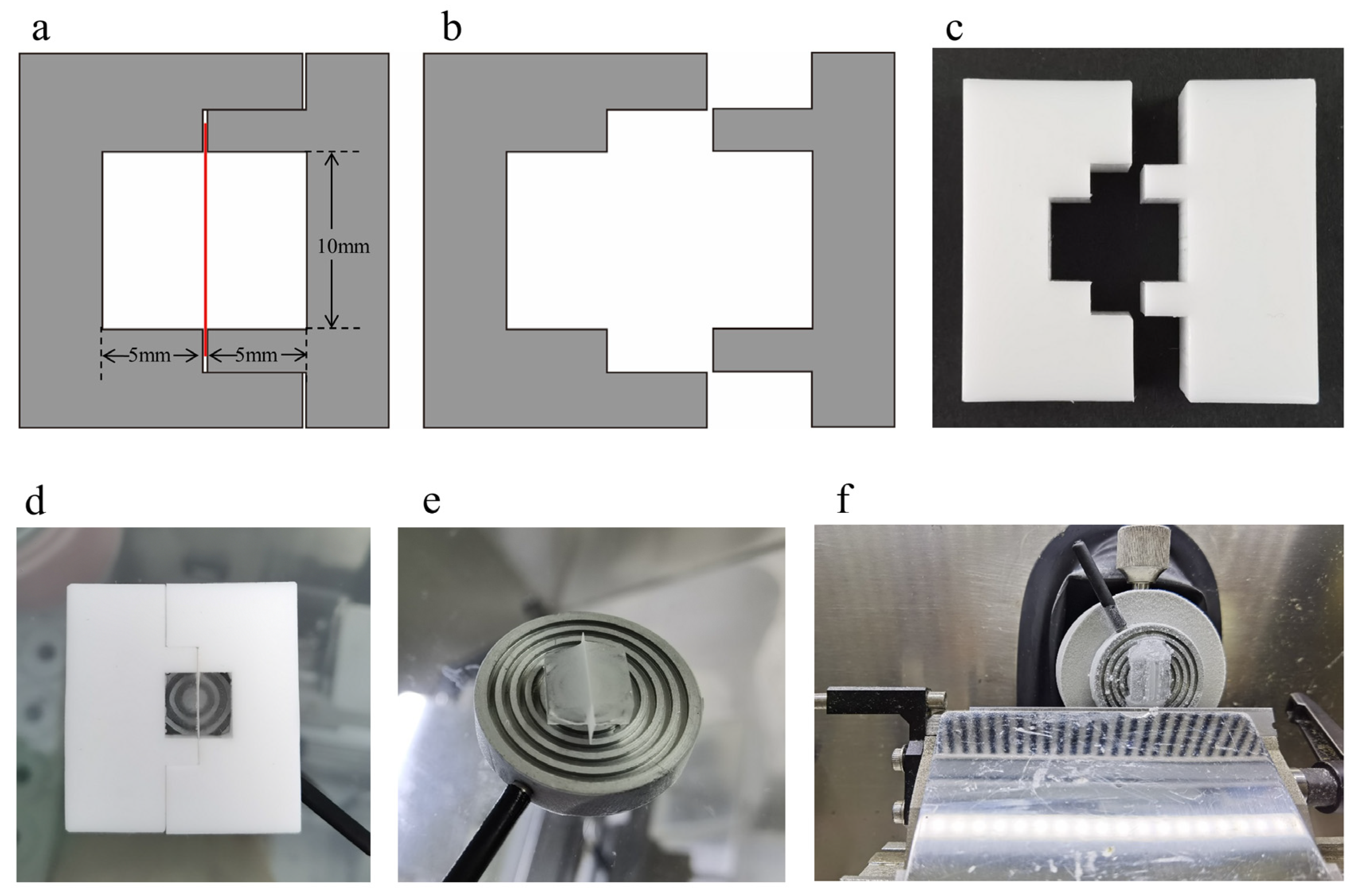
2.4.1. Fabrication of the Embedding Mold
2.4.2. Use of the Embedding Mold and Preparation of Membrane Cross-Sections
2.5. SEM Observation and EDS Analysis
3. Results and Discussion
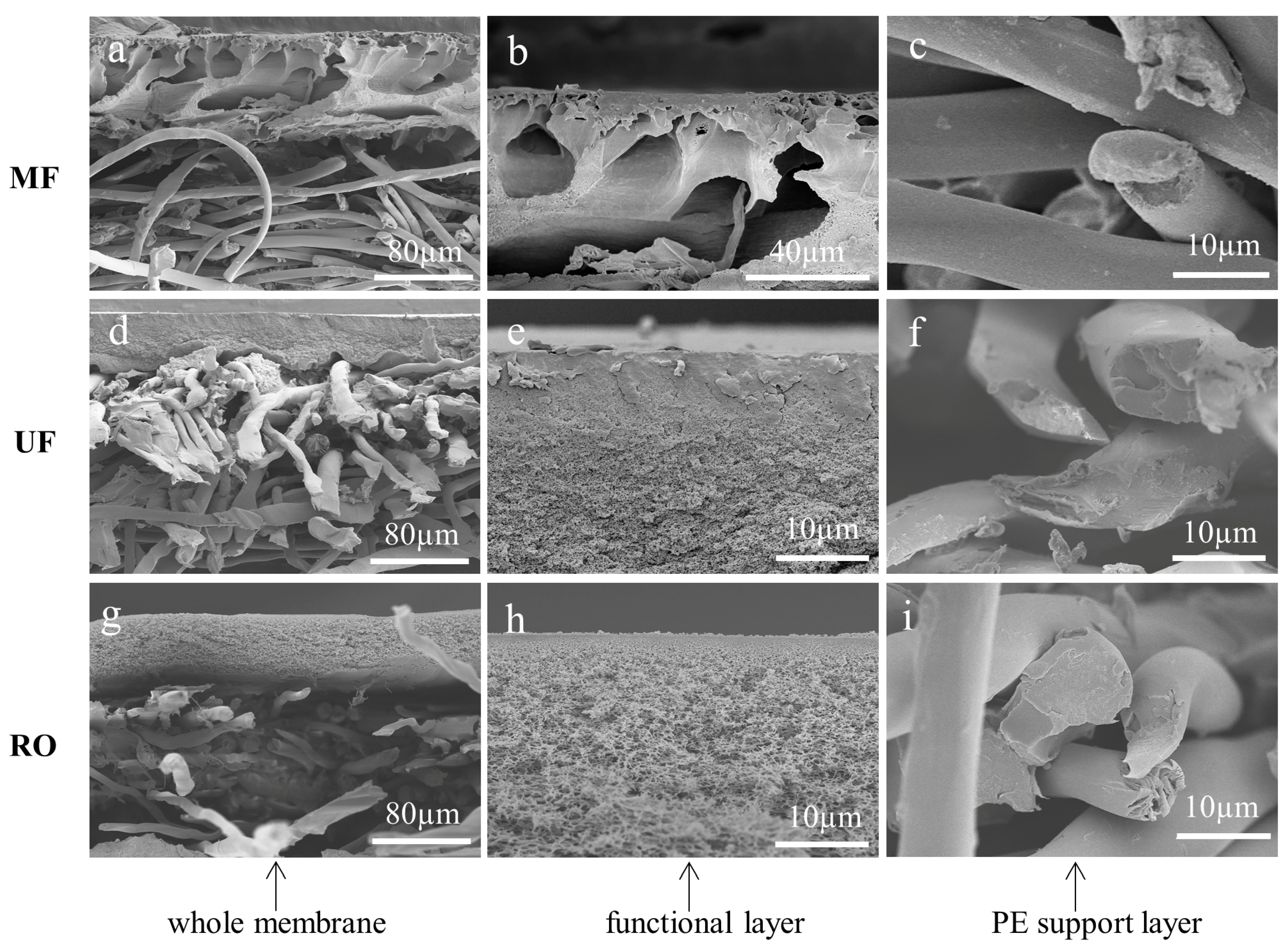
3.1. Cross-Sections of Membranes Cryogenically Fractured with Liquid Nitrogen
3.2. Cross-Sections of Membranes Prepared by BIB Polishing
3.3. Cross-Sections of Membranes Prepared by Frozen Section Technique
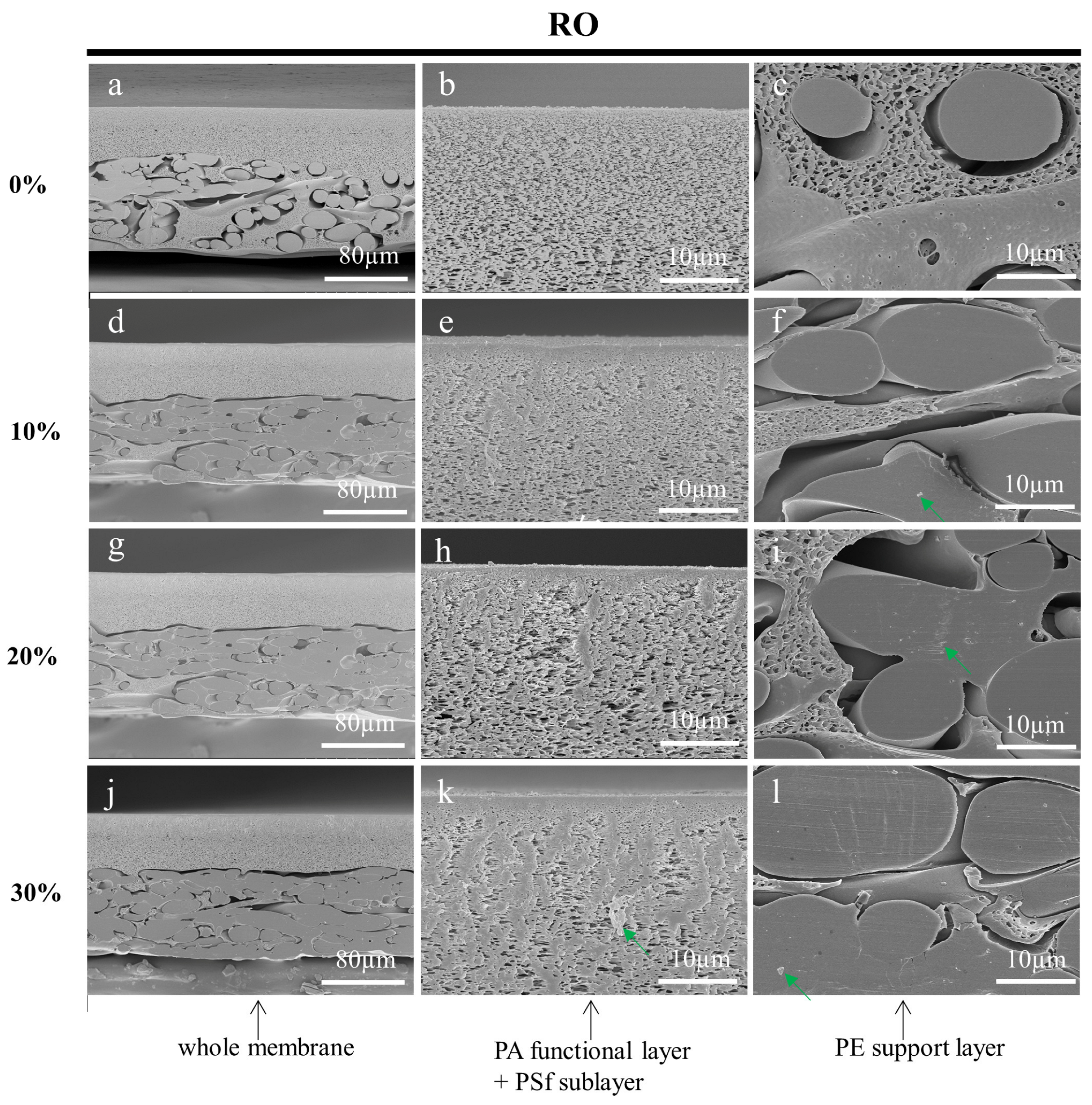
3.3.1. Effect of Embedding Medium on the Cross-Sections of Prepared Membranes
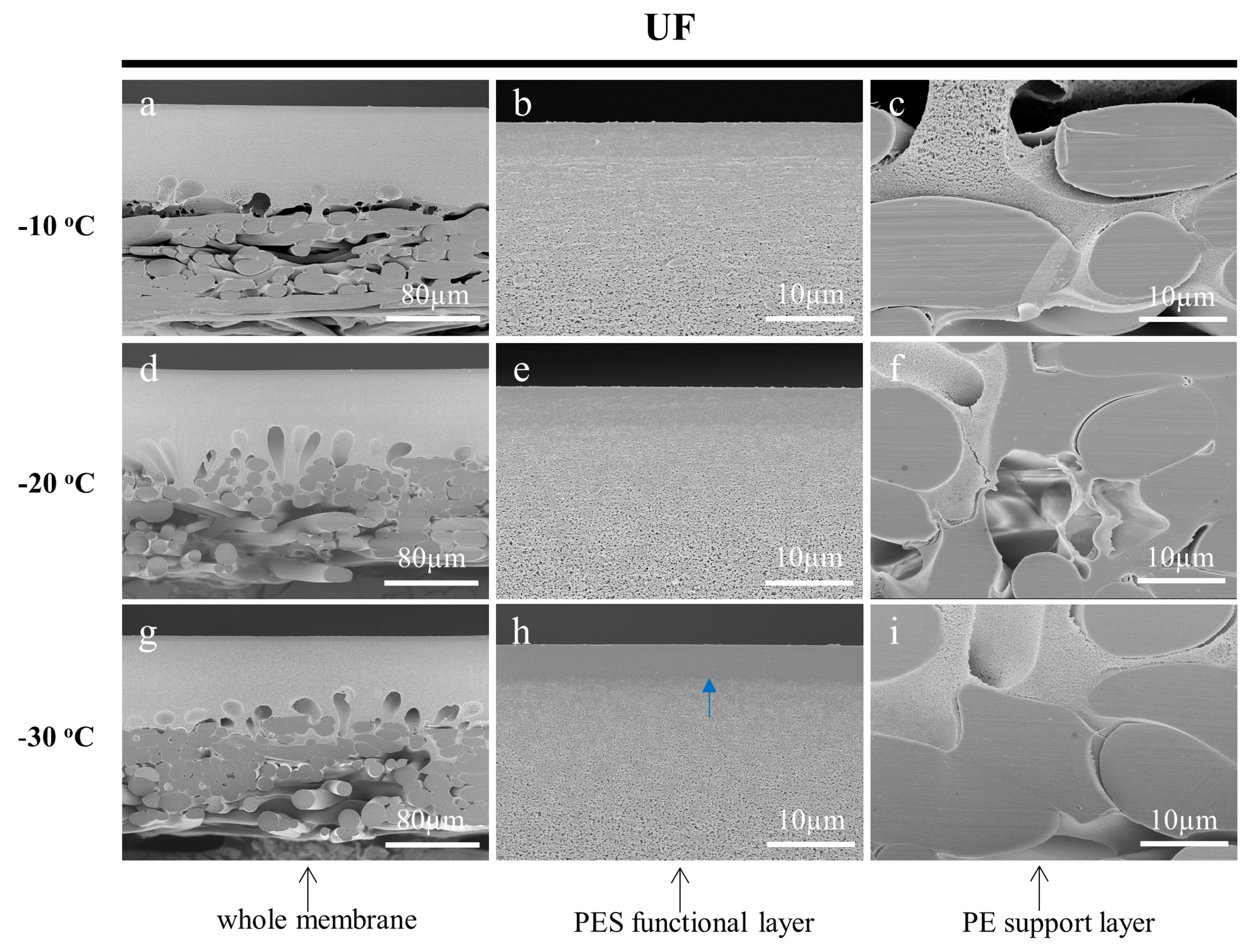
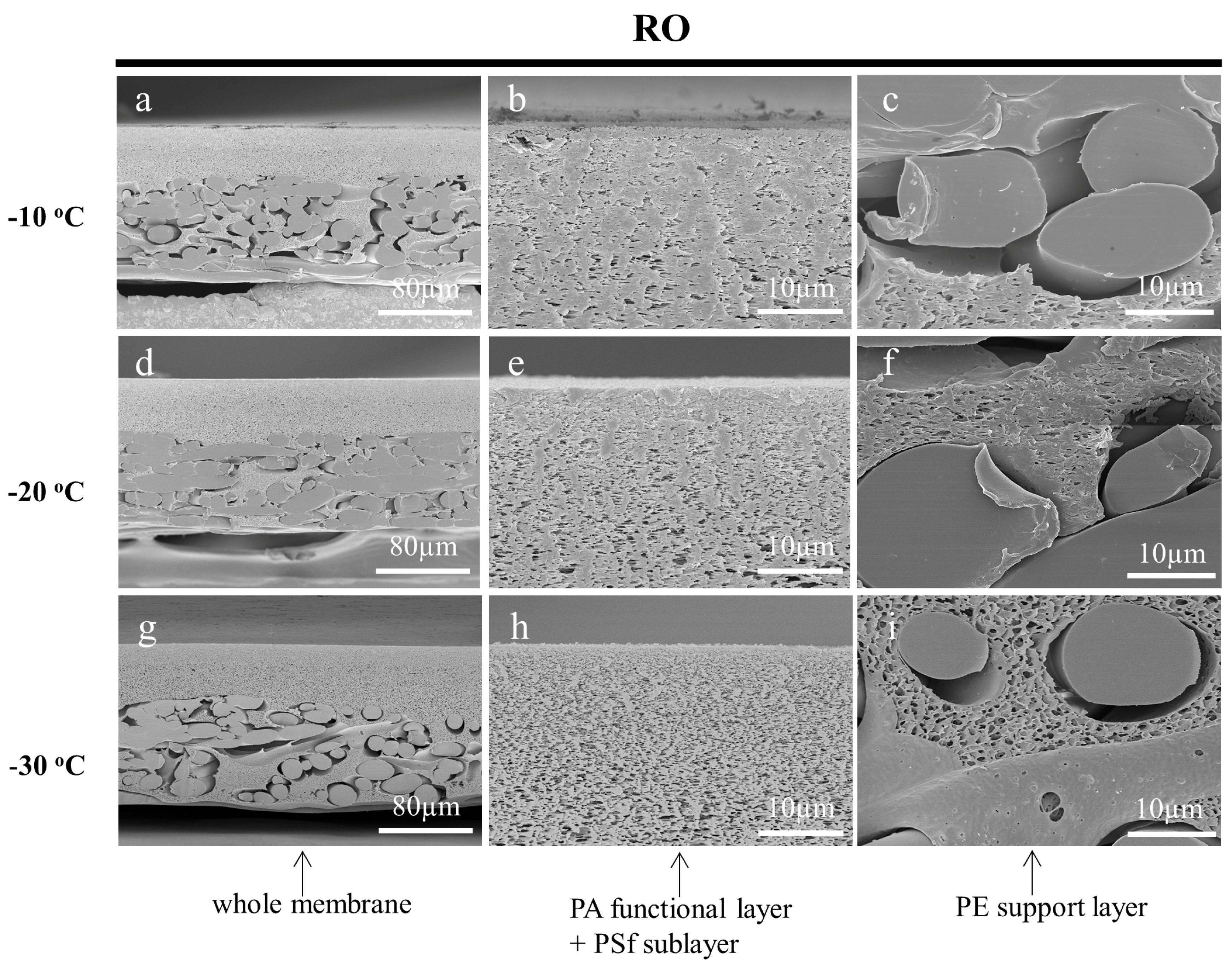
3.3.2. Effect of Working Temperature on the Cross-Sections of Prepared Membranes
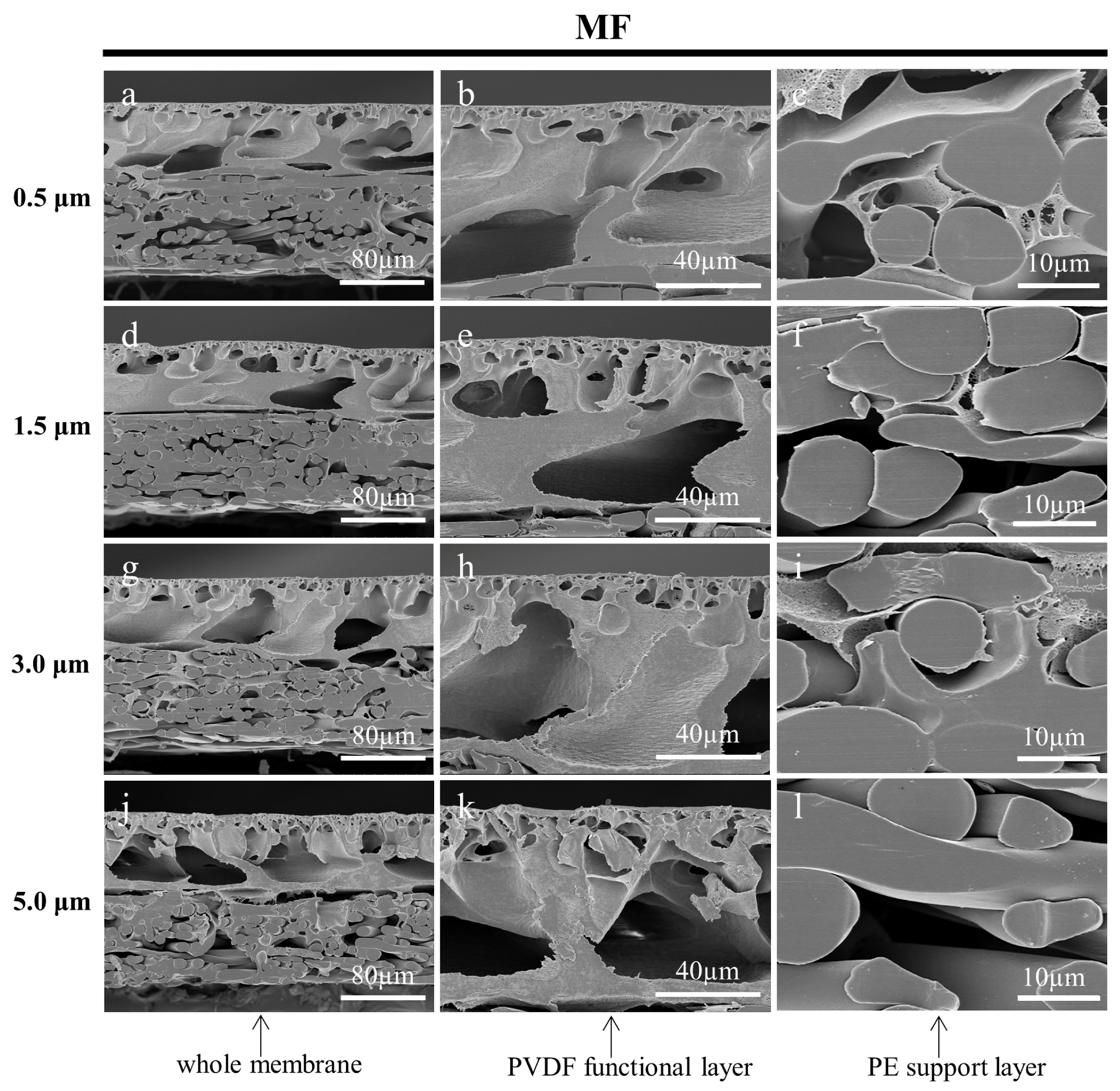
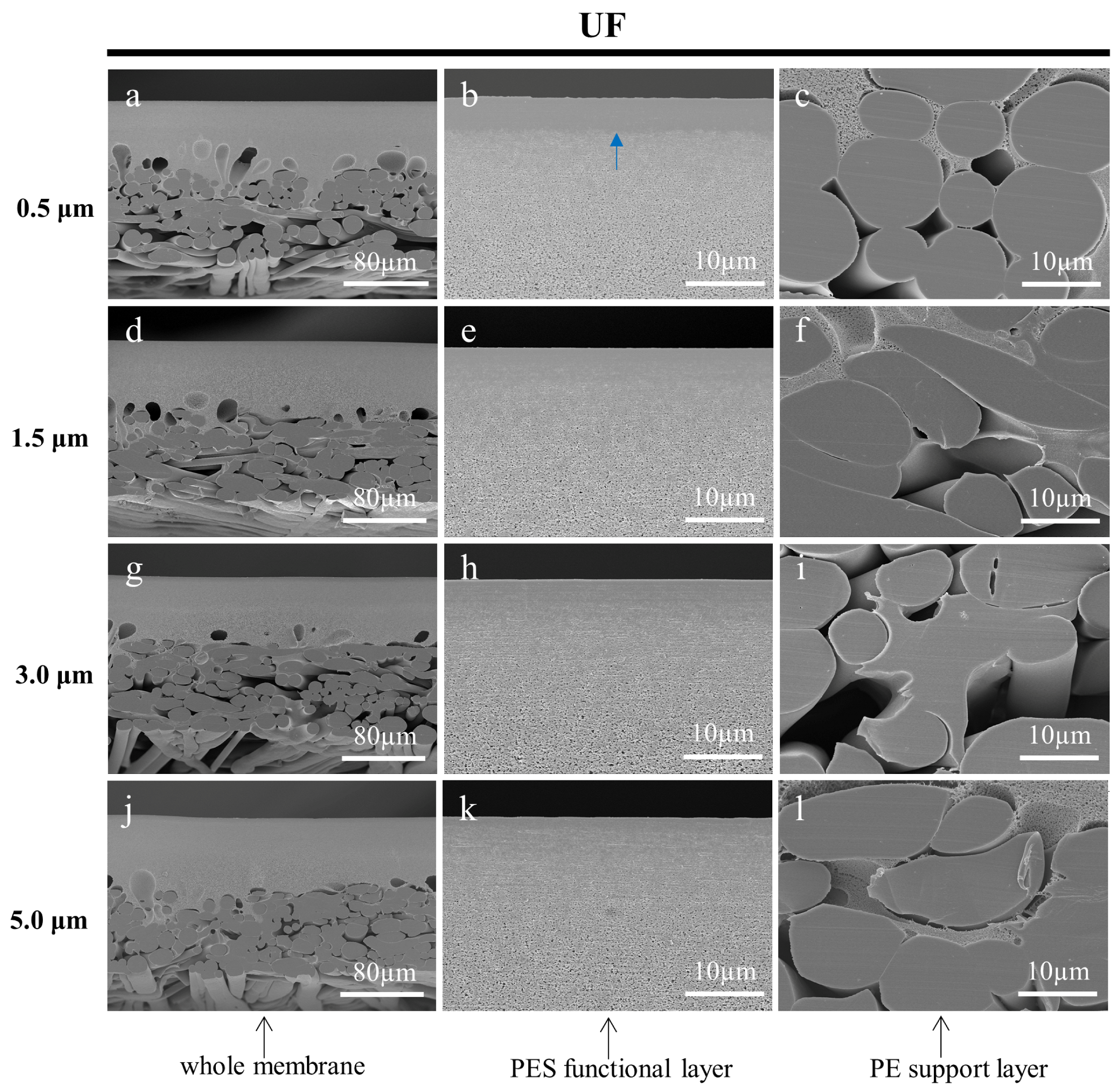
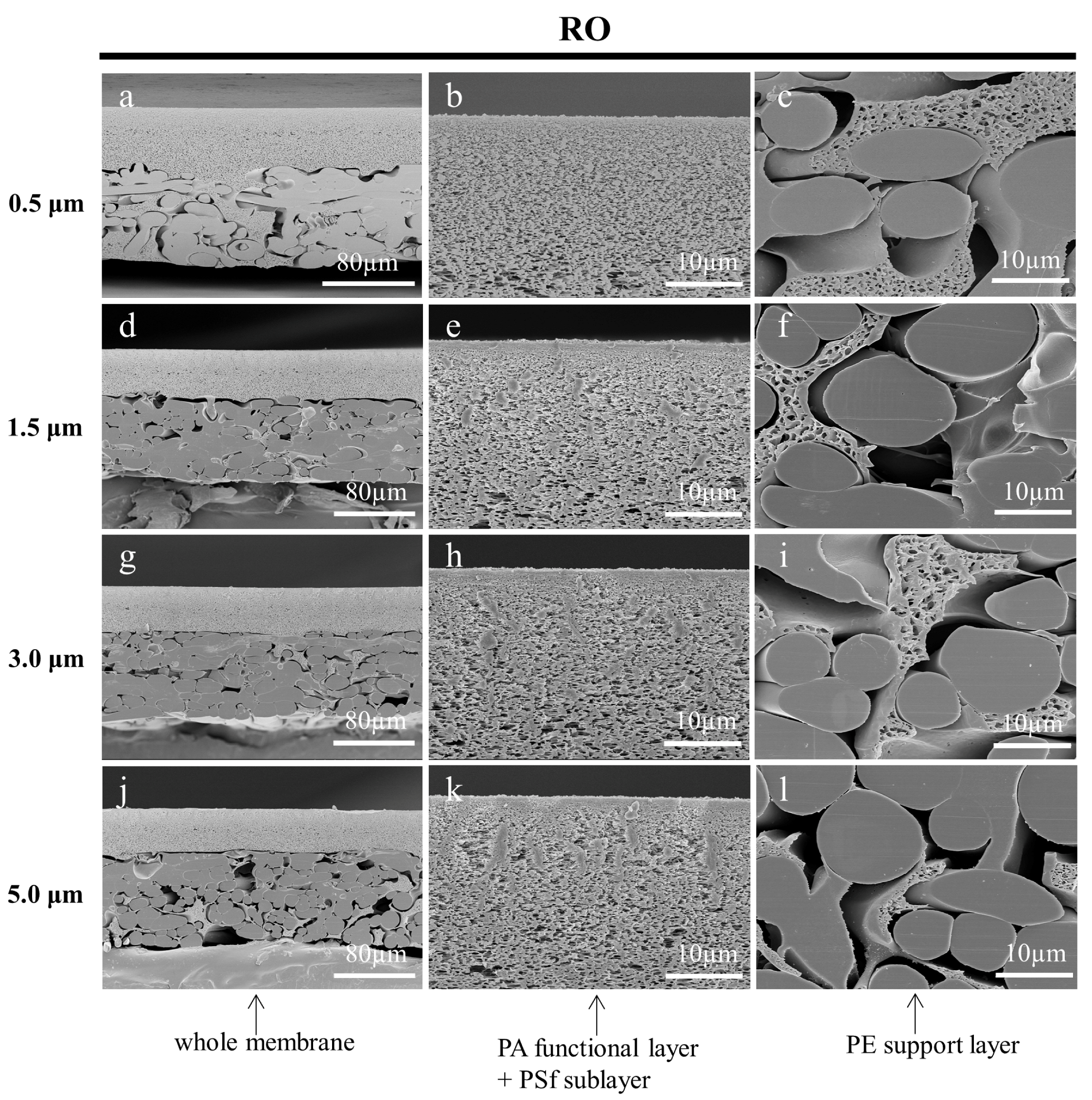
3.3.3. Effect of Fine Cutting Thickness on the Cross-Sections of Prepared Membranes
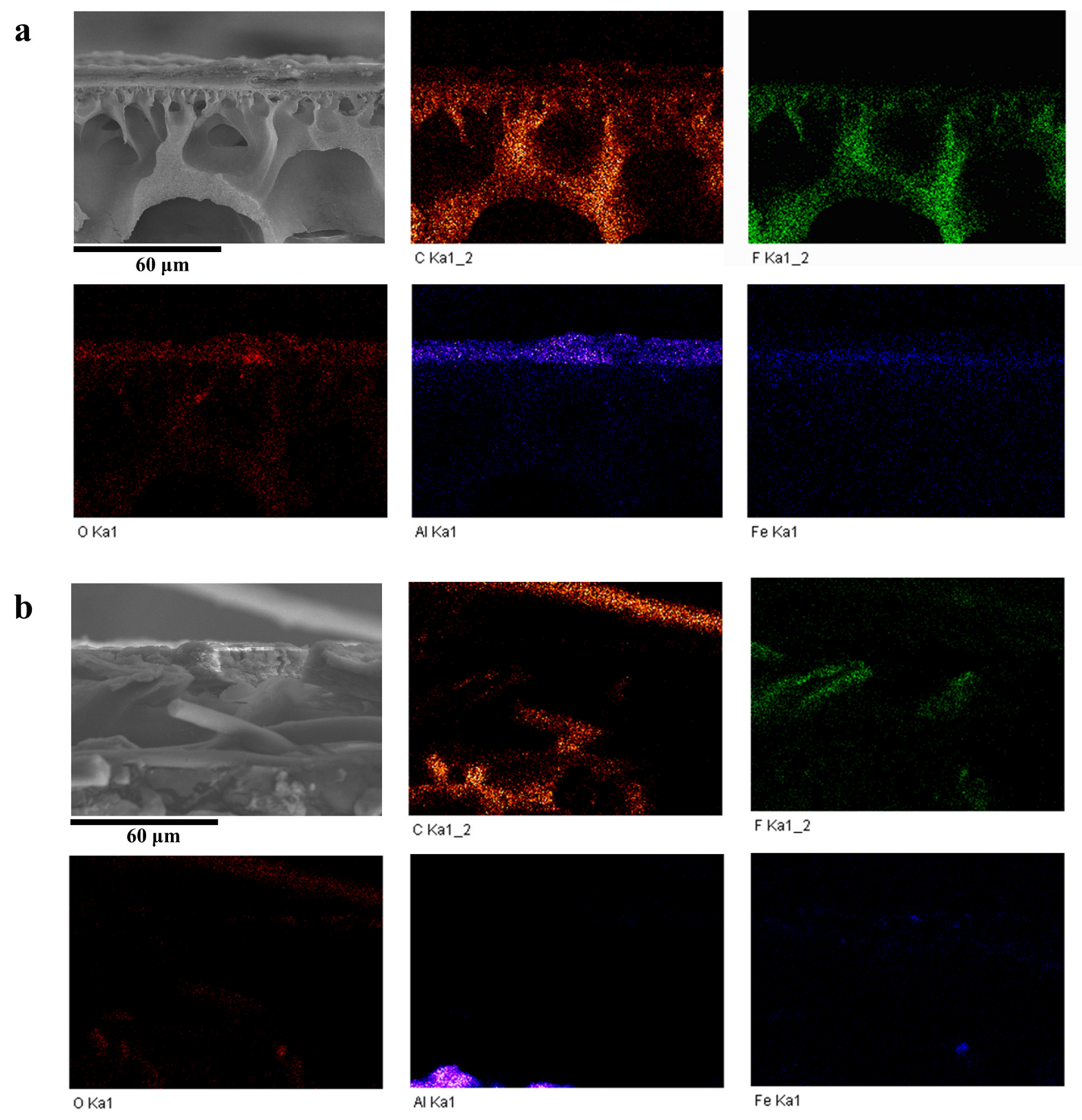
3.4. EDS Elemental Analysis
4. Economic Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Warsinger, D.M.; Chakraborty, S.; Tow, E.W.; Plumlee, M.H.; Bellona, C.; Loutatidou, S.; Karimi, L.; Mikelonis, A.M.; Achilli, A.; Ghassemi, A.; et al. A review of polymeric membranes and processes for potable water reuse. Prog. Polym. Sci. 2018, 81, 209–237. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Ahmed, S.K.M. Application of Membrane Technology in Food Processing. In Food Processing: Strategies for Quality Assessment; Malik, A., Erginkaya, Z., Ahmad, S., Erten, H., Eds.; Springer: New York, NY, USA, 2014; pp. 379–394. [Google Scholar]
- Agboola, O.; Fayomi, O.S.I.; Ayodeji, A.; Ayeni, A.O.; Alagbe, E.E.; Sanni, S.E.; Okoro, E.E.; Moropeng, L.; Sadiku, R.; Kupolati, K.W.; et al. A Review on Polymer Nanocomposites and Their Effective Applications in Membranes and Adsorbents for Water Treatment and Gas Separation. Membranes 2021, 11, 139. [Google Scholar] [CrossRef]
- Bera, S.P.; Godhaniya, M.; Kothari, C. Emerging and advanced membrane technology for wastewater treatment: A review. J. Basic Microbiol. 2021, 62, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Sadrzadeh, M.; Mohammadi, T. Nanocomposite Membranes for Water and Gas Separation; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Sagle, A.; Freeman, B. Fundamentals of membranes for water treatment. Future Desalin. Tex. 2004, 2, 137. [Google Scholar]
- Baker, R.W. Membrane Technology and Applications; Wiley Online Library: Hoboken, NJ, USA, 2012. [Google Scholar]
- Lalia, B.S.; Kochkodan, V.; Hashaikeh, R.; Hilal, N. A review on membrane fabrication: Structure, properties and performance relationship. Desalination 2013, 326, 77–95. [Google Scholar] [CrossRef]
- Yang, S.; Yang, D.; Ren, W.; Liu, J.; Mou, J.; Wang, Y. Analysis of Research Status of Modified PVDF Ultrafiltration Membrane. IOP Conf. Ser. Earth Environ. Sci. 2020, 585, 012190. [Google Scholar] [CrossRef]
- Tian, H.; Wu, X.; Zhang, K. Polydopamine-Assisted Two-Dimensional Molybdenum Disulfide (MoS2)-Modified PES Tight Ultrafiltration Mixed-Matrix Membranes: Enhanced Dye Separation Performance. Membranes 2021, 11, 96. [Google Scholar] [CrossRef]
- Zeng, H.; Yu, Z.; Shao, L.; Li, X.; Zhu, M.; Liu, Y.; Feng, X.; Zhu, X. A novel strategy for enhancing the performance of membranes for dyes separation: Embedding PAA@ UiO-66-NH2 between graphene oxide sheets. Chem. Eng. J. 2021, 403, 126281. [Google Scholar] [CrossRef]
- Gholami, S.; Llacuna, J.L.; Vatanpour, V.; Dehqan, A.; Paziresh, S.; Cortina, J.L. Impact of a new functionalization of multiwalled carbon nanotubes on antifouling and permeability of PVDF nanocomposite membranes for dye wastewater treatment. Chemosphere 2022, 294, 133699. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, H.; Yang, Y.; Zhu, L.; Zeng, Z.; Liu, S.; Li, Y.; Liang, Z. Poly (vinylidene fluoride) membranes with underwater superoleophobicity for highly efficient separation of oil-in-water emulsions in resisting fouling. Sep. Purif. Technol. 2022, 285, 120298. [Google Scholar] [CrossRef]
- Grzebyk, K.; Armstrong, M.D.; Coronell, O. Accessing greater thickness and new morphology features in polyamide active layers of thin-film composite membranes by reducing restrictions in amine monomer supply. J. Memb. Sci. 2022, 644, 120112. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Zhang, Y.Z.; Ding, X.L.; Lin, L.G.; Li, H. Preparation and Characterization of PES/SPSF Blend Ultrafiltration Membrane. Adv. Mater. Res. 2011, 221, 37–42. [Google Scholar] [CrossRef]
- Zheng, H.; Zhou, Y.; Wang, D.; Zhu, M.; Sun, X.; Jiang, S.; Fan, Y.; Zhang, D.; Zhang, L. Surface-functionalized PVDF membranes by facile synthetic Cu-MOF-74 for enhanced contaminant degradation and antifouling performance. Colloids Surf. A Physicochem. Eng. Asp. 2022, 651, 129640. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, C.; Yan, H.; Pan, G.; Guo, M.; Na, H.; Liu, Y. Highly chlorine-resistant multilayer reverse osmosis membranes based on sulfonated poly (arylene ether sulfone) and poly (vinyl alcohol). Desalination 2014, 336, 58–63. [Google Scholar] [CrossRef]
- Grobe, A.; Schmatz, J.; Littke, R.; Klaver, J.; Urai, J.L. Enhanced surface flatness of vitrinite particles by broad ion beam polishing and implications for reflectance measurements. Int. J. Coal Geol. 2017, 180, 113–121. [Google Scholar] [CrossRef]
- Jiang, R.; Li, M.; Yao, Y.; Guan, J.; Lu, H. Application of BIB polishing technology in cross-section preparation of porous, layered and powder materials: A review. Front. Mater. Sci. 2019, 13, 107–125. [Google Scholar] [CrossRef]
- Takahashi, H.; Sato, A.; Takakura, M.; Mori, N.; Boerder, J.; Knoll, W.; Critchell, J. A New Method of Surface Preparation for High Spatial Resolution EPMA/SEM with an Argon Ion Beam. Microchim. Acta 2006, 155, 295–300. [Google Scholar] [CrossRef]
- Brodusch, N.; Yourdkhani, M.; Hubert, P.; Gauvin, R. Efficient cross-section preparation method for high-resolution imaging of hard polymer composites with a scanning electron microscope. J. Microsc. 2015, 260, 117–124. [Google Scholar] [CrossRef]
- Erdman, N.; Campbell, R.; Asahina, S. Precise SEM Cross Section Polishing via Argon Beam Milling. Microsc. Today 2006, 14, 22–25. [Google Scholar] [CrossRef]
- Desbois, G.; Urai, J.L.; Houben, M.E.; Sholokhova, Y. Typology, morphology and connectivity of pore space in claystones from reference site for research using BIB, FIB and cryo-SEM methods. EPJ Web Conf. 2010, 6, 22005. [Google Scholar] [CrossRef]
- Nagamoto-Combs, K.; Manocha, G.D.; Puig, K.; Combs, C.K. An improved approach to align and embed multiple brain samples in a gelatin-based matrix for simultaneous histological processing. J. Neurosci. Methods 2016, 261, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Arcega, R.S.; Woo, J.S.; Xu, H. Performing and Cutting Frozen Sections. In Biobanking: Methods and Protocols; Yong, W.H., Ed.; Springer: New York, NJ, USA, 2019; pp. 279–288. [Google Scholar]
- Costello, M.J. Cryo-electron microscopy of biological samples. Ultrastruct. Pathol. 2006, 30, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.R. Embedding of Tissue for Frozen Section. In A Practical Guide to Frozen Section Technique; Peters, S.R., Ed.; Springer: New York, NY, USA, 2010; pp. 37–74. [Google Scholar]
- Chen, L.; Qiu, G. One simple physical embedding technique for the polymer film to be cryoultramicrotomed. J. Microsc. Ultrastruct. 2014, 2, 117–120. [Google Scholar] [CrossRef]
- Pierson, J.; Fernandez, J.J.; Bos, E.; Amini, S.; Gnaegi, H.; Vos, M.; Bel, B.; Adolfsen, F.; Carrascosa, J.L.; Peters, P.J. Improving the technique of vitreous cryo-sectioning for cryo-electron tomography: Electrostatic charging for section attachment and implementation of an anti-contamination glove box. J. Struct. Biol. 2010, 169, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, B.R.; Chan, W.; Cheney, K.L.; Sullivan, R.K.P.; Floetenmeyer, M.; Garson, M.J.; Wepf, R. Cryo-ultramicrotomy and Mass Spectrometry Imaging Analysis of Nudibranch Microstructures. J. Am. Soc. Mass. Spectrom. 2022, 33, 592–597. [Google Scholar] [CrossRef] [PubMed]
- Grabenbauer, M.; Han, H.M.; Huebinger, J. Cryo-fixation by self-pressurized rapid freezing. In Methods Mol. Biol.; Kuo, J., Ed.; Humana Press: Totowa, NJ, USA, 2014; pp. 173–191. [Google Scholar]
- Peters, S.R. The Art of Embedding Tissue for Frozen Section. Part I: A System for Precision Face Down Cryoembedding of Tissues Using Freezing Temperature-Embedding Wells. J. Histotechnol. 2003, 26, 11–19. [Google Scholar] [CrossRef]
- Paterson, S.M.; Casadio, Y.S.; Brown, D.H.; Shaw, J.A.; Chirila, T.V.; Baker, M.V. Laser scanning confocal microscopy versus scanning electron microscopy for characterization of polymer morphology: Sample preparation drastically distorts morphologies of poly (2-hydroxyethyl methacrylate)-based hydrogels. J. Appl. Polym. Sci. 2013, 127, 4296–4304. [Google Scholar] [CrossRef]
- Trieu, H.H.; Qutubuddin, S. Polyvinyl alcohol hydrogels I. Microscopic structure by freeze-etching and critical point drying techniques. Colloid Polym. Sci. 1994, 3, 301–309. [Google Scholar] [CrossRef]
- Kaberova, Z.; Karpushkin, E.; Nevoralova, M.; Vetrik, M.; Slouf, M.; Duskova-Smrckova, M. Microscopic Structure of Swollen Hydrogels by Scanning Electron and Light Microscopies: Artifacts and Reality. Polymers 2020, 12, 578. [Google Scholar] [CrossRef]
- Wypych, G. Handbook of Polymers, 3rd ed.; Elsevier: Toronto, ON, Canada, 2022. [Google Scholar]
- Kuo, J. Processing plant tissues for ultrastructural study. In Methods in Molecular Biology 1117, 3rd ed.; Kuo, J., Ed.; Humana Press: Totowa, NJ, USA, 2014; pp. 39–55. [Google Scholar]
- Webster, P. Microwave-assisted processing and embedding for transmission electron microscopy. In Methods in Molecular Biology 1117; Kuo, J., Ed.; Humana Press: Totowa, NJ, USA, 2014; pp. 21–37. [Google Scholar]
- Mueller, J.; Meyer-Lueckel, H.; Paris, S.; Hopfenmuller, W.; Kielbassa, A.M. Inhibition of lesion progression by the penetration of resins in vitro: Influence of the application procedure. Oper. Dent. 2006, 31, 338–345. [Google Scholar] [CrossRef]
- Sands, G.B.; Gerneke, D.A.; Hooks, D.A.; Green, C.R.; Smaill, B.H.; Legrice, I.J. Automated imaging of extended tissue volumes using confocal microscopy. Microsc. Res. Tech. 2005, 67, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Saga, K. Application of cryofixation and cryoultramicrotomy for biological electron microscopy. Med. Mol. Morphol. 2005, 38, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Webster, P.; Webster, A. Cryosectioning fixed and cryoprotected biological material for immunocytochemistry. In Methods in Molecular Biology 1117; Kuo, J., Ed.; Humana Press: Totowa, NJ, USA, 2014; pp. 273–313. [Google Scholar]
- Peters, S.R. Variables Affecting the Cutting Properties of Tissues and the Resulting Artifacts. In A Practical Guide to Frozen Section Technique; Peters, S.R., Ed.; Springer: New York, NY, USA, 2010; pp. 97–116. [Google Scholar]
- Cocco, C.; Melis, G.V.; Ferri, G.L. Embedding media for cryomicrotomy: An applicative reappraisal. Appl. Immunohistochem. Mol. Morphol. 2003, 11, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Echlin, P. Handbook of Sample Preparation for Scanning Electron Microscopy and X-ray Microanalysis; Springer: New York, NY, USA, 2009. [Google Scholar]





| Method | Instrument Price ($) | Sample Preparation Price ($) | Processing Time (h) |
|---|---|---|---|
| Liquid nitrogen cryogenic fracture | / | / | 0.25 |
| BIB polishing | 120,000~290,000 | 140~220 | 3~5 |
| Frozen section technique | 25,000~38,000 | 7~15 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, H.; Zhang, X.; Li, Y.; Zhang, D.; Huang, F.; Zhang, Z. Preparation of Cross-Sectional Membrane Samples for Scanning Electron Microscopy Characterizations Using a New Frozen Section Technique. Membranes 2023, 13, 634. https://doi.org/10.3390/membranes13070634
Ren H, Zhang X, Li Y, Zhang D, Huang F, Zhang Z. Preparation of Cross-Sectional Membrane Samples for Scanning Electron Microscopy Characterizations Using a New Frozen Section Technique. Membranes. 2023; 13(7):634. https://doi.org/10.3390/membranes13070634
Chicago/Turabian StyleRen, Hongyun, Xian Zhang, Yi Li, Dandan Zhang, Fuyi Huang, and Zixing Zhang. 2023. "Preparation of Cross-Sectional Membrane Samples for Scanning Electron Microscopy Characterizations Using a New Frozen Section Technique" Membranes 13, no. 7: 634. https://doi.org/10.3390/membranes13070634
APA StyleRen, H., Zhang, X., Li, Y., Zhang, D., Huang, F., & Zhang, Z. (2023). Preparation of Cross-Sectional Membrane Samples for Scanning Electron Microscopy Characterizations Using a New Frozen Section Technique. Membranes, 13(7), 634. https://doi.org/10.3390/membranes13070634






