Hypo-Osmotic Stress and Pore-Forming Toxins Adjust the Lipid Order in Sheep Red Blood Cell Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Red Blood Cell Preparation
2.2. Pore-Forming Toxins
2.3. Hypo-Osmotic Shock Exposure
2.4. Fluorescence Measurements
3. Results and Discussion
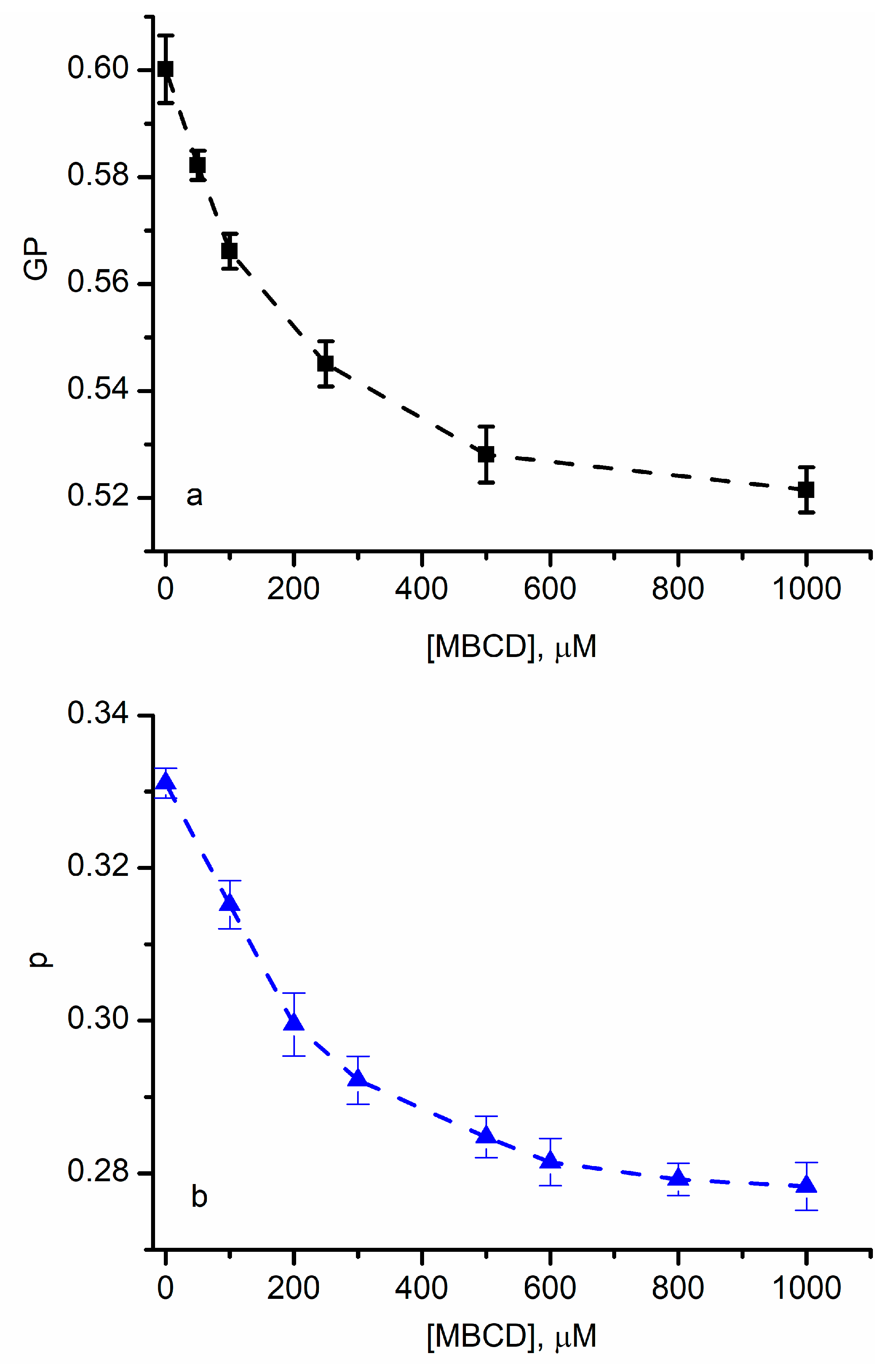
3.1. Cholesterol Depletion in Red Blood Cell Membranes Modulates Lipid Ordering
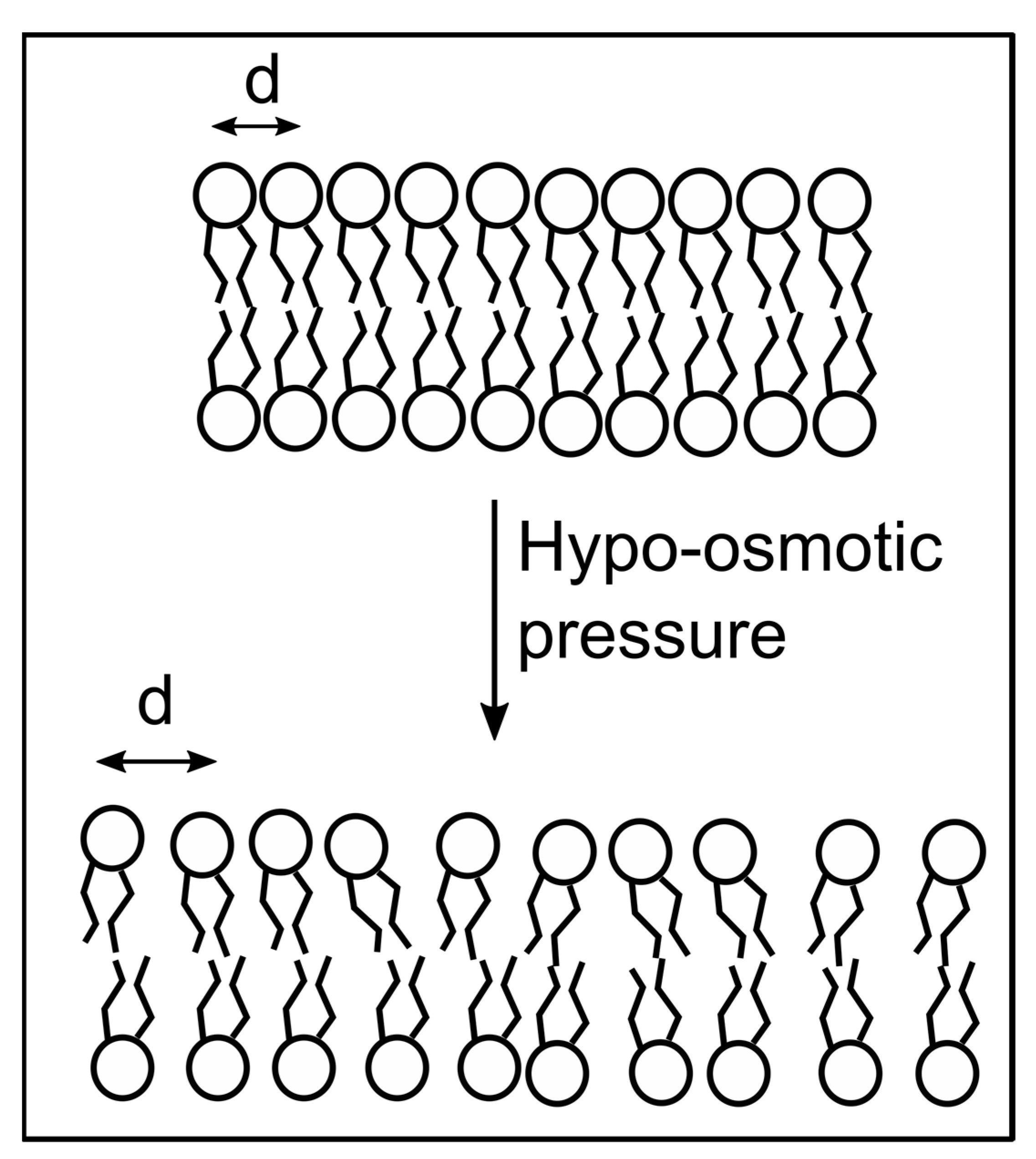
3.2. Hypo-Osmotic Shock Modulates the Optical Responses of Environment-Sensitive Membrane Probes
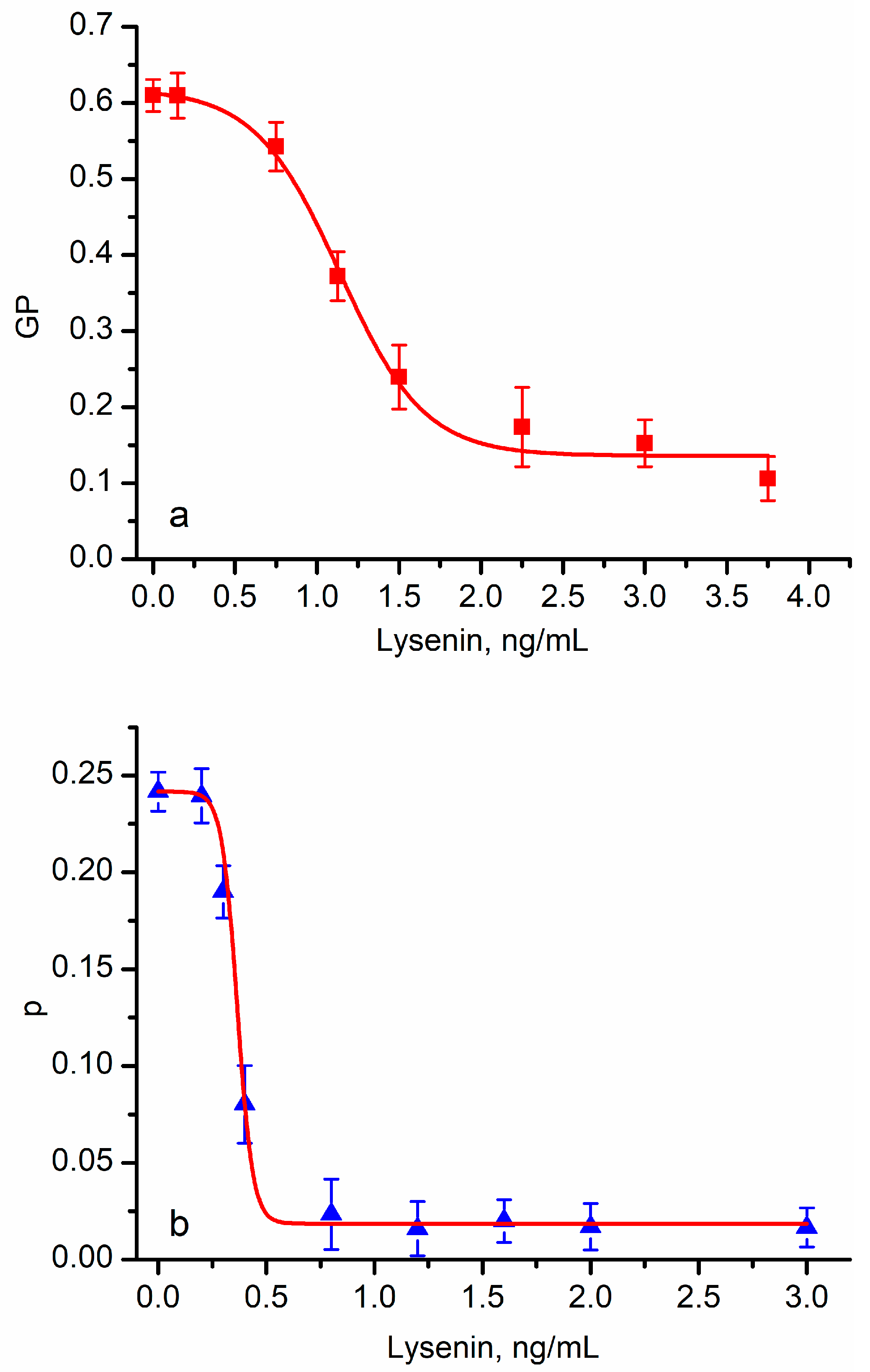
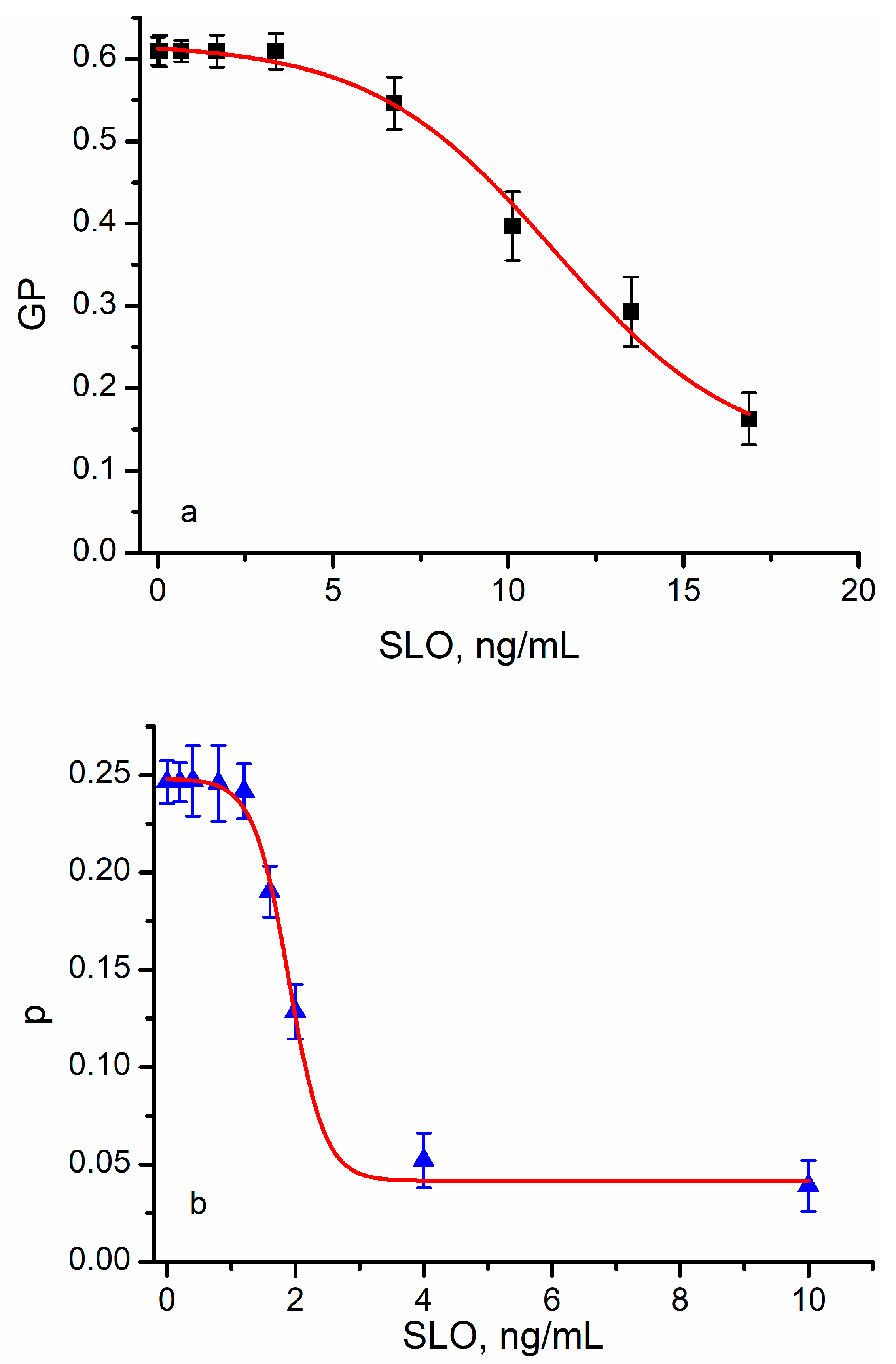
3.3. Pore-Forming Toxins (PFTs) Adjust the Lipid Order in Target Membranes
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Suzuki, K.G.N.; Kusumi, A. Refinement of Singer-Nicolson fluid-mosaic model by microscopy imaging: Lipid rafts and actin-induced membrane compartmentalization. Biochim. Biophys. Acta Biomembr. 2023, 1865, 184093. [Google Scholar] [CrossRef] [PubMed]
- Jouhet, J. Importance of the hexagonal lipid phase in biological membrane organization. Front. Plant. Sci. 2013, 4, 494. [Google Scholar] [CrossRef]
- Goñi, F.M. The basic structure and dynamics of cell membranes: An update of the Singer-Nicolson model. Biochim. Biophys. Acta 2014, 1838, 1467–1476. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, H.-J.; Lingwood, D.; Levental, I.; Sampaio, J.L.; Kalvodova, L.; Rajendran, L.; Simons, K. Order of lipid phases in model and plasma membranes. Proc. Natl. Acad. Sci. USA 2009, 106, 16645–16650. [Google Scholar] [CrossRef] [PubMed]
- Klymchenko, A.S.; Kreder, R. Fluorescent Probes for Lipid Rafts: From Model Membranes to Living Cells. Chem. Biol. 2014, 21, 97–113. [Google Scholar] [CrossRef]
- Nicolson, G.L. Update of the 1972 Singer-Nicolson Fluid-Mosaic Model of Membrane Structure. Discoveries 2013, 1, e3. [Google Scholar] [CrossRef]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef]
- Shaw, T.R.; Ghosh, S.; Veatch, S.L. Critical Phenomena in Plasma Membrane Organization and Function. Annu. Rev. Phys. Chem. 2021, 72, 51–72. [Google Scholar] [CrossRef]
- Lingwood, D.; Simons, K. Lipid Rafts As a Membrane-Organizing Principle. Science 2010, 327, 46–50. [Google Scholar] [CrossRef]
- London, E. Insights into lipid raft structure and formation from experiments in model membranes. Curr. Opin. Struct. Biol. 2002, 12, 480–486. [Google Scholar] [CrossRef]
- London, E. Ordered Domain (Raft) Formation in Asymmetric Vesicles and Its Induction upon Loss of Lipid Asymmetry in Artificial and Natural Membranes. Membranes 2022, 12, 870. [Google Scholar] [CrossRef]
- Murata, M.; Matsumori, N.; Kinoshita, M.; London, E. Molecular substructure of the liquid-ordered phase formed by sphingomyelin and cholesterol: Sphingomyelin clusters forming nano-subdomains are a characteristic feature. Biophys. Rev. 2022, 14, 655–678. [Google Scholar] [CrossRef]
- Gaibelet, G.; Tercé, F.; Allart, S.; Lebrun, C.; Collet, X.; Jamin, N.; Orlowski, S. Fluorescent probes for detecting cholesterol-rich ordered membrane microdomains: Entangled relationships between structural analogies in the membrane and functional homologies in the cell. AIMS Biophys. 2017, 4, 121–151. [Google Scholar] [CrossRef]
- Gibbons, E.; Pickett, K.R.; Streeter, M.C.; Warcup, A.O.; Nelson, J.; Judd, A.M.; Bell, J.D. Molecular details of membrane fluidity changes during apoptosis and relationship to phospholipase A2 activity. Biochim. Biophys. Acta 2013, 1828, 887–895. [Google Scholar] [CrossRef]
- Bálint, Š.; Dustin, M.L. Localizing order to boost signaling. Elife 2017, 6, e25375. [Google Scholar] [CrossRef]
- Dustin, M.L.; Muller, J. Liquidity in immune cell signaling. Science 2016, 352, 516–517. [Google Scholar] [CrossRef]
- Stone, M.B.; Shelby, S.A.; Núñez, M.F.; Wisser, K.; Veatch, S.L. Protein sorting by lipid phase-like domains supports emergent signaling function in B lymphocyte plasma membranes. eLife 2017, 6, e19891. [Google Scholar] [CrossRef]
- Su, X.; Ditlev, J.A.; Hui, E.; Xing, W.; Banjade, S.; Okrut, J.; King, D.S.; Taunton, J.; Rosen, M.K.; Vale, R.D. Phase separation of signaling molecules promotes T cell receptor signal transduction. Science 2016, 352, 595–599. [Google Scholar] [CrossRef]
- Noutsi, P.; Gratton, E.; Chaieb, S. Assessment of Membrane Fluidity Fluctuations during Cellular Development Reveals Time and Cell Type Specificity. PLoS ONE 2016, 11, e0158313. [Google Scholar] [CrossRef]
- Gupta, A.; Dey, S.; Bhowmik, D.; Maiti, S. Coexisting Ordered and Disordered Membrane Phases Have Distinct Modes of Interaction with Disease-Associated Oligomers. J. Phys. Chem. B 2022, 126, 1016–1023. [Google Scholar] [CrossRef]
- Gimpl, G.; Fahrenholz, F. Human oxytocin receptors in cholesterol-rich vs. cholesterol-poor microdomains of the plasma membrane. Eur. J. Biochem. 2000, 267, 2483–2497. [Google Scholar] [CrossRef]
- Urbančič, I.; Schiffelers, L.; Jenkins, E.; Gong, W.; Santos, A.M.; Schneider, F.; O’Brien-Ball, C.; Vuong, M.T.; Ashman, N.; Sezgin, E.; et al. Aggregation and mobility of membrane proteins interplay with local lipid order in the plasma membrane of T cells. FEBS Lett. 2021, 595, 2127–2146. [Google Scholar] [CrossRef]
- Gonzalez, L.J.; Gibbons, E.; Bailey, R.W.; Fairbourn, J.; Nguyen, T.; Smith, S.K.; Best, K.B.; Nelson, J.; Judd, A.M.; Bell, J.D. The influence of membrane physical properties on microvesicle release in human erythrocytes. PMC Biophys. 2009, 2, 7. [Google Scholar] [CrossRef]
- Sengupta, S.; Karsalia, R.; Morrissey, A.; Bamezai, A.K. Cholesterol-dependent plasma membrane order (Lo) is critical for antigen-specific clonal expansion of CD4+ T cells. Sci. Rep. 2021, 11, 13970. [Google Scholar] [CrossRef]
- Orlikowska-Rzeznik, H.; Krok, E.; Chattopadhyay, M.; Lester, A.; Piatkowski, L. Laurdan discerns lipid membrane hydration and cholesterol content. bioRxiv 2022, 2022, 514927. [Google Scholar] [CrossRef]
- Färber, N.; Westerhausen, C. Broad lipid phase transitions in mammalian cell membranes measured by Laurdan fluorescence spectroscopy. Biochim. Biophys. Acta 2022, 1864, 183794. [Google Scholar] [CrossRef]
- Neunert, G.; Tomaszewska-Gras, J.; Baj, A.; Gauza-Włodarczyk, M.; Witkowski, S.; Polewski, K. Phase Transitions and Structural Changes in DPPC Liposomes Induced by a 1-Carba-Alpha-Tocopherol Analogue. Molecules 2021, 26, 2851. [Google Scholar] [CrossRef]
- Havlíková, M.; Szabová, J.; Mravcová, L.; Venerová, T.; Chang, C.-H.; Pekař, M.; Jugl, A.; Mravec, F. Cholesterol Effect on Membrane Properties of Cationic Ion Pair Amphiphile Vesicles at Different Temperatures. Langmuir 2021, 37, 2436–2444. [Google Scholar] [CrossRef]
- Kure, T.; Sakai, H. Transmembrane Difference in Colloid Osmotic Pressure Affects the Lipid Membrane Fluidity of Liposomes Encapsulating a Concentrated Protein Solution. Langmuir 2017, 33, 1533–1540. [Google Scholar] [CrossRef]
- Bagatolli, L.A.; Maggio, B.; Aguilar, F.; Sotomayor, C.P.; Fidelio, G.D. Laurdan properties in glycosphingolipid-phospholipid mixtures: A comparative fluorescence and calorimetric study. Biochim. Biophys. Acta 1997, 1325, 80–90. [Google Scholar] [CrossRef]
- Parasassi, T.; Di Stefano, M.; Loiero, M.; Ravagnan, G.; Gratton, E. Influence of cholesterol on phospholipid bilayers phase domains as detected by Laurdan fluorescence. Biophys. J. 1994, 66, 120–132. [Google Scholar] [CrossRef]
- Parasassi, T.; De Stasio, G.; Ravagnan, G.; Rusch, R.M.; Gratton, E. Quantitation of lipid phases in phospholipid vesicles by the generalized polarization of Laurdan fluorescence. Biophys. J. 1991, 60, 179–189. [Google Scholar] [CrossRef]
- Moulton, E.R.; Hirsche, K.J.; Hobbs, M.L.; Schwab, J.M.; Bailey, E.G.; Bell, J.D. Examining the effects of cholesterol on model membranes at high temperatures: Laurdan and Patman see it differently. Biochim. Biophys. Acta 2018, 1860, 1571–1579. [Google Scholar] [CrossRef]
- Pokorna, S.; Ventura, A.E.; Santos, T.C.B.; Hof, M.; Prieto, M.; Futerman, A.H.; Silva, L.C. Laurdan in live cell imaging: Effect of acquisition settings, cell culture conditions and data analysis on generalized polarization measurements. J. Photochem. Photobiol. B Biol. 2022, 228, 112404. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Frangos, J.A.; Chachisvilis, M. Laurdan fluorescence senses mechanical strain in the lipid bilayer membrane. Biochem. Biophys. Res. Commun. 2006, 347, 838–841. [Google Scholar] [CrossRef]
- do Canto, A.M.T.M.; Robalo, J.R.; Santos, P.D.; Carvalho, A.J.P.; Ramalho, J.P.P.; Loura, L.M.S. Diphenylhexatriene membrane probes DPH and TMA-DPH: A comparative molecular dynamics simulation study. Biochim. Biophys. Acta 2016, 1858, 2647–2661. [Google Scholar] [CrossRef]
- Mykytczuk, N.C.S.; Trevors, J.T.; Leduc, L.G.; Ferroni, G.D. Fluorescence polarization in studies of bacterial cytoplasmic membrane fluidity under environmental stress. Prog. Biophys. Mol. Biol. 2007, 95, 60–82. [Google Scholar] [CrossRef]
- Bondelli, G.; Paternò, G.M.; Lanzani, G. Fluorescent probes for optical investigation of the plasma membrane. Opt. Mater. X 2021, 12, 100085. [Google Scholar] [CrossRef]
- Sanchez, S.A.; Tricerri, M.A.; Gratton, E. Laurdan generalized polarization fluctuations measures membrane packing micro-heterogeneity in vivo. Proc. Natl. Acad. Sci. USA 2012, 109, 7314–7319. [Google Scholar] [CrossRef]
- Ariola, F.S.; Li, Z.; Cornejo, C.; Bittman, R.; Heikal, A.A. Membrane Fluidity and Lipid Order in Ternary Giant Unilamellar Vesicles Using a New Bodipy-Cholesterol Derivative. Biophys. J. 2009, 96, 2696–2708. [Google Scholar] [CrossRef]
- Blicher, A.; Wodzinska, K.; Fidorra, M.; Winterhalter, M.; Heimburg, T. The Temperature Dependence of Lipid Membrane Permeability, its Quantized Nature, and the Influence of Anesthetics. Biophys. J. 2009, 96, 4581–4591. [Google Scholar] [CrossRef]
- Frallicciardi, J.; Melcr, J.; Siginou, P.; Marrink, S.J.; Poolman, B. Membrane thickness, lipid phase and sterol type are determining factors in the permeability of membranes to small solutes. Nat. Commun. 2022, 13, 1605. [Google Scholar] [CrossRef] [PubMed]
- Kraske, W.V.; Mountcastle, D.B. Effects of cholesterol and temperature on the permeability of dimyristoylphosphatidylcholine bilayers near the chain melting phase transition. Biochim. Biophys. Acta 2001, 1514, 159–164. [Google Scholar] [CrossRef]
- Ma, Y.; Benda, A.; Kwiatek, J.; Owen, D.M.; Gaus, K. Time-Resolved Laurdan Fluorescence Reveals Insights into Membrane Viscosity and Hydration Levels. Biophys. J. 2018, 115, 1498–1508. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Tristram-Nagle, S.; Kučerka, N.; Nagle, J.F. Temperature Dependence of Structure, Bending Rigidity, and Bilayer Interactions of Dioleoylphosphatidylcholine Bilayers. Biophys. J. 2008, 94, 117–124. [Google Scholar] [CrossRef]
- Steinkühler, J.; Sezgin, E.; Urbančič, I.; Eggeling, C.; Dimova, R. Mechanical properties of plasma membrane vesicles correlate with lipid order, viscosity and cell density. Commun. Biol. 2019, 2, 337. [Google Scholar] [CrossRef]
- Hamada, N.; Longo, M.L. Characterization of phase separation phenomena in hybrid lipid/block copolymer/cholesterol bilayers using laurdan fluorescence with log-normal multipeak analysis. Biochim. Biophys. Acta 2022, 1864, 183887. [Google Scholar] [CrossRef]
- Stott, B.M.; Vu, M.P.; McLemore, C.O.; Lund, M.S.; Gibbons, E.; Brueseke, T.J.; Wilson-Ashworth, H.A.; Bell, J.D. Use of fluorescence to determine the effects of cholesterol on lipid behavior in sphingomyelin liposomes and erythrocyte membranes. J. Lipid Res. 2008, 49, 1202–1215. [Google Scholar] [CrossRef]
- Ingólfsson, H.I.; Melo, M.N.; van Eerden, F.J.; Arnarez, C.; Lopez, C.A.; Wassenaar, T.A.; Periole, X.; de Vries, A.H.; Tieleman, D.P.; Marrink, S.J. Lipid Organization of the Plasma Membrane. J. Am. Chem. Soc. 2014, 136, 14554–14559. [Google Scholar] [CrossRef]
- Luchini, A.; Vitiello, G. Mimicking the Mammalian Plasma Membrane: An Overview of Lipid Membrane Models for Biophysical Studies. Biomimetics 2021, 6, 3. [Google Scholar] [CrossRef]
- Róg, T.; Pasenkiewicz-Gierula, M.; Vattulainen, I.; Karttunen, M. Ordering effects of cholesterol and its analogues. Biochim. Biophys. Acta 2009, 1788, 97–121. [Google Scholar] [CrossRef]
- de Meyer, F.; Smit, B. Effect of cholesterol on the structure of a phospholipid bilayer. Proc. Natl. Acad. Sci. USA 2009, 106, 3654–3658. [Google Scholar] [CrossRef]
- Los, D.A.; Murata, N. Membrane fluidity and its roles in the perception of environmental signals. Biochim. Biophys. Acta 2004, 1666, 142–157. [Google Scholar] [CrossRef] [PubMed]
- Söderlund, T.; Alakoskela, J.-M.I.; Pakkanen, A.L.; Kinnunen, P.K.J. Comparison of the Effects of Surface Tension and Osmotic Pressure on the Interfacial Hydration of a Fluid Phospholipid Bilayer. Biophys. J. 2003, 85, 2333–2341. [Google Scholar] [CrossRef] [PubMed]
- Nomoto, T.; Takahashi, M.; Fujii, T.; Chiari, L.; Toyota, T.; Fujinami, M. Effects of Cholesterol Concentration and Osmolarity on the Fluidity and Membrane Tension of Free-standing Black Lipid Membranes. Anal. Sci. 2018, 34, 1237–1242. [Google Scholar] [CrossRef]
- Laroche, C.; Beney, L.; Marechal, P.; Gervais, P. The effect of osmotic pressure on the membrane fluidity of Saccharomyces cerevisiae at different physiological temperatures. Appl. Microbiol. Biotechnol. 2001, 56, 249–254. [Google Scholar] [CrossRef]
- Bogard, A.; Finn, P.W.; McKinney, F.; Flacau, I.M.; Smith, A.R.; Whiting, R.; Fologea, D. The Ionic Selectivity of Lysenin Channels in Open and Sub-Conducting States. Membranes 2021, 11, 897. [Google Scholar] [CrossRef]
- Amaro, M.; Reina, F.; Hof, M.; Eggeling, C.; Sezgin, E. Laurdan and Di-4-ANEPPDHQ probe different properties of the membrane. J. Phys. D. Appl. Phys. 2017, 50, 134004. [Google Scholar] [CrossRef]
- Weber, P.; Wagner, M.; Schneckenburger, H. Fluorescence imaging of membrane dynamics in living cells. J. Biomed. Opt. 2010, 15, 046017. [Google Scholar] [CrossRef]
- Golfetto, O.; Hinde, E.; Gratton, E. Laurdan Fluorescence Lifetime Discriminates Cholesterol Content from Changes in Fluidity in Living Cell Membranes. Biophys. J. 2013, 104, 1238–1247. [Google Scholar] [CrossRef]
- Horváth, Á.; Erostyák, J.; Szőke, É. Effect of Lipid Raft Disruptors on Cell Membrane Fluidity Studied by Fluorescence Spectroscopy. Int. J. Mol. Sci. 2022, 23, 13729. [Google Scholar] [CrossRef]
- dos Santos, A.G.; Bayiha, J.C.; Dufour, G.; Cataldo, D.; Evrard, B.; Silva, L.C.; Deleu, M.; Mingeot-Leclercq, M.-P. Changes in membrane biophysical properties induced by the Budesonide/Hydroxypropyl-β-cyclodextrin complex. Biochim. Biophys. Acta 2017, 1859, 1930–1940. [Google Scholar] [CrossRef]
- Xü, Y.H.; Gietzen, K.; Galla, H.J.; Sackmann, E. A simple assay to study protein-mediated lipid exchange by fluorescence polarization. Biochem. J. 1983, 209, 257–260. [Google Scholar] [CrossRef]
- Nagamachi, E.; Hirai, Y.; Tomochika, K.; Kanemasa, Y. Studies on Osmotic Stability of Liposomes Prepared with Bacterial Membrane Lipids by Carboxyfluorescein Release. Microbiol. Immunol. 1992, 36, 231–241. [Google Scholar] [CrossRef]
- Reddy, A.S.; Warshaviak, D.T.; Chachisvilis, M. Effect of membrane tension on the physical properties of DOPC lipid bilayer membrane. Biochim. Biophys. Acta 2012, 1818, 2271–2281. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.C.; Lovrien, R.E. Human red cell hemolysis rates in the subsecond to seconds range. An analysis. Biophys. J. 1977, 20, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.M.; Taylor, G.; Meyer, D.B. Shape and volume changes in erythrocyte ghosts and spectrin-actin networks. J. Cell Biol. 1980, 86, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Beattie, M.E.; Veatch, S.L.; Stottrup, B.L.; Keller, S.L. Sterol Structure Determines Miscibility versus Melting Transitions in Lipid Vesicles. Biophys. J. 2005, 89, 1760–1768. [Google Scholar] [CrossRef]
- Boyd, M.A.; Kamat, N.P. Visualizing Tension and Growth in Model Membranes Using Optical Dyes. Biophys. J. 2018, 115, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.; Ursell, T.; Wiggins, P.; Sens, P. Emerging roles for lipids in shaping membrane-protein function. Nature 2009, 459, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Ide, T.; Aoki, T.; Takeuchi, Y.; Yanagida, T. Lysenin forms a voltage-dependent channel in artificial lipid bilayer membranes. Biochem. Biophys. Res. Commun. 2006, 346, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Kulma, M.; Hereć, M.; Grudziński, W.; Anderluh, G.; Gruszecki, W.I.; Kwiatkowska, K.; Sobota, A. Sphingomyelin-rich domains are sites of lysenin oligomerization: Implications for raft studies. Biochim. Biophys. Acta 2010, 1798, 471–481. [Google Scholar] [CrossRef]
- Yilmaz, N.; Yamaji-Hasegawa, A.; Hullin-Matsuda, F.; Kobayashi, T. Molecular mechanisms of action of sphingomyelin-specific pore-forming toxin, lysenin. Semin. Cell Dev. Biol. 2018, 73, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Feil, S.C.; Ascher, D.B.; Kuiper, M.J.; Tweten, R.K.; Parker, M.W. Structural Studies of Streptococcus pyogenes Streptolysin O Provide Insights into the Early Steps of Membrane Penetration. J. Mol. Biol. 2014, 426, 785–792. [Google Scholar] [CrossRef]
- Miller, H.; Song, W. Use of Streptolysin O-Induced Membrane Damage as a Method of Studying the Function of Lipid Rafts During B Cell Activation. In B Cell Receptor Signaling: Methods and Protocols; Liu, C., Ed.; Springer: New York, NY, USA, 2018; pp. 235–241. [Google Scholar]
- Tweten, R.K.; Hotze, E.M.; Wade, K.R. The Unique Molecular Choreography of Giant Pore Formation by the Cholesterol-Dependent Cytolysins of Gram-Positive Bacteria. Annu. Rev. Microbiol. 2015, 69, 323–340. [Google Scholar] [CrossRef]
- Giddings, K.S.; Johnson, A.E.; Tweten, R.K. Redefining cholesterol′s role in the mechanism of the cholesterol-dependent cytolysins. Proc. Natl. Acad. Sci. USA 2003, 100, 11315. [Google Scholar] [CrossRef] [PubMed]
- Andersen, O.S.; Koeppe, R.E. Bilayer Thickness and Membrane Protein Function: An Energetic Perspective. Annu. Rev. Biophys. Biomol. Struct. 2007, 36, 107–130. [Google Scholar] [CrossRef]
- Bavi, O.; Vossoughi, M.; Naghdabadi, R.; Jamali, Y. The Combined Effect of Hydrophobic Mismatch and Bilayer Local Bending on the Regulation of Mechanosensitive Ion Channels. PLoS ONE 2016, 11, e0150578. [Google Scholar] [CrossRef] [PubMed]
- Gahbauer, S.; Böckmann, R.A. Membrane-Mediated Oligomerization of G Protein Coupled Receptors and Its Implications for GPCR Function. Front. Physiol. 2016, 7, 494. [Google Scholar] [CrossRef]
- Kondrashov, O.V.; Kuzmin, P.I.; Akimov, S.A. Hydrophobic Mismatch Controls the Mode of Membrane-Mediated Interactions of Transmembrane Peptides. Membranes 2022, 12, 89. [Google Scholar] [CrossRef]
- Piknova, B.; Perochon, E.; Tocanne, J.-F. Hydrophobic mismatch and long-range protein/lipid interactions in bacteriorhodopsin/phosphatidylcholine vesicles. Eur. J. Biochem. 1993, 218, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Startek, J.B.; Boonen, B.; Talavera, K.; Meseguer, V. TRP Channels as Sensors of Chemically-Induced Changes in Cell Membrane Mechanical Properties. Int. J. Mol. Sci. 2019, 20, 371. [Google Scholar] [CrossRef] [PubMed]
- Bokori-Brown, M.; Martin, T.G.; Naylor, C.E.; Basak, A.K.; Titball, R.W.; Savva, C.G. Cryo-EM structure of lysenin pore elucidates membrane insertion by an aerolysin family protein. Nat. Commun. 2016, 7, 11293. [Google Scholar] [CrossRef] [PubMed]
- De Colibus, L.; Sonnen, A.F.P.; Morris, K.J.; Siebert, A.C.; Abrusci, P.; Plitzko, J.; Hodnik, V.; Leippe, M.; Volpi, E.; Anderluh, G.; et al. Structures of lysenin reveal a shared evolutionary origin for pore-forming proteins and its mode of sphingomyelin recognition. Structure 2012, 20, 1498–1507. [Google Scholar] [CrossRef] [PubMed]
- Podobnik, M.; Savory, P.; Rojko, N.; Kisovec, M.; Wood, N.; Hambley, R.; Pugh, J.; Wallace, E.J.; McNeill, L.; Bruce, M.; et al. Crystal structure of an invertebrate cytolysin pore reveals unique properties and mechanism of assembly. Nat. Commun. 2016, 7, 11598. [Google Scholar] [CrossRef]
- Tweten, R.K. Cholesterol-Dependent Cytolysins, a Family of Versatile Pore-Forming Toxins. Infect. Immun. 2005, 73, 6199. [Google Scholar] [CrossRef]
- Borghi, N.; Kremer, S.; Askovic, V.; Brochard-Wyart, F. Tube extrusion from permeabilized giant vesicles. Europhys. Lett. 2006, 75, 666. [Google Scholar] [CrossRef]
- Krueger, E.; Bryant, S.; Shrestha, N.; Clark, T.; Hanna, C.; Pink, D.; Fologea, D. Intramembrane congestion effects on lysenin channel voltage-induced gating. Eur. Biophys. J. 2016, 45, 187–194. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Whiting, R.; Stanton, S.; Kucheriava, M.; Smith, A.R.; Pitts, M.; Robertson, D.; Kammer, J.; Li, Z.; Fologea, D. Hypo-Osmotic Stress and Pore-Forming Toxins Adjust the Lipid Order in Sheep Red Blood Cell Membranes. Membranes 2023, 13, 620. https://doi.org/10.3390/membranes13070620
Whiting R, Stanton S, Kucheriava M, Smith AR, Pitts M, Robertson D, Kammer J, Li Z, Fologea D. Hypo-Osmotic Stress and Pore-Forming Toxins Adjust the Lipid Order in Sheep Red Blood Cell Membranes. Membranes. 2023; 13(7):620. https://doi.org/10.3390/membranes13070620
Chicago/Turabian StyleWhiting, Rose, Sevio Stanton, Maryna Kucheriava, Aviana R. Smith, Matt Pitts, Daniel Robertson, Jacob Kammer, Zhiyu Li, and Daniel Fologea. 2023. "Hypo-Osmotic Stress and Pore-Forming Toxins Adjust the Lipid Order in Sheep Red Blood Cell Membranes" Membranes 13, no. 7: 620. https://doi.org/10.3390/membranes13070620
APA StyleWhiting, R., Stanton, S., Kucheriava, M., Smith, A. R., Pitts, M., Robertson, D., Kammer, J., Li, Z., & Fologea, D. (2023). Hypo-Osmotic Stress and Pore-Forming Toxins Adjust the Lipid Order in Sheep Red Blood Cell Membranes. Membranes, 13(7), 620. https://doi.org/10.3390/membranes13070620






