Role of Calcium-Activated Potassium Channels in Proliferation, Migration and Invasion of Human Chronic Myeloid Leukemia K562 Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Reagents
2.2. Electrophysiology
2.3. Solutions
2.4. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.5. Immunofluorescence
2.6. Cell Proliferation and Viability Assays
2.7. Transwell Migration and Invasion Assays
2.8. Ca2+ Imaging
2.9. Statistics
3. Results
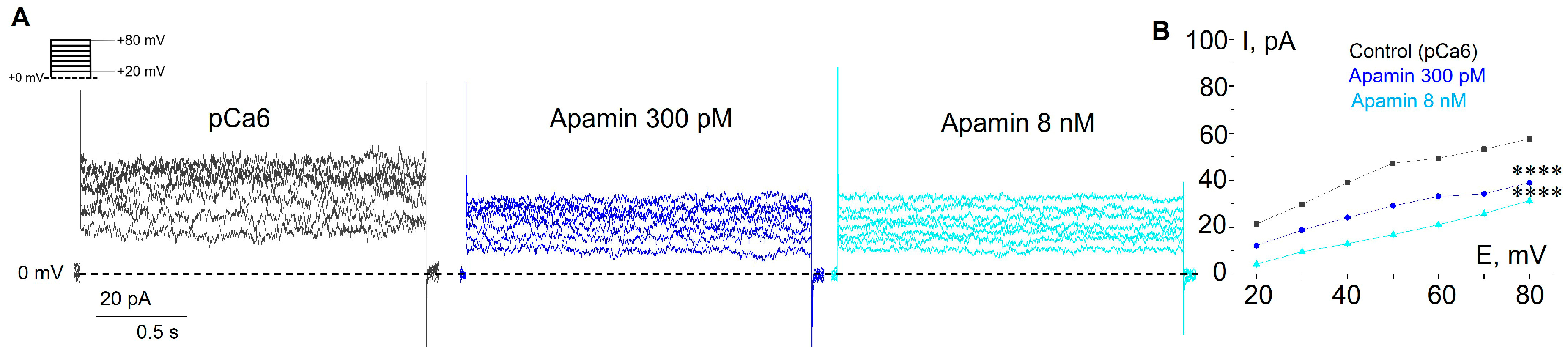
3.1. Selective SK and IK Channel Blockers Inhibit Whole-Cell Currents in the Plasma Membrane of Human Myeloid Leukemia Cells
3.2. Functional Expression of SK2, SK3 and IK Channels in K562 Cells
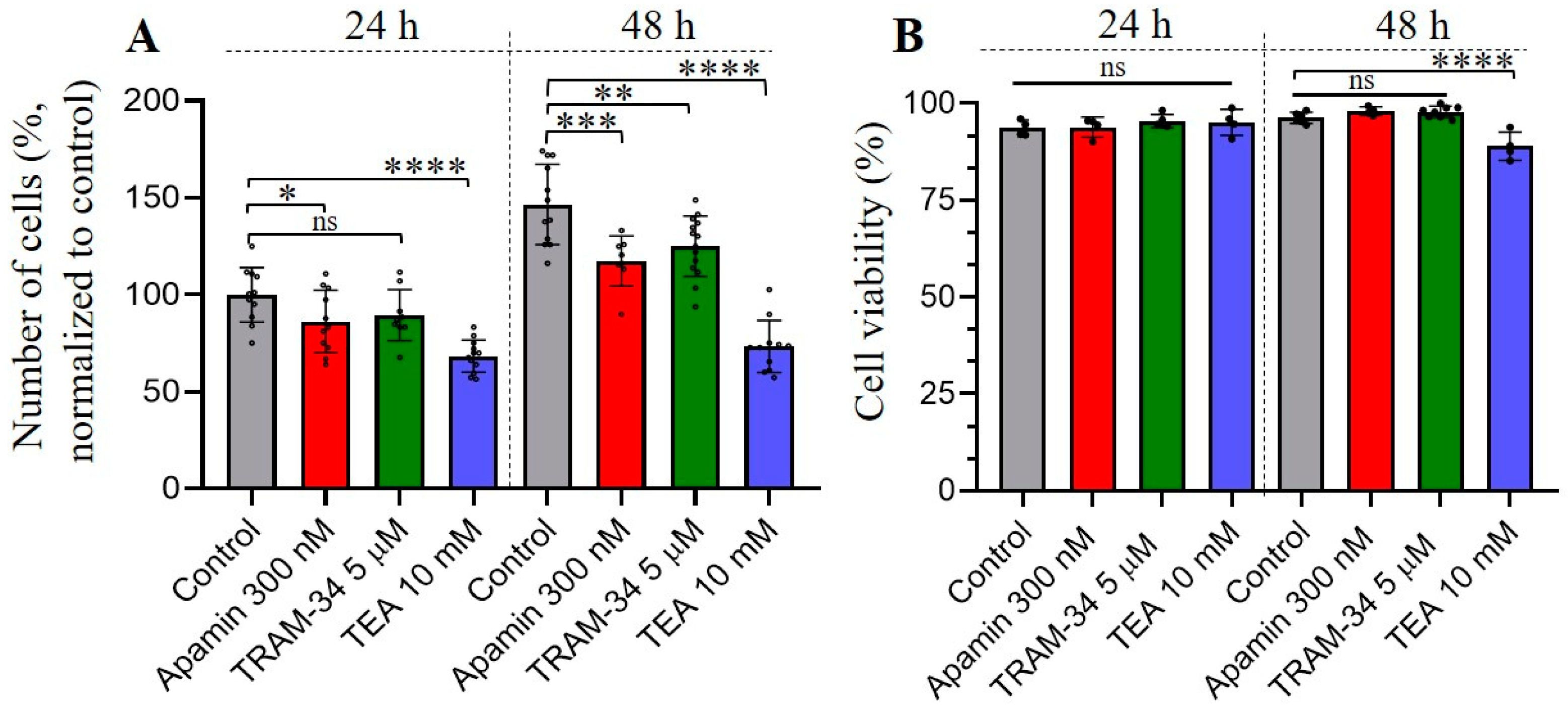
3.3. The Effects of Selective SK and IK Channel Inhibitors on Cell Proliferation and Viability
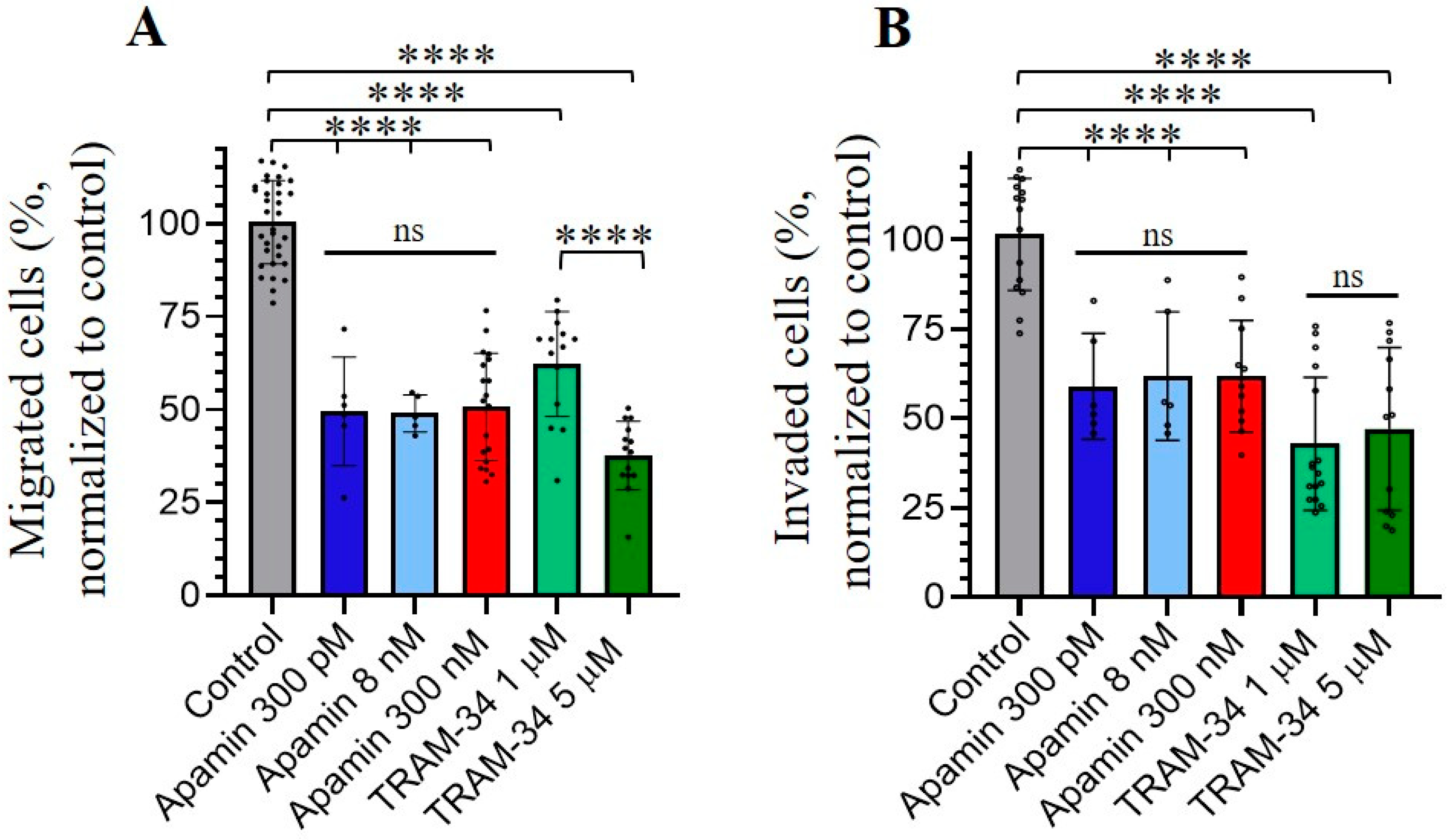
3.4. Role of SK and IK Channels in Migration and Invasion of Chronic Myeloid Leukemia K562 Cells
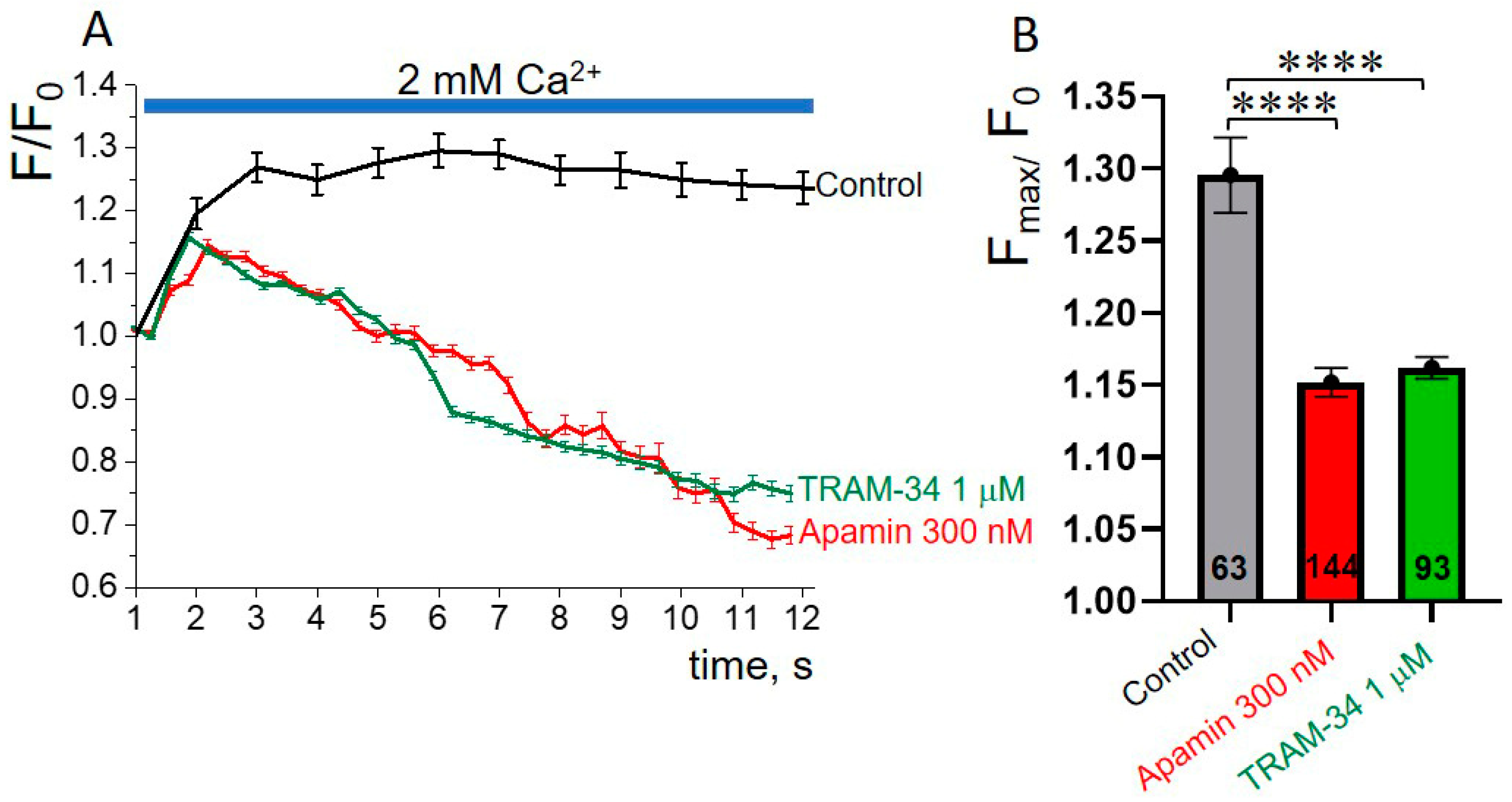
3.5. SK or IK Channel Inhibitors Decrease Capacitative Ca2+ Entry in K562 Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Arcangeli, A.; Becchetti, A. Novel perspectives in cancer therapy: Targeting ion channels. Drug Resist. Updat. 2015, 21–22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Rafieemehr, H.; Samimi, A.; Maleki Behzad, M.; Ghanavat, M.; Shahrabi, S. Altered expression and functional role of ion channels in leukemia: Bench to bedside. Clin. Transl. Oncol. 2020, 22, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Köhler, M.; Hirschberg, B.; Bond, C.T.; Kinzie, J.M.; Marrion, N.V.; Maylie, J.; Adelman, J.P. Small-conductance, calcium-activated potassium channels from mammalian brain. Science 1996, 273, 1709–1714. [Google Scholar] [CrossRef]
- Pedarzani, P.; Stocker, M. Molecular and cellular basis of small- and intermediate-conductance, calcium-activated potassium channel function in the brain. Cell. Mol. Life Sci. 2008, 65, 3196–3217. [Google Scholar] [CrossRef]
- Brown, B.M.; Shim, H.; Christophersen, P.; Wulff, H. Pharmacology of Small- and Intermediate-Conductance Calcium-Activated Potassium Channels. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 219–240. [Google Scholar] [CrossRef] [PubMed]
- Kuzmenkov, A.I.; Peigneur, S.; Nasburg, J.A.; Mineev, K.S.; Nikolaev, M.V.; Pinheiro-Junior, E.L.; Arseniev, A.S.; Wulff, H.; Tytgat, J.; Vassilevski, A.A. Apamin structure and pharmacology revisited. Front. Pharmacol. 2022, 13, 977440. [Google Scholar] [CrossRef]
- Agarwal, J.J.; Zhu, Y.; Zhang, Q.Y.; Mongin, A.A.; Hough, L.B. TRAM-34, a putatively selective blocker of intermediate-conductance, calcium-activated potassium channels, inhibits cytochrome P450 activity. PLoS ONE 2013, 8, e63028. [Google Scholar] [CrossRef]
- Wulff, H.; Kolski-Andreaco, A.; Sankaranarayanan, A.; Sabatier, J.M.; Shakkottai, V. Modulators of small- and intermediate-conductance calcium-activated potassium channels and their therapeutic indications. Curr. Med. Chem. 2007, 14, 1437–1457. [Google Scholar] [CrossRef]
- Gardos, G. The function of calcium in the potassium permeability of human erythrocytes. BiochimBiophys. Acta 1958, 30, 653–654. [Google Scholar] [CrossRef]
- Fanger, C.M.; Rauer, H.; Neben, A.L.; Miller, M.J.; Rauer, H.; Wulff, H.; Rosa, J.C.; Ganellin, C.R.; Chandy, K.G.; Cahalan, M.D. Calcium-activated potassium channels sustain calcium signaling in T lymphocytes. Selective blockers and manipulated channel expression levels. J. Biol. Chem. 2001, 276, 12249–12256. [Google Scholar] [CrossRef]
- Feske, S.; Skolnik, E.Y.; Prakriya, M. Ion channels and transporters in lymphocyte function and immunity. Nat. Rev. Immunol. 2012, 12, 532–547. [Google Scholar] [CrossRef] [PubMed]
- Valle-Reyes, S.; Valencia-Cruz, G.; Liñan-Rico, L.; Pottosin, I.; Dobrovinskaya, O. Differential Activity of Voltage- and Ca2+-Dependent Potassium Channels in Leukemic T Cell Lines: Jurkat Cells Represent an Exceptional Case. Front. Physiol. 2018, 9, 499. [Google Scholar] [CrossRef] [PubMed]
- Kaestner, L.; Bogdanova, A.; Egee, S. Calcium Channels and Calcium-Regulated Channels in Human Red Blood Cells. Adv. Exp. Med. Biol. 2020, 1131, 625–648. [Google Scholar] [CrossRef]
- Fermo, E.; Bogdanova, A.; Petkova-Kirova, P.; Zaninoni, A.; Marcello, A.P.; Makhro, A.; Hänggi, P.; Hertz, L.; Danielczok, J.; Vercellati, C.; et al. ‘Gardos Channelopathy’: A variant of hereditary Stomatocytosis with complex molecular regulation. Sci. Rep. 2017, 7, 1744. [Google Scholar] [CrossRef] [PubMed]
- Dyrda, A.; Cytlak, U.; Ciuraszkiewicz, A.; Lipinska, A.; Cueff, A.; Bouyer, G.; Egée, S.; Bennekou, P.; Lew, V.L.; Thomas, S.L. Local membrane deformations activate Ca2+-dependent K+ and anionic currents in intact human red blood cells. PLoS ONE 2010, 5, e9447. [Google Scholar] [CrossRef] [PubMed]
- Cahalan, S.M.; Lukacs, V.; Ranade, S.S.; Chien, S.; Bandell, M.; Patapoutian, A. Piezo1 links mechanical forces to red blood cell volume. Elife 2015, 4, e07370. [Google Scholar] [CrossRef]
- Staruschenko, A.V.; Vedernikova, E.A. Mechanosensitive cation channels in human leukaemia cells: Calcium permeation and blocking effect. J. Physiol. 2002, 541 Pt 1, 81–90. [Google Scholar] [CrossRef]
- Staruschenko, A.; Negulyaev, Y.A.; Morachevskaya, E.A. Actin cytoskeleton disassembly affects conductive properties of stretch-activated cation channels in leukaemia cells. Biochim. Biophys. Acta 2005, 1669, 53–60. [Google Scholar] [CrossRef]
- Chubinskiy-Nadezhdin, V.I.; Negulyaev, Y.A.; Morachevskaya, E.A. Cholesterol depletion-induced inhibition of stretch-activated channels is mediated via actin rearrangement. Biochem. Biophys. Res. Commun. 2011, 412, 80–85. [Google Scholar] [CrossRef]
- Chubinskiy-Nadezhdin, V.I.; Negulyaev, Y.A.; Morachevskaya, E.A. Functional Coupling of Ion Channels in the Process of Mechano-Dependent Activation in the Membrane of K562 Cells. Cell Tiss. Biol. 2019, 13, 470–477. [Google Scholar] [CrossRef]
- Guéguinou, M.; Chantôme, A.; Fromont, G.; Bougnoux, P.; Vandier, C.; Potier-Cartereau, M. KCa and Ca(2+) channels: The complex thought. Biochim. Biophys. Acta 2014, 1843, 2322–2333. [Google Scholar] [CrossRef] [PubMed]
- Weatherall, K.L.; Seutin, V.; Liégeois, J.F.; Marrion, N.V. Crucial role of a shared extracellular loop in apamin sensitivity and maintenance of pore shape of small-conductance calcium-activated potassium (SK) channels. Proc. Natl. Acad. Sci. USA 2011, 108, 18494–18499. [Google Scholar] [CrossRef]
- Lamy, C.; Goodchild, S.J.; Weatherall, K.L.; Jane, D.E.; Liégeois, J.F.; Seutin, V.; Marrion, N.V. Allosteric block of KCa2 channels by apamin. J. Biol. Chem. 2010, 285, 27067–27077. [Google Scholar] [CrossRef] [PubMed]
- Wulff, H.; Gutman, G.A.; Cahalan, M.D.; Chandy, K.G. Delineation of the clotrimazole/TRAM-34 binding site on the intermediate conductance calcium-activated potassium channel, IKCa1. J. Biol. Chem. 2001, 276, 32040–32045. [Google Scholar] [CrossRef] [PubMed]
- Maylie, J.; Bond, C.T.; Herson, P.S.; Lee, W.S.; Adelman, J.P. Small conductance Ca2+-activated K+ channels and calmodulin. J Physiol 2004, 554 Pt 2, 255–261. [Google Scholar] [CrossRef]
- Qi, M.M.; Qian, L.L.; Wang, R.X. Modulation of SK Channels: Insight into Therapeutics of Atrial Fibrillation. Heart Lung Circ. 2021, 30, 1130–1139. [Google Scholar] [CrossRef]
- Bong, A.H.L.; Monteith, G.R. Calcium signaling and the therapeutic targeting of cancer cells. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865 Pt B, 1786–1794. [Google Scholar] [CrossRef]
- Wu, L.; Lian, W.; Zhao, L. Calcium signaling in cancer progression and therapy. FEBS J. 2021, 288, 6187–6205. [Google Scholar] [CrossRef]
- Khodakhah, K.; Melishchuk, A.; Armstrong, C.M. Killing K channels with TEA+. Proc. Natl. Acad. Sci. USA 1997, 94, 13335–13338. [Google Scholar] [CrossRef]
- Lallet-Daher, H.; Roudbaraki, M.; Bavencoffe, A.; Mariot, P.; Gackière, F.; Bidaux, G.; Urbain, R.; Gosset, P.; Delcourt, P.; Fleurisse, L.; et al. Intermediate-conductance Ca2+-activated K+ channels (IKCa1) regulate human prostate cancer cell proliferation through a close control of calcium entry. Oncogene 2009, 28, 1792–1806. [Google Scholar] [CrossRef]
- Faouzi, M.; Hague, F.; Geerts, D.; Ay, A.S.; Potier-Cartereau, M.; Ahidouch, A.; Ouadid-Ahidouch, H. Functional cooperation between KCa3.1 and TRPC1 channels in human breast cancer: Role in cell proliferation and patient prognosis. Oncotarget 2016, 7, 36419–36435. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.M.; Fakler, B.; Rivard, A.; Wayman, G.; Johnson-Pais, T.; Keen, J.E.; Ishii, T.; Hirschberg, B.; Bond, C.T.; Lutsenko, S.; et al. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature 1998, 395, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Joiner, W.J.; Wang, L.Y.; Tang, M.D.; Kaczmarek, L.K. hSK4, a member of a novel subfamily of calcium-activated potassium channels. Proc. Natl. Acad. Sci. USA 1997, 94, 11013–11018. [Google Scholar] [CrossRef] [PubMed]
- Strassmaier, T.; Bond, C.T.; Sailer, C.A.; Knaus, H.G.; Maylie, J.; Adelman, J.P. A novel isoform of SK2 assembles with other SK subunits in mouse brain. J. Biol. Chem. 2005, 280, 21231–21236. [Google Scholar] [CrossRef]
- Girault, A.; Haelters, J.P.; Potier-Cartereau, M.; Chantôme, A.; Jaffrés, P.A.; Bougnoux, P.; Joulin, V.; Vandier, C. Targeting SKCa channels in cancer: Potential new therapeutic approaches. Curr. Med. Chem. 2012, 19, 697–713. [Google Scholar] [CrossRef]
- Weaver, A.K.; Bomben, V.C.; Sontheimer, H. Expression and function of calcium-activated potassium channels in human glioma cells. Glia 2006, 54, 223–233. [Google Scholar] [CrossRef]
- Checchetto, V.; Teardo, E.; Carraretto, L.; Leanza, L.; Szabo, I. Physiology of intracellular potassium channels: A unifying role as mediators of counterion fluxes? Biochim. Et Biophys. Acta 2016, 1857, 1258–1266. [Google Scholar] [CrossRef]
- Capera, J.; Serrano-Novillo, C.; Navarro-Pérez, M.; Cassinelli, S.; Felipe, A. The Potassium Channel Odyssey: Mechanisms of Traffic and Membrane Arrangement. Int. J. Mol. Sci. 2019, 20, 734. [Google Scholar] [CrossRef]
- Bonito, B.; Sauter, D.R.; Schwab, A.; Djamgoz, M.B.; Novak, I. KCa3.1 (IK) modulates pancreatic cancer cell migration, invasion and proliferation: Anomalous effects on TRAM-34. Pflugers Arch. 2016, 468, 1865–1875. [Google Scholar] [CrossRef]
- Lai, W.; Chen, S.; Wu, H.; Guan, Y.; Liu, L.; Zeng, Y.; Zhao, H.; Jiang, J.; Chu, Z. PRL-3 promotes the proliferation of LoVo cells via the upregulation of KCNN4 channels. Oncol. Rep. 2011, 26, 909–917. [Google Scholar] [CrossRef]
- Bulk, E.; Ay, A.S.; Hammadi, M.; Ouadid-Ahidouch, H.; Schelhaas, S.; Hascher, A.; Rohde, C.; Thoennissen, N.H.; Wiewrodt, R.; Schmidt, E.; et al. Epigenetic dysregulation of KCa 3.1 channels induces poor prognosis in lung cancer. Int. J. Cancer 2015, 137, 1306–1317. [Google Scholar] [CrossRef] [PubMed]
- Mohr, C.J.; Steudel, F.A.; Gross, D.; Ruth, P.; Lo, W.Y.; Hoppe, R.; Schroth, W.; Brauch, H.; Huber, S.M.; Lukowski, R. Cancer-Associated Intermediate Conductance Ca2+-Activated K⁺ Channel KCa3.1. Cancers 2019, 11, 109. [Google Scholar] [CrossRef] [PubMed]
- Millership, J.E.; Devor, D.C.; Hamilton, K.L.; Balut, C.M.; Bruce, J.I.; Fearon, I.M. Calcium-activated K+ channels increase cell proliferation independent of K+ conductance. Am. J. Physiol. Cell Physiol. 2011, 300, C792–C802. [Google Scholar] [CrossRef] [PubMed]
- Potier, M.; Joulin, V.; Roger, S.; Besson, P.; Jourdan, M.L.; Leguennec, J.Y.; Bougnoux, P.; Vandier, C. Identification of SK3 channel as a new mediator of breast cancer cell migration. Mol. Cancer Ther. 2006, 5, 2946–2953. [Google Scholar] [CrossRef]
- Steinestel, K.; Eder, S.; Ehinger, K.; Schneider, J.; Genze, F.; Winkler, E.; Wardelmann, E.; Schrader, A.J.; Steinestel, J. The small conductance calcium-activated potassium channel 3 (SK3) is a molecular target for Edelfosine to reduce the invasive potential of urothelial carcinoma cells. Tumour. Biol. 2016, 37, 6275–6283, Erratum in Tumour Biol. 2016, 37, 2773. [Google Scholar] [CrossRef]
- Tajima, N.; Schönherr, K.; Niedling, S.; Kaatz, M.; Kanno, H.; Schönherr, R.; Heinemann, S.H. Ca2+-activated K+ channels in human melanoma cells are up-regulated by hypoxia involving hypoxia-inducible factor-1alpha and the von Hippel-Lindau protein. J. Physiol. 2006, 571 Pt 2, 349–359. [Google Scholar] [CrossRef]
- Huang, L.; Huang, Q.Y.; Huang, H.Q. The evidence of HeLa cell apoptosis induced with tetraethylammonium using proteomics and various analytical methods. J. Biol. Chem. 2014, 289, 2217–2229. [Google Scholar] [CrossRef]
- ErdemKış, E.; Tiftik, R.N.; Al Hennawi, K.; Ün, İ. The role of potassium channels in the proliferation and migration of endometrial adenocarcinoma HEC1-A cells. Mol. Biol. Rep. 2022, 49, 7447–7454. [Google Scholar] [CrossRef]
- Yang, K.B.; Zhao, S.G.; Liu, Y.H.; Hu, E.X.; Liu, B.X. Tetraethylammonium inhibits glioma cells via increasing production of intracellular reactive oxygen species. Chemotherapy 2009, 55, 372–380. [Google Scholar] [CrossRef]
- Han, X.B.; Wang, F.; Yao, W.X.; Liu, Y.Q.; Wang, G.; Ma, D. Inhibitory effects of tetraethylammonium on proliferation and voltage-gated potassium channels in human cervical carcinoma cell line SiHa. Ai Zheng 2006, 25, 451–455, In Chinese. [Google Scholar]
- Bi, D.; Toyama, K.; Lemaître, V.; Takai, J.; Fan, F.; Jenkins, D.P.; Wulff, H.; Gutterman, D.D.; Park, F.; Miura, H. The intermediate conductance calcium-activated potassium channel KCa3.1 regulates vascular smooth muscle cell proliferation via controlling calcium-dependent signaling. J. Biol. Chem. 2013, 288, 15843–15853. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, H.; Tian, J.B.; Zhu, M.X.; O’Neil, R.G. Dynamic coupling between TRPV4 and Ca2+-activated SK1/3 and IK1 K+ channels plays a critical role in regulating the K+-secretory BK channel in kidney collecting duct cells. Am. J. Physiol. Ren. Physiol. 2017, 312, F1081–F1089. [Google Scholar] [CrossRef] [PubMed]
- Roach, K.M.; Bradding, P. Ca2+signalling in fibroblasts and the therapeutic potential of KCa3.1 channel blockers in fibrotic diseases. Br. J. Pharmacol. 2020, 177, 1003–1024. [Google Scholar] [CrossRef]
- Wojtulewski, J.A.; Gow, P.J.; Walter, J.; Grahame, R.; Gibson, T.; Panayi, G.S.; Mason, J. Clotrimazole in rheumatoid arthritis. Ann. Rheum. Dis. 1980, 39, 469–472. [Google Scholar] [CrossRef]
- Brugnara, C.; Gee, B.; Armsby, C.C.; Kurth, S.; Sakamoto, M.; Rifai, N.; Alper, S.L.; Platt, O.S. Therapy with oral clotrimazole induces inhibition of the Gardos channel and reduction of erythrocyte dehydration in patients with sickle cell disease. J. Clin. Investig. 1996, 97, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Ataga, K.I.; Smith, W.R.; De Castro, L.M.; Swerdlow, P.; Saunthararajah, Y.; Castro, O.; Vichinsky, E.; Kutlar, A.; Orringer, E.P.; Rigdon, G.C.; et al. ICA-17043-05 Investigators. Efficacy and safety of the Gardos channel blocker, senicapoc (ICA-17043), in patients with sickle cell anemia. Blood 2008, 111, 3991–3997. [Google Scholar] [CrossRef]
- Wulff, H.; Miller, M.J.; Hansel, W.; Grissmer, S.; Cahalan, M.D.; Chandy, K.G. Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: A potential immunosuppressant. Proc. Natl. Acad. Sci. USA 2000, 97, 8151–8156. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhang, L.; Shi, Y.; Yi, H.; Zhao, Y.; Chen, J.; Pollock, C.A.; Chen, X.M. The KCa3.1 blocker TRAM34 reverses renal damage in a mouse model of established diabetic nephropathy. PLoS ONE 2018, 13, e0192800. [Google Scholar] [CrossRef]
- Chen, Y.J.; Raman, G.; Bodendiek, S.; O’Donnell, M.E.; Wulff, H. The KCa3.1 blocker TRAM-34 reduces infarction and neurological deficit in a rat model of ischemia/reperfusion stroke. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2011, 31, 2363–2374. [Google Scholar] [CrossRef]
- Gu, H.; Han, S.M.; Park, K.K. Therapeutic Effects of Apamin as a Bee Venom Component for Non-Neoplastic Disease. Toxins 2020, 12, 195. [Google Scholar] [CrossRef]
- McGahon, A.J.; Brown, D.G.; Martin, S.J.; Amarante-Mendes, G.P.; Cotter, T.G.; Cohen, G.M.; Green, D.R. Downregulation of Bcr-Abl in K562 cells restores susceptibility to apoptosis: Characterization of the apoptotic death. Cell Death Differ. 1997, 4, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, E.; Kantarjian, H. Chronic myeloid leukemia: 2022 update on diagnosis, therapy, and monitoring. Am. J. Hematol. 2022, 97, 1236–1256. [Google Scholar] [CrossRef] [PubMed]
- Younes, S.; Ismail, M.A.; Al-Jurf, R.; Ziyada, A.; Nasrallah, G.K.; Abdulrouf, P.V.; Nagy, M.; Zayed, H.; Farrell, T.; Sorio, C.; et al. Management of chronic myeloid leukaemia: Current treatment options, challenges, and future strategies. Hematology 2023, 28, 2196866. [Google Scholar] [CrossRef] [PubMed]
- Vasileva, V.Y.; Chubinskiy-Nadezhdin, V.I. Local mechano-dependent calcium influx controls the activity of calcium-dependent potassium channels of big and small conductance in human lymphoma cells. Cell Tiss Biol. 2022, 16, 150–156. [Google Scholar] [CrossRef]


| Gene (KCa Type) | Forward Primer Reverse Primer | Predicted Product Size, bp |
|---|---|---|
| hKCNN1 (KCa2.1, SK1) | 3′-AGA ACA GCA AGA CAT ATC CG-5′ 5′-ATT GTA GCT GTG GCT GTT CA-3′ | 282 |
| hKCNN2 (KCa2.2, SK2) | 3′-CTT ATC AGT CTC TCC ACG ATC-5′ 5′-TAC AGT TCC TGG GCA TAT AG-3′ | 423 |
| hKCNN3 (KCa2.3, SK3) | 3′-CGA CTG AGT GAC TAT GCT C-5′ 5′-GTG GAC AGA CTG ATA AGG C-3′ | 137 |
| hKCNN4 (KCa3.1, IK) | 5′-GGC CAA GCT TTA CAT GAA C-3′ 3′-ATC ATG AAG TTG TGC ACG TG-5′ | 325 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasileva, V.Y.; Khairullina, Z.M.; Sudarikova, A.V.; Chubinskiy-Nadezhdin, V.I. Role of Calcium-Activated Potassium Channels in Proliferation, Migration and Invasion of Human Chronic Myeloid Leukemia K562 Cells. Membranes 2023, 13, 583. https://doi.org/10.3390/membranes13060583
Vasileva VY, Khairullina ZM, Sudarikova AV, Chubinskiy-Nadezhdin VI. Role of Calcium-Activated Potassium Channels in Proliferation, Migration and Invasion of Human Chronic Myeloid Leukemia K562 Cells. Membranes. 2023; 13(6):583. https://doi.org/10.3390/membranes13060583
Chicago/Turabian StyleVasileva, Valeria Y., Zuleikha M. Khairullina, Anastasia V. Sudarikova, and Vladislav I. Chubinskiy-Nadezhdin. 2023. "Role of Calcium-Activated Potassium Channels in Proliferation, Migration and Invasion of Human Chronic Myeloid Leukemia K562 Cells" Membranes 13, no. 6: 583. https://doi.org/10.3390/membranes13060583
APA StyleVasileva, V. Y., Khairullina, Z. M., Sudarikova, A. V., & Chubinskiy-Nadezhdin, V. I. (2023). Role of Calcium-Activated Potassium Channels in Proliferation, Migration and Invasion of Human Chronic Myeloid Leukemia K562 Cells. Membranes, 13(6), 583. https://doi.org/10.3390/membranes13060583








