Abstract
Ion exchange membranes, especially cation exchange membranes (CEMs), are an important component in membrane-based energy generation and storage because of their ability to transport cations via the electrochemical potential gradient while preventing electron transport. However, developing a CEM with low areal resistance, high permselectivity, and stability remains difficult. In this study, electrospun graphene oxide/polyethersulfone (GO/PES) composite nanofibers were prepared with varying concentrations of GO. To fabricate a CEM, the pores of the electrospun GO/PES nanofiber substrates were filled with a Nafion ionomer. The pore-filled PES nanofiber loaded with 1% GO revealed a noticeable improvement in hydrophilicity, structural morphology, and mechanical properties. The 1% GO/PES pore-filled CEM was compared to a Nafion membrane of a varying thickness and without a nanofiber substrate. The CEM with a nanofiber substrate showed permselectivity of 85.75%, toughness of 111 J/m3, and areal resistance of 3.7 Ω cm2, which were 12.8%, 4.3 times, and 4.0 times better, respectively, than those of the Nafion membrane at the same thickness. The development of a reinforced concrete-like GO/PES nanofiber structure containing stretchable ionomer-enhanced membrane surfaces exhibited suitable areal resistance and reduced the thickness of the composite membrane without compromising the mechanical strength, suggesting its potential application as a cation exchange membrane in electrochemical membrane-based systems.
1. Introduction
The demand for green energy is increasing due to the environmental drawbacks associated with fossil fuels, such as greenhouse gas emissions [1,2]. Since the emergence of the concept of water security, the need for a safe and reliable water supply of quality and quantity has also gained steady attention [3,4,5]. Membrane-based electrochemical systems, under-researched and commercialized in water and energy applications, are the one option to solve the water and energy demands of the world. For water-based systems, efforts are underway to obtain industrial ultrapure water through electrodialysis (ED) and capacitive deionization (CDI), which utilize electrical forces to separate impurities in water, across ion exchange membranes (IEMs) [6,7,8,9]. For the energy production and storage system, one of the most active studied areas, efforts are underway regarding reverse electrodialysis (RED), which generates electricity through the movement of ions, fuel cell (FC), which converts chemical potential into electrical energy, microbial fuel cell (MFC), which reacts with micro-organisms to generate electricity, redox flow battery (RFB), which is attracting interest in the large-scale energy storage system, and electrolysis, which researches for hydrogen production [10,11,12,13,14,15,16,17]. They have a significantly lower carbon footprint than conventional methods and a high potential for stable and efficient energy generation and water production [18,19,20,21,22,23].
Electrochemical cells, IEMs, transport ions selectively based on charge and the relative size of the ions compared to the membrane. The development of novel IEMs entails incorporating high-selectivity (permselectivity), low-resistance, and anti-fouling properties while maintaining a high stability [10,24,25]. However, at a fixed thickness, there is a directly proportional relationship between resistance and permselectivity [11,12,13]. As a result, decreasing membrane thickness will reduce resistance to maintaining high permselectivity. Because thickness and mechanical strength are proportional, decreasing the thickness reduces mechanical strength and reduces the likelihood of successful application to electrochemical systems. Since membrane properties are interdependent, it is difficult to manipulate them separately. Nonetheless, increasing permselectivity and mechanical strength is critical for the broad practical application of IEMs. Therefore, researchers are investigating reducing membrane resistance through a material combination in order to form mixed-matrix membranes [14,15]. When two or more materials with dissimilar properties are combined, the benefits of each of the component materials are realized [26,27]. For example, combining polymeric materials with inorganic materials could provide benefits, such as ease of processing from the polymeric components and selectivity enhancement from the inorganic components. So, it could be possible to fabricate organic–inorganic hybrid membranes adjusted to the feasible condition. However, some combination between organic and inorganic materials means a negative interaction could occur, cross-linking could be reduced, or a non-uniform matrix could be produced due to the agglomeration of the membrane’s performance. For this reason, polyethersulfone and graphene oxide were hired as well as a known positive to composite.
Polyethersulfone (PES) is one of the most commonly used polymers for mixed-matrix membrane fabrication because of its good chemical and mechanical stability and the fact that it contains a sulfone functional group and ether linkage, but it has poor hydrophilicity [21,28,29]. To overcome this drawback, inorganic additives, such as graphene oxide (GO), can be used to improve the membrane’s properties. GO has recently garnered great attention for use in both electrochemical processes and desalination applications because of its structural and electronic properties [30,31,32,33]. GO is employed to improve the hydrophilicity, mechanical strength, and ion conductivity of membranes through its large surface area, hydrophilic nature, high electrochemical properties, and tensile modulus [34,35]. GO has become an appealing candidate for use in cation conducting systems due to its stability and variety of oxygen functional groups within its structure, which could assist in the development of hydrogen-bonded channels for cation transport [36]. Due to its interactions with the PES polymeric matrix, GO has been shown to improve the cation conductivity of polymeric IEMs [37,38].
To fabricate nanofibrous substrate, we hired the electrospinning method. Electro-spinning is a versatile technique used to prepare nanofibers that inject from the needle to collection plate in a high voltage [39,40]. The polymer solution is sprayed through a metallic needle, and the sprayed droplets are electrified and stretched and then obtained on a collecting plate in a fibrous structure due to electrostatic repulsion and coulomb forces [41,42,43]. These electrospun fibers could be fabricated uniformly with nanoscale diameters. This versatility allows the large-scale fabrication of natural, synthetic, and mixed-matrix nanofibers to take place. Electrospinning is rapidly emerging as a simple and reliable technique for producing various materials with adjustable morphologies, textures, thicknesses, mechanical properties, and porous structures from a variety of polymers [39,44]. It could be compared with other methods that fabricate nanofiber mats, such as melt-blowing, ultrasonic blowing, and self-assembly as well as a nanopore structure, such as phase inversion and stretching [45,46,47,48,49,50,51]. Compared to other methods, electrospinning can provide uniform nanoscale fibers, higher porosity, a large surface area, and a tunable structure by forming a pore structure in which the nanofibers are interconnected [52,53]. Also, these can be produced in a simple set-up, at a low cost, and whilst reducing waste materials or water [41,48].
Based on the electrospun nanofiber substrate, pore-filling was prepared to fabricate IEM. Pore-filling is one of the techniques used to fabricate IEM by filling nanometric size pores with conductive polymers such as Nafion, Aciplex, and Flemion [54,55,56]. The polymeric electrolyte can have an affinity for the pore-filled substrate with a high flexibility and even distribution with a uniform structure. In this study, Nafion ionomer, one of the most attractive commercialized materials for the electrochemical system, was employed for polymeric electrolyte solution [54,55,57]. This pre-fluorinated sulfonic acid polymer-based electrolyte has a high ion conductivity and good mechanical and chemical stability. So, the Nafion ionomer fully filled the void of the substrate, among electrospun nanofibers, forming a reinforced concrete-like structure.
In this study, our hypothesis is that the incorporation of GO into PES will enhance the mechanical strength and hydrophilicity of the resulting membrane. Additionally, we propose that pore-filling the membrane with Nafion ionomer will enhance its performance by creating a fully filled void structure, thereby improving ion transport and over-all membrane properties. The high mechanical strength provided by the incorporation of GO will allow a reduction in membrane thickness to occur without compromising its strength, leading to a further reduction in membrane resistance.
2. Materials and Methods
2.1. Materials
Polyethersulfone (BASF Co., Germany) was used after drying at 120 °C for at least 24 h. Graphite power (325 mesh) was purchased from Alfa Aesar, USA. Nafion solution (D2021, dispersion alcohol-based 1100 EW at 20 wt%, Fuel Cell Store, USA) was employed as the pore-filling ionomer. Deionized water (DI) was obtained from Milli-Q System (Millipore, USA). Dimethylacetamide (DMAc), hydrochloric acid (HCl, mass fraction of 35%), sulfuric acid (H2SO4, mass fraction of 98%), potassium permanganate (KMnO4), hydrogen peroxide (H2O2, mass fraction of 30%), sodium chloride (NaCl), and sodium hydroxide (NaOH) were provided by Daejung Chemical, Republic of Korea. All chemicals were used without further purification.
2.2. Nanofiber Preparation
The GO was prepared using a modified Hummers method, as described in [58,59,60]. Briefly, graphite powder was thoroughly mixed with H2SO4. The mixture was placed in an ice bath to neutralize the temperature of the exothermic reaction, after which KMnO4 was added and stirred for at least an hour until it reached a homogenous state. The solution was placed in a 35 °C water bath and stirred for 24 h. Next, H2O2 was dripped into the solution. During this process, the mixture’s color gradually changed from yellow to brown, and gas was produced as a result of oxidation. Following oxidation, the solution was centrifuged and repeatedly washed with 10% HCl solution and DI water. After washing, the solution was dialyzed for 24 h to remove impurities before being dried at 60 °C to produce dark brown GO. Figure 1a provides a concise and straight forward illustration of the concept.
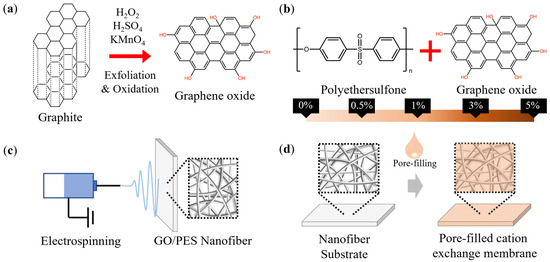
Figure 1.
The process of membrane fabrication: (a) exfoliation and oxidation of graphite to graphene oxide, (b) preparation of a GO and PES mixture, (c) electrospinning, and (d) pore-filling.
The polymer mixtures used for electrospinning were prepared by mixing the PES solution with different concentrations of the obtained GO (0, 0.5, 1, 3, 5%) to obtain five types of nanofibers (N-0, N-0.5, N-1, N-3, N-3, respectively). The mixture solutions were prepared solute/solvent w/v% as the 25 w/v%, and the percentage of GO was calculated as the weight ratio between GO. The brief scheme of the GO/PES mixture and fabrication of the nanofiber are explained in Figure 1b,c. The spinning conditions were a temperature of 20 ± 2 °C, a humidity of 50% ± 5%, and a spinning time of 40 min. Nanofibers were collected on a drum with an aluminum plate that rotated at 200 rpm. A lab-scale electrospinning setup (NanoNC Co., Ltd., Republic of Korea) was used to fabricate the nanofibers.
2.3. Pore-Filling
The pore-filling of the nanofiber is simply shown as Figure 1d. The Nafion ionomer was used for pore-filling after dilution with ethanol at a 1:3 v/v ratio. The nanofiber mat was placed in a Petri dish, and the prepared Nafion solution was poured slowly and evenly over the top. The membrane was heat treated on a hot plate at 50 °C for 12 h and then at 80 °C for one hour. The pore-filled membrane was named “pore-filled cation exchange membrane (PFM)” for this study. Three kinds of nanofiber-free cast Nafion membranes (CNM-1, CNM-2, CNM-3) were fabricated to compare the relationship between thickness and pore-filling. CNM-1 was fabricated with the same amount of Nafion solution that was used to prepare PFM. CNM-2 and CNM-3 were prepared with an additional 5 mL and 10 mL of Nafion solution compared to CNM-1, respectively. Except for the amount of Nafion solution used, the entire fabrication process was identical to that of PFM.
2.4. Characterization
The morphology of the GO material and membranes was observed using a field emission scanning electron microscope (FE-SEM, Hitachi, S-4700, Japan), and the fiber diameter was calculated using image software (https://imagej.nih.gov/ij/download.html, accessed on 25 April 2023). The water contact angle (WCA) was measured with a contact goniometer (Phoenix 300, Surface Electro Optics Co., Ltd., Republic of Korea), which dropped a single water droplet onto the dried samples and took images in five seconds. Toughness was analyzed to determine the mechanical strength of the material. The definition of toughness is the ability to withstand elastic deformation, a measure of the amount of energy a material can absorb before actual cracking or fracture occurs. Based on this definition, a universal testing machine (Instron 5567, USA) was used to simultaneously measure the tensile strength and strain to obtain a graph, which was then integrated to determine the amount of energy required to fracture the membrane. The thermogravimetric analyzer (TGA, TGA8000, Perkin Elmer, USA) was measured for thermal stability until 700 °C by 20 °C/min in N2 atmosphere. The composition of the nanofibers was analyzed using an X-ray diffraction (XRD) spectroscope (D/Max-2500, Rigaku, Japan) and Raman spectroscopy (LabRAM HR Evolution, Horiba, France). A zeta potential analyzer (ELSZ-2000, Otsuka Electronics Corp., Japan) was also used to measure the zeta potential of the membrane surface.
A two-compartment cell experiment with direct current was used to measure permselectivity (α). The electrolyte solutions were prepared with 0.1 M and 0.5 M sodium chloride concentrations, and a membrane was placed between the two compartments as described in Figure 2. The solutions were continuously stirred, and voltage (∆Vmeasured) was measured with a multimeter and an Ag/AgCl electrode. As a result, permselectivity was calculated using Equation (1), where F is the Faraday constant, R is the gas constant, T is the absolute temperature, z is the valence of the ions, and CH and CL are the high and low salinity concentrations, respectively.
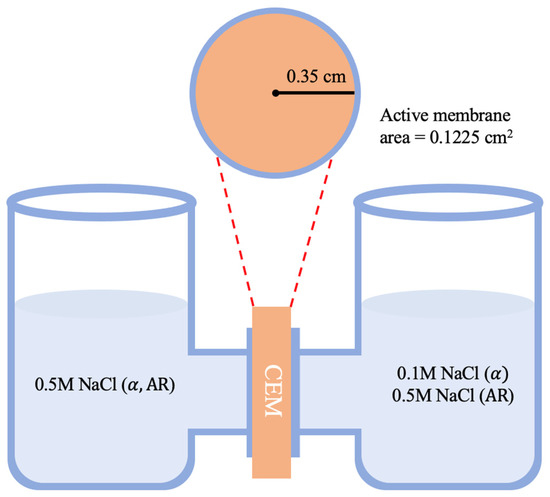
Figure 2.
Schematic diagram of two-compartment cell experiments for permselectivity and areal resistance.
The two-compartment cell was also used to estimate the areal resistance as described in Figure 2. The electrolyte was 0.5 M NaCl solution, and the active membrane area was 0.1225 cm2. The calculation was performed using Equation (4).
The weight difference between dry and wet membranes was used to calculate the water uptake (WU). The wet membrane was dried for at least 24 h at 80 °C in a dry oven then soaked in water for 24 h to ensure the complete absorption of water molecules into the membrane. Any excess water on the membrane’s surface was removed. The WU was calculated using Equation (5), where wwet and wdry represent the membrane weight in wet and dry states, respectively.
Titrations with NaCl and NaOH solutions were used to calculate the ion exchange capacity (IEC). After immersing the membrane in a 2 M NaCl solution for 24 h, the solution containing the membrane was titrated with a 0.1 M NaOH solution until a pH of 7 was reached. Therefore, the calculation was performed as shown in Equation (6), where CNaOH and VNaOH are the concentration and volume of the titrated NaOH solution, respectively, and mdry is the weight of the dry membrane.
3. Results
3.1. Effect of GO on the Electrospun PES Nanofiber
Figure 3 depicts the digital image, SEM image, and XRD data of the GO prepared using a modified Hummers method. The SEM image revealed a wrinkled and multi-layered surface for the GO nanosheets. Furthermore, XRD data indicated that the prepared GO had a significant peak near 2θ = 10° [61]. Evidently, as-prepared GO was exfoliated from graphite and successfully oxidized.
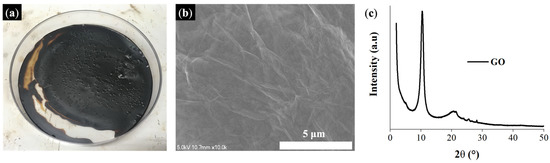
Figure 3.
Prepared GO: (a) digital picture of GO, (b) SEM image of GO, and (c) XRD analysis of GO.
Figure 4 shows the digital pictures, SEM images, and thickness profiles of electrospun nanofibers at various GO concentrations. The brown color of the GO in nanofiber membranes became more pronounced (i.e., from white to brown) as the concentration of GO increased from left to right in the digital images. As shown in the SEM images of each nanofiber and its diameter distribution, there was no evidence of a significant change in the nanofiber diameter and structure due to a change in the GO concentration, implying that adding GO had no effect on the fiber’s condition.
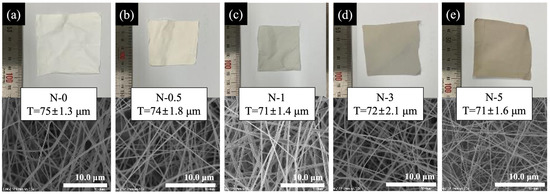
Figure 4.
Digital picture and SEM image; thickness profiles of electrospun nanofibers: (a) N-0, (b) N-0.5, (c) N-1, (d) N-3, (e) N-5.
The thickness of the nanofiber mats was consistent because the same volume of solution was injected for the same amount of time. Figure 5 depicts an XRD and Raman spectroscopy analysis of nanofibers to confirm the status of composition between GO and PES. In the XRD analysis, the peak of N-5 containing 5% GO was similar to the peak of GO at 2θ = 10°. In the Raman spectroscopy, N-1 showed a higher intensity near 1500 and 2800 cm−1 where the GO peaks compared to N-0. The results imply that GO and PES were successfully combined.
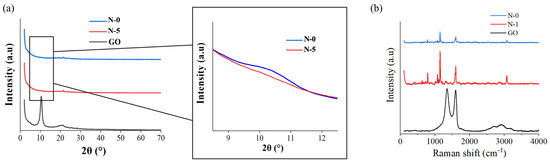
Figure 5.
XRD and Raman spectroscopy comparison among GO and nanofibers: (a) XRD data of N-0, N-5, GO; (b) Raman data of N-0, N-1, GO.
When GO was added to PES, the properties changed. As shown in Table 1, the WCA of the nanofibers with GO decreased by approximately 10° compared to that of the pure PES nanofiber, reaching a minimum of 63.41° at N-5. Therefore, regardless of the concentration, the hydrophilicity of the nanofiber membrane was improved when GO was present. As also shown in Table 1, adding GO improved the toughness over pristine PES nanofibers. Among the nanofibers, N-1 with 1% GO demonstrated the highest mechanical strength. It was deduced that when GO and PES were combined, a strong bond was formed between the functional groups of the materials, increasing the strength of the membrane [59,60].

Table 1.
Properties of nanofibers.
Furthermore, the electrochemical properties of GO may aid in drawing the electrospinning jet more evenly and uniformly than with pristine PES, thereby improving the mechanical strength of the nanofiber [43,62]. However, when the amount of GO exceeded the binding stoichiometry between GO and PES, the mechanical strength of the membrane decreased, as shown in Table 1 and Figure 6, with N-3 and N-5 being weaker than N-1. Moreover, when the nanofibers were spun, excess GO coagulated, caused by a higher concentration of GO. The coagulated GO regions acted as a defect, reducing the mechanical strength. Since tensile strength and strain have the same tendency as toughness, it was concluded that N-1 had the best mechanical strength.
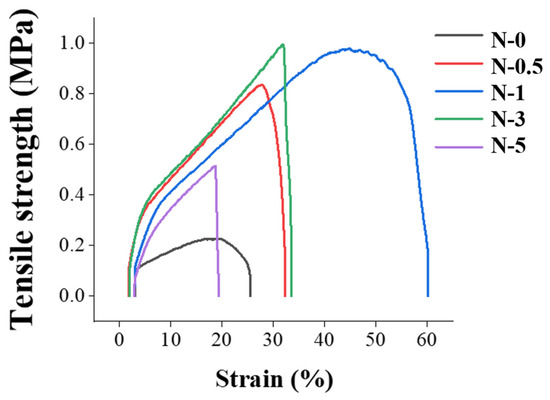
Figure 6.
Tensile strength–strain curves of nanofibers.
3.2. Effects of the Pore-Filled Structure Design on Membrane Performance
Following the comparison of nanofibers, N-1 was chosen as the pore-filling substrate. Therefore, PFM was fabricated using the smooth and polymeric Nafion ionomer solution as the pore-filling electrolyte, which filled the pores of the N-1 nanofiber, as shown in the SEM image in Figure 7. The Nafion ionomer fully filled the void of the substrate, among electrospun nanofibers, forming a reinforced concrete-like structure. It was confirmed that there were no voids inside the PFM through SEM images. Thus, the pore-filling procedure was completed successfully.
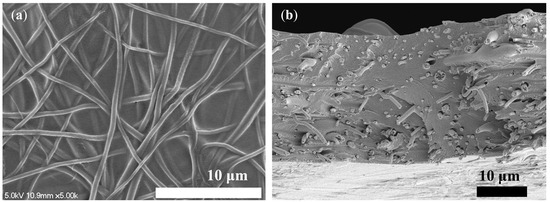
Figure 7.
SEM image of the pore-filled membrane. (a) top view; (b) cross-sectional view.
Figure 8a shows the tensile strength–strain curves of PFM, CNM-1, CNM-2, and CNM-3. PFM and CNM-1 had the same thickness because the same volume of pore-filling electrolyte was used. As the volume of the pore-filling solution increased, the thickness of CNM-2 and CNM-3 increased. Correspondingly, membranes could be compared in terms of the mechanical properties, such as toughness, in relation to thickness differences. The curves were increased by the thicker casted Nafion membrane, respectively. The toughness of cast Nafion membranes without a nanofiber mat was proportional to thickness. Casted Nafion membranes showed a higher strain than PFM; however, they were not enduring the tensile. So, PFM, the thinnest membrane with a nanofiber substrate, had the highest toughness overall. The structure improved via pore-filling with Nafion ionomer into the nanofiber with a higher mechanical stability. This is analogous to the formation of a reinforced concrete structure [63,64]. Therefore, improving the membrane structure may result in increased toughness despite the lower thickness.
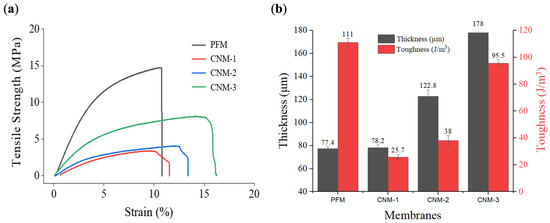
Figure 8.
Mechanical stability of membranes: (a) tensile strength–strain curves of membranes; (b) relationship between thickness and toughness of membranes.
Figure 9 shows the TGA curves of the membranes. The casted Nafion membranes show that decreases in similar curves started near 400 °C and dramatically dropped near 500 °C. However, PFM, containing the GO/PES composite nanofiber, loses weight faster than casted Nafion membranes. GO does not perform in a way which ensures a high-temperature membrane, as the references reported [65,66]. PES has a higher thermal stability than other materials (GO, Nafion); however, its weight ratio of PES in the membrane, mostly composed by Nafion, is not enough to induce an increase in the thermal stability [67,68].
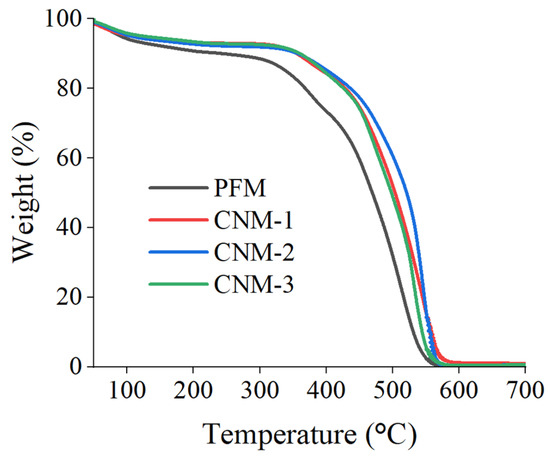
Figure 9.
TGA curves of membranes.
Table 2 shows the physical and electrochemical properties of the membranes. PFM had a lower surface charge than the CNMs because of the GO which was added to the nanofiber. PES had a surface charge of approximately −30 mV and GO had a surface charge of approximately −50 mV at a pH of 7 [69,70]. Therefore, incorporating GO into the nanofiber resulted in a difference in the zeta potential. Higher WU and permselectivity could be caused by the negative surface charge. In the case of the CNMs, the WU was proportional to membrane thickness, implying that the thicker the membrane, the more water molecules which can be contained. In the case of PFM, WU was improved by 12% when compared to CNM-1 at the same thickness. This result was caused by the negative surface charge of PFM, which attracted water molecules and thus increased hydrophilicity [60,71]. The lower WCA of PFM compared to the CNMs demonstrated increased hydrophilicity. The permselectivity of PFM was 13% higher than that of CNM-1 and slightly higher than CNM-2. The lower zeta potential, which could effectively reject co-ions while allowing counter-ions, explained the high permselectivity at the same thickness. The higher hydrophilicity and WU of PFM, in accordance with the general ion transfer mechanism inside the membranes, can induce more effective ion transport.

Table 2.
Physical and electrochemical properties of the membranes.
According to the IEC, the number of ions that can be contained by a thicker membrane is proportional to its thickness. PFM had an IEC of 0.88 meq/gdry, which is 3.3% lower than CNM-1′s IEC of 0.91 meq/gdry. The structure of PFM could hinder ion exchange due to the presence of a nanofiber substrate inside the membrane. This substrate may act as a barrier and create a lack of electrolyte-filled pores, which could impede the movement of ions across the membrane. PFM had up to four times the areal resistance of CNM-1, the thinnest membrane, and six times the areal resistance of CNM-3, the thickest membrane. This may be explained similarly to permselectivity. The area resistance is proportional to the thickness; as the thickness increases, the ion passage decreases, and the area resistance increases. The high WU of PFM allowed effective ion transport to take place, and the negative surface charge promoted counter-ion penetration through the membrane [13]. Therefore, PFM, the thinnest cation exchange membrane, outperforms the thickness-controlled Nafion membranes.
4. Conclusions
In this study, the cation exchange membrane was fabricated by the sequence of electrospinning for nanofiber substrate and pore-filling. An electrospun nanofiber was selected from five different concentrations of GO based on the PES polymer. The addition of GO increased the electrochemical and mechanical properties of the PES nanofiber due to the more negatively charged surface that induced a hydrophilic atmosphere and bonding force between the polymer molecules. Furthermore, by combining PES and 1% GO, the nanofiber achieved a toughness of up to 39.9 J/m3. Based on this substrate, for pore-filling followed by the Nafion ionomer, we fabricated a pore-filled cation exchange membrane with electrolyte solution which fully filled the void of the substrate. This was compared with three different recast Nafion membranes in order to demonstrate it structurally modified formation, high strength, high permselectivity, high hydrophilicity, and low areal resistance. The mechanical strength was greatly improved up to 111.4 J/m3 compared to that of the nanofiber mat alone and the cast Nafion membrane. The strength could be further improved by combining high-tensile-strength nanofibers with stretchable Nafion electrolyte. This structural engineering resulted in an IEM with a reinforced concrete-like structure. Even as its thickness decreased, the IEM exhibited improved mechanical strength and resistance due to the smoother flow of ions through ion channel structures provided by the nanofibers. Furthermore, because GO increased the membrane’s zeta potential, a more negatively charged membrane surface effectively selected ions to pass or reject, increasing permselectivity up to 85.75% and decreasing areal resistance up to 3.7 Ω cm2. In conclusion, the membrane designed in this paper improved mechanical strength, permselectivity, and areal resistance through material combination via electrospinning and structure modification with pore-filling and has a promising future applied to system adaptation.
Author Contributions
Conceptualization, J.J., Y.K. and I.S.K.; formal analysis, J.J. and Y.K.; investigation, S.K. and A.B.A.; methodology, A.B.A. and E.Y.; supervision, M.-H.H. and K.-J.C.; writing—original draft, S.K. and T.E.; writing—review and editing, A.B.A., M.-H.H. and K.-J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Korea Institute of Marine Science & Technology Promotion (KIMST) funded by the Ministry of Oceans and Fisheries, Republic of Korea (20210500).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- Tian, M.; Wang, R.; Goh, K.; Liao, Y.; Fane, A.G. Synthesis and Characterization of High-Performance Novel Thin Film Nanocomposite PRO Membranes with Tiered Nanofiber Support Reinforced by Functionalized Carbon Nanotubes. J. Membr. Sci. 2015, 486, 151–160. [Google Scholar] [CrossRef]
- Hickenbottom, K.L.; Vanneste, J.; Elimelech, M.; Cath, T.Y. Assessing the Current State of Commercially Available Membranes and Spacers for Energy Production with Pressure Retarded Osmosis. Desalination 2016, 389, 108–118. [Google Scholar] [CrossRef]
- ESCAP U.N. Water Security & the Global Water Agenda: A UN-Water Analytical Brief; United Nations University (UNU): Tokyo, Japan, 2013; ISBN 9280860380. [Google Scholar]
- Griffiths, J.; Lambert, R. Free Flow: Reaching Water Security through Cooperation; UNESCO: London, UK, 2013; ISBN 9231042564. [Google Scholar]
- Gerlak, A.K.; House-Peters, L.; Varady, R.G.; Albrecht, T.; Zúñiga-Terán, A.; de Grenade, R.R.; Cook, C.; Scott, C.A. Water Security: A Review of Place-Based Research. Environ. Sci. Policy 2018, 82, 79–89. [Google Scholar] [CrossRef]
- Campione, A.; Gurreri, L.; Ciofalo, M.; Micale, G.; Tamburini, A.; Cipollina, A. Electrodialysis for Water Desalination: A Critical Assessment of Recent Developments on Process Fundamentals, Models and Applications. Desalination 2018, 434, 121–160. [Google Scholar] [CrossRef]
- Oren, Y. Capacitive Deionization (CDI) for Desalination and Water Treatment—Past, Present and Future (a Review). Desalination 2008, 228, 10–29. [Google Scholar] [CrossRef]
- AlMarzooqi, F.A.; Al Ghaferi, A.A.; Saadat, I.; Hilal, N. Application of Capacitive Deionisation in Water Desalination: A Review. Desalination 2014, 342, 3–15. [Google Scholar] [CrossRef]
- Al-Amshawee, S.; Yunus, M.Y.B.M.; Azoddein, A.A.M.; Hassell, D.G.; Dakhil, I.H.; Hasan, H.A. Electrodialysis Desalination for Water and Wastewater: A Review. Chem. Eng. J. 2020, 380, 122231. [Google Scholar] [CrossRef]
- Park, S.G.; Rajesh, P.P.; Hwang, M.H.; Chu, K.H.; Cho, S.; Chae, K.J. Long-Term Effects of Anti-Biofouling Proton Exchange Membrane Using Silver Nanoparticles and Polydopamine on the Performance of Microbial Electrolysis Cells. Int. J. Hydrogen Energy 2021, 46, 11345–11356. [Google Scholar] [CrossRef]
- Geise, G.M.; Hickner, M.A.; Logan, B.E. Ionic Resistance and Permselectivity Tradeoffs in Anion Exchange Membranes. ACS Appl. Mater. Interfaces 2013, 5, 10294–10301. [Google Scholar] [CrossRef]
- Kim, T.N.; Lee, J.; Choi, J.H.; Ahn, J.H.; Yang, E.; Hwang, M.H.; Chae, K.J. Tunable Atomic Level Surface Functionalization of a Multi-Layered Graphene Oxide Membrane to Break the Permeability-Selectivity Trade-off in Salt Removal of Brackish Water. Sep. Purif. Technol. 2021, 274, 119047. [Google Scholar] [CrossRef]
- Kallem, P.; Yanar, N.; Choi, H. Nanofiber-Based Proton Exchange Membranes: Development of Aligned Electrospun Nanofibers for Polymer Electrolyte Fuel Cell Applications. ACS Sustain. Chem. Eng. 2019, 7, 1808–1825. [Google Scholar] [CrossRef]
- Siddique, T.; Dutta, N.K.; Choudhury, N.R. Mixed-Matrix Membrane Fabrication for Water Treatment. Membranes 2021, 11, 557. [Google Scholar] [CrossRef]
- Muntha, S.T.; Kausar, A.; Siddiq, M. A Review Featuring Fabrication, Properties, and Application of Polymeric Mixed Matrix Membrane Reinforced with Different Fillers. Polym. Plast. Technol. Eng. 2017, 56, 2043–2064. [Google Scholar] [CrossRef]
- Mulder, F.M.; Weninger, B.M.H.; Middelkoop, J.; Ooms, F.G.B.; Schreuders, H. Efficient Electricity Storage with a Battolyser, an Integrated Ni–Fe Battery and Electrolyser. Energy Environ. Sci. 2017, 10, 756–764. [Google Scholar] [CrossRef]
- Schalenbach, M.; Tjarks, G.; Carmo, M.; Lueke, W.; Mueller, M.; Stolten, D. Acidic or Alkaline? Towards a New Perspective on the Efficiency of Water Electrolysis. J. Electrochem. Soc. 2016, 163, F3197. [Google Scholar] [CrossRef]
- Długołęcki, P.; Nymeijer, K.; Metz, S.; Wessling, M. Current Status of Ion Exchange Membranes for Power Generation from Salinity Gradients. J. Membr. Sci. 2008, 319, 214–222. [Google Scholar] [CrossRef]
- Logan, B.E.; Elimelech, M. Membrane-Based Processes for Sustainable Power Generation Using Water. Nature 2012, 488, 313–319. [Google Scholar] [CrossRef]
- Khoiruddin; Aryanti, P.T.P.; Hakim, A.N.; Wenten, I.G. The Role of Ion-Exchange Membrane in Energy Conversion. AIP Conf. Proc. 2017, 1840, 090006. [Google Scholar] [CrossRef]
- Ran, J.; Wu, L.; He, Y.; Yang, Z.; Wang, Y.; Jiang, C.; Ge, L.; Bakangura, E.; Xu, T. Ion Exchange Membranes: New Developments and Applications. J. Membr. Sci. 2017, 522, 267–291. [Google Scholar] [CrossRef]
- Di Noto, V.; Vezzù, K.; Crivellaro, G.; Pagot, G.; Sun, C.; Meda, L.; Rutkowska, I.A.; Kulesza, P.J.; Zawodzinski, T.A. A General Electrochemical Formalism for Vanadium Redox Flow Batteries. Electrochim. Acta 2022, 408, 139937. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, C.; Ge, M. Review of the Research Status of Cost-Effective Zinc–Iron Redox Flow Batteries. Batteries 2022, 8, 202. [Google Scholar] [CrossRef]
- Kim, B.; Yang, E.; Kim, B.; Obaid, M.; Jang, J.K.; Chae, K.J. Recent Application of Nanomaterials to Overcome Technological Challenges of Microbial Electrolysis Cells. Nanomaterials 2022, 12, 1316. [Google Scholar] [CrossRef]
- Yang, E.; Alayande, A.B.; Goh, K.; Kim, C.M.; Chu, K.H.; Hwang, M.H.; Ahn, J.H.; Chae, K.J. 2D Materials-Based Membranes for Hydrogen Purification: Current Status and Future Prospects. Int. J. Hydrogen Energy 2021, 46, 11389–11410. [Google Scholar] [CrossRef]
- Yin, J.; Deng, B. Polymer-Matrix Nanocomposite Membranes for Water Treatment. J. Membr. Sci. 2015, 479, 256–275. [Google Scholar] [CrossRef]
- Casado-Coterillo, C. Mixed Matrix Membranes. Membranes 2019, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Fahmi, M.Z.; Wathoniyyah, M.; Khasanah, M.; Rahardjo, Y.; Wafiroh, S.; Abdulloh. Incorporation of Graphene Oxide in Polyethersulfone Mixed Matrix Membranes to Enhance Hemodialysis Membrane Performance. RSC Adv. 2018, 8, 931–937. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, H.; Hsiao, B.S.; Chu, B.; Tsou, A.H. Improvement of Meltdown Temperature of Lithium-Ion Battery Separator Using Electrospun Polyethersulfone Membranes. Polymer 2016, 107, 163–169. [Google Scholar] [CrossRef]
- Gahlot, S.; Sharma, P.P.; Gupta, H.; Kulshrestha, V.; Jha, P.K. Preparation of Graphene Oxide Nano-Composite Ion-Exchange Membranes for Desalination Application. RSC Adv. 2014, 4, 24662–24670. [Google Scholar] [CrossRef]
- Leong, Z.Y.; Lu, G.; Yang, H.Y. Three-Dimensional Graphene Oxide and Polyvinyl Alcohol Composites as Structured Activated Carbons for Capacitive Desalination. Desalination 2019, 451, 172–181. [Google Scholar] [CrossRef]
- Abdelkader, B.A.; Antar, M.A.; Laoui, T.; Khan, Z. Development of Graphene Oxide-Based Membrane as a Pretreatment for Thermal Seawater Desalination. Desalination 2019, 465, 13–24. [Google Scholar] [CrossRef]
- Giwa, A.; Hasan, S.W. Novel Polyethersulfone-Functionalized Graphene Oxide (PES-FGO) Mixed Matrix Membranes for Wastewater Treatment. Sep. Purif. Technol. 2020, 241, 116735. [Google Scholar] [CrossRef]
- Movil, O.; Soc, J.E.; Movil, O.; Frank, L.; Staser, J.A. Graphene Oxide—Polymer Nanocomposite Anion-Exchange Membranes. J. Electrochem. Soc. 2015, 162, F419–F426. [Google Scholar] [CrossRef]
- Theophile, N.; Jeong, H.K. Electrochemical Properties of Poly (Vinyl Alcohol) and Graphene Oxide Composite for Supercapacitor Applications. Chem. Phys. Lett. 2017, 669, 125–129. [Google Scholar] [CrossRef]
- Karim, M.R.; Hatakeyama, K.; Matsui, T.; Takehira, H.; Taniguchi, T.; Koinuma, M.; Matsumoto, Y.; Akutagawa, T.; Nakamura, T.; Noro, S.; et al. Graphene Oxide Nanosheet with High Proton Conductivity. J. Am. Chem. Soc. 2013, 135, 8097–8100. [Google Scholar] [CrossRef]
- Kim, Y.; Ketpang, K.; Jaritphun, S.; Park, J.S.; Shanmugam, S. A Polyoxometalate Coupled Graphene Oxide–Nafion Composite Membrane for Fuel Cells Operating at Low Relative Humidity. J. Mater. Chem. A Mater. 2015, 3, 8148–8155. [Google Scholar] [CrossRef]
- Oh, K.; Son, B.; Sanetuntikul, J.; Shanmugam, S. Polyoxometalate Decorated Graphene Oxide/Sulfonated Poly(Arylene Ether Ketone) Block Copolymer Composite Membrane for Proton Exchange Membrane Fuel Cell Operating under Low Relative Humidity. J. Membr. Sci. 2017, 541, 386–392. [Google Scholar] [CrossRef]
- Zhang, C.L.; Yu, S.H. Nanoparticles Meet Electrospinning: Recent Advances and Future Prospects. Chem. Soc. Rev. 2014, 43, 4423–4448. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A Fascinating Method for the Preparation of Ultrathin Fibers. Angew. Chem.-Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef]
- Zhang, W.; He, Z.; Han, Y.; Jiang, Q.; Zhan, C.; Zhang, K.; Li, Z.; Zhang, R. Structural Design and Environmental Applications of Electrospun Nanofibers. Compos. Part A Appl. Sci. Manuf. 2020, 137, 106009. [Google Scholar] [CrossRef]
- Ma, X.H.; Yang, Z.; Yao, Z.K.; Guo, H.; Xu, Z.L.; Tang, C.Y. Interfacial Polymerization with Electrosprayed Microdroplets: Toward Controllable and Ultrathin Polyamide Membranes. Environ. Sci. Technol. Lett. 2018, 5, 117–122. [Google Scholar] [CrossRef]
- Barakat, N.A.M.; Kanjwal, M.A.; Sheikh, F.A.; Kim, H.Y. Spider-Net within the N6, PVA and PU Electrospun Nanofiber Mats Using Salt Addition: Novel Strategy in the Electrospinning Process. Polymer 2009, 50, 4389–4396. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Lalia, B.S.; Hashaikeh, R. A Review on Electrospinning for Membrane Fabrication: Challenges and Applications. Desalination 2015, 356, 15–30. [Google Scholar] [CrossRef]
- Jung, J.T.; Kim, J.F.; Wang, H.H.; di Nicolo, E.; Drioli, E.; Lee, Y.M. Understanding the Non-Solvent Induced Phase Separation (NIPS) Effect during the Fabrication of Microporous PVDF Membranes via Thermally Induced Phase Separation (TIPS). J. Membr. Sci. 2016, 514, 250–263. [Google Scholar] [CrossRef]
- Wang, H.; Ding, S.; Zhu, H.; Wang, F.; Guo, Y.; Zhang, H.; Chen, J. Effect of Stretching Ratio and Heating Temperature on Structure and Performance of PTFE Hollow Fiber Membrane in VMD for RO Brine. Sep. Purif. Technol. 2014, 126, 82–94. [Google Scholar] [CrossRef]
- Hartgerink, J.D.; Beniash, E.; Stupp, S.I. Self-Assembly and Mineralization of Peptide-Amphiphile Nanofibers. Science 2001, 294, 1684–1688. [Google Scholar] [CrossRef]
- Odularu, A.T. Basic Principles of Electrospinning, Mechanisms, Nanofibre Production, and Anticancer Drug Delivery. J. Chem. 2022, 2022, 9283325. [Google Scholar] [CrossRef]
- Sinha-Ray, S.; Zhang, W.; Sahu, R.P.; Sinha-Ray, S.; Yarin, A.L. Pool Boiling of Novec 7300 and DI Water on Nano-Textured Heater Covered with Supersonically-Blown or Electrospun Polymer Nanofibers. Int. J. Heat Mass Transf. 2017, 106, 482–490. [Google Scholar] [CrossRef]
- Hassan, M.A.; Yeom, B.Y.; Wilkie, A.; Pourdeyhimi, B.; Khan, S.A. Fabrication of Nanofiber Meltblown Membranes and Their Filtration Properties. J. Membr. Sci. 2013, 427, 336–344. [Google Scholar] [CrossRef]
- Duraikkannu, S.L.; Castro-Muñoz, R.; Figoli, A. A Review on Phase-Inversion Technique-Based Polymer Microsphere Fabrication. Colloid Interface Sci. Commun. 2021, 40, 100329. [Google Scholar] [CrossRef]
- Kumar, V.V.; Balaganesan, G.; Lee, J.K.Y.; Neisiany, R.E.; Surendran, S.; Ramakrishna, S. A Review of Recent Advances in Nanoengineered Polymer Composites. Polymers 2019, 11, 644. [Google Scholar] [CrossRef]
- Chae, K.-J.; Kim, K.-Y.; Choi, M.-J.; Yang, E.; Kim, I.S.; Ren, X.; Lee, M. Sulfonated Polyether Ether Ketone (SPEEK)-Based Composite Proton Exchange Membrane Reinforced with Nanofibers for Microbial Electrolysis Cells. Chem. Eng. J. 2014, 254, 393–398. [Google Scholar] [CrossRef]
- Ramya, K.; Velayutham, G.; Subramaniam, C.K.; Rajalakshmi, N.; Dhathathreyan, K.S. Effect of Solvents on the Characteristics of Nafion®/PTFE Composite Membranes for Fuel Cell Applications. J. Power Sources 2006, 160, 10–17. [Google Scholar] [CrossRef]
- Rodgers, M.P.; Berring, J.; Holdcroft, S.; Shi, Z. The Effect of Spatial Confinement of Nafion® in Porous Membranes on Macroscopic Properties of the Membrane. J. Membr. Sci. 2008, 321, 100–113. [Google Scholar] [CrossRef]
- Kim, K.; Kim, S.-K.; Park, J.O.; Choi, S.-W.; Kim, K.-H.; Ko, T.; Pak, C.; Lee, J.-C. Highly Reinforced Pore-Filling Membranes Based on Sulfonated Poly(Arylene Ether Sulfone)s for High-Temperature/Low-Humidity Polymer Electrolyte Membrane Fuel Cells. J. Membr. Sci. 2017, 537, 11–21. [Google Scholar] [CrossRef]
- Gloukhovski, R.; Freger, V.; Tsur, Y. Understanding Methods of Preparation and Characterization of Pore-Filling Polymer Composites for Proton Exchange Membranes: A Beginner’s Guide. Rev. Chem. Eng. 2018, 34, 455–479. [Google Scholar] [CrossRef]
- Jang, J.; Park, I.; Chee, S.S.; Song, J.H.; Kang, Y.; Lee, C.; Lee, W.; Ham, M.H.; Kim, I.S. Graphene Oxide Nanocomposite Membrane Cooperatively Cross-Linked by Monomer and Polymer Overcoming the Trade-off between Flux and Rejection in Forward Osmosis. J. Membr. Sci. 2019, 470, 117684. [Google Scholar] [CrossRef]
- Kang, Y.; Obaid, M.; Jang, J.; Kim, I.S. Sulfonated Graphene Oxide Incorporated Thin Film Nanocomposite Nanofiltration Membrane to Enhance Permeation and Antifouling Properties. Desalination 2019, 470, 114125. [Google Scholar] [CrossRef]
- Kang, Y.; Obaid, M.; Jang, J.; Ham, M.H.; Kim, I.S. Novel Sulfonated Graphene Oxide Incorporated Polysulfone Nanocomposite Membranes for Enhanced-Performance in Ultrafiltration Process. Chemosphere 2018, 207, 581–589. [Google Scholar] [CrossRef]
- Rag, S.A.; Selvakumar, M.; Bhat, S.; Chidangil, S.; De, S. Synthesis and Characterization of Reduced Graphene Oxide for Supercapacitor Application with a Biodegradable Electrolyte. J. Electron. Mater. 2020, 49, 985–994. [Google Scholar] [CrossRef]
- Chen, H.; Elabd, Y.A. Polymerized Ionic Liquids: Solution Properties and Electrospinning. Macromolecules 2009, 42, 3368–3373. [Google Scholar] [CrossRef]
- Jang, J.; Kang, Y.; Kim, K.; Kim, S.; Son, M.; Chee, S.S.; Kim, I.S. Concrete-Structured Nafion@MXene/Cellulose Acetate Cation Exchange Membrane for Reverse Electrodialysis. J. Membr. Sci. 2022, 646, 120239. [Google Scholar] [CrossRef]
- Hollaway, L.C. High Performance Fibre-Reinforced Composites for Sustainable Energy Applications. In High Performance Textiles and Their Applications; Lawrence, C.A., Ed.; Woodhead Publishing Series in Textiles; Woodhead Publishing: Amsterdam, The Netherlands, 2014; pp. 366–417. ISBN 9780857099075. [Google Scholar]
- Kuila, T.; Mishra, A.K.; Khanra, P.; Kim, N.H.; Lee, J.H. Recent Advances in the Efficient Reduction of Graphene Oxide and Its Application as Energy Storage Electrode Materials. Nanoscale 2013, 5, 52–71. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, D.; Li, B.; Wang, J.; Han, S.; Liu, L.; Wei, H. A Bio-Inspired Nacre-like Layered Hybrid Structure of Calcium Carbonate under the Control of Carboxyl Graphene. CrystEngComm 2015, 17, 520–525. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Mohshim, D.F.; Nasir, R.; Mannan, H.A.; Mukhtar, H. Effect of Solvents on the Morphology and Performance of Polyethersulfone (PES) Polymeric Membranes Material for CO2/CH4 Separation. IOP Conf. Ser. Mater. Sci. Eng. 2018, 290, 012074. [Google Scholar] [CrossRef]
- Mollá, S.; Compañ, V. Polyvinyl Alcohol Nanofiber Reinforced Nafion Membranes for Fuel Cell Applications. J. Membr. Sci. 2011, 372, 191–200. [Google Scholar] [CrossRef]
- Salinas-Rodriguez, S.G.; Amy, G.L.; Schippers, J.C.; Kennedy, M.D. The Modified Fouling Index Ultrafiltration Constant Flux for Assessing Particulate/Colloidal Fouling of RO Systems. Desalination 2015, 365, 79–91. [Google Scholar] [CrossRef]
- Konkena, B.; Vasudevan, S. Understanding Aqueous Dispersibility of Graphene Oxide and Reduced Graphene Oxide through PKa Measurements. J. Phys. Chem. Lett. 2012, 3, 867–872. [Google Scholar] [CrossRef]
- Alipour Moghaddam, J.; Parnian, M.J.; Rowshanzamir, S. Preparation, Characterization, and Electrochemical Properties Investigation of Recycled Proton Exchange Membrane for Fuel Cell Applications. Energy 2018, 161, 699–709. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).