Ion–Conducting Ceramic Membrane Reactors for the Conversion of Chemicals
Abstract
1. Introduction
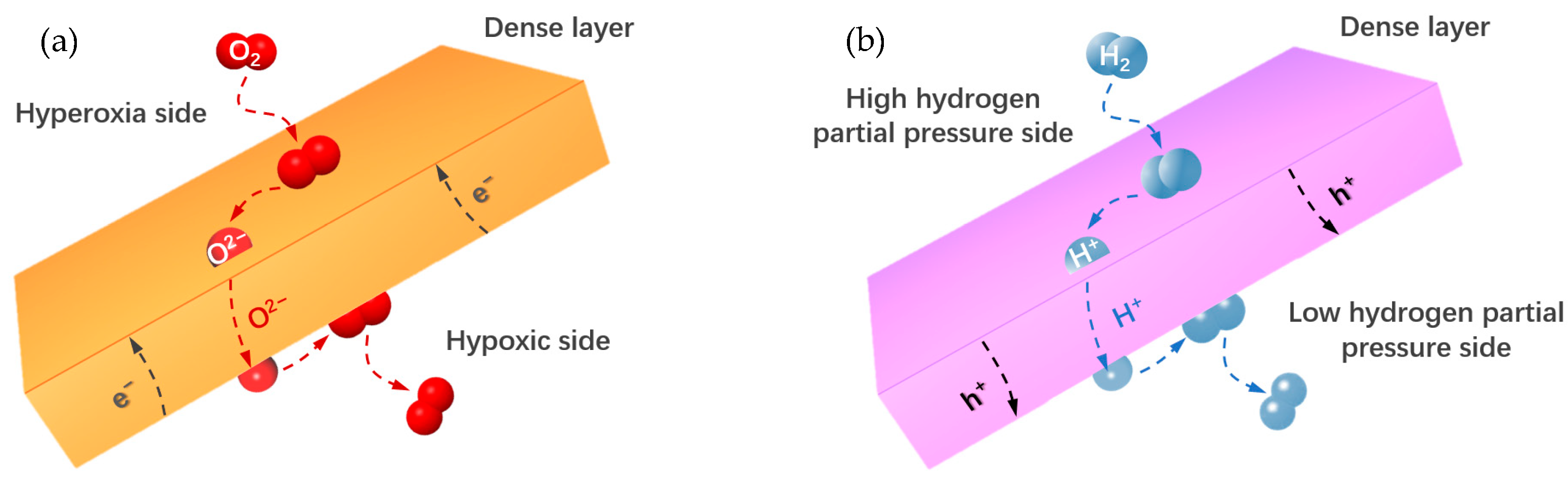
2. Fundamentals
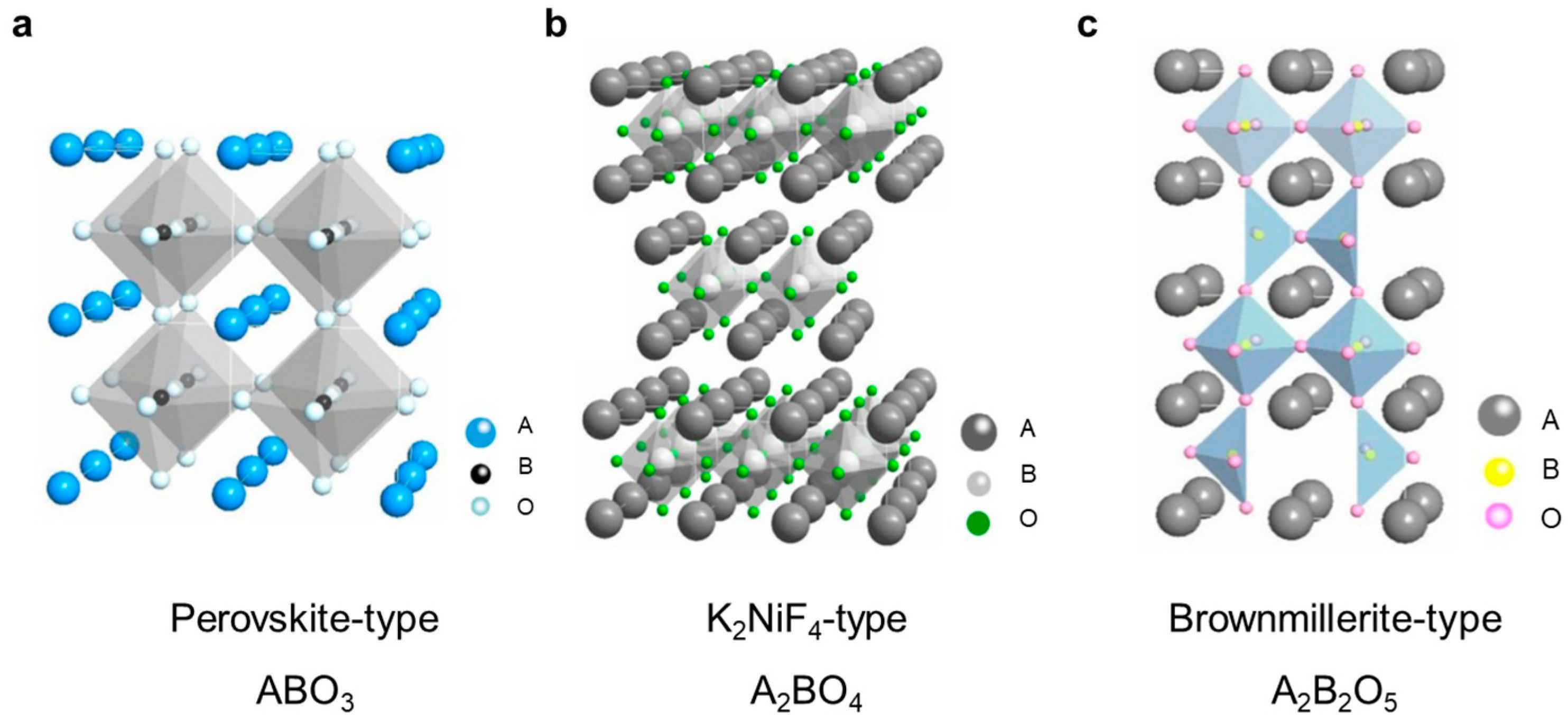
3. Classification of Mixed Conducting Membranes
3.1. Single–Phase Oxygen–Permeable Membranes
3.2. Dual–Phase Oxygen–Permeable membranes
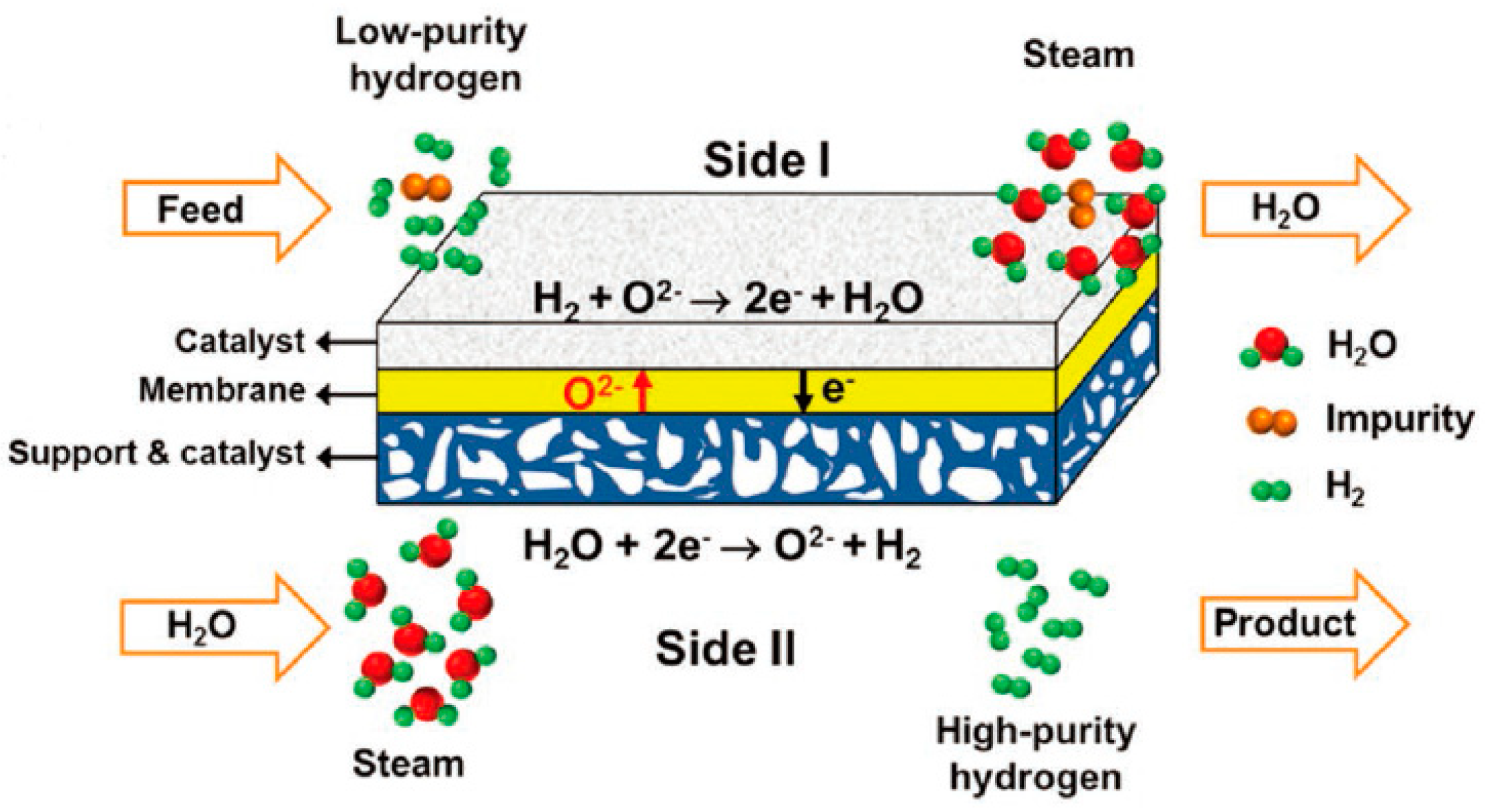
3.3. Hydrogen–Permeable Membranes
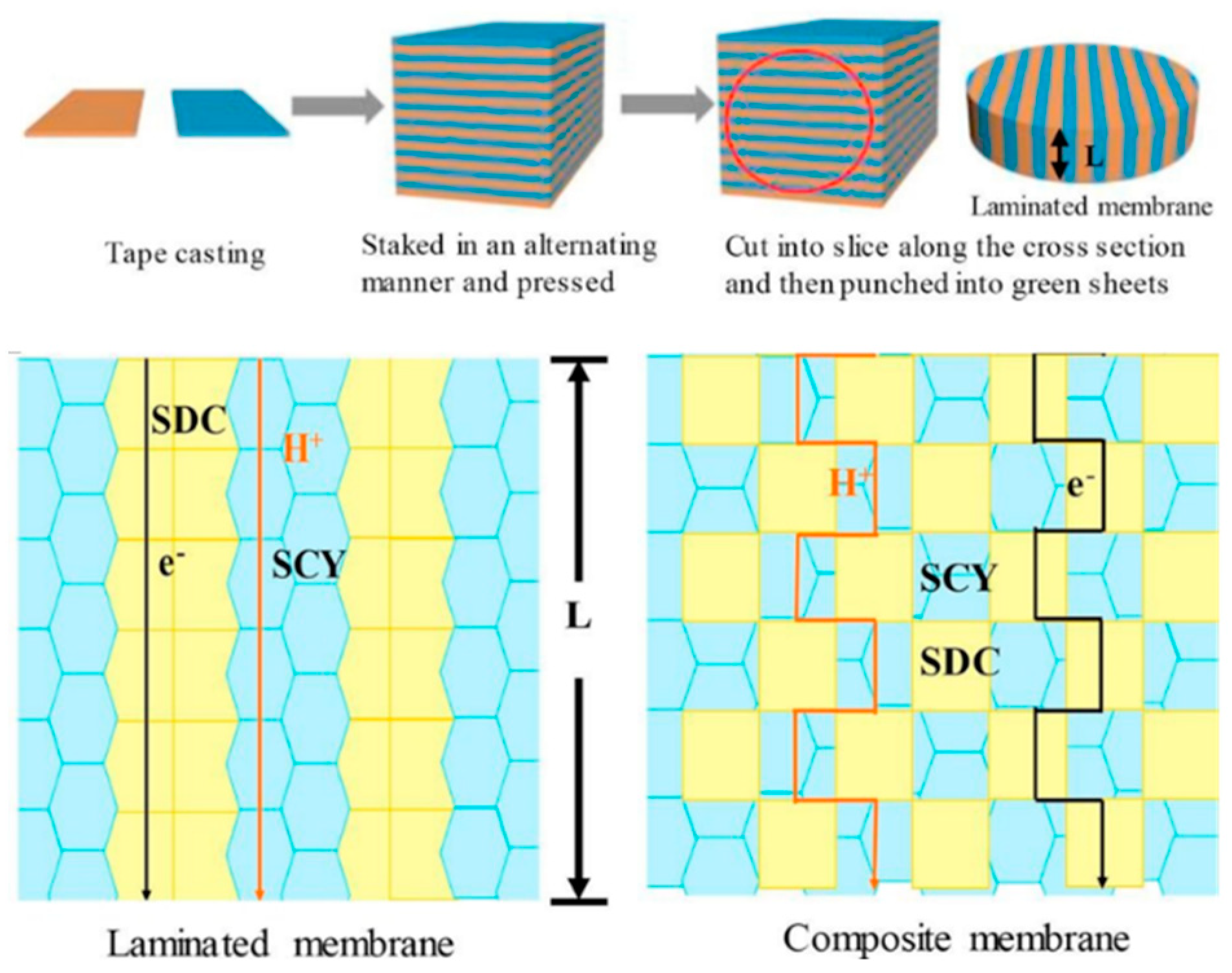
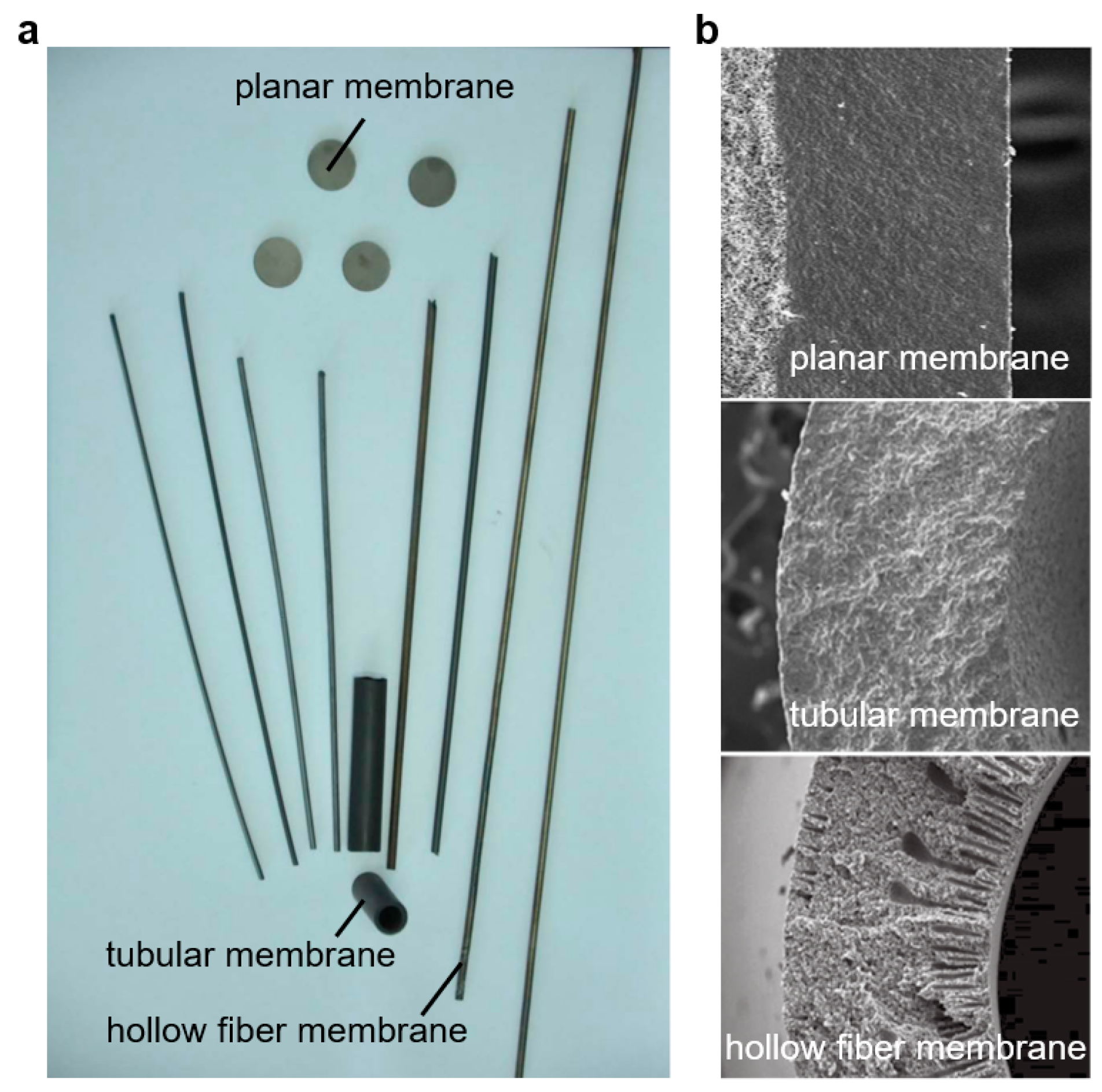
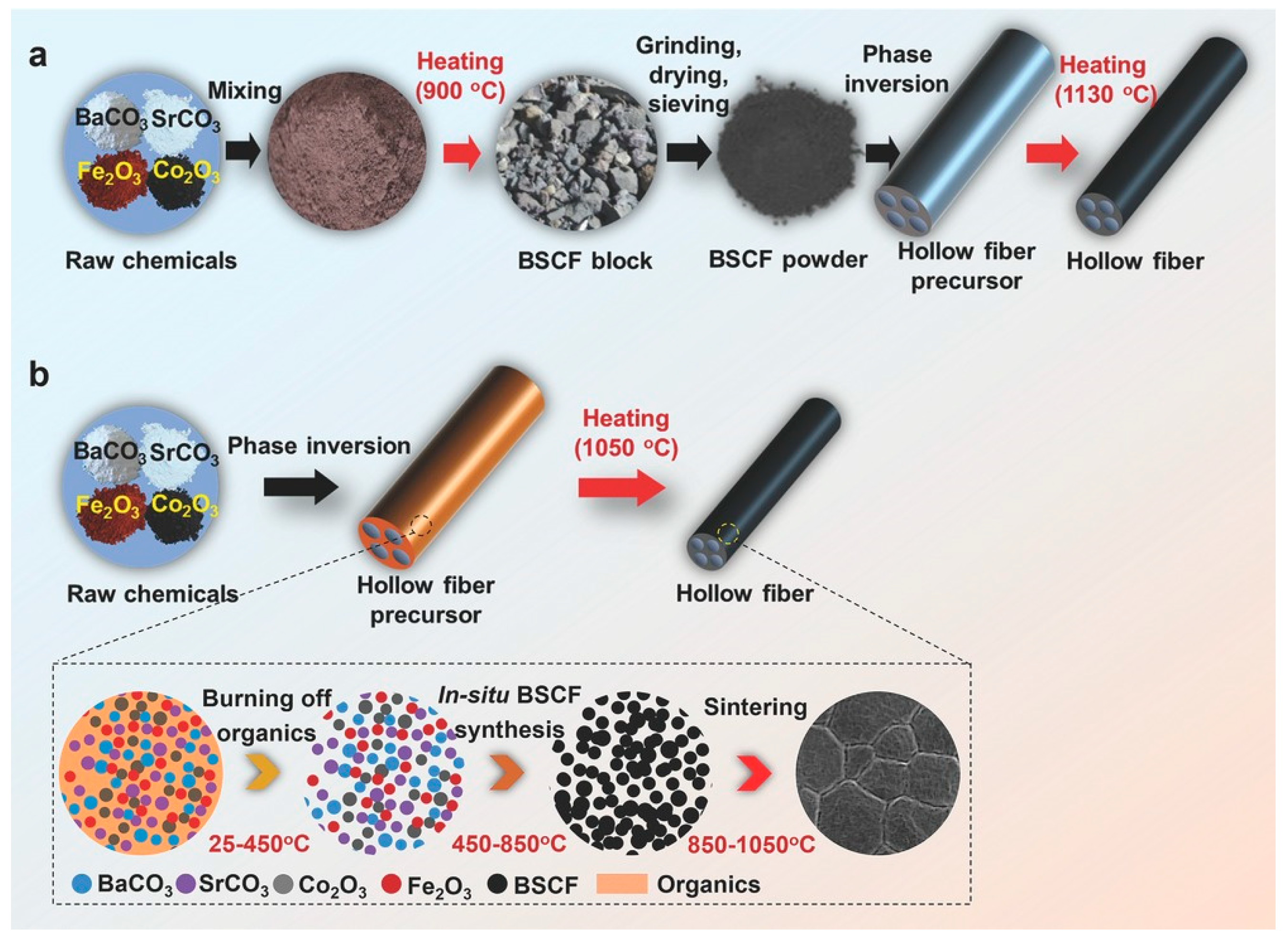
4. Membrane Architecture
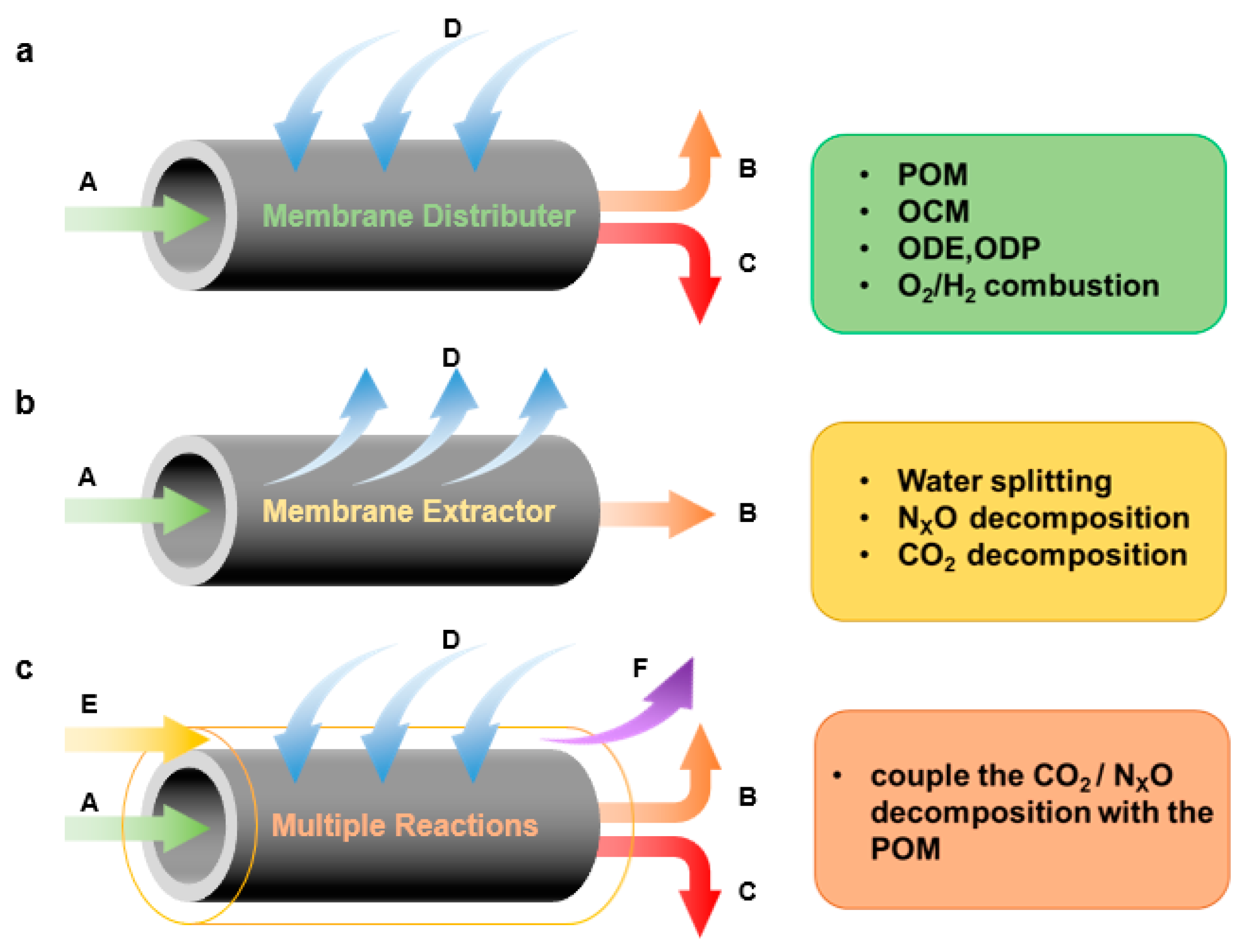
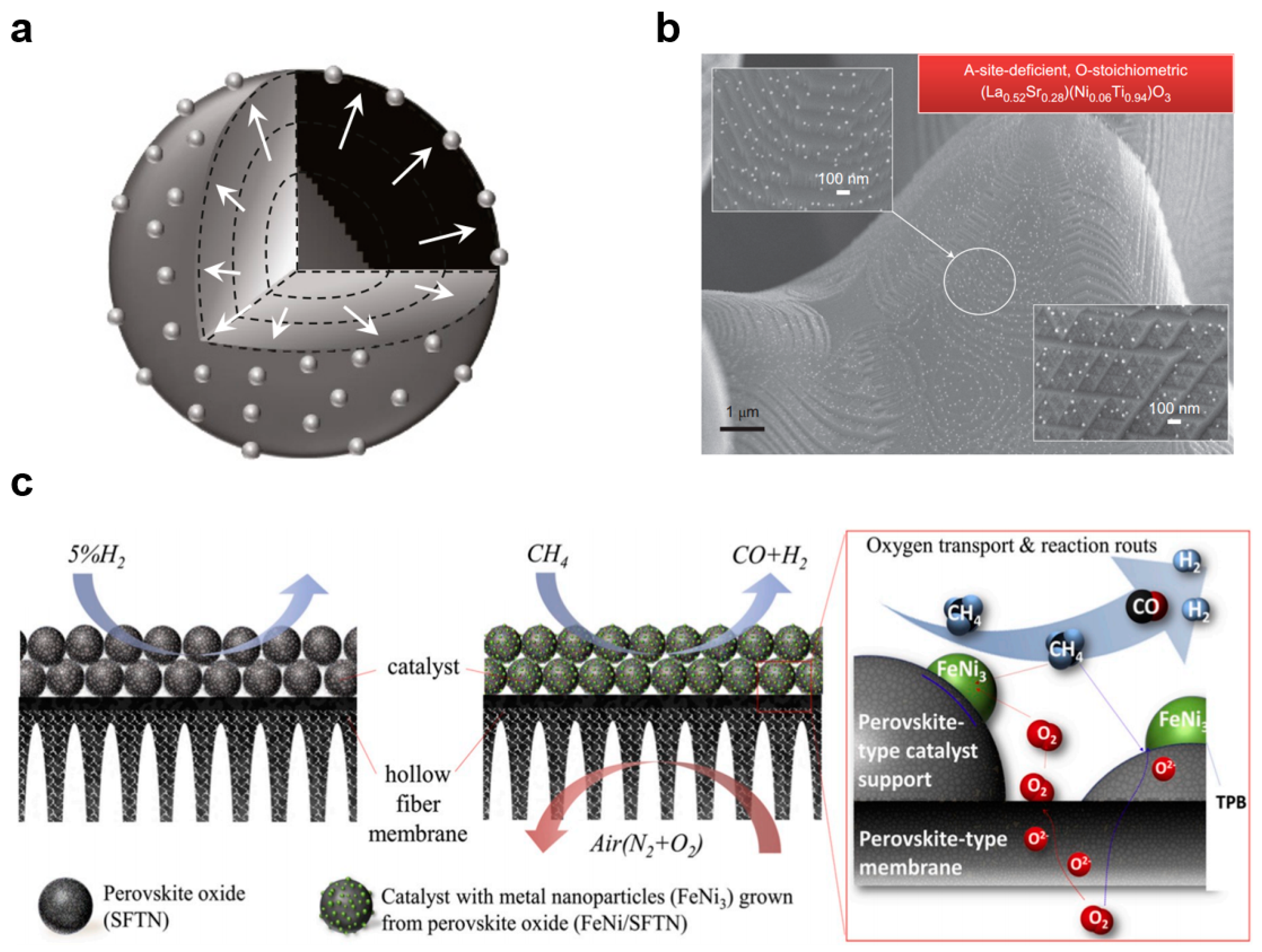
5. Functions of the Membrane in CMR
6. Applications of CMRs
6.1. Partial Oxidation of Methane
6.2. Oxidative Coupling of Methane
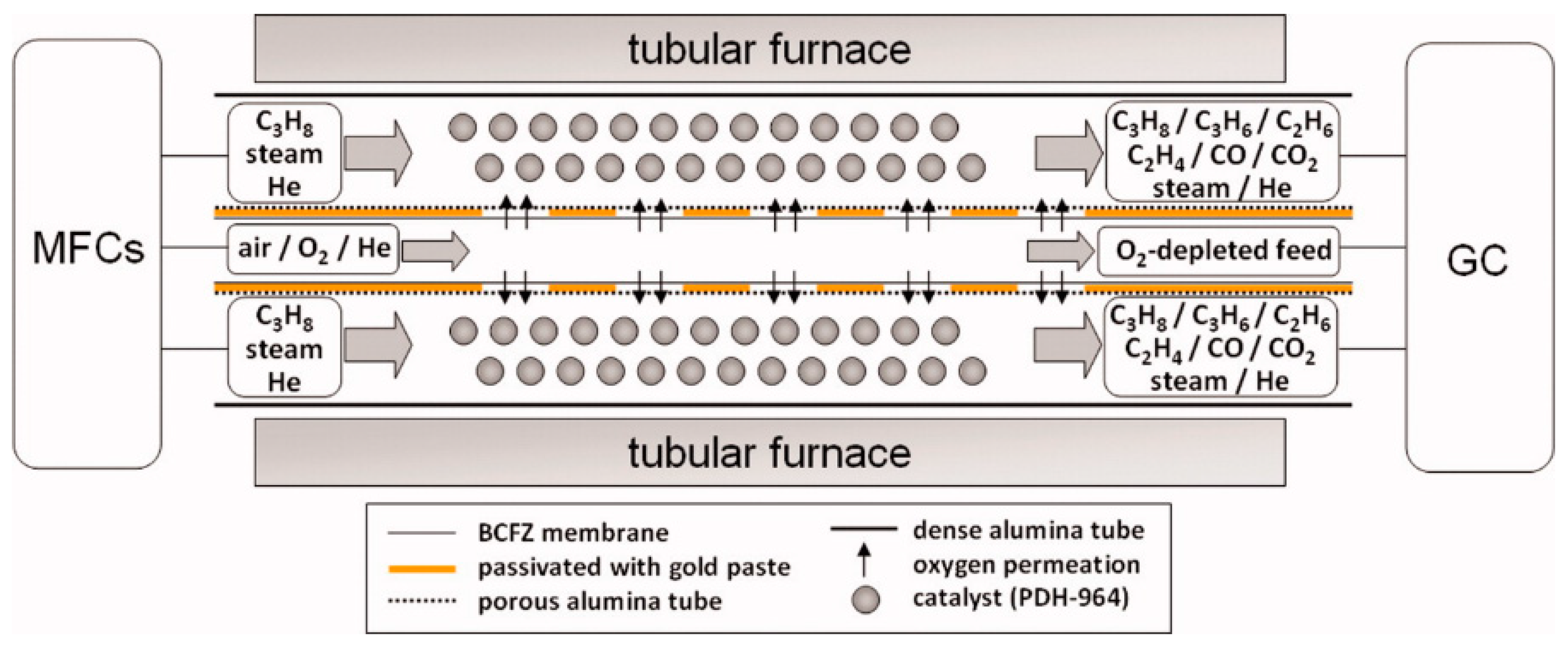
6.3. Oxidative Dehydrogenation of Hydrocarbons
6.4. Water Splitting Reaction
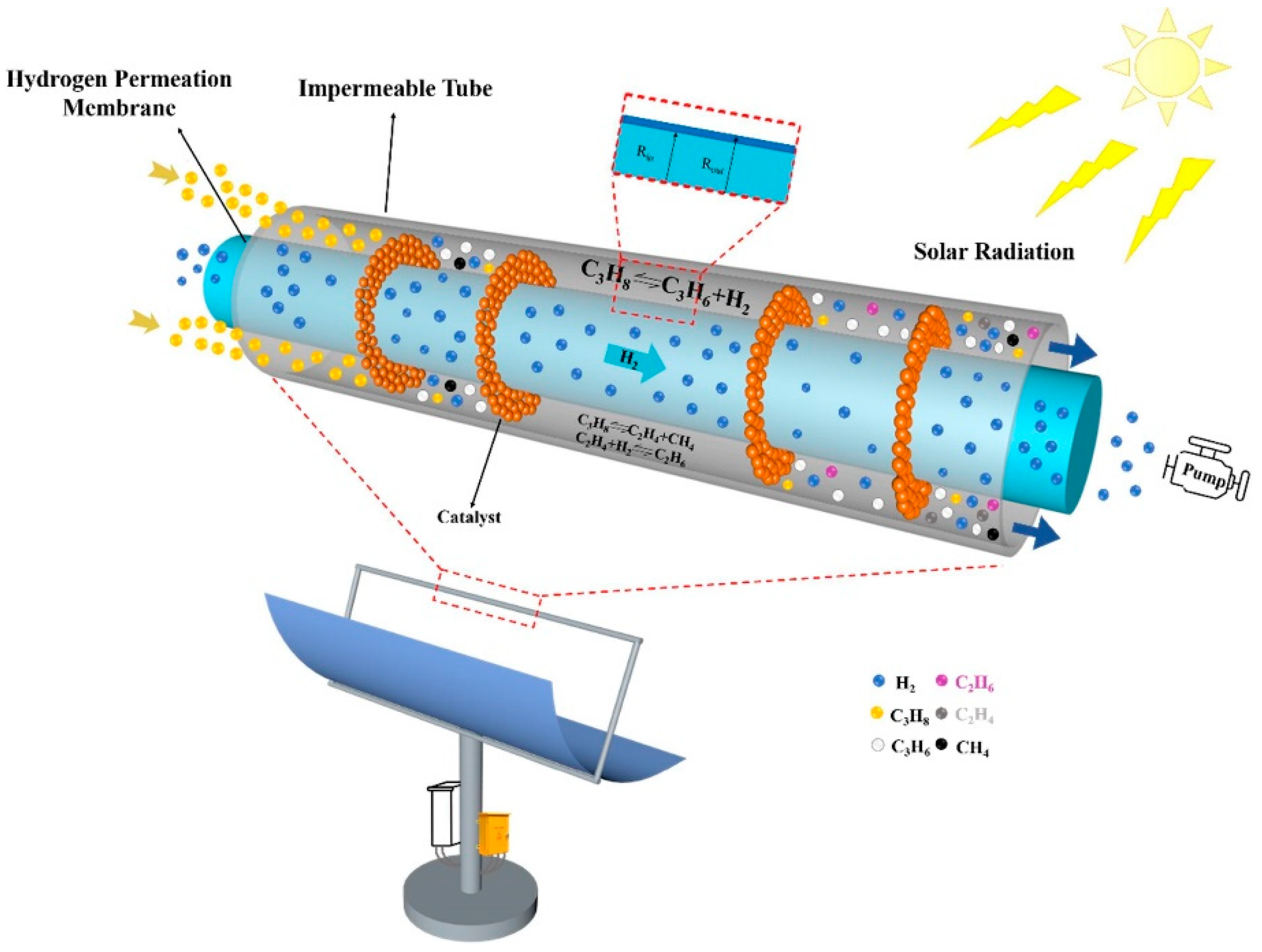
6.5. Alkane to Olefin Reaction
6.6. Coupling of Reactions
6.6.1. CO2 Decomposition
6.6.2. NOx Decomposition
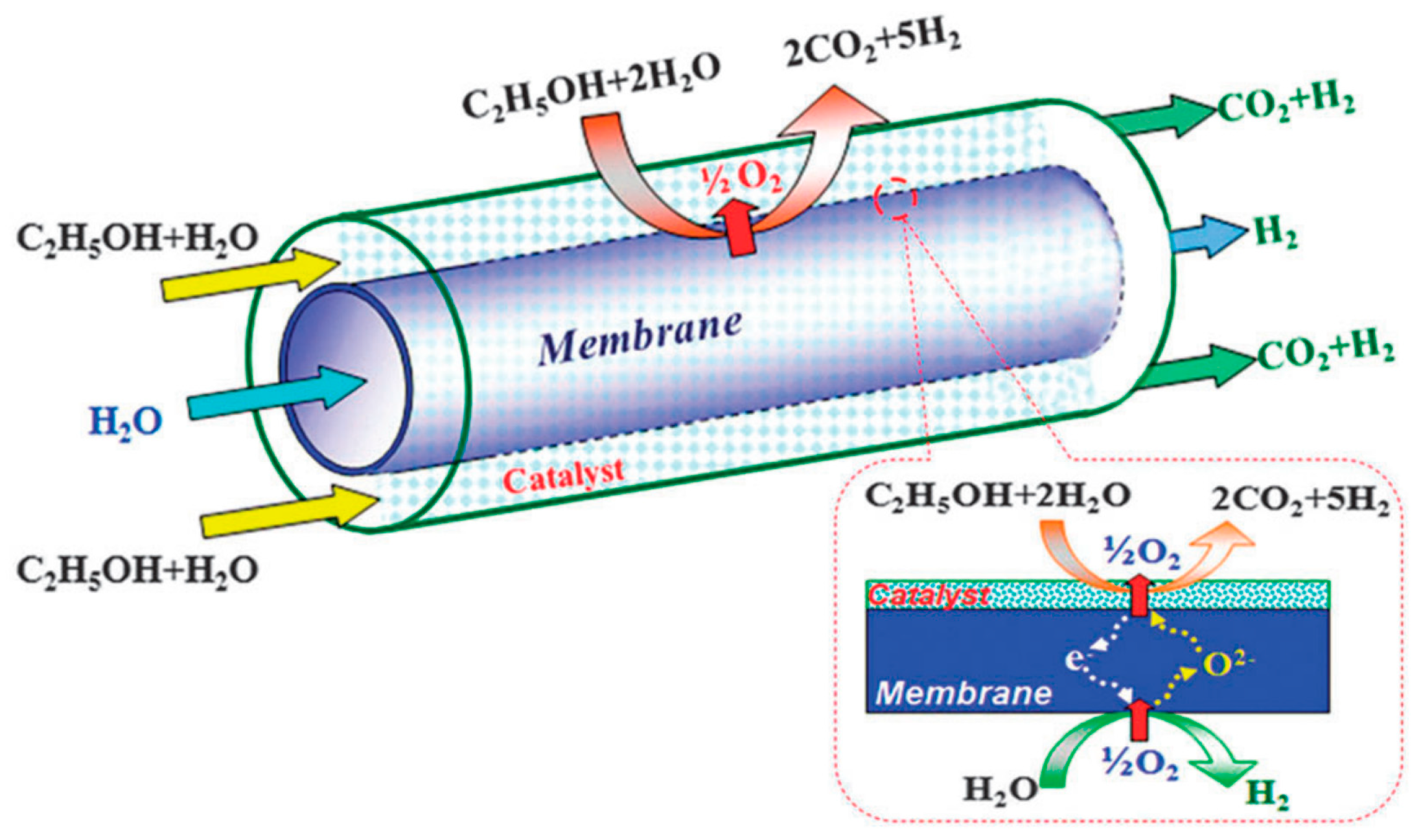
6.6.3. Water Splitting
7. Challenges
8. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dong, X.L.; Jin, W.Q.; Xu, N.P.; Li, K. Dense ceramic catalytic membranes and membrane reactors for energy and environmental applications. Chem. Commun. 2011, 47, 10886–10902. [Google Scholar] [CrossRef]
- Yi, J.; Schroeder, M.; Martin, M. CO2–Tolerant and Cobalt–Free SrFe0.8Nb0.2O3−δ Perovskite Membrane for Oxygen Separation. Chem. Mater. 2013, 25, 815–817. [Google Scholar] [CrossRef]
- Ning, H.; Wei, Z.; Wei, G.; Sijie, X.; Chi, Z.; Xuan, Z.; Jan, F.; Shaomin, L. Novel oxygen permeable hollow fiber perovskite membrane with surface wrinkles. Sep. Purif. Technol. 2021, 261, 118295. [Google Scholar]
- Zhu, Y.; Lei, J.; Liu, J.; Tan, J.; Zhang, G.; Liu, Z.; Jin, W. Fabrication of CO2–tolerant SrFe0.8Nb0.2O3−δ/SrCo0.9Nb0.1O3−δ dual–layer 7–channel hollow fiber membrane by co–spinning and one–step thermal process. J. Membr. Sci. 2023, 670, 121346. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Ning, K.; Xu, Z.; Zheng, Q.K.; Tan, J.K.; Liu, Z.K.; Wu, Z.T.; Zhang, G.R.; Jin, W.Q. Highly efficient preparation of Ce0.8Sm0.2O2−δ SrCo0.9Nb0.1O3−δ dual–phase four–channel hollow fiber membrane via one–step thermal processing approach. J. Membr. Sci. 2021, 620, 118752. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Wang, G.; Li, Y.; Bai, M.; Hall, D.; Dou, R. A case study of the effect of Ni substitution on the sintering behaviours of Ba0.5Sr0.5Co0.8Fe0.2O3−δ oxygen transport membranes. Adv. Appl. Ceram. 2018, 117, 269–278. [Google Scholar] [CrossRef]
- Song, J.; Hei, Y.; Li, C.; Yang, N.; Meng, B.; Tan, X.; Sunarso, J.; Liu, S. Dehydrogenation Coupling of Methane Using Catalyst–Loaded Proton–Conducting Perovskite Hollow Fiber Membranes. Membranes 2022, 12, 191. [Google Scholar] [CrossRef]
- Fairuzov, D.; Gerzeliev, I.; Maximov, A.; Naranov, E. Catalytic Dehydrogenation of Ethane: A Mini Review of Recent Advances and Perspective of Chemical Looping Technology. Catalysts 2021, 11, 833. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, S.Z.; Xie, K. Conversion of Methane to Ethylene with BaCe0.9Y0.1CoxO3−δ Hydrogen Permeation Membrane. Chin. J. Struct. Chem. 2021, 40, 901–907. [Google Scholar]
- Mohanty, U.S.; Ali, M.; Azhar, M.R.; Al–Yaseri, A.; Keshavarz, A.; Iglauer, S. Current advances in syngas (CO+H2) production through bi–reforming of methane using various catalysts: A review. Int. J. Hydrogen Energy 2021, 46, 32809–32845. [Google Scholar] [CrossRef]
- Schmeda–Lopez, D.R.; Nunes, E.H.M.; Vasconcelos, D.; Vasconcelos, W.L.; Meulenberg, W.A.; Diniz da Costa, J.C. The Neck to Particle Ratio Effect on the Mechanical and Morphological Sintering Features of Porous Stainless Steel (SS) Hollow Fibers. Adv. Eng. Mater. 2018, 20, 1800045. [Google Scholar] [CrossRef]
- Gui, T.; Zhang, F.; Li, Y.Q.; Cui, X.; Wu, X.W.; Zhu, M.H.; Hu, N.; Chen, X.S.; Kita, H.; Kondo, M. Scale–up of NaA zeolite membranes using reusable stainless steel tubes for dehydration in an industrial plant. J. Membr. Sci. 2019, 583, 180–189. [Google Scholar] [CrossRef]
- Shin, J.H.; Yu, H.J.; An, H.; Lee, A.S.; Hwang, S.S.; Lee, S.Y.; Lee, J.S. Rigid double–stranded siloxane–induced high–flux carbon molecular sieve hollow fiber membranes for CO2/CH4 separation. J. Membr. Sci. 2019, 570, 504–512. [Google Scholar] [CrossRef]
- Wang, D.Q.; Gao, Y.F.; Gao, S.J.; Huang, H.K.; Min, F.; Li, Y.X.; Seeger, S.; Jin, J.; Chu, Z.L. Antifouling superhydrophilic porous glass membrane based on sulfobetaine prepared by thiol–ene click chemistry for high–efficiency oil/ water separation. J. Membr. Sci. 2023, 670, 121336. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Tan, X.Y.; Li, K. Mixed conducting ceramics for catalytic membrane processing. Catal. Rev. Sci. Eng. 2006, 48, 145–198. [Google Scholar] [CrossRef]
- Bouwmeester, H.J.M.; Burggraaf, A.J. Dense ceramic membranes for oxygen separation. Membr. Sci. Technol. 1996, 4, 435–528. [Google Scholar]
- Kharton, V.V.; Yaremchenko, A.A.; Kovalevsky, A.V.; Viskup, A.P.; Naumovich, E.N.; Kerko, P.F. Perovskite–type oxides for high–temperature oxygen separation membranes. J. Membr. Sci. 1999, 163, 307–317. [Google Scholar] [CrossRef]
- Al, S.; Zhang, G. The Role of Metal Catalyst on Water Permeation and Stability of BaCe0.8Y0.2O3−δ. J. Electrochem. Sci. Technol. 2018, 9, 212–219. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Han, J.-J.; Huang, Y.; Chen, Y.; Yan, X.; Lang, W.-Z. Effect of Ba non–stoichiometry in Ba1−xZr0.1Ce0.7Y0.2O3−δ on its structure defect, sinterability and hydrogen permeability. Ceram. Int. 2020, 46, 19564–19573. [Google Scholar] [CrossRef]
- Tao, Z.; Yan, L.; Qiao, J.; Wang, B.; Zhang, L.; Zhang, J. A review of advanced proton–conducting materials for hydrogen separation. Prog. Mater. Sci. 2015, 74, 1–50. [Google Scholar] [CrossRef]
- Yaremchenko, A.; Kharton, V.; Valente, A.; Veniaminov, S.; Belyaev, V.; Sobyanin, V.; Marques, F. Methane oxidation over mixed–conducting SrFe(Al)O3−δ–SrAl2O4 composite. Phys. Chem. Chem. Phys. 2007, 9, 2744–2752. [Google Scholar] [CrossRef]
- Tan, X.; Li, K. Design of mixed conducting ceramic membranes/reactors for the partial oxidation of methane to syngas. AIChE J. 2009, 55, 2675–2685. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, G.; Liu, Z.; Zhong, Z.; Jin, W.; Xu, N. CO2–tolerant mixed conducting oxide for catalytic membrane reactor. J. Membr. Sci. 2009, 340, 141–147. [Google Scholar] [CrossRef]
- Zhu, X.; Li, Q.; He, Y.; Cong, Y.; Yang, W. Oxygen permeation and partial oxidation of methane in dual–phase membrane reactors. J. Membr. Sci. 2010, 360, 454–460. [Google Scholar] [CrossRef]
- Olivier, L.; Haag, S.; Mirodatos, C.; van Veen, A.C. Oxidative coupling of methane using catalyst modified dense perovskite membrane reactors. Catal. Today 2009, 142, 34–41. [Google Scholar] [CrossRef]
- Czuprat, O.; Schiestel, T.; Voss, H.; Caro, J. Oxidative Coupling of Methane in a BCFZ Perovskite Hollow Fiber Membrane Reactor. Ind. Eng. Chem. Res. 2010, 49, 10230–10236. [Google Scholar] [CrossRef]
- Wei, Y.Y.; Yang, W.S.; Caro, J.; Wang, H.H. Dense ceramic oxygen permeable membranes and catalytic membrane reactors. Chem. Eng. J. 2013, 220, 185–203. [Google Scholar] [CrossRef]
- Hashim, S.S.; Somalu, M.R.; Loh, K.S.; Liu, S.M.; Zhou, W.; Sunarso, J. Perovskite–based proton conducting membranes for hydrogen separation: A review. Int. J. Hydrogen Energy 2018, 43, 15281–15305. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, W. Microstructural and Interfacial Designs of Oxygen–Permeable Membranes for Oxygen Separation and Reaction–Separation Coupling. Adv. Mater. 2019, 31, e19025472019. [Google Scholar] [CrossRef]
- Jin, W.X.; Nanping. Mixed–Conducting Oxygen–Permeation Materials (Book); Science Press: Beijing, China, 2013. [Google Scholar]
- Zhang, K.; Sunarso, J.; Shao, Z.; Zhou, W.; Sun, C.; Wang, S.; Liu, S. Research progress and materials selection guidelines on mixed conducting perovskite–type ceramic membranes for oxygen production. RSC Adv. 2011, 1, 1661–1676. [Google Scholar] [CrossRef]
- Tejuca, L.G.; Fierro, J.L.G.; Tascón, J.M.D. Structure and Reactivity of Perovskite–Type Oxides. Adv. Catal. 1989, 36, 237–328. [Google Scholar]
- Kharton, V.; Viskup, A.; Naumovich, E.; Lapchuk, N. Mixed electronic and ionic conductivity of LaCo(M)O3 (M=Ga, Cr, Fe or Ni): I. Oxygen transport in perovskites LaCoO3–LaGaO3. Solid State Ion. 1997, 104, 67–78. [Google Scholar] [CrossRef]
- Lin, Y.; Zeng, Y. Catalytic properties of oxygen semipermeable perovskite–type ceramic membrane materials for oxidative coupling of methane. J. Catal. 1996, 164, 220–231. [Google Scholar] [CrossRef]
- Lu, Y.; Dixon, A.G.; Moser, W.R.; Ma, Y.H.; Balachandran, U. Oxygen–permeable dense membrane reactor for the oxidative coupling of methane. J. Membr. Sci. 2000, 170, 27–34. [Google Scholar] [CrossRef]
- Jin, W.; Gu, X.; Li, S.; Huang, P.; Xu, N.; Shi, J. Experimental and simulation study on a catalyst packed tubular dense membrane reactor for partial oxidation of methane to syngas. Chem. Eng. Sci. 2000, 55, 2617–2625. [Google Scholar] [CrossRef]
- Jin, W.; Li, S.; Huang, P.; Xu, N.; Shi, J.; Lin, Y. Tubular lanthanum cobaltite perovskite–type membrane reactors for partial oxidation of methane to syngas. J. Membr. Sci. 2000, 166, 13–22. [Google Scholar] [CrossRef]
- Shao, Z.; Dong, H.; Xiong, G.; Cong, Y.; Yang, W. Performance of a mixed–conducting ceramic membrane reactor with high oxygen permeability for methane conversion. J. Membr. Sci. 2001, 183, 181–192. [Google Scholar] [CrossRef]
- Shao, Z.; Xiong, G.; Dong, H.; Yang, W.; Lin, L. Synthesis, oxygen permeation study and membrane performance of a Ba0. 5Sr0. 5Co0. 8Fe0. 2O3−δ oxygen–permeable dense ceramic reactor for partial oxidation of methane to syngas. Sep. Purif. Technol. 2001, 25, 97–116. [Google Scholar] [CrossRef]
- Itoh, N.; Sanchez, M.A.; Xu, W.C.; Haraya, K.; Hongo, M. Application of a membrane reactor system to thermal–decomposition of CO2. J. Membr. Sci. 1993, 77, 245–253. [Google Scholar] [CrossRef]
- Han, N.; Wei, Q.; Zhang, S.G.; Yang, N.T.; Liu, S.M. Rational design via tailoring Mo content in La2Ni1−xMoxO4+delta to improve oxygen permeation properties in CO2 atmosphere. J. Alloys Compd. 2019, 806, 153–162. [Google Scholar] [CrossRef]
- Halat, D.M.; Dunstan, M.T.; Gaultois, M.W.; Britto, S.; Grey, C.P. Study of Defect Chemistry in the System La2−xSrxNiO4+delta by O–17 Solid–State NMR Spectroscopy and Ni K–Edge XANES. Chem. Mater. 2018, 30, 4556–4570. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, S.; Meng, B.; Ding, J.; Tan, X. Preparation and the superior oxygen permeability of a new CO2–resistant Ruddlesden–Popper composite oxide Pr2Ni0.9Mo0.1O4+δ. J. Alloys Compd. 2018, 742, 966–976. [Google Scholar] [CrossRef]
- Teraoka, Y.; Zhang, H.M.; Furukawa, S.; Yamazoe, N. Oxygen permeation through perovskite–type oxides. Chem. Lett. 1985, 11, 1743–1746. [Google Scholar] [CrossRef]
- Akin, F.; Lin, J.Y. Oxygen permeation through oxygen ionic or mixed–conducting ceramic membranes with chemical reactions. J. Membr. Sci. 2004, 231, 133–146. [Google Scholar] [CrossRef]
- Lin, Y.S. Microporous and dense inorganic membranes: Current status and prospective. Sep. Purif. Technol. 2001, 25, 39–55. [Google Scholar] [CrossRef]
- Shao, Z.P.; Yang, W.S.; Cong, Y.; Dong, H.; Tong, J.H.; Xiong, G.X. Investigation of the permeation behavior and stability of a Ba0.5Sr0.5Co0.8Fe0.2O3−delta oxygen membrane. J. Membr. Sci. 2000, 172, 177–188. [Google Scholar] [CrossRef]
- Kharton, V.; Naumovich, E.; Kovalevsky, A.; Viskup, A.; Figueiredo, F.; Bashmakov, I.; Marques, F. Mixed electronic and ionic conductivity of LaCo(M)O3 (M=Ga, Cr, Fe or Ni): IV. Effect of preparation method on oxygen transport in LaCoO3−δ. Solid State Ion. 2000, 138, 135–148. [Google Scholar] [CrossRef]
- Li, S.; Jin, W.; Huang, P.; Xu, N.; Shi, J.; Hu, M.Z.-C.; Payzant, E.A.; Ma, Y.H. Perovskite–related ZrO2–doped SrCo0.4Fe0.6O3−δ membrane for oxygen permeation. AIChE J. 1999, 45, 276–284. [Google Scholar] [CrossRef]
- Mazanec, T.J.; Cable, T.L.; Frye, J.G.; Kliewer, W.R. Solid multi–component membranes, electrochemical reactor components, electrochemical reactors and use of membranes, reactor components, and reactor for oxidation reactions. J. Membr. Sci. 1999, 163, 307–317. [Google Scholar]
- Wang, H.H.; Yang, W.S.; Cong, Y.; Zhu, X.F.; Lin, Y.S. Structure and oxygen permeability of a dual–phase membrane. J. Membr. Sci. 2003, 224, 107–115. [Google Scholar] [CrossRef]
- Kharton, V.; Kovalevsky, A.; Viskup, A.; Shaula, A.; Figueiredo, F.; Naumovich, E.; Marques, F. Oxygen transport in Ce0.8Gd0.2O2−δ–based composite membranes. Solid State Ion. 2003, 160, 247–258. [Google Scholar] [CrossRef]
- Fang, W.; Liang, F.; Cao, Z.; Steinbach, F.; Feldhoff, A. A Mixed Ionic and Electronic Conducting Dual–Phase Membrane with High Oxygen Permeability. Angew. Chem. Int. Ed. 2015, 54, 4847–4850. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Steinbach, F.; Chen, C.; Feldhoff, A. An approach to enhance the CO2 tolerance of fluorite–perovskite dual–phase oxygen–transporting membrane. Chem. Mater. 2015, 27, 7820–7826. [Google Scholar] [CrossRef]
- Yasutake, T.; Takashi, N.; Noboru, Y. Effect of Cation Substitution on the Oxygen Semipermeability of Perovskite–type Oxides. Chem. Lett. 1988, 17, 503–506. [Google Scholar]
- Li, S.; Jin, W.; Xu, N.; Shi, J. Synthesis and oxygen permeation properties of La0.2Sr0.8Co0.2Fe0.8O3−δ membranes. Solid State Ion. 1999, 124, 161–170. [Google Scholar] [CrossRef]
- Tan, L.; Yang, L.; Gu, X.; Jin, W.; Zhang, L.; Xu, N. Influence of the size of doping ion on phase stability and oxygen permeability of SrCo0.8Fe0.2O3−δ oxide. J. Membr. Sci. 2004, 230, 21–27. [Google Scholar] [CrossRef]
- Liu, S.; Gavalas, G. Oxygen selective ceramic hollow fiber membranes. J. Membr. Sci. 2005, 246, 103–108. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, H.; Yang, W. Novel cobalt–free oxygen permeable membrane. Chem. Commun. 2004, 9, 1103–1131. [Google Scholar] [CrossRef]
- Zhu, X.; Cong, Y.; Yang, W. Oxygen permeability and structural stability of BaCe0.15Fe0.85O3−δ membranes. J. Membr. Sci. 2006, 283, 38–44. [Google Scholar] [CrossRef]
- Shao, Z.; Xiong, G.; Cong, Y.; Yang, W. Synthesis and oxygen permeation study of novel perovskite–type BaBixCo0.2Fe0 8−xO3−δ ceramic membranes. J. Membr. Sci. 2000, 164, 167–176. [Google Scholar] [CrossRef]
- Nagai, T.; Ito, W.; Sakon, T. Relationship between cation substitution and stability of perovskite structure in SrCoO3−δ−based mixed conductors. Solid State Ion. 2007, 177, 3433–3444. [Google Scholar] [CrossRef]
- Huang, L.; Wei, Y.; Wang], H. Tantalum stabilized SrCoO3−δ perovskite membrane for oxygen separation. J. Membr. Sci. 2011, 368, 159–164. [Google Scholar]
- Serra, J.M.; Lobera, M.P.; Schulze–Küppers, F.; Meulenberg], W.A. Ultrahigh oxygen permeation flux through supported Ba0.5Sr0.5Co0.8Fe0.2O3−δ membranes. J. Membr. Sci. 2011, 377, 198–205. [Google Scholar]
- Zhu, J.; Dong, Z.; Liu, Z.; Zhang, K.; Zhang, G.; Jin, W. Multichannel mixed–conducting hollow fiber membranes for oxygen separation. AIChE J. 2014, 60, 1969–1976. [Google Scholar] [CrossRef]
- Zhu, J.; Guo, S.; Chu, Z.; Jin, W. CO2–tolerant oxygen–permeable perovskite–type membranes with high permeability. J. Mater. Chem. A 2015, 3, 22564–22573. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, Z.; Zhang, G.; Liu, Z.; Zhu, J.; Jin, W. Effects of polymer binders on separation performance of the perovskite–type 4–bore hollow fiber membranes. Sep. Purif. Technol. 2017, 187, 294–302. [Google Scholar] [CrossRef]
- Iwahara, H.; Esaka, T.; Uchida, H.; Maeda, N. Proton conduction in sintered oxides and its application to steam electrolysis for hydrogen production. Solid State Ion. 1981, 3, 359–363. [Google Scholar] [CrossRef]
- Iwahara, H. Proton conducting ceramics and their applications. Solid State Ion. 1996, 86, 9–15. [Google Scholar] [CrossRef]
- Escolástico, S.; Solís, C.; Scherb, T.; Schumacher, G.; Serra, J.M. Hydrogen separation in La5.5WO11.25–δ membranes. J. Membr. Sci. 2013, 444, 276–284. [Google Scholar] [CrossRef]
- Chen, T.; Zhao, H.; Xie, Z.; Xu, N.; Lu, Y. Oxygen permeability of Ce0.8Sm0.2O2−δ–LnBaCo2O5+δ (Ln=La, Nd, Sm, and Y) dual–phase ceramic membranes. Ionics 2015, 21, 1683–1692. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, S.; Chen, L.; Wei, Y.; Ashman, P.J.; Wang, H. Niobium and molybdenum co–doped La5.5WO11.25−δ membrane with improved hydrogen permeability. J. Membr. Sci. 2016, 510, 155–163. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, H.; Zhuang, L.; Wei, Y.; Wang, H. Hydrogen permeability through Nd5.5W0.35Mo0.5Nb0.15O11.25−δ mixed protonic–electronic conducting membrane. J. Membr. Sci. 2019, 579, 33–39. [Google Scholar] [CrossRef]
- Siriwardane, R.; Poston Jr, J.; Fisher, E.; Lee, T.; Dorris, S.; Balachandran, U. Characterization of ceramic hydrogen separation membranes with varying nickel concentrations. Appl. Surf. Sci. 2000, 167, 34–50. [Google Scholar] [CrossRef]
- Zhang, G.; Dorris, S.E.; Balachandran, U.; Liu, M. Interfacial resistances of Ni–BCY mixed–conducting membranes for hydrogen separation. Solid State Ion. 2003, 159, 121–134. [Google Scholar] [CrossRef]
- Meng, B.; Wang, H.N.; Cheng, H.D.; Wang, X.B.; Meng, X.X.; Sunarso, J.; Tan, X.Y.; Liu, S.M. Hydrogen permeation performance of dual–phase protonic–electronic conducting ceramic membrane with regular and independent transport channels. Sep. Purif. Technol. 2019, 213, 515–523. [Google Scholar] [CrossRef]
- Yang, M.; He, F.; Zhou, C.; Dong, F.; Yang, G.; Zhou, W.; Shao, Z. New perovskite membrane with improved sintering and self–reconstructed surface for efficient hydrogen permeation. J. Membr. Sci. 2021, 620, 118980. [Google Scholar] [CrossRef]
- Heidari, M.; Safekordi, A.; Zamaniyan, A.; Ganji Babakhani, E.; Amanipour, M. Comparison of microstructure and hydrogen permeability of perovskite type ACe0.9Y0.1O3−δ (A is Sr, Ba, La, and BaSr) membranes. Int. J. Hydrogen Energy 2015, 40, 6559–6565. [Google Scholar] [CrossRef]
- Song, J.; Meng, B.; Tan, X.; Liu, S. Surface–modified proton conducting perovskite hollow fibre membranes by Pd–coating for enhanced hydrogen permeation. Int. J. Hydrogen Energy 2015, 40, 6118–6127. [Google Scholar] [CrossRef]
- Song, J.; Li, L.; Tan, X.; Li, K. BaCe0.85Tb0.05Co0.1O3−δ perovskite hollow fibre membranes for hydrogen/oxygen permeation. Int. J. Hydrogen Energy 2013, 38, 7904–7912. [Google Scholar] [CrossRef]
- Cai, M.; Liu, S.; Efimov, K.; Caro, J.; Feldhoff, A.; Wang, H. Preparation and hydrogen permeation of BaCe0.95Nd0.05O3−δ membranes. J. Membr. Sci. 2009, 343, 90–96. [Google Scholar] [CrossRef]
- Zhang, K.; Sunarso, J.; Pham, G.H.; Wang, S.; Liu, S. External short circuit–assisted proton conducting ceramic membrane for H2 permeation. Ceram. Int. 2014, 40, 791–797. [Google Scholar] [CrossRef]
- Jin, W.Q.; Li, S.G.; Huang, P.; Xu, N.P.; Shi, J. Preparation of an asymmetric perovskite–type membrane and its oxygen permeability. J. Membr. Sci. 2001, 185, 237–243. [Google Scholar] [CrossRef]
- Kovalevsky, A.; Kharton, V.; Maxim, F.; Shaula, A.; Frade, J. Processing and characterization of La0. 5Sr0. 5FeO3–supported Sr1−xFe (Al)O3–SrAl2O4 composite membranes. J. Membr. Sci. 2006, 278, 162–172. [Google Scholar] [CrossRef]
- Feng, J.; Fan, Y.; Qi, H.; Xu, N. Co–sintering synthesis of tubular bilayer α−alumina membrane. J. Membr. Sci. 2007, 288, 20–27. [Google Scholar] [CrossRef]
- Watenabe, K.; Yuasa, M.; Kida, T.; Teraoka, Y.; Yamazoe, N.; Shimanoe, K. High-Performance Oxygen-Permeable Membranes with an Asymmetric Structure Using Ba0.95La0.05FeO3−δ Perovskite-Type Oxide. Adv. Mater. 2010, 22, 2367–2370. [Google Scholar] [CrossRef] [PubMed]
- Li, S.G.; Jin, W.Q.; Huang, P.; Xu, N.P.; Shi, J.; Lin, Y.S. Tubular lanthanum cobaltite perovskite type membrane for oxygen permeation. J. Membr. Sci. 2000, 166, 51–61. [Google Scholar] [CrossRef]
- Tan, X.Y.; Liu, S.M.; Li, K. Preparation and characterization of inorganic hollow fiber membranes. J. Membr. Sci. 2001, 188, 87–95. [Google Scholar] [CrossRef]
- Khayet, M. The effects of air gap length on the internal and external morphology of hollow fiber membranes. Chem. Eng. Sci. 2003, 58, 3091–3104. [Google Scholar] [CrossRef]
- Pan, X.L.; Stroh, N.; Brunner, H.; Xiong, G.X.; Sheng, S.S. Pd/ceramic hollow fibers for H2 separation. Sep. Purif. Technol. 2003, 32, 265–270. [Google Scholar] [CrossRef]
- De Jong, J.; Benes, N.E.; Koops, G.H.; Wessling, M. Towards single step production of multi–layer inorganic hollow fibers. J. Membr. Sci. 2004, 239, 265–269. [Google Scholar] [CrossRef]
- Li, D.F.; Chung, T.S.; Rong, W. Morphological aspects and structure control of dual–layer asymmetric hollow fiber membranes formed by a simultaneous co–extrusion approach. J. Membr. Sci. 2004, 243, 155–175. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, B.; Li, K. A novel dual–layer ceramic hollow fibre membrane reactor for methane conversion. J. Membr. Sci. 2010, 352, 63–70. [Google Scholar] [CrossRef]
- Zhu, J.W.; Guo, S.B.; Liu, G.P.; Liu, Z.K.; Zhang, Z.C.; Jin, W.Q. A robust mixed–conducting multichannel hollow fiber membrane reactor. Aiche J. 2015, 61, 2592–2599. [Google Scholar] [CrossRef]
- Zhu, J.W.; Wang, T.L.; Song, Z.; Liu, Z.K.; Zhang, G.R.; Jin, W.Q. Enhancing oxygen permeation via multiple types of oxygen transport paths in hepta–bore perovskite hollow fibers. Aiche J. 2017, 63, 4273–4277. [Google Scholar] [CrossRef]
- Wang, T.L.; Liu, Z.K.; Xu, X.L.; Zhu, J.W.; Zhang, G.R.; Jin, W.Q. Insights into the design of nineteen–channel perovskite hollow fiber membrane and its oxygen transport behaviour. J. Membr. Sci. 2020, 595, 117600. [Google Scholar] [CrossRef]
- Tan, X.Y.; Thursfield, A.; Metcalfe, I.S.; Li, K. Analysis of a perovskite ceramic hollow fibre membrane reactor for the partial oxidation of methane to syngas. Asia Pac. J. Chem. Eng. 2009, 4, 251–258. [Google Scholar] [CrossRef]
- Kleinert, A.; Feldhoff, A.; Schiestel, T.; Caro, J.G. Novel hollow fibre membrane reactor for the partial oxidation of methane. Catal. Today 2006, 118, 44–51. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, S.; Jin, Y.; Song, J.; Meng, X.; Meng, B.; Yang, N.; Tan, X.; Zhu, Z.; Liu, S. One stone two birds: Simultaneous realization of partial oxidation of methane to syngas and N2 purification via robust ceramic oxygen–permeable membrane reactors. Chem. Eng. J. 2021, 419, 129462. [Google Scholar] [CrossRef]
- Babakhani, E.G.; Towfighi, J.; Taheri, Z.; Pour, A.N.; Zekordi, M.; Taheri, A. Partial oxidation of methane in Ba0.5Sr0.5Co0.8Fe0.1Ni0.1O3−δ ceramic membrane reactor. J. Nat. Gas Chem. 2012, 21, 519–525. [Google Scholar] [CrossRef]
- Wei, Y.; Liao, Q.; Li, Z.; Wang, H.; Feldhoff, A.; Caro, J. Partial Oxidation of Methane in Hollow–Fiber Membrane Reactors Based on Alkaline–Earth Metal–Free CO2–Tolerant Oxide. Aiche J. 2014, 60, 3587–3595. [Google Scholar] [CrossRef]
- Wang, H.; Tablet, C.; Schiestel, T.; Werth, S.; Caro, J. Partial oxidation of methane to syngas in a perovskite hollow fiber membrane reactor. Catal. Commun. 2006, 7, 907–912. [Google Scholar] [CrossRef]
- Czuprat, O.; Werth, S.; Schirrmeister, S.; Schiestel, T.; Caro, J. Olefin production by a multistep oxidative dehydrogenation in a perovskite hollow-fiber membrane reactor. ChemCatChem 2009, 1, 401–405. [Google Scholar] [CrossRef]
- Czuprat, O.; Werth, S.; Caro, J.; Schiestel, T. Oxidative dehydrogenation of propane in a perovskite membrane reactor with multi-step oxygen insertion. AIChE J. 2010, 56, 2390–2396. [Google Scholar] [CrossRef]
- Kleiminger, L.; Li, T.; Li, K.; Kelsall, G.H. CO2 splitting into CO and O2 in micro–tubular solid oxide electrolysers. Rsc. Adv. 2014, 4, 50003–50016. [Google Scholar] [CrossRef]
- Tan, X.Y.; Capar, G.; Li, K. Analysis of dissolved oxygen removal in hollow fibre membrane modules: Effect of water vapour. J. Membr. Sci. 2005, 251, 111–119. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Z.; Cui, Y.; Chen, T.; Hu, J.; Kawi, S. Highly Efficient NO Decomposition via Dual–Functional Catalytic Perovskite Hollow Fiber Membrane Reactor Coupled with Partial Oxidation of Methane at Medium–Low Temperature. Environ. Sci. Technol. 2019, 53, 9937–9946. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, Y.; Tan, X.; Gao, J.; Liu, S. Nickel hollow fiber membranes for hydrogen separation from reformate gases and water gas shift reactions operated at high temperatures. J. Membr. Sci. 2019, 575, 89–97. [Google Scholar] [CrossRef]
- Cheng, H.; Meng, B.; Li, C.; Wang, X.; Meng, X.; Sunarso, J.; Tan, X.; Liu, S. Single–step synthesized dual–layer hollow fiber membrane reactor for on–site hydrogen production through ammonia decomposition. Int. J. Hydrogen Energy 2020, 45, 7423–7432. [Google Scholar] [CrossRef]
- Dittrich, C.J. The role of heat transfer on the feasibility of a packed–bed membrane reactor for propane dehydrogenation. Chem. Eng. J. 2020, 381, 122492. [Google Scholar] [CrossRef]
- Kyriakou, V.; Garagounis, I.; Vourros, A.; Vasileiou, E.; Manerbino, A.; Coors, W.G.; Stoukides, M. Methane steam reforming at low temperatures in a BaZr0.7Ce0.2Y0.1O2.9 proton conducting membrane reactor. Appl. Catal. B Environ. 2016, 186, 1–9. [Google Scholar] [CrossRef]
- Xue, J.; Chen, Y.; Wei, Y.; Feldhoff, A.; Wang, H.; Caro, J. Gas to Liquids: Natural Gas Conversion to Aromatic Fuels and Chemicals in a Hydrogen–Permeable Ceramic Hollow Fiber Membrane Reactor. ACS Catal. 2016, 6, 2448–2451. [Google Scholar] [CrossRef]
- Morejudo, S.H.; Zanon, R.; Escolastico, S.; Yuste–Tirados, I.; Malerod–Fjeld, H.; Vestre, P.K.; Coors, W.G.; Martinez, A.; Norby, T.; Serra, J.M.; et al. Direct conversion of methane to aromatics in a catalytic co–ionic membrane reactor. Science 2016, 353, 563–566. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.T.; Wang, B.; Li, K. Functional LSM–ScSZ/NiO–ScSZ dual–layer hollow fibres for partial oxidation of methane. Int. J. Hydrogen Energy 2011, 36, 5334–5341. [Google Scholar] [CrossRef]
- Kathiraser, Y.; Kawi, S. La0.6Sr0.4Co0.8Ga0.2O3−delta (LSCG) Hollow Fiber Membrane Reactor: Partial Oxidation of Methane at Medium Temperature. Aiche J. 2013, 59, 3874–3885. [Google Scholar] [CrossRef]
- Li, W.; Cao, Z.; Zhu, X.; Yang, W. High-rate hydrogen separation using an MIEC oxygen permeable membrane reactor. AIChE J. 2017, 63, 1278–1286. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, G.; Liu, Z.; Dong, X.; Jin, W.; Liu, S.; Gur, T. A Comparative Study of the Performance of SrCo0.76Fe0.19Al0.1Ox and (SrCo0.8Fe0.2O3−δ)0.95(SrAl2O4)0.05 Mixed–Conducting Membranes. J. Am. Ceram. Soc. 2013, 96, 1285–1291. [Google Scholar] [CrossRef]
- Zhu, N.; Dong, X.; Liu, Z.; Zhang, G.; Jin, W.; Xu, N. Toward highly–effective and sustainable hydrogen production: Bio–ethanol oxidative steam reforming coupled with water splitting in a thin tubular membrane reactor. Chem. Commun. 2012, 48, 7137–7139. [Google Scholar] [CrossRef]
- Shao, Z.P.; Xiong, G.X.; Tong, J.H.; Dong, H.; Yang, W.S. Ba effect in doped Sr(Co0.8Fe0.2)O3−delta on the phase structure and oxygen permeation properties of the dense ceramic membranes. Sep. Purif. Technol. 2001, 25, 419–429. [Google Scholar] [CrossRef]
- Tong, J.H.; Yang, W.S.; Cai, R.; Zhu, B.C.; Lin, L.W. Novel and ideal zirconium–based dense membrane reactors for partial oxidation of methane to syngas. Catal. Lett. 2002, 78, 129–137. [Google Scholar] [CrossRef]
- Bouwmeester, H.J.M. Dense ceramic membranes for methane conversion. Catal. Today 2003, 82, 141–150. [Google Scholar] [CrossRef]
- Dong, X.L.; Liu, Z.K.; Jin, W.Q.; Xu, N.P. A self–catalytic mixed–conducting membrane reactor for effective production of hydrogen from methane. J. Power Sources 2008, 185, 1340–1347. [Google Scholar] [CrossRef]
- Dong, X.L.; Zhang, C.; Chang, X.F.; Jin, W.Q.; Xu, N.P. A self–catalytic membrane reactor based on a supported mixed–conducting membrane. Aiche J. 2008, 54, 1678–1680. [Google Scholar] [CrossRef]
- Wang, H.; Tablet, C.; Feldhoff, A.; Caro, J. A Cobalt-Free Oxygen-Permeable Membrane Based on the Perovskite-Type Oxide Ba0.5Sr0.5Zn0.2Fe0.8O3–δ. Adv. Mater. 2005, 17, 1785–1788. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, X.; He, Y.; Yang, W. Partial oxidation of methane in BaCe0.1Co0.4Fe0.5O3−δ membrane reactor. Catal. Today 2010, 149, 185–190. [Google Scholar] [CrossRef]
- Gong, Z.; Hong, L. Integration of air separation and partial oxidation of methane in the La0.4Ba0.6Fe0.8Zn0.2O3−δ membrane reactor. J. Membr. Sci. 2011, 380, 81–86. [Google Scholar] [CrossRef]
- Song, S.; Zhang, P.; Han, M.; Singhal, S.C. Oxygen permeation and partial oxidation of methane reaction in Ba0.9Co0.7Fe0.2Nb0.1O3−δ oxygen permeation membrane. J. Membr. Sci. 2012, 415–416, 654–662. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, G.; Liu, Z.; Zhang, K.; Jin, W. A Novel Porous–Dense Dual–Layer Composite Membrane Reactor with Long–Term Stability. Aiche J. 2013, 59, 4355–4363. [Google Scholar] [CrossRef]
- Liao, Q.; Chen, Y.; Wei, Y.; Zhou, L.; Wang, H. Performance of U–shaped BaCo0.7Fe0.2Ta0.1O3−δ hollow–fiber membranes reactor with high oxygen permeation for methane conversion. Chem. Eng. J. 2014, 237, 146–152. [Google Scholar] [CrossRef]
- Song, S.; Zhang, P.; Zhang, X.; Han, M. Partial oxidation of methane reaction in Ba0.9Co0.7Fe0.2Nb0.1O3−δ oxygen permeation membrane with three–layer structure. Int. J. Hydrogen Energy 2015, 40, 10894–10901. [Google Scholar] [CrossRef]
- Meng, X.; Bi, X.; Meng, B.; Yang, N.; Tan, X.; Liu, L.; Liu, S. H2/CH4/CO2–tolerant properties of SrCo0.8Fe0.1Ga0.1O3−δ hollow fiber membrane reactors for methane partial oxidation to syngas. Fuel Process. Technol. 2017, 161, 265–272. [Google Scholar] [CrossRef]
- Wang, Z.; Ashok, J.; Pu, Z.; Kawi, S. Low temperature partial oxidation of methane via BaBi0.05Co0.8Nb0.15O3−δ−Ni phyllosilicate catalytic hollow fiber membrane reactor. Chem. Eng. J. 2017, 315, 315–323. [Google Scholar] [CrossRef]
- Akin, F.; Lin, Y. Oxidative coupling of methane in dense ceramic membrane reactor with high yields. AIChE J. 2002, 48, 2298–2306. [Google Scholar] [CrossRef]
- Othman, N.H.; Wu, Z.; Li, K. An oxygen permeable membrane microreactor with an in–situ deposited Bi1.5Y0.3Sm0.2O3−delta catalyst for oxidative coupling of methane. J. Membr. Sci. 2015, 488, 182–193. [Google Scholar] [CrossRef]
- Cavani, F.; Ballarini, N.; Cericola, A. Oxidative dehydrogenation of ethane and propane: How far from commercial implementation? Catal. Today 2007, 127, 113–131. [Google Scholar] [CrossRef]
- Czuprat, O.; Arnold, M.; Schirrmeister, S.; Schiestel, T.; Caro, J. Influence of CO2 on the oxygen permeation performance of perovskite–type BaCoxFeyZrzO3−delta hollow fiber membranes. J. Membr. Sci. 2010, 364, 132–137. [Google Scholar] [CrossRef]
- Wang, Z.; Bian, Z.; Dewangan, N.; Xu, J.; Kawi, S. High–performance catalytic perovskite hollow fiber membrane reactor for oxidative propane dehydrogenation. J. Membr. Sci. 2019, 578, 36–42. [Google Scholar] [CrossRef]
- Jin, Y.; Meng, X.X.; Meng, B.; Yang, N.T.; Sunarso, J.; Liu, S.M. Parametric modeling study of oxidative dehydrogenation of propane in La0.6Sr0.4Co0.2Fe0.8O3−delta hollow fiber membrane reactor. Catal. Today 2019, 330, 135–141. [Google Scholar] [CrossRef]
- Wang, H.; Cong, Y.; Yang, W. Oxidative coupling of methane in Ba0.5Sr0.5Co0.8Fe0.2O3−δ tubular membrane reactors. Catal. Today 2005, 104, 160–167. [Google Scholar] [CrossRef]
- Bhatia, S.; Thien, C.Y.; Mohamed, A.R. Oxidative coupling of methane (OCM) in a catalytic membrane reactor and comparison of its performance with other catalytic reactors. Chem. Eng. J. 2009, 148, 525–532. [Google Scholar] [CrossRef]
- Igenegbai, V.O.; Almallahi, R.; Meyer, R.J.; Linic, S. Oxidative Coupling of Methane over Hybrid Membrane/Catalyst Active Centers: Chemical Requirements for Prolonged Lifetime. ACS Energy Lett. 2019, 4, 1465–1470. [Google Scholar] [CrossRef]
- Wang, H.; Cong, Y.; Yang, W. Continuous Oxygen Ion Transfer Medium as a Catalyst for High Selective Oxidative Dehydrogenation of Ethane. Catal. Lett. 2002, 84, 101–106. [Google Scholar] [CrossRef]
- Wang, H.; Tablet, C.; Schiestel, T.; Caro, J. Hollow fiber membrane reactors for the oxidative activation of ethane. Catal. Today 2006, 118, 98–103. [Google Scholar] [CrossRef]
- Liang, F.Y.; He, G.H.; Jia, L.J.; Jiang, H.Q. Cobalt–free dual–phase oxygen transporting membrane reactor for the oxidative dehydrogenation of ethane. Sep. Purif. Technol. 2019, 211, 966–971. [Google Scholar] [CrossRef]
- Balachandran, U.; Lee, T.; Wang, S.; Dorris, S. Use of mixed conducting membranes to produce hydrogen by water dissociation. Int. J. Hydrogen Energy 2004, 29, 291–296. [Google Scholar] [CrossRef]
- Park, C.; Lee, T.; Dorris, S.; Balachandran, U. Hydrogen production from fossil and renewable sources using an oxygen transport membrane. Int. J. Hydrogen Energy 2010, 35, 4103–4110. [Google Scholar] [CrossRef]
- Park, C.; Lee, T.; Dorris, S.; Balachandran, U. A cobalt–free oxygen transport membrane, BaFe0.9Zr0.1O3−δ, and its application for producing hydrogen. Int. J. Hydrogen Energy 2013, 38, 6450–6459. [Google Scholar] [CrossRef]
- Li, L.; Borry, R.W.; Iglesia, E. Design and optimization of catalysts and membrane reactors for the non–oxidative conversion of methane. Chem. Eng. Sci. 2002, 57, 4595–4604. [Google Scholar] [CrossRef]
- Liu, S.M.; Tan, X.Y.; Li, K.; Hughes, R. Methane coupling using catalytic membrane reactors. Catal. Rev. Sci. Eng. 2001, 43, 147–198. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, G.; Liu, Z.; Zhu, J.; Zhu, N.; Jin, W. Enhanced stability of membrane reactor for thermal decomposition of CO2 via porous–dense–porous triple–layer composite membrane. J. Membr. Sci. 2014, 471, 9–15. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, C.; Chang, X.; Fan, Y.; Xing, W.; Xu, N. Efficient catalytic decomposition of CO2 to CO and O2 over Pd/mixed–conducting oxide catalyst in an oxygen–permeable membrane reactor. Environ. Sci. Technol. 2008, 42, 3064–3068. [Google Scholar] [CrossRef]
- Zhang, C.; Jin, W.Q.; Yang, C.; Xu, N.P. Decomposition of CO2 coupled with POM in a thin tubular oxygen–permeable membrane reactor. Catal. Today 2009, 148, 298–302. [Google Scholar] [CrossRef]
- Jiang, H.; Xing, L.; Czuprat, O.; Wang, H.; Schirrmeister, S.; Schiestel, T.; Caro, J. Highly effective NO decomposition by in situ removal of inhibitor oxygen using an oxygen transporting membrane. Chem. Commun. 2009, 44, 6738–6740. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, H.; Liang, F.; Werth, S.; Schiestel, T.; Caro, J. Direct decomposition of nitrous oxide to nitrogen by in situ oxygen removal with a perovskite membrane. Angew. Chem. Int. Ed. 2009, 48, 2983–2986. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, H.; Werth, S.; Schiestel, T.; Caro, J. Simultaneous production of hydrogen and synthesis gas by combining water splitting with partial oxidation of methane in a hollow–fiber membrane reactor. Angew. Chem. Int. Ed. 2008, 47, 9341–9344. [Google Scholar] [CrossRef]
- Cao, Z.; Jiang, H.; Luo, H.; Baumann, S.; Meulenberg, W.A.; Voss, H.; Caro, J. Simultaneous overcome of the equilibrium limitations in BSCF oxygen–permeable membrane reactors: Water splitting and methane coupling. Catal. Today 2012, 193, 2–7. [Google Scholar] [CrossRef]
- Alvarez Feijoo, M.A.; Arce Farina, M.E.; Suarez–Garcia, A.; Gonzalez–Pena, D.; Diez–Mediavilla, M. Compounds with Epoxy Resins and Phase Change Materials for Storage in Solar Applications. Materials 2019, 12, 3522. [Google Scholar] [CrossRef]
- Zhao, L.; Qi, Y.; Song, L.; Ning, S.; Ouyang, S.; Xu, H.; Ye, J. Solar–Driven Water–Gas Shift Reaction over CuOx/Al2O3 with 1.1% of Light–to–Energy Storage. Angew. Chem. Int. Ed. Engl. 2019, 58, 7708–7712. [Google Scholar] [CrossRef]
- Wang, F.Q.; Tan, J.Y.; Jin, H.J.; Yu, L. Thermochemical performance analysis of solar driven CO2 methane reforming. Energy 2015, 91, 645–654. [Google Scholar]
- Chuayboon, S.; Abanades, S. An overview of solar decarbonization processes, reacting oxide materials, and thermochemical reactors for hydrogen and syngas production. Int. J. Hydrogen Energy 2020, 45, 25783–25810. [Google Scholar] [CrossRef]
- Li, W.; Zhu, X.; Cao, Z.; Wang, W.; Yang, W. Mixed ionic–electronic conducting (MIEC) membranes for hydrogen production from water splitting. Int. J. Hydrogen Energy 2015, 40, 3452–3461. [Google Scholar] [CrossRef]
- Wu, X.-Y.; Ghoniem, A.F. Mixed ionic–electronic conducting (MIEC) membranes for thermochemical reduction of CO2: A review. Prog. Energy Combust. Sci. 2019, 74, 1–30. [Google Scholar] [CrossRef]
- Wang, H.; Wang, B.; Qi, X.; Wang, J.; Yang, R.; Li, D.; Hu, X. Innovative non–oxidative methane dehydroaromatization via solar membrane reactor. Energy 2021, 216, 119265. [Google Scholar] [CrossRef]
- Tou, M.; Michalsky, R.; Steinfeld, A. Solar–Driven Thermochemical Splitting of CO2 and In Situ Separation of CO and O2 across a Ceria Redox Membrane Reactor. Joule 2017, 1, 146–154. [Google Scholar] [CrossRef] [PubMed]
- He, R.J.; Wang, Y.P.; Wang, H.S.; Lundin, S.T.B.; Wang, B.Z.; Kong, H.; Lu, X.F.; Wang, J.; Li, W.J. A mid/low–temperature solar–driven integrated membrane reactor for the dehydrogenation of propane–A thermodynamic assessment. Appl. Therm. Eng. 2021, 193, 116952. [Google Scholar] [CrossRef]
- Kothari, M.; Jeon, Y.; Miller, D.N.; Pascui, A.E.; Kilmartin, J.; Wails, D.; Ramos, S.; Chadwick, A.; Irvine, J.T.S. Platinum incorporation into titanate perovskites to deliver emergent active and stable platinum nanoparticles. Nat. Chem. 2021, 13, 677–682. [Google Scholar] [CrossRef]
- Madsen, B.D.; Kobsiriphat, W.; Wang, Y.; Marks, L.D.; Barnett, S.A. Nucleation of nanometer–scale electrocatalyst particles in solid oxide fuel cell anodes. J. Power Sources 2007, 166, 64–67. [Google Scholar] [CrossRef]
- Neagu, D.; Tsekouras, G.; Miller, D.N.; Menard, H.; Irvine, J.T. In situ growth of nanoparticles through control of non–stoichiometry. Nat. Chem. 2013, 5, 916–923. [Google Scholar] [CrossRef]
- Kousi, K.; Neagu, D.; Metcalfe, I.S. Combining Exsolution and Infiltration for Redox, Low Temperature CH4 Conversion to Syngas. Catalysts 2020, 10, 468. [Google Scholar] [CrossRef]
- Neagu, D.; Irvine, J.T.S. Structure and Properties of La0.4Sr0.4TiO3 Ceramics for Use as Anode Materials in Solid Oxide Fuel Cells. Chem. Mater. 2010, 22, 5042–5053. [Google Scholar] [CrossRef]
- Myung, J.H.; Neagu, D.; Miller, D.N.; Irvine, J.T. Switching on electrocatalytic activity in solid oxide cells. Nature 2016, 537, 528–531. [Google Scholar] [CrossRef]
- Jiang, K.; Liu, Z.; Zhang, G.; Jin, W. A novel catalytic membrane reactor with homologous exsolution–based perovskite catalyst. J. Membr. Sci. 2020, 608, 118213. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, G.; Liu, G.; Liu, Z.; Jin, W.; Xu, N. Perovskite Hollow Fibers with Precisely Controlled Cation Stoichiometry via One–Step Thermal Processing. Adv. Mater. 2017, 29, 1606377. [Google Scholar] [CrossRef] [PubMed]


| Material | Membrane Type | Thickness (mm) | Feed Gas | Sweep Gas | Temperature (°C) | Oxygen Flux () | Stability | Ref. |
|---|---|---|---|---|---|---|---|---|
| SrCo0.8Fe0.2O3−δ | disk | 1.0 | Air | He | 877 | 3.1 | / | [44] |
| SrCo0.4Fe0.6O3−δ | disk | 1.0 | Air | He | 877 | 2.4 | / | [44] |
| La0.2Sr0.8Co0.4Fe0.6O3−δ | disk | 1.0 | Air | He | 877 | 0.6 | / | [44] |
| La0.6Sr0.4CoO3−δ | disk | 1.5 | Air | He | 860 | 1.0 | / | [55] |
| La0.6Sr0.4Co0.8Fe0.2O3−δ | disk | 1.5 | Air | He | 860 | 0.62 | / | [55] |
| La0.6Sr0.4Co0.8Mn0.2O3−δ | disk | 1.5 | Air | He | 860 | 0.50 | / | [55] |
| La0.6Sr0.4Co0.8Ni0.2O3−δ | disk | 1.5 | Air | He | 860 | 1.44 | / | [55] |
| La0.2Sr0.8Co0.2Fe0.8O3−δ | disk | 2 | Air | He | 850 | 0.32 | / | [56] |
| SrCo0.8Fe0.2O3−δ | disk | 1 | Air | Inert gas | 900 | 4.24 | / | [57] |
| Ba0.5Sr0.5Co0.8Fe0.2O3−δ | hollow fiber | 0.22 | Air | He | 900 | 3.80 | / | [58] |
| BaCe0.15Fe0.85O3−δ | disk | 1 | Air | He | 900 | 0.418 | / | [59] |
| BaCe0.15Fe0.85O3−δ | disk | 1.4 | Air | He | 940 | 0.47 | 1 h in 5% H2 + Ar | [60] |
| 0.56 | 0.81 | |||||||
| BaBi0.3Co0.2Fe0.5O3−δ | disk | 1.5 | Air | He | 900 | 0.5 | / | [61] |
| 0.66 | 0.85 | |||||||
| SrCo0.9Nb0.1O3−δ | disk | 1 | 21% oxygen−He | Ar | 900 | 4.24 | 200 days | [62] |
| SrCo0.9Ta0.1O3−δ | disk | 0.65 | Air | He | 900 | 1.83 | 520 h | [63] |
| Ba0.5Sr0.5Co0.8Fe0.2O3−δ | disk | 0.9 (0.07 dense) | Air | Ar | 900 | 10.0 | / | [64] |
| SrCo0.8Fe0.2O3−δ +0.5%wt Nb2O5 | 4−channel hollow fiber | 0.2 (0.01 dense) | Air | He | 900 | 1.6 | 500 h | [65] |
| SrFe0.8Nb0.2O3−δ | disk | 1 | Air | CO2 | 900 | 0.25 | 210 h | [2] |
| SrFe0.9Ta0.1O3−δ | disk | 1 | Air | CO2 | 900 | 0.30 | 130 h | [66] |
| SrFe0.9Ta0.1O3−δ | 4−channel hollow fiber | 0.25 | Air | CO2 | 900 | 1.15 | 130 h | [66] |
| BaCo0.7Fe0.22Nb0.08O3−δ | 4−channel hollow fiber | 0.13 | Air | He | 900 | 8.1 | / | [67] |
| 0.13 | 650 | 1.15 | 300 h | [67] |
| Material | Membrane Type | Thickness (mm) | Feed Gas | Sweep Gas | Temperature (°C) | Oxygen Flux () | Stability | Ref. |
|---|---|---|---|---|---|---|---|---|
| Ba(Ce0.7Zr0.1Y0.1Yb0.1)0.95Ni0.05O3−δ | disk | 0.45 | 10% H2−N2 | Ar | 800 | 0.42 | Stable at 700 °C for 200 h | [77] |
| 0.6 | 0.24 | |||||||
| SrCe0.9Y0.1O3−δ | disk | 0.8 | 40% H2−N2 | Ar | 900 | 0.10 | / | [78] |
| BaCe0.9Y0.1O3−δ | disk | 0.8 | 40% H2−N2 | Ar | 900 | 0.75 | / | [78] |
| Ba0.5Sr0.5Ce0.9Y0.1O3−δ | disk | 0.8 | 40% H2−N2 | Ar | 900 | 1.60 | / | [78] |
| LaCe0.9Y0.1O3−δ | disk | 0.8 | 40% H2−N2 | Ar | 900 | 0.02 | / | [78] |
| Pd modified−BaCe0.95Tb0.05O3−δ | hollow fiber | 0.26 | 50% H2−Ne | N2 | 900 | 0.27 | / | [79] |
| BaCe0.85Tb0.05Co0.1O3−δ | hollow fiber | 0.75 | 50% H2−Ne | N2 | 1000 | 0.38 | / | [80] |
| BaCe0.95Nd0.05O3−δ | disk | 0.7 | 80% H2+He+H2O | Ar | 925 | 0.026 | / | [81] |
| BaZr0.1Ce0.2Y0.7O3−δ | disk | 50% H2−N2 | Ar | 900 | 0.018 | Stable at 850 °C for 2 h | [82] | |
| BaZr0.3Ce0.6Y0.1Zn0.05 O3−δ | disk | 50% H2−N2 | Ar | 900 | 0.012 | Stable at 850 °C for 4 h | [82] |
| Reaction | Reaction Equation | (−1) | Membrane Transport | Temperature () |
|---|---|---|---|---|
| Partial oxidation of methane | −36 | O2−/e− | 850–900 | |
| Oxidative coupling of methane | −177 −282 | O2−/e− | 688–955 | |
| Oxidative dehydrogenation of hydrocarbons | −105 | O2−/e− | 700–850 | |
| Water splitting reaction | −242 | O2−/e− H+/e− | 900 | |
| Alkane to olefin reaction | 71.1 57.2 | H+/e− | 750–900 | |
| Carbon dioxide decomposition | 552 | O2−/e− | >900 | |
| Steam reforming | 210 256 | O2−/e− | 800 | |
| Dry reforming | 260.5 | O2−/e− | 700–1000 | |
| Dehydroaromation of methane | 531 −1846 | H+/e− O2−/e− | 700–750 |
| Material | Membrane Type | Thickness (mm) | Feed Gas | Reaction Gas | Temp. (°C) | Oxygen Flux () | CH4 Conversion (%) | CO Selectivity (%) | Stability | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Ba0.5Sr0.5Co0.8Fe0.2O3−δ | disk | 1.50 | Air | 50% CH4+He | 875 | 11.50 | 98.2 | 96.0 | 500 h | [39] |
| BaCo0.4Fe0.4Zr0.2O3−δ | disk | 1.0 | Air | 50% CH4+He | 850 | 5.7 | 97.5 | 98.5 | 2200 h | [120] |
| Ba0.5Sr0.5Zn0.2Fe0.8O3−δ | disk | 1.25 | Air | 50% CH4+He | 950 | 2.55 | 60.0 | 98.3 | 65 h | [124] |
| BaCe0.1Co0.4Fe0.5O3−δ | disk | 1.0 | Air | CH4 | 875 | 8.9 | >97.0 | >97.0 | 1000 h | [125] |
| 75wt.% Ce 0.85Sm 0.15O1.925 –25 wt.% Sm0.6Sr0.4FeO3−δ | disk | 0.6 | Air | CH4 | 950 | 4.2 | >98.0 | >98.0 | 650 h | [24] |
| La0.4Ba0.6Fe1−xZnxO3−δ | disk | 0.5 | Air | 17.5% CH4+He | 950 | 7.80 | 100 | 72 | 500 h | [126] |
| 100% CH4 | 950 | 11.8 | 55 | 100 | 180 h | [126] | ||||
| Ba0.9Co0.7Fe0.2Nb0.1O3−δ | disk | 1 | Air | 30% CH4+He | 875 | 7.10 | 93.4 | 94.5 | 400 h | [127] |
| Ba0.5Sr0.5Co0.8Fe0.1Ni0.1O3−δ | disk | Not | Air | 50% CH4+He | 850 | 12.00 | 98.0 | 97.5 | 120 h | [100] |
| SrCo0.8Fe0.2NbO3−δ (Ba0.3Sr0.7Fe0.9Mo0.1O3−δ) | disk | 1.0 | Air | 13% CH4+He | 850 | 13.0 | 80.0 | 98.84 | 1500 h | [128] |
| BaCo0.7Fe0.2Ta0.1O3−δ | hollow fiber | 0.23 | Air | 55.8% CH4+He | 875 | 20.0 | 96 | 99 | 83 h | [129] |
| (Pr0.9La0.1)2 (Ni0.74Cu0.21Ga0.05)O4+δ | hollow fiber | 0.19 | Air | 20.3% CH4+He | 900 | 10.5 | 97 | 99.5 | 140 h | [101] |
| Ba0.9Co0.7Fe0.2Nb0.1O3−δ | disk | 0.12 | Air | 30% CH4+Ar | 875 | 16.00 | 96.66 | 78.7 | 100 h | [130] |
| SrFe0.8Nb0.2O3−δ | 4−channel hollow fiber | 0.08 | Air | 17% CH4+He | 900 | 19.2 | 94.6 | 99 | 120 h | [94] |
| SrCo0.8Fe0.1Ga0.1O3−δ | hollow fiber | 0.21 | Air | 20% CH4+He | 800 | 4.14 | 100 | 33 | 220 h | [131] |
| BaBi0.05Co0.8Nb0.15O3−δ | hollow fiber | 0.13 | Air | 50% CH4+He | 730 | 15.05 | 80 | 85 | 5 h | [132] |
| Material | Membrane Type | Thickness (mm) | Feed Gas | Reaction Gas | Temp. (°C) | Oxygen Flux () | Conversion (%) | Selectivity (%) | Yield (%) | Reaction & Stability | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BaCe0.8Gd0.2O3−δ | tube | 0.7 | Air | 3% CH4+He | 780 | 0.1 | 26 (CH4) | 62 (C2) | >16 (C2) | OCM | [35] |
| Ba0.5Sr0.5Co0.8Fe0.2O3−δ | tube | 1.7 | Air | 10% CH4+He | 850 | 1.50 | 5 (CH4) | 62 (C2) | 15 (C2) | OCM | [139] |
| Ba0.5Sr0.5Co0.8Fe0.2O3−δ | disk | 1.0 | Air | 34% CH4+He | 900 | 7.0 | 25 (CH4) | 70 (C2) | 18 (C2) | OCM | [25] |
| Ba0.5Sr0.5Co0.8Fe0.2O3−δ | disk | 1.0 | Air | 11% CH4+He | 900 | 2.00 | 23 (CH4) | 63 (C2) | 15 (C2) | OCM | [25] |
| Ba0.5Ce0.4Gd0.1Co0.8Fe0.2O3−δ | tube | 3.0 | Air | 50% CH4+He | 850 | 1.40 | 51.6 (CH4) | 67.4 (C2) | 34.7 (C2) | OCM | [140] |
| La0.6Sr0.4Co0.2Fe0.8O3−δ | hollow fiber | 0.25 | Air | 75% CH4+Ar | 900 | 8.73 | 49 (CH4) | 79.5 (C2) | 39 (C2) | OCM (18 h) | [134] |
| BaCe0.8Gd0.2O3−δ | disk | 0.5 | Air | 95% CH4+He | 880 | 2.1 | / | 80 (C2) | / | OCM (25 h) | [141] |
| Ni–La0.8Sr0.2Ga0.8Mg0.2O3−δ | disk | 0.5 | Air | 95% CH4+He | 880 | 0.9 | / | 30 (C2) | / | OCM (25 h) | [141] |
| Ba0.5Sr0.5Co0.8Fe0.2O3−δ | planar | 1.0 | Air | 10% C2H6+He | 800 | 1.75 | 84 (C2H6) | 80 (C2) | / | ODH (100 h) | [142] |
| BaCoxFeyZrzO3−δ | hollow fiber | 0.1 | Air | 10% C2H6+He | 800 | 1.15 | 89.6 (C2H6) | 39.9 (C2) | / | ODH | [143] |
| BaCoxFeyZrzO3−δ | hollow fiber | 0.14 | Air | 10% C3H8+ 20% H2O+He | 675 | / | 48 (C3H8) | 59 (C3H6) | 28 (C2) | ODH | [104] |
| Ce0.9Gd0.1O2−δ–BaFe0.9Mg0.05Ce0.05O3−δ | disk | 0.5 | Air | 5% C2H6+He | 725 | / | 74 (C2H6) | 82 (C2H4) | 63 (C2) | ODH (200 h) | [144] |
| BaBi0.05Co0.8Nb0.15O3−δ | hollow fiber | 0.12 | Air | C3H8 | 650 | / | 62–75 (C3H8) | 68–74 (C3H6) | 46–51 (C3H6) | ODH (50 h) | [137] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Zhou, W.; Wang, T.; Gu, Z.; Zhu, Y.; Liu, Z.; Wu, Z.; Zhang, G.; Jin, W. Ion–Conducting Ceramic Membrane Reactors for the Conversion of Chemicals. Membranes 2023, 13, 621. https://doi.org/10.3390/membranes13070621
Zhang Z, Zhou W, Wang T, Gu Z, Zhu Y, Liu Z, Wu Z, Zhang G, Jin W. Ion–Conducting Ceramic Membrane Reactors for the Conversion of Chemicals. Membranes. 2023; 13(7):621. https://doi.org/10.3390/membranes13070621
Chicago/Turabian StyleZhang, Zhicheng, Wanglin Zhou, Tianlei Wang, Zhenbin Gu, Yongfan Zhu, Zhengkun Liu, Zhentao Wu, Guangru Zhang, and Wanqin Jin. 2023. "Ion–Conducting Ceramic Membrane Reactors for the Conversion of Chemicals" Membranes 13, no. 7: 621. https://doi.org/10.3390/membranes13070621
APA StyleZhang, Z., Zhou, W., Wang, T., Gu, Z., Zhu, Y., Liu, Z., Wu, Z., Zhang, G., & Jin, W. (2023). Ion–Conducting Ceramic Membrane Reactors for the Conversion of Chemicals. Membranes, 13(7), 621. https://doi.org/10.3390/membranes13070621







