Role of Fe/Co Ratio in Dual Phase Ce0.8Gd0.2O2−δ–Fe3−xCoxO4 Composites for Oxygen Separation
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterization Methods
2.2.1. Crystal Structure
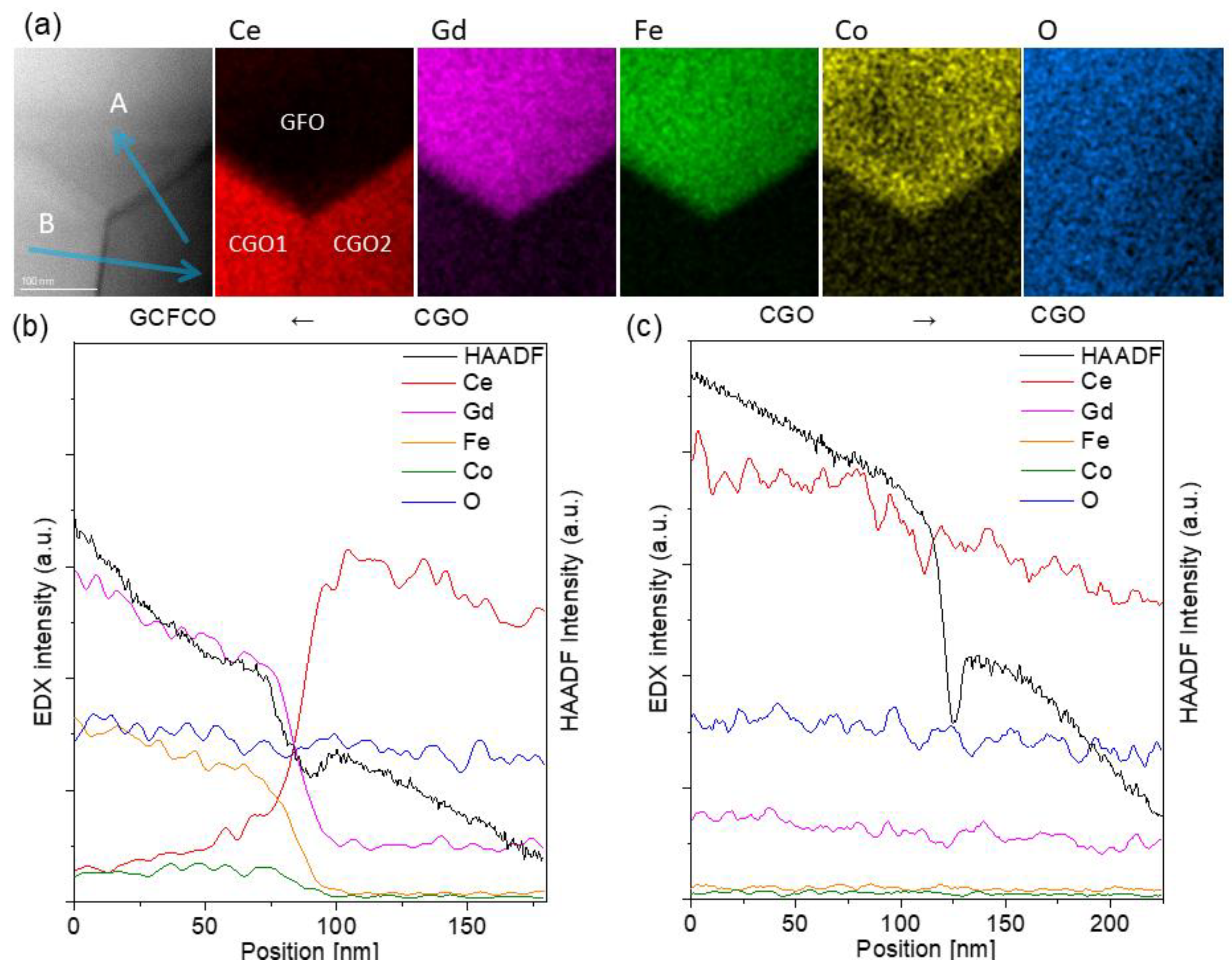
2.2.2. Microscopy
2.2.3. Electrical Conductivity
2.2.4. Oxygen Permeation Measurements
3. Results and Discussion
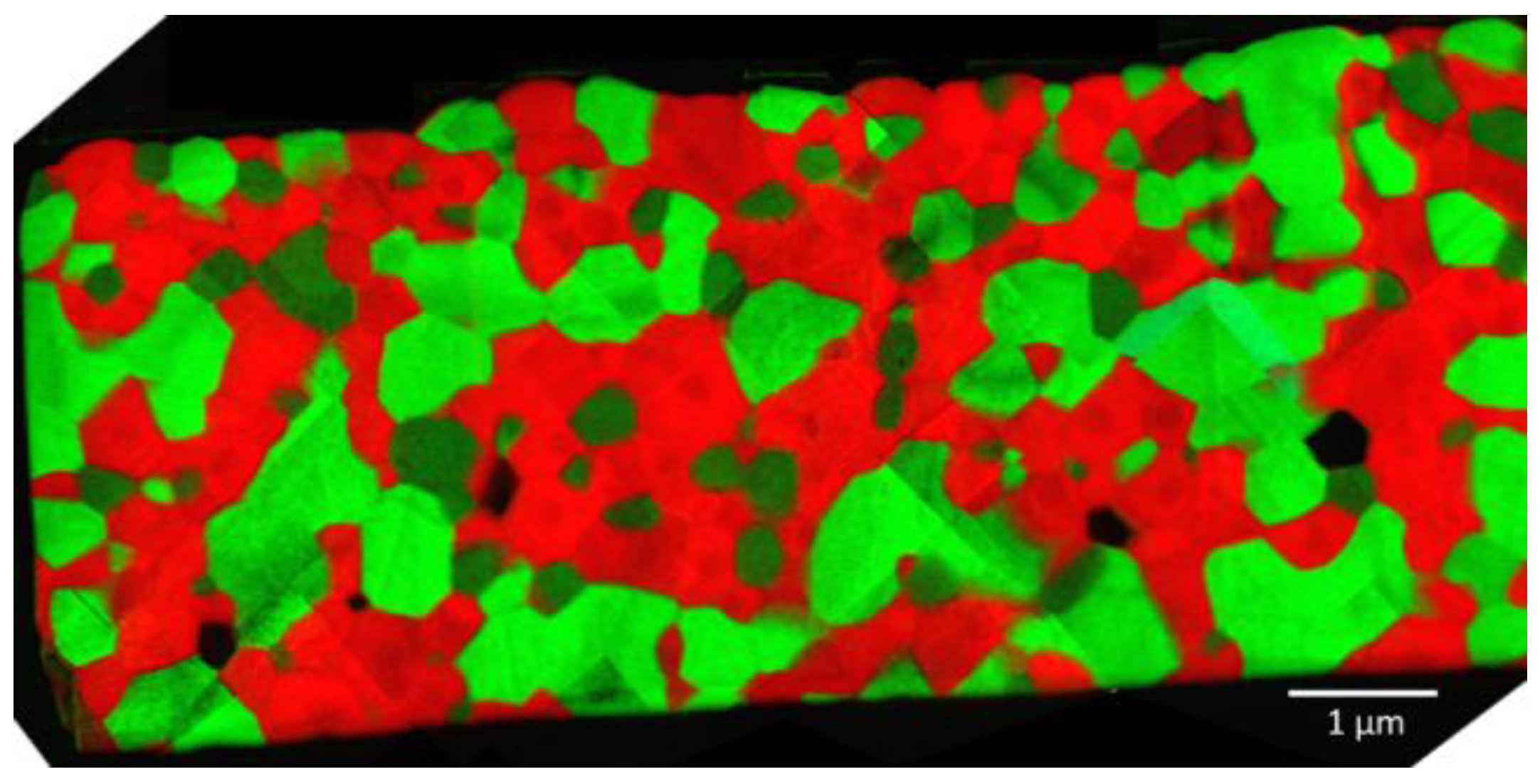
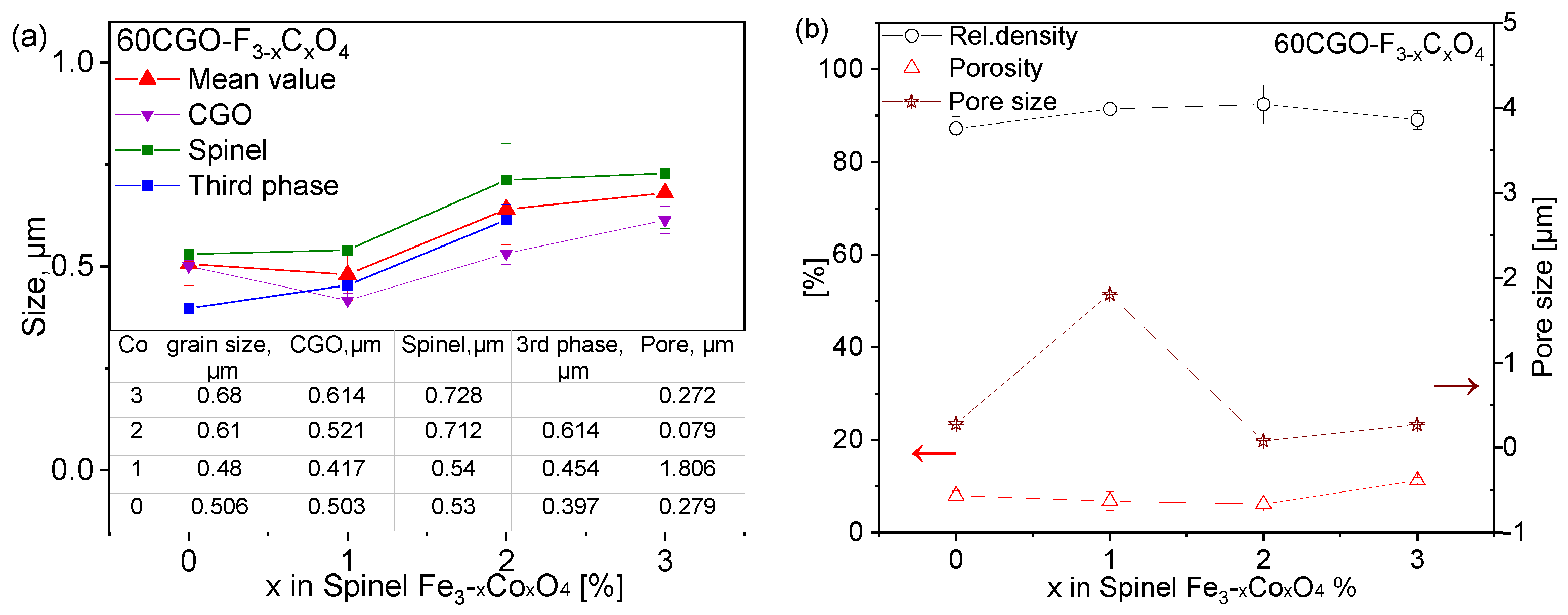
3.1. Microstructure Evolution
3.1.1. Fe-Free Composites (x = 3 in Fe3−xCoxO4)
3.1.2. Co-Free Composite (x = 0 in Fe3−xCoxO4)
3.1.3. Iron Rich Spinel (x = 1 in Fe3−xCoxO4)
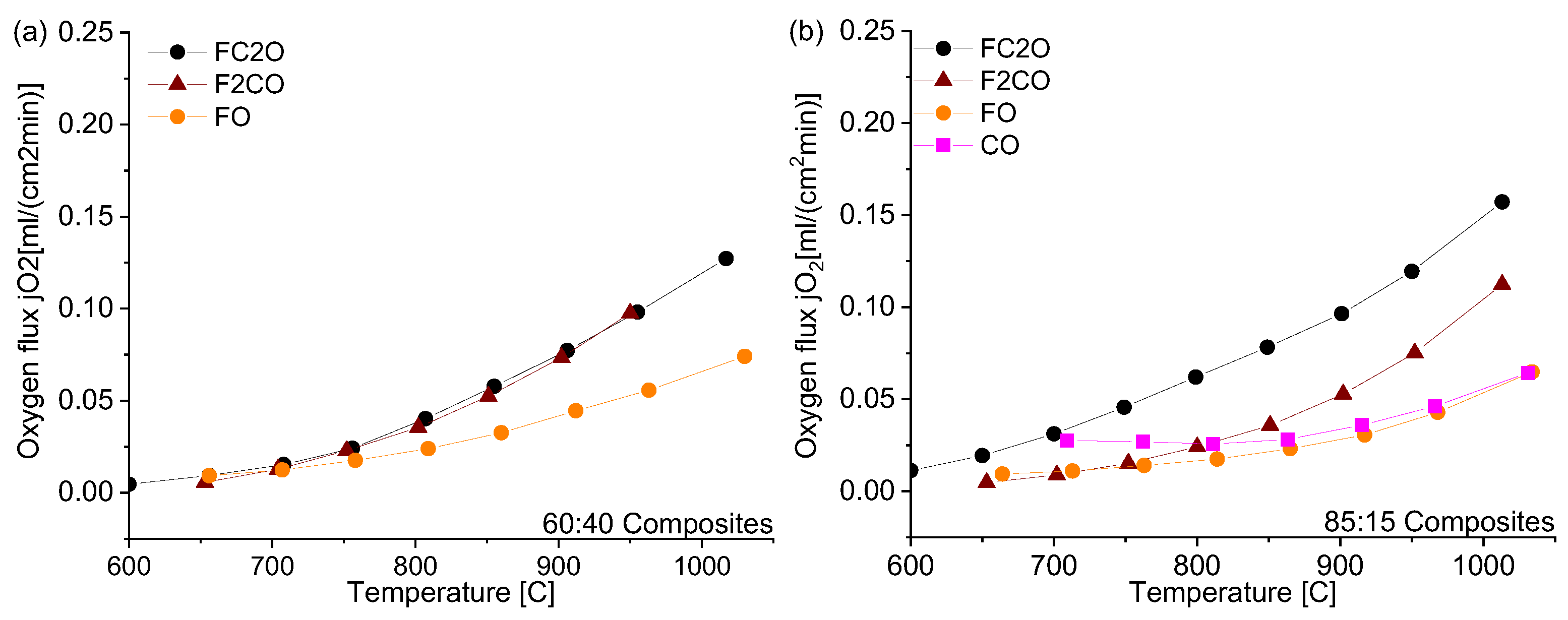
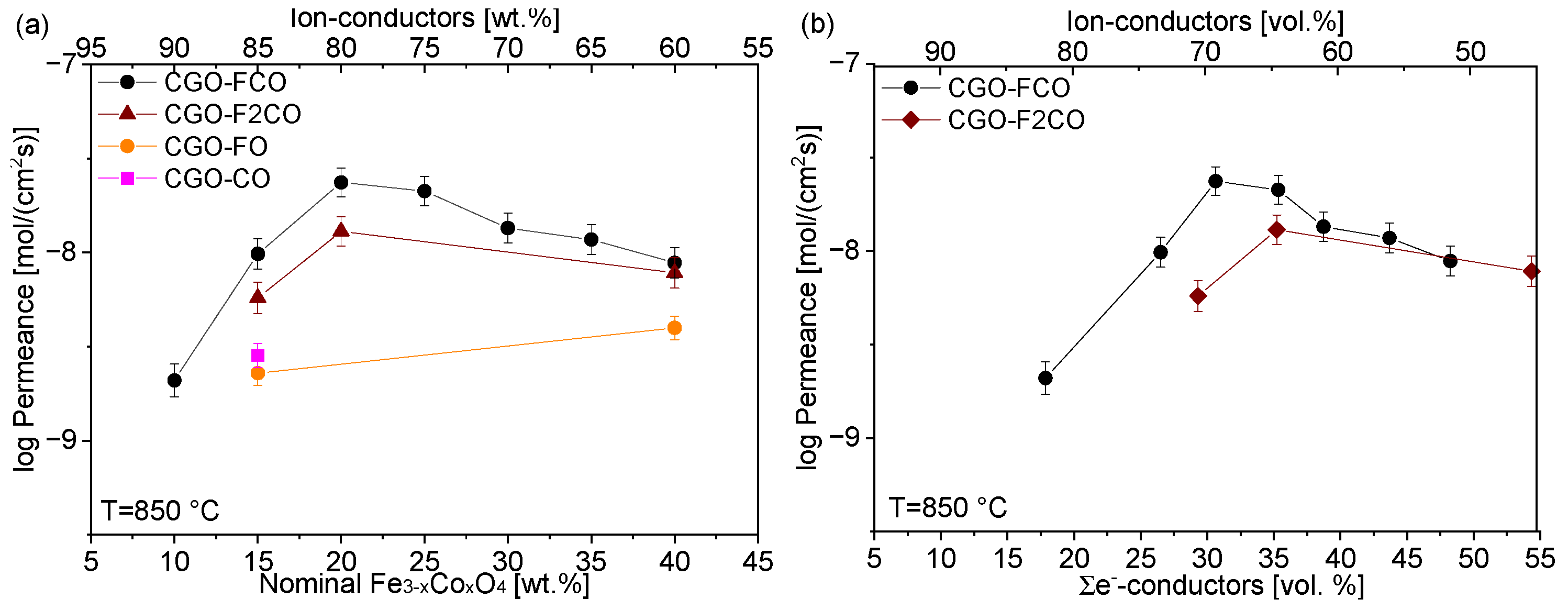
3.2. Permeation of the Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sunarso, J.; Baumann, S.; Serra, J.M.; Meulenberg, W.A.; Liu, S.; Lin, Y.S.; Diniz da Costa, J.C. Mixed ionic–electronic conducting (MIEC) ceramic-based membranes for oxygen separation. J. Membr. Sci. 2008, 320, 13–41. [Google Scholar] [CrossRef]
- Kiebach, R.; Pirou, S.; Martinez Aguilera, L.; Haugen, A.B.; Kaiser, A.; Hendriksen, P.V.; Balaguer, M.; García-Fayos, J.; Serra, J.M.; Schulze-Küppers, F.; et al. A review on dual-phase oxygen transport membranes: From fundamentals to commercial deployment. J. Mater. Chem. A 2022, 10, 2152–2195. [Google Scholar] [CrossRef]
- Garcia-Fayos, J.; Balaguer, M.; Baumann, S.; Serra, J.M. Dual-phase membrane based on LaCo0.2Ni0.4Fe0.4O3−x-Ce0.8Gd0.2O2−δ composition for oxygen permeation under CO2/SO2-rich gas environments. J. Membr. Sci. 2018, 548, 117–124. [Google Scholar] [CrossRef]
- Takamura, H.; Okumura, K.; Koshino, Y.; Kamegawa, A.; Okada, M. Oxygen Permeation Properties of Ceria-Ferrite-Based Composites. J. Electroceram. 2004, 13, 613–618. [Google Scholar] [CrossRef]
- Balaguer, M.; García-Fayos, J.; Solís, C.; Serra, J.M. Fast Oxygen Separation Through SO2- and CO2-Stable Dual-Phase Membrane Based on NiFe2O4–Ce0.8Tb0.2O2-δ. Chem. Mater. 2013, 25, 4986–4993. [Google Scholar] [CrossRef]
- Czyperek, M.; Zapp, P.; Bouwmeester, H.; Modigell, M.; Peinemann, K.-V.; Voigt, I.; Meulenberg, W.A.; Singheiser, L.; Stöver, D. MEM-BRAIN gas separation membranes for zero-emission fossil power plants. Energy Procedia 2009, 1, 303–310. [Google Scholar] [CrossRef]
- Wang, S.; Shi, L.; Xie, Z.; He, Y.; Yan, D.; Li, M.-R.; Caro, J.; Luo, H. High-flux dual-phase percolation membrane for oxygen separation. J. Eur. Ceram. Soc. 2019, 39, 4882–4890. [Google Scholar] [CrossRef]
- Ramasamy, M.; Baumann, S.; Opitz, A.; Iskandar, R.; Mayer, J.; Udomsilp, D.; Breuer, U.; Bram, M. Phase Interaction and Distribution in Mixed Ionic Electronic Conducting Ceria-Spinel Composites. In Advances in Solid Oxide Fuel Cells and Electronic Ceramics II; Kusnezoff, M., Bansal, N.P., Shimamura, K., Fukushima, M., Gyekenyesi, A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 99–112. [Google Scholar]
- Fischer, L.; Neuhaus, K.; Schmidt, C.; Ran, K.; Behr, P.; Baumann, S.; Mayer, J.; Meulenberg, W.A. Phase formation and performance of solid state reactive sintered Ce0.8Gd0.2O2−δ-FeCo2O4 composites. J. Mater. Chem. A 2022, 10, 2412–2420. [Google Scholar] [CrossRef]
- Bouwmeester, H.; Burggraaf, A.J. Membrane Science and Technology: Chapter 10 Dense Ceramic Membranes for Oxygen Separation; Springer: Amsterdam, The Netherlands, 1996. [Google Scholar]
- Joo, J.H.; Park, G.S.; Yoo, C.-Y.; Yu, J.H. Contribution of the surface exchange kinetics to the oxygen transport properties in Ce0.9Gd0.1O2−δ–La0.6Sr0.4Co0.2Fe0.8O3−δ dual-phase membrane. Solid State Ion. 2013, 253, 64–69. [Google Scholar] [CrossRef]
- Chen, G.; Feldhoff, A.; Weidenkaff, A.; Li, C.; Liu, S.; Zhu, X.; Sunarso, J.; Huang, K.; Wu, X.-Y.; Ghoniem, A.F.; et al. Roadmap for Sustainable Mixed Ionic-Electronic Conducting Membranes. Adv. Funct. Mater. 2022, 32, 2105702. [Google Scholar] [CrossRef]
- Bai, W.; Feng, J.; Luo, C.; Zhang, P.; Wang, H.; Yang, Y.; Zhao, Y.; Fan, H. A comprehensive review on oxygen transport membranes: Development history, current status, and future directions. Int. J. Hydrogen Energy 2021, 46, 36257–36290. [Google Scholar] [CrossRef]
- Ramasamy, M.; Persoon, E.S.; Baumann, S.; Schroeder, M.; Schulze-Küppers, F.; Görtz, D.; Bhave, R.; Bram, M.; Meulenberg, W.A. Structural and chemical stability of high performance Ce0.8Gd0.2O2-FeCo2O4dual phase oxygen transport membranes. J. Membr. Sci. 2017, 544, 278–286. [Google Scholar] [CrossRef]
- Lin, Y.; Fang, S.; Su, D.; Brinkman, K.S.; Chen, F. Enhancing grain boundary ionic conductivity in mixed ionic-electronic conductors. Nat. Commun. 2015, 6, 6824. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Malzbender, J.; Baumann, S.; Schulze-Küppers, F.; Krüger, M.; Nijmeijer, A.; Guillon, O.; Meulenberg, W.A. Micromechanical Characterization of Ce0.8Gd0.2O2−δ-FeCo2O4 Dual Phase Oxygen Transport Membranes. Adv. Eng. Mater. 2020, 22, 1901558. [Google Scholar] [CrossRef]
- Wang, S.; Shi, L.; Boubeche, M.; Wang, H.; Xie, Z.; Tan, W.; He, Y.; Yan, D.; Luo, H. The effect of Fe/Co ratio on the structure and oxygen permeability of Ca-containing composite membranes. Inorg. Chem. Front. 2019, 6, 2885–2893. [Google Scholar] [CrossRef]
- Tong, J.; Yang, W.; Cai, R.; Zhu, B.; Xiong, G.; Lin, L. Investigation on the structure stability and oxygen permeability of titanium-doped perovskite-type oxides of BaTi0.2CoxFe0.8−xO3−δ (x=0.2–0.6). Sep. Purif. Technol. 2003, 32, 289–299. [Google Scholar] [CrossRef]
- Shao, Z.; Xiong, G.; Dong, H.; Yang, W.; Lin, L. Synthesis, oxygen permeation study and membrane performance of a Ba0.5Sr0.5Co0.8Fe0.2O3−δ oxygen-permeable dense ceramic reactor for partial oxidation of methane to syngas. Sep. Purif. Technol. 2001, 25, 97–116. [Google Scholar] [CrossRef]
- Bahlawane, N.; Ngamou, P.H.T.; Vannier, V.; Kottke, T.; Heberle, J.; Kohse-Höinghaus, K. Tailoring the properties and the reactivity of the spinel cobalt oxide. Phys. Chem. Chem. Phys. PCCP 2009, 11, 9224–9232. [Google Scholar] [CrossRef]
- Ramasamy, M. Dual Phase Oxygen Transport Membrane for Efficient Oxyfuel Combustion; Forschungszentrum Jülich GmbH: Jülich, Germany, 2016. [Google Scholar]
- Zhang, T. Iron oxide as an effective sintering aid and a grain boundary scavenger for ceria-based electrolytes. Solid State Ion. 2004, 167, 203–207. [Google Scholar] [CrossRef]
- Kiefer, T. Entwicklung Neuer Schutz- und Kontaktierungsschichten für Hochtemperatur-Brennstoffzellen; Forschungszentrum, Zentralbibliothek: Jülich, Germany, 2008. [Google Scholar]
- Ramasamy, M.; Baumann, S.; Palisaitis, J.; Schulze-Küppers, F.; Balaguer, M.; Kim, D.; Meulenberg, W.A.; Mayer, J.; Bhave, R.; Guillon, O.; et al. Influence of Microstructure and Surface Activation of Dual-Phase Membrane Ce0.8Gd0.2O2−δ FeCo2O4 on Oxygen Permeation. J. Am. Ceram. Soc. 2016, 99, 349–355. [Google Scholar] [CrossRef]
- Zeng, F.; Malzbender, J.; Baumann, S.; Nijmeijer, A.; Winnubst, L.; Ziegner, M.; Guillon, O.; Schwaiger, R.; Meulenberg, W.A. Optimization of sintering conditions for improved microstructural and mechanical properties of dense Ce0.8Gd0.2O2–FeCo2O4 oxygen transport membranes. J. Eur. Ceram. Soc. 2021, 41, 509–516. [Google Scholar] [CrossRef]
- Ferreira, T.; Waerenborgh, J.C.; Mendonça, M.; Nunes, M.R.; Costa, F.M. Structural and morphological characterization of FeCo2O4 and CoFe2O4 spinels prepared by a coprecipitation method. Solid State Sci. 2003, 5, 383–392. [Google Scholar] [CrossRef]
- Ji, Y.; Kilner, J.; Carolan, M. Electrical conductivity and oxygen transfer in gadolinia-doped ceria (CGO)–Co3O4−δ composites. J. Eur. Ceram. Soc. 2004, 24, 3613–3616. [Google Scholar] [CrossRef]
- Lewis, G. Effect of Co addition on the lattice parameter, electrical conductivity and sintering of gadolinia-doped ceria. Solid State Ion. 2002, 152–153, 567–573. [Google Scholar] [CrossRef]
- Zhang, T.; Hing, P.; Huang, H.; Kilner, J. Densification, microstructure and grain growth in the CeO2–Fe2O3 system (0 ≤ Fe/Ce ≤ 20%). J. Eur. Ceram. Soc. 2001, 21, 2221–2228. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhou, M.; Ge, L.; Li, S.; Chen, H.; Guo, L. Effect of Fe2O3 on Sm-doped ceria system solid electrolyte for IT-SOFCs. J. Alloy. Compd. 2011, 509, 546–550. [Google Scholar] [CrossRef]
- Buchheit, A.; Teßmer, B.; Ran, K.; Mayer, J.; Wiemhöfer, H.-D.; Neuhaus, K. The Impact of Fe Addition on the Electronic Conductivity of Gadolinium Doped Ceria. ECS J. Solid State Sci. Technol. 2019, 8, P41–P50. [Google Scholar] [CrossRef]
- Matovic, B.; Dohcevic-Mitrovic, Z.; Radovic, M.; Brankovic, Z.; Brankovic, G.; Boskovic, S.; Popovic, Z.V. Synthesis and characterization of ceria based nanometric powders. J. Power Sources 2009, 193, 146–149. [Google Scholar] [CrossRef]
- Neuhaus, K.; Dolle, R.; Wiemhöfer, H.-D. The Effect of Transition Metal Oxide Addition on the Conductivity of Commercially Available Gd-Doped Ceria. J. Electrochem. Soc. 2020, 167, 44507. [Google Scholar] [CrossRef]
- Zhang, T. Preparation and mechanical properties of dense Ce0.8Gd0.2O2−δ ceramics. Solid State Ion. 2004, 167, 191–196. [Google Scholar] [CrossRef]
- Zeng, F.; Baumann, S.; Malzbender, J.; Nijmeijer, A.; Winnubst, L.; Guillon, O.; Schwaiger, R.; Meulenberg, W.A. Enhancing oxygen permeation of solid-state reactive sintered Ce0.8Gd0.2O2-FeCo2O4 composite by optimizing the powder preparation method. J. Membr. Sci. 2021, 628, 119248. [Google Scholar] [CrossRef]
- Ge, M.; Huang, X.; Yan, H.; Gursoy, D.; Meng, Y.; Zhang, J.; Ghose, S.; Chiu, W.K.S.; Brinkman, K.S.; Chu, Y.S. Three-dimensional imaging of grain boundaries via quantitative fluorescence X-ray tomography analysis. Commun. Mater. 2022, 3, 37. [Google Scholar] [CrossRef]
- Kovács, A.; Schierholz, R.; Tillmann, K. FEI Titan G2 80-200 CREWLEY. JLSRF J. Large-Scale Res. Facil. 2016, 2, A43. [Google Scholar] [CrossRef]
- Hansson, A.N.; Linderoth, S.; Mogensen, M.; Somers, M.A. X-ray diffraction investigation of phase stability in the Co–Cr–O and the Fe–Co–Cr–O systems in air at 1323K. J. Alloys Compd. 2005, 402, 194–200. [Google Scholar] [CrossRef]
- Smith, P.A.; Spencer, C.D.; Stillwell, R.P. Co57 and Fe57 mössbauer studies of the spinels FeCo2O4 and Fe0. 5Co2.5O4. J. Phys. Chem. Solids 1978, 39, 107–111. [Google Scholar] [CrossRef]
- Raval, A.; Panchal, N.; Jotania, R. Structural properties and mictostructure of cobalt ferrite particles synthesized by a sol-gel autocombustion method. Int. J. Mod. Phys. Conf. Ser. 2013, 22, 558–563. [Google Scholar] [CrossRef]
- Fritsch, D.; Ederer, C. Epitaxial strain effects in the spinel ferrites CoFe2O4and NiFe2O4 from first principles. Phys. Rev. B 2010, 82, 137. [Google Scholar] [CrossRef]
- Zhao, Q.; Yan, Z.; Chen, C.; Chen, J. Spinels: Controlled Preparation, Oxygen Reduction/Evolution Reaction Application, and Beyond. Chem. Rev. 2017, 117, 10121–10211. [Google Scholar] [CrossRef]
- Zeng, F.; Malzbender, J.; Baumann, S.; Zhou, W.; Ziegner, M.; Nijmeijer, A.; Guillon, O.; Schwaiger, R.; Meulenberg, W.A. Mechanical reliability of Ce0.8Gd0.2O2−δ-FeCo2O4 dual phase membranes synthesized by one-step solid-state reaction. J. Am. Ceram. Soc. 2021, 104, 1814–1830. [Google Scholar] [CrossRef]
- Buscaglia, V. Growth of ternary oxides in the Gd2O3–Fe2O3 system. A diffusion couple study. Solid State Ion. 2002, 146, 257–271. [Google Scholar] [CrossRef]
- Kharton, V.V.; Shaula, A.L.; Naumovich, E.N.; Vyshatko, N.P.; Marozau, I.P.; Viskup, A.P.; Marques, F.M.B. Ionic Transport in Gd3Fe5O12- and Y3Fe5O12-Based Garnets. J. Electrochem. Soc. 2003, 150, J33. [Google Scholar] [CrossRef]
- Shaula, A.L.; Kharton, V.V.; Marques, F. Mixed conductivity of garnet phases based on gadolinium ferrite. J. Eur. Ceram. Soc. 2004, 24, 1309–1312. [Google Scholar] [CrossRef]
- Hou, Y.H.; Zhao, Y.J.; Liu, Z.W.; Yu, H.Y.; Zhong, X.C.; Qiu, W.Q.; Zeng, D.C.; Wen, L.S. Structural, electronic and magnetic properties of partially inverse spinel CoFe2O4: A first-principles study. J. Mater. Sci. 2010, 43, 445003. [Google Scholar] [CrossRef]
- Kharton, V.V.; Kovalevsky, A.V.; Viskup, A.P.; Figueiredo, F.M.; Yaremchenko, A.A.; Naumovich, E.N.; Marques, F.M.B. Oxygen Permeability of Ce0.8Gd0.2O2−δ-La0.7Sr0.3MnO3−δ Composite Membranes. J. Electrochem. Soc. 2000, 147, 2814. [Google Scholar] [CrossRef]
- Hong, S.J.; Virkar, A.V. Lattice Parameters and Densities of Rare-Earth Oxide Doped Ceria Electrolytes. J. Am. Ceram. Soc. 1995, 78, 433–439. [Google Scholar] [CrossRef]
- Koettgen, J.; Martin, M. The ionic conductivity of Sm-doped ceria. J. Am. Ceram. Soc. 2020, 103, 3776–3787. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Jiang, H.; Efimov, K.; Liang, F.; Wang, H.; Caro, J. CO2-Tolerant Oxygen-Permeable Fe2O3-Ce0.9Gd0.1O2-δ Dual Phase Membranes. Ind. Eng. Chem. Res. 2011, 50, 13508–13517. [Google Scholar] [CrossRef]
- Takamura, H.; Kawai, M.; Okumura, K.; Kamegawa, A.; Okada, M. Preparation and Oxygen Permeability of Gd-Doped Ceria and Spinel-Type Ferrite Composites. MRS Proc. 2002, 756, EE8.11. [Google Scholar] [CrossRef]
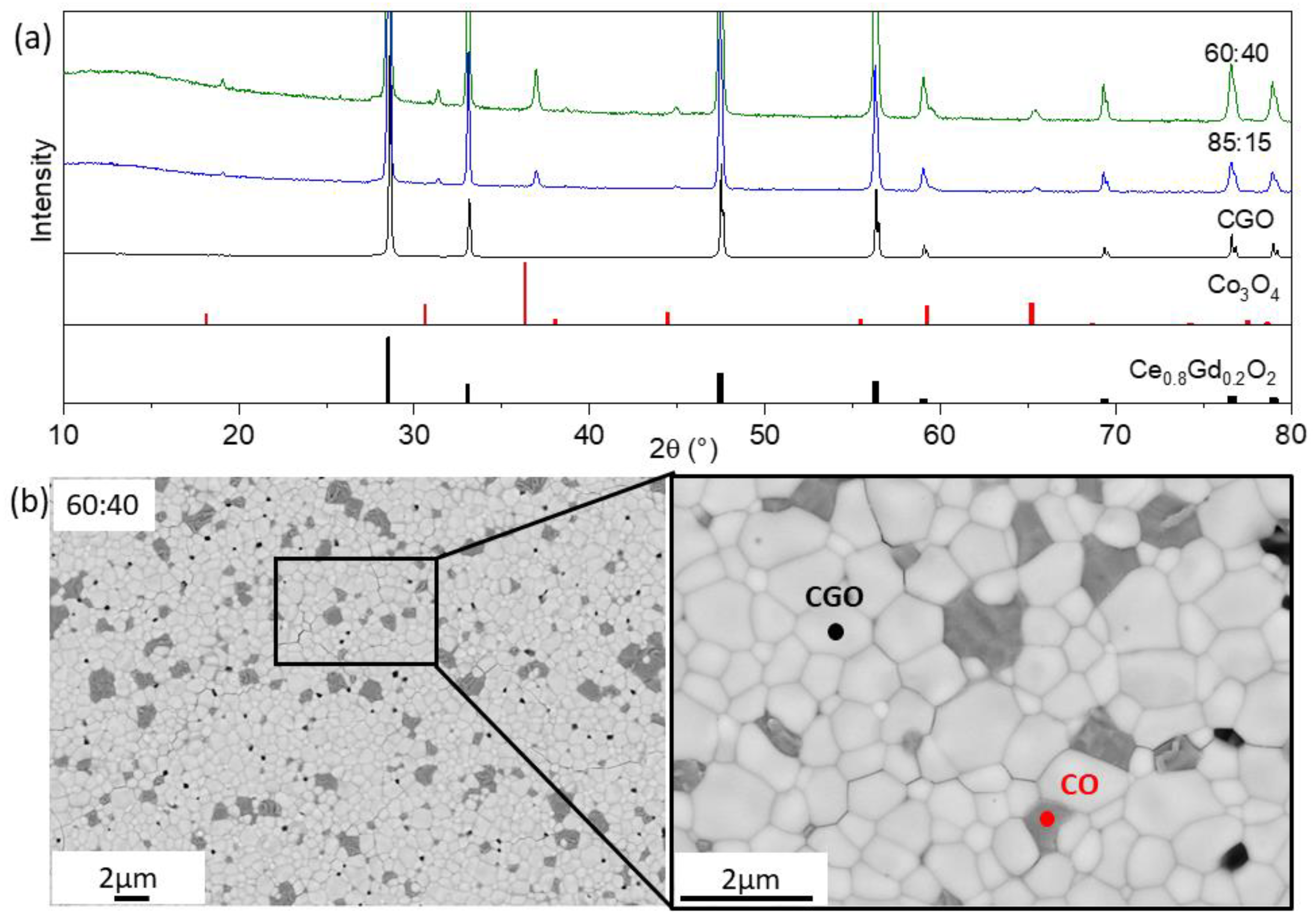
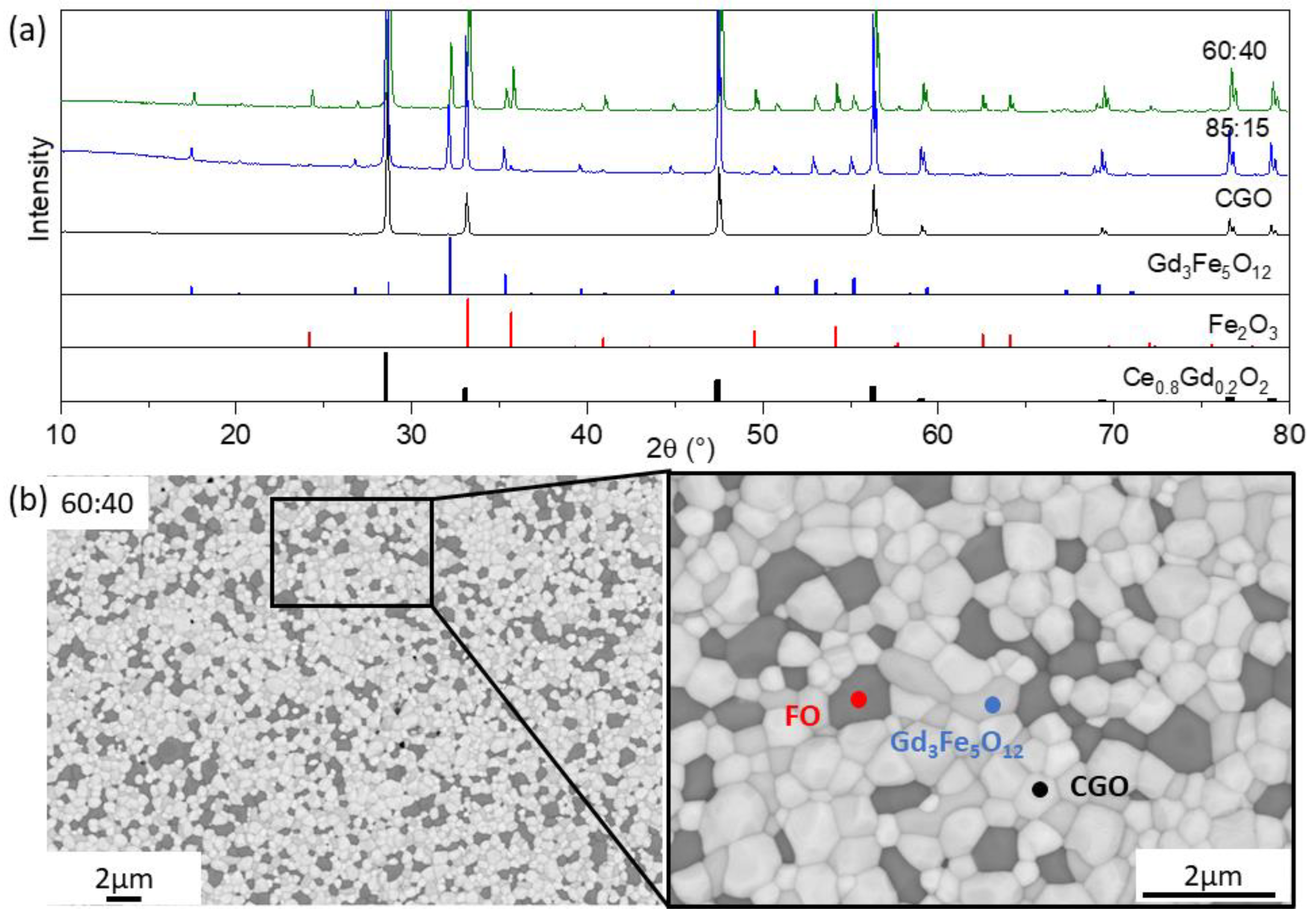
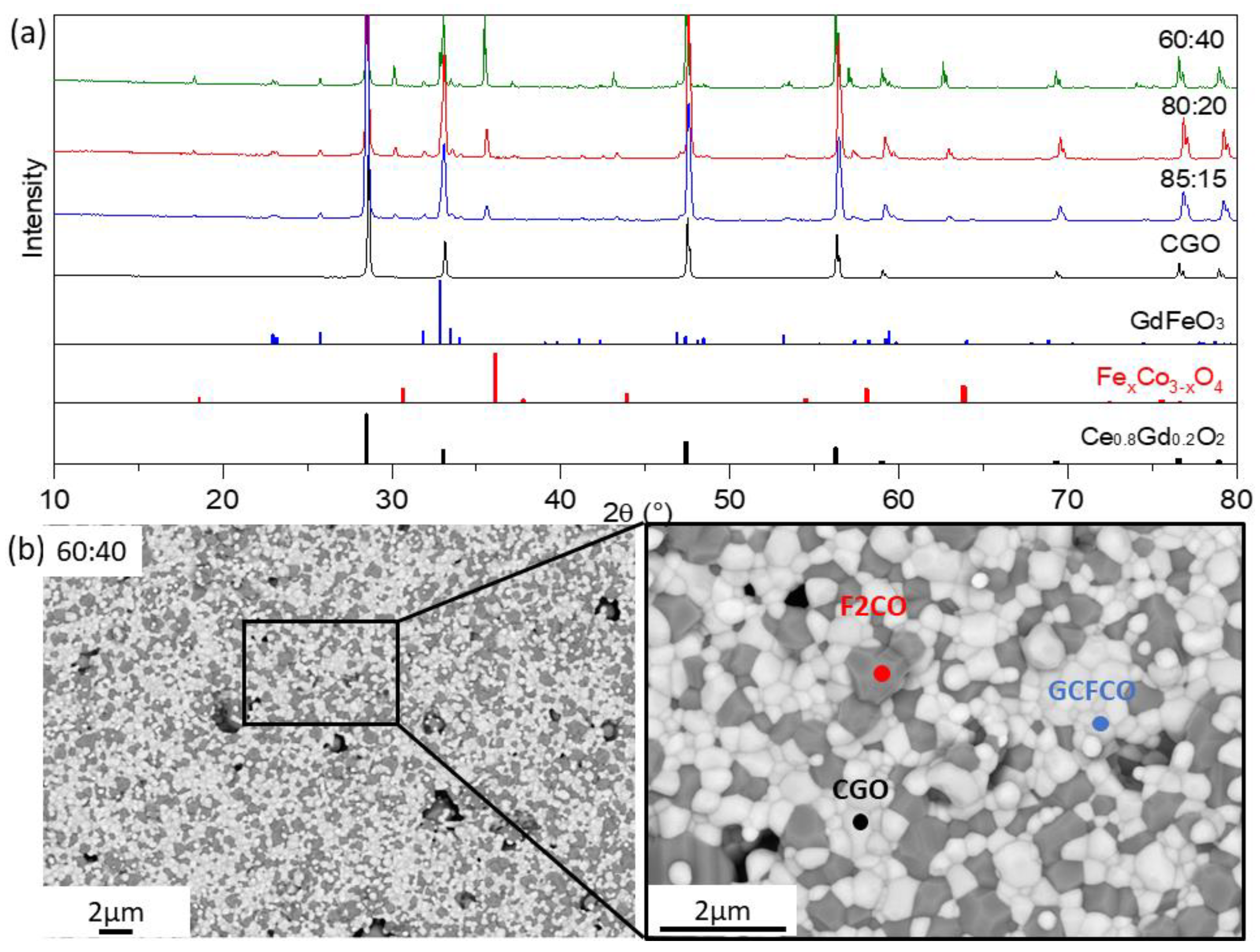





| FCO | CGO | Co3O4 | ||
|---|---|---|---|---|
| wt.% | F, wt.% | a = b = c, Å | F, wt.% | a = b = c, Å |
| 0 | 100.00 | 5.4246 [0] | 0 | - |
| 15 | 83.50 [5] | 5.4249 [0] | 16.50 [5] | 8.0808 [5] |
| FCO | CGO | Gd3Fe5O12 | Fe2O3 | ||||
|---|---|---|---|---|---|---|---|
| wt.% | F, wt.% | a = b = c, Å | F, wt.% | a = b = c, Å | F, wt.% | a = b, Å | c, Å |
| 0 | 100.00 | 5.4246 [0] | 0 | - | 0 | - | - |
| 15 | 74.30 [3] | 5.4154 [5] | 20.75 [2] | 12.47 [2] | 4.96 [2] | 5.0351 [4] | 13.7358 [0] |
| 40 | 55.00 [3] | 5.4155 [4] | 15.33 [2] | 12.47 [2] | 30.7 [3] | 5.0366 [6] | 13.7359 [0] |
| FCO | CGO | F2CO | CGFCO | |||||
|---|---|---|---|---|---|---|---|---|
| wt.% | F, wt.% | a = b = c, Å | F, wt.% | a = b = c, Å | F, wt.% | a, Å | b, Å | c, Å |
| 0 | 100.00 | 5.4246 [0] | 0 | - | 0 | - | - | - |
| 15 | 74.00 [1] | 5.4185 [1] | 13.50 [1] | 8.3688 [2] | 12.30 [2] | 5.3429 [8] | 5.6099 [1] | 7.658 [1] |
| 20 | 69.00 [2] | 5.4181 [8] | 18.90 [1] | 8.3699 [1] | 12.00 [1] | 5.3433 [8] | 5.6097 [1] | 7.662 [5] |
| 40 | 51.60 [1] | 5.4179 [4] | 37.40 [9] | 8.3835 [1] | 11.00 [9] | 5.3485 [2] | 5.6103 [5] | 7.669 [3] |
| Membrane Material | Weight Ratio | T, (°C) | Thickness (mm) | Atmosphere | Synthesis | Coating | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Ce0.8Gd0.2O2d–FeCo2O4 | 85:15 | 0.16 | 850 | 1 | Air/Ar | one-pot | + | [24] |
| Ce0.8Gd0.2O2d–CoFe2O4 | 60:40 | 0.1 | 850 | 1 | Air/He | SSRS | + | [15] |
| Ce0.9Gd0.1O2d–Fe2O3 | 60:40 | 0.06 | 900 | 0.5 | Air/He | Pechini | [52] | |
| Ce0.8Gd0.2O2d–CoFe2O4 | 75:25 | 0.28 | 1000 | 1 | Air/He | Pechini | [53] | |
| Ce0.8Gd0.2O2d–FeCo2O4 | 85:15 | 0.11 | 850 | 1 | Air/Ar | SSRS | + | [35] |
| Ce0.8Gd0.2O2d–Fe2CoO4 | 80:20 | 0.08 | 850 | 1 | Air/Ar | SSRS | + | This work |
| Ce0.8Gd0.2O2d–Fe2CoO4 | 80:20 | 0.16 | 1000 | 1 | Air/Ar | SSRS | + | This work |
| Ce0.8Gd0.2O2d–FeCo2O4 | 80:20 | 0.11 | 850 | 1 | Air/Ar | SSRS | + | This work |
| Ce0.8Gd0.2O2d–FeCo2O4 | 80:20 | 0.20 | 1000 | 1 | Air/Ar | SSRS | + | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fischer, L.; Ran, K.; Schmidt, C.; Neuhaus, K.; Baumann, S.; Behr, P.; Mayer, J.; Bouwmeester, H.J.M.; Nijmeijer, A.; Guillon, O.; et al. Role of Fe/Co Ratio in Dual Phase Ce0.8Gd0.2O2−δ–Fe3−xCoxO4 Composites for Oxygen Separation. Membranes 2023, 13, 482. https://doi.org/10.3390/membranes13050482
Fischer L, Ran K, Schmidt C, Neuhaus K, Baumann S, Behr P, Mayer J, Bouwmeester HJM, Nijmeijer A, Guillon O, et al. Role of Fe/Co Ratio in Dual Phase Ce0.8Gd0.2O2−δ–Fe3−xCoxO4 Composites for Oxygen Separation. Membranes. 2023; 13(5):482. https://doi.org/10.3390/membranes13050482
Chicago/Turabian StyleFischer, Liudmila, Ke Ran, Christina Schmidt, Kerstin Neuhaus, Stefan Baumann, Patrick Behr, Joachim Mayer, Henny J. M. Bouwmeester, Arian Nijmeijer, Olivier Guillon, and et al. 2023. "Role of Fe/Co Ratio in Dual Phase Ce0.8Gd0.2O2−δ–Fe3−xCoxO4 Composites for Oxygen Separation" Membranes 13, no. 5: 482. https://doi.org/10.3390/membranes13050482
APA StyleFischer, L., Ran, K., Schmidt, C., Neuhaus, K., Baumann, S., Behr, P., Mayer, J., Bouwmeester, H. J. M., Nijmeijer, A., Guillon, O., & Meulenberg, W. A. (2023). Role of Fe/Co Ratio in Dual Phase Ce0.8Gd0.2O2−δ–Fe3−xCoxO4 Composites for Oxygen Separation. Membranes, 13(5), 482. https://doi.org/10.3390/membranes13050482







