Novel Electrospun Composite Membranes Based on Polyhydroxybutyrate and Poly(vinyl formate) Loaded with Protonated Montmorillonite for Organic Dye Removal: Kinetic and Isotherm Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Characterization
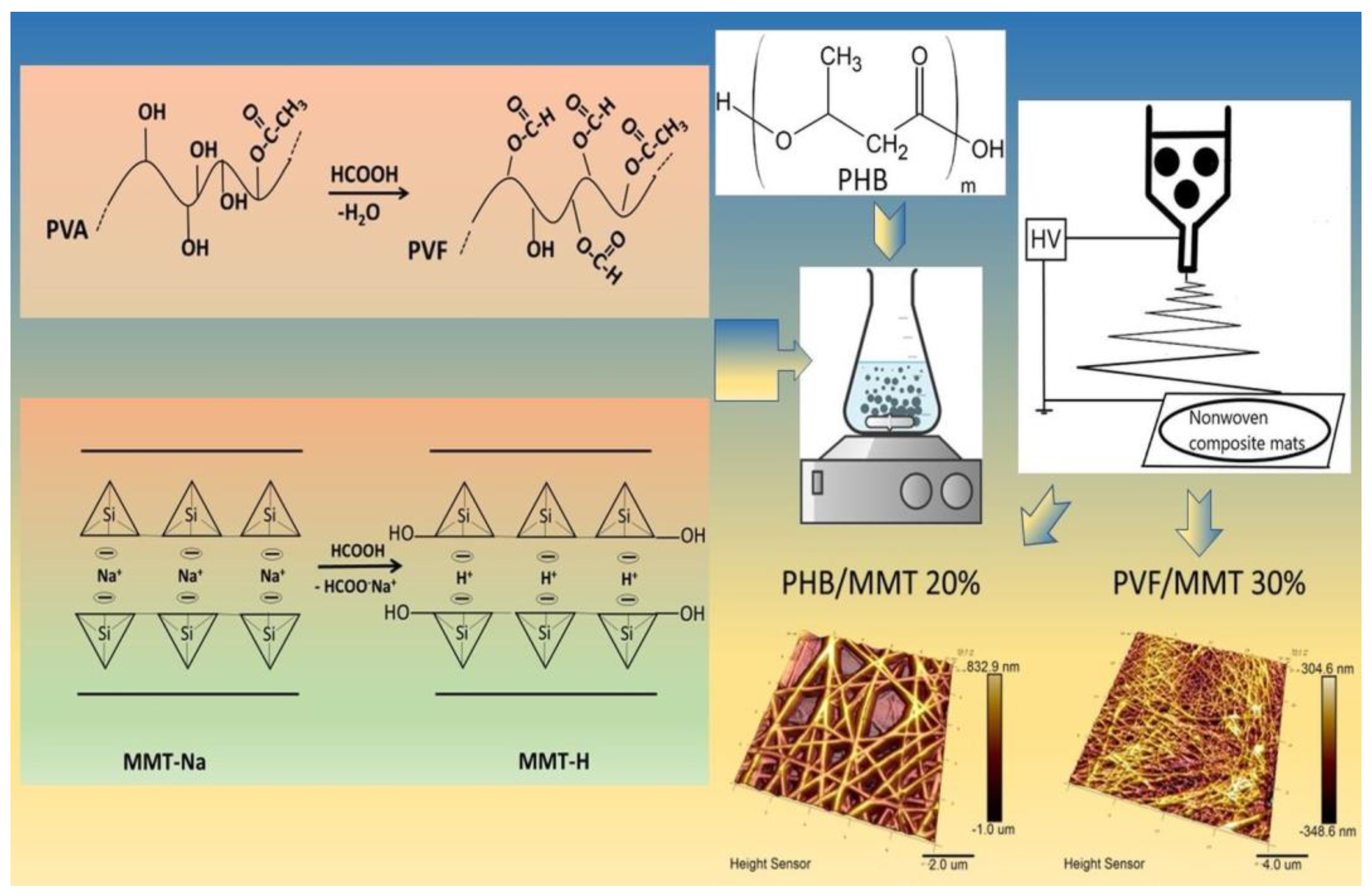
2.2. Preparation of PVF- and PHB/MMT-H Spinning Dispersions
2.3. Electrospinning Process
2.4. Dye Sorption and Kinetics Studies of the Composite Membranes
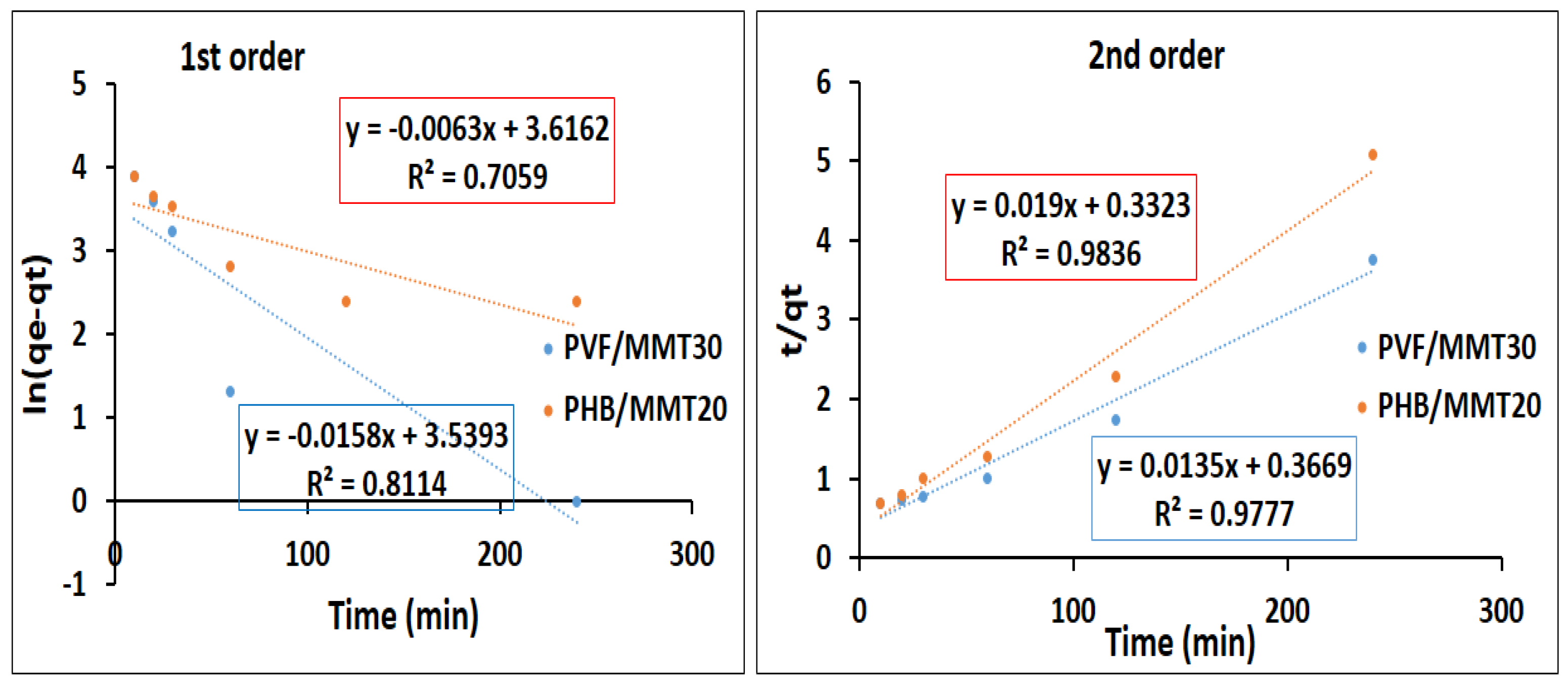
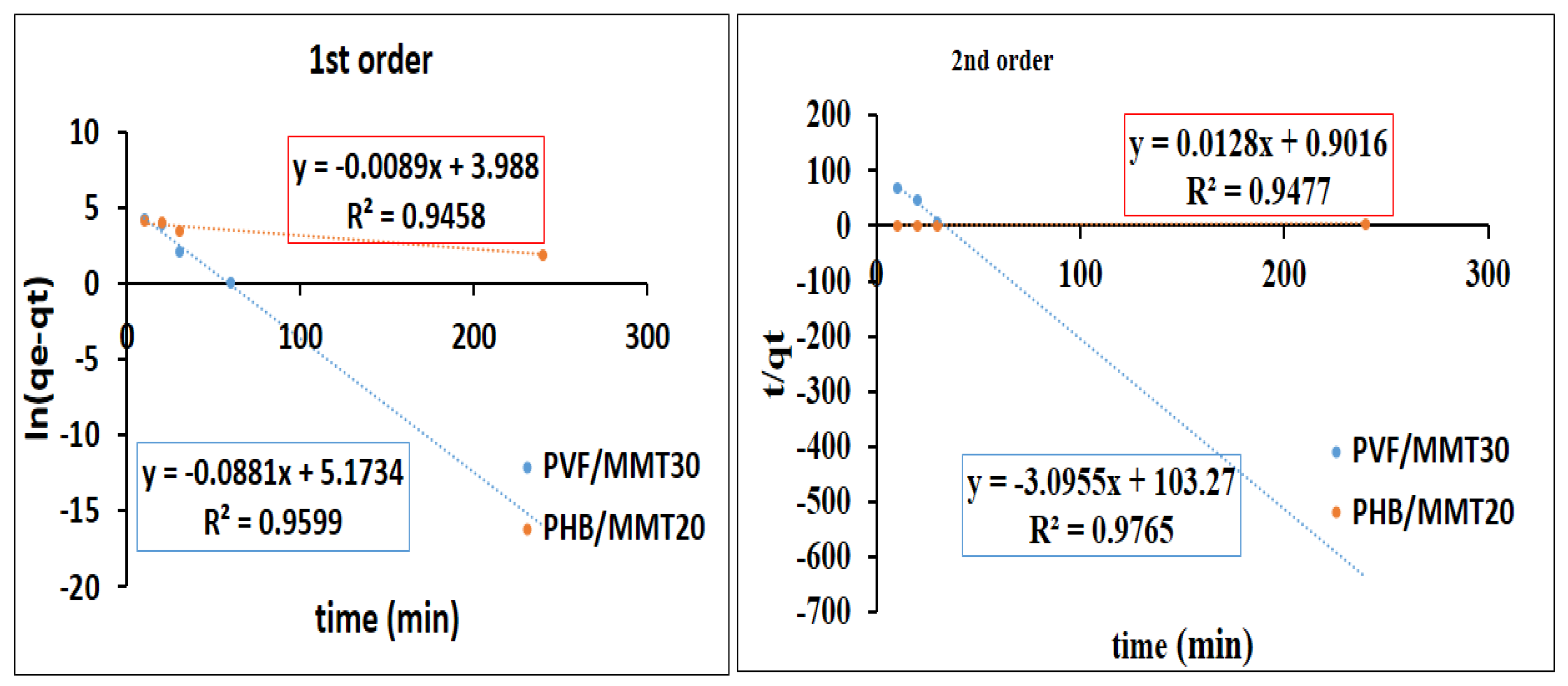
Adsorption Kinetics
3. Results and Discussion
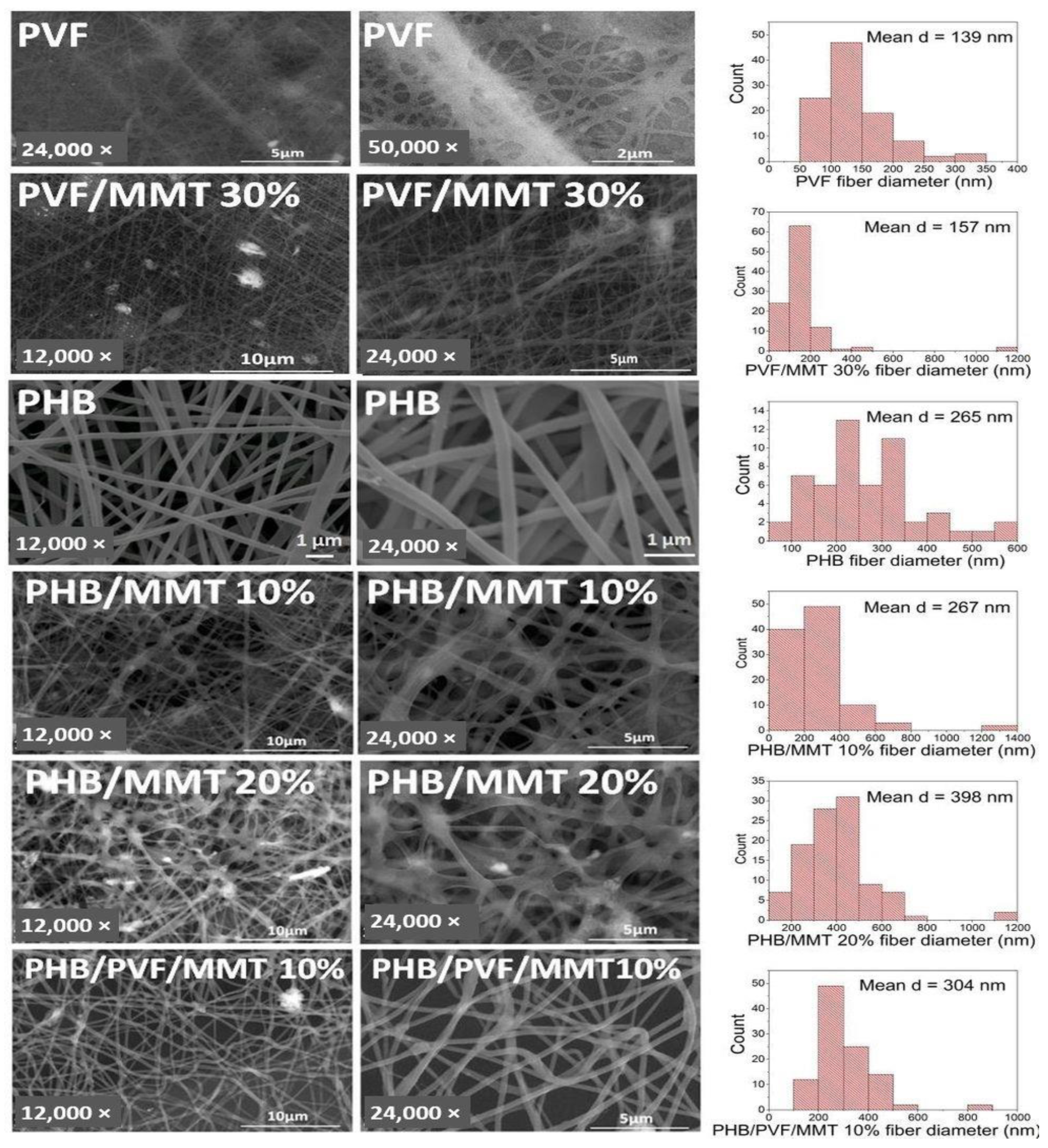
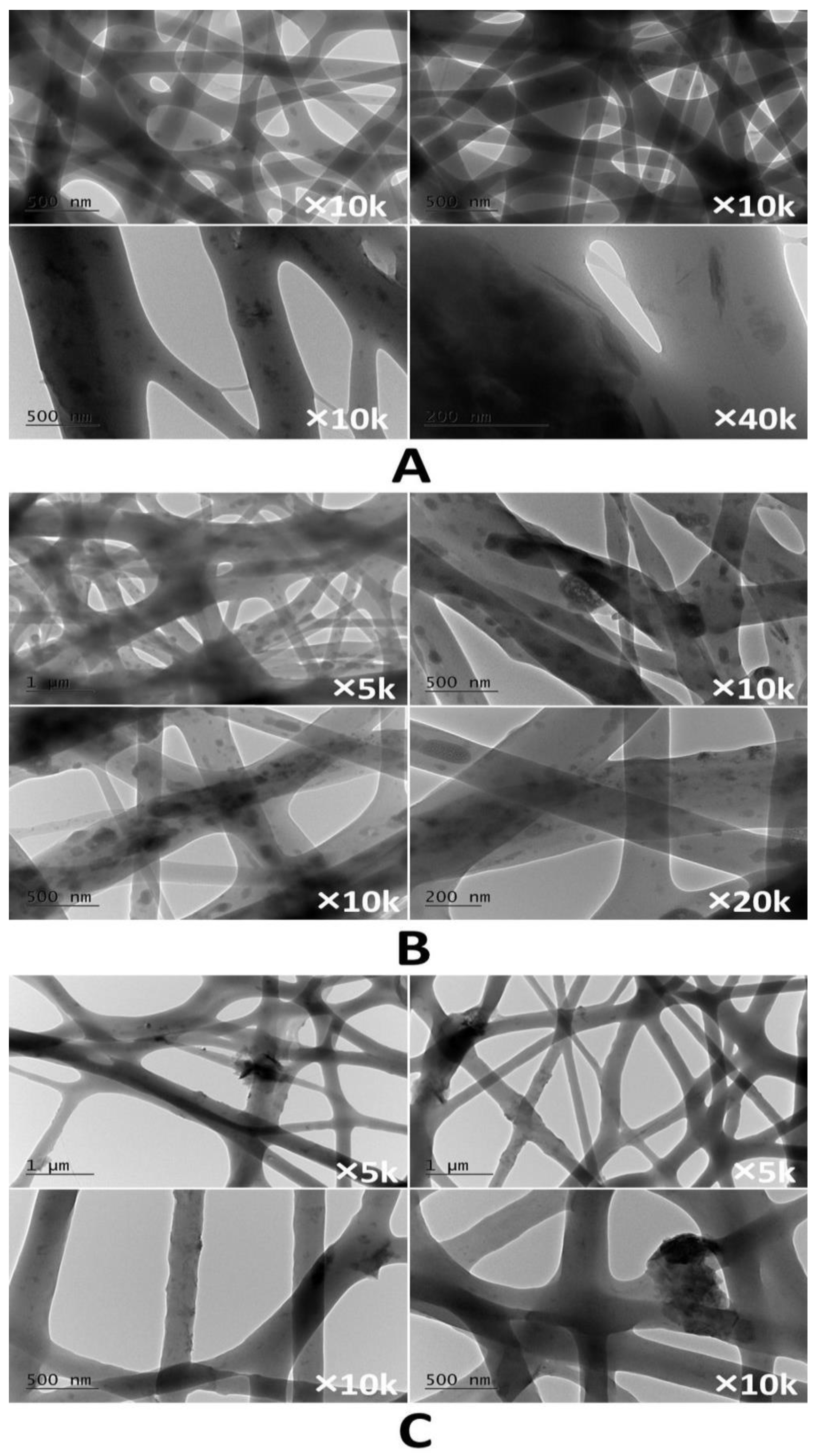
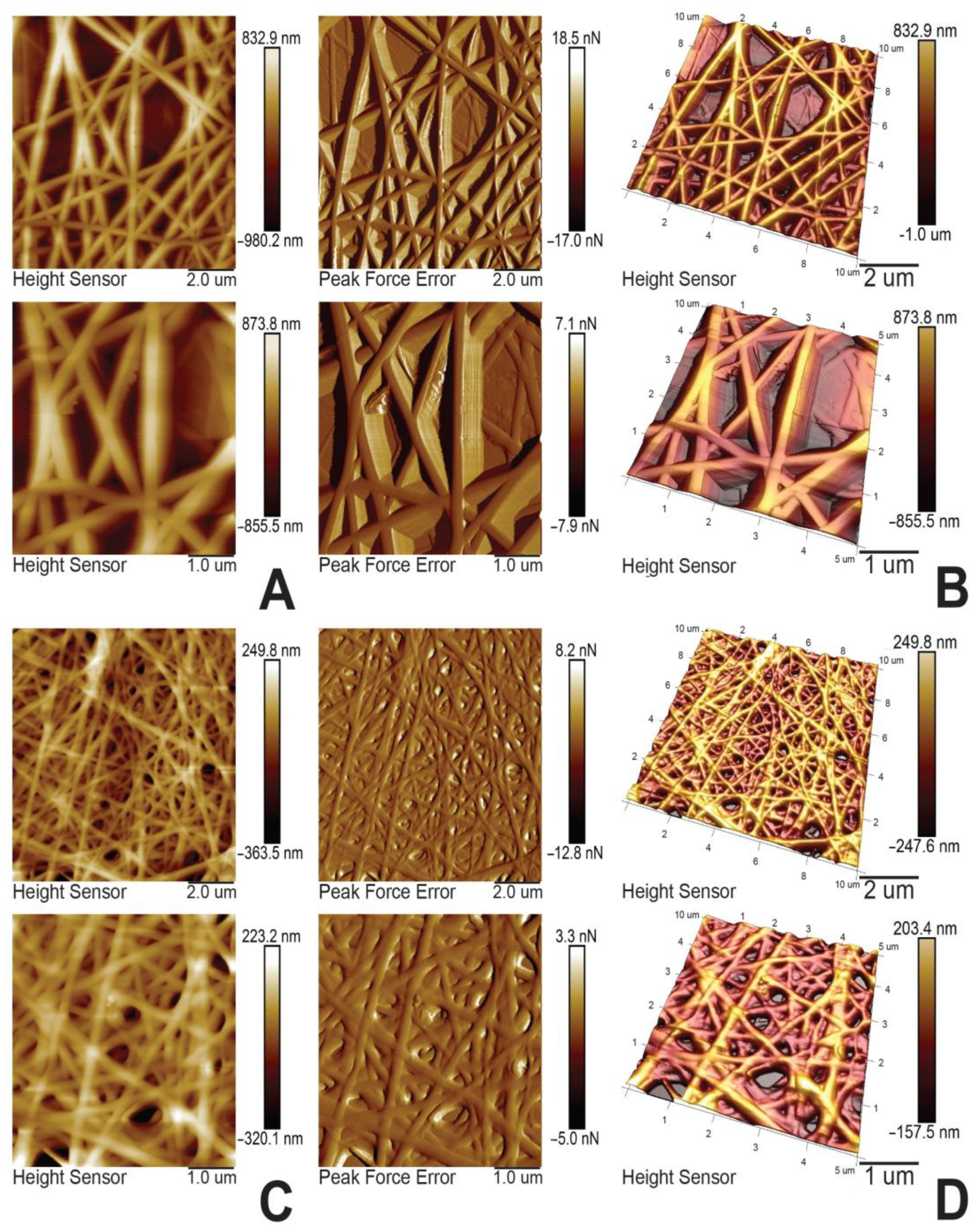
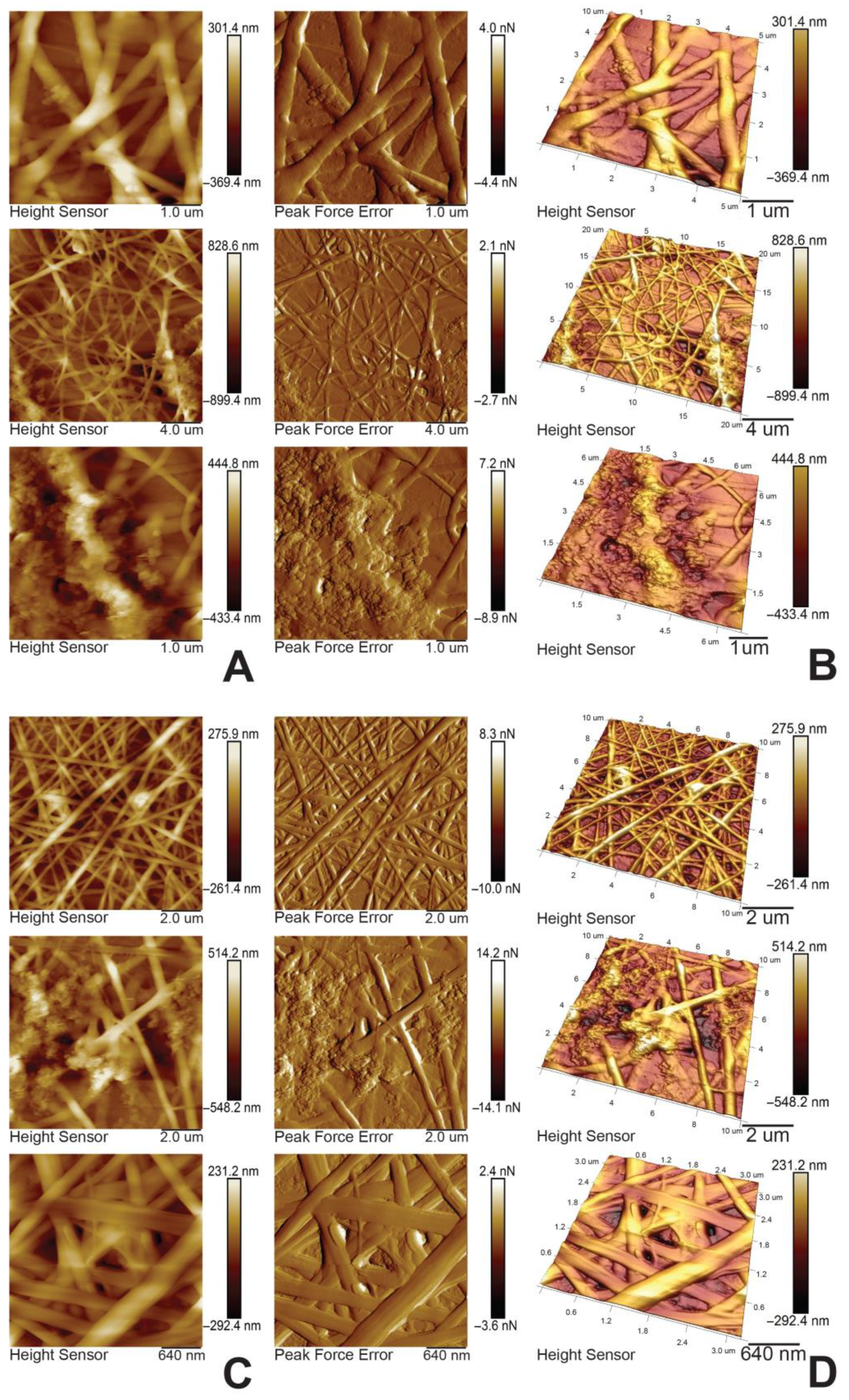
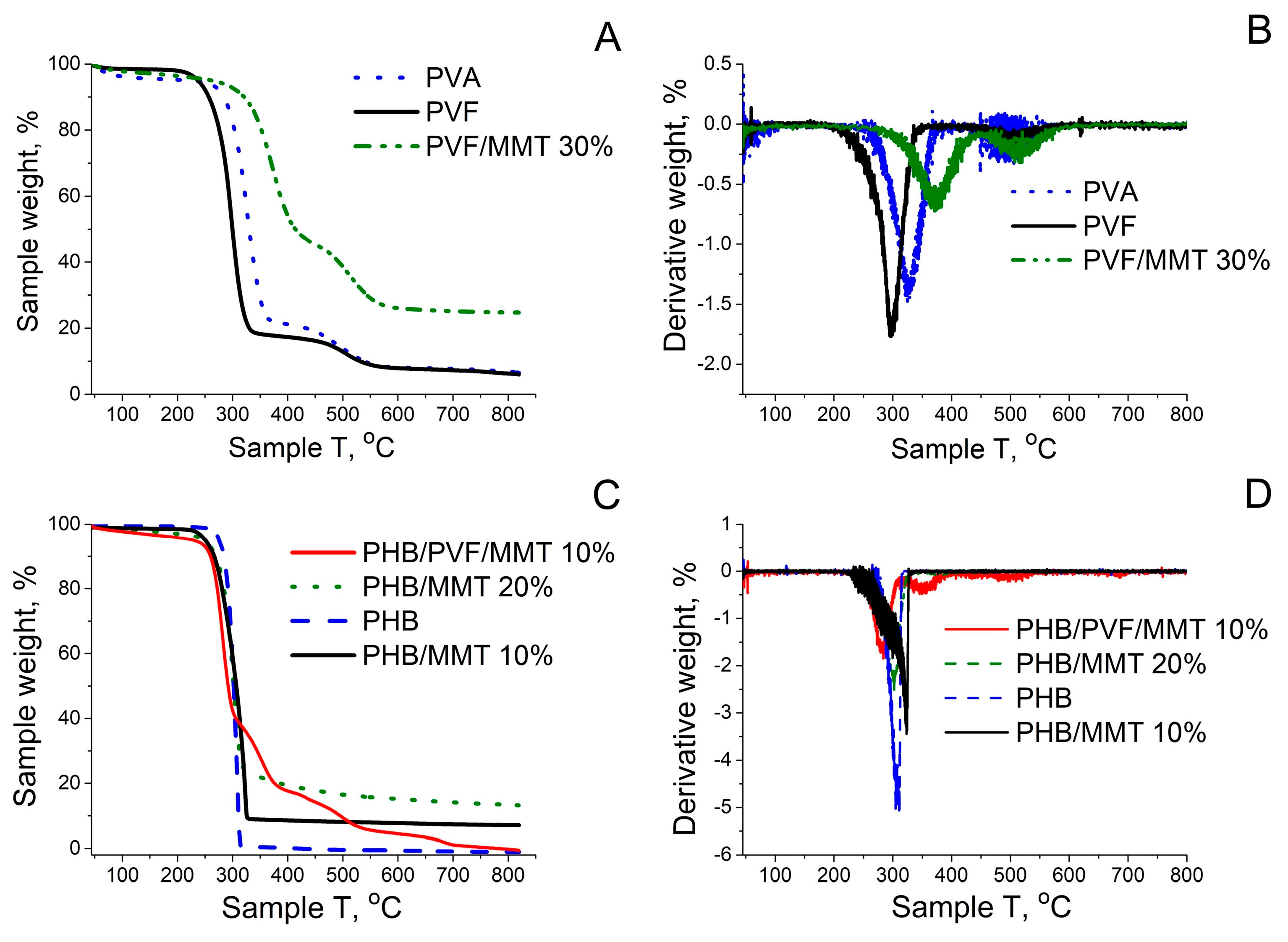
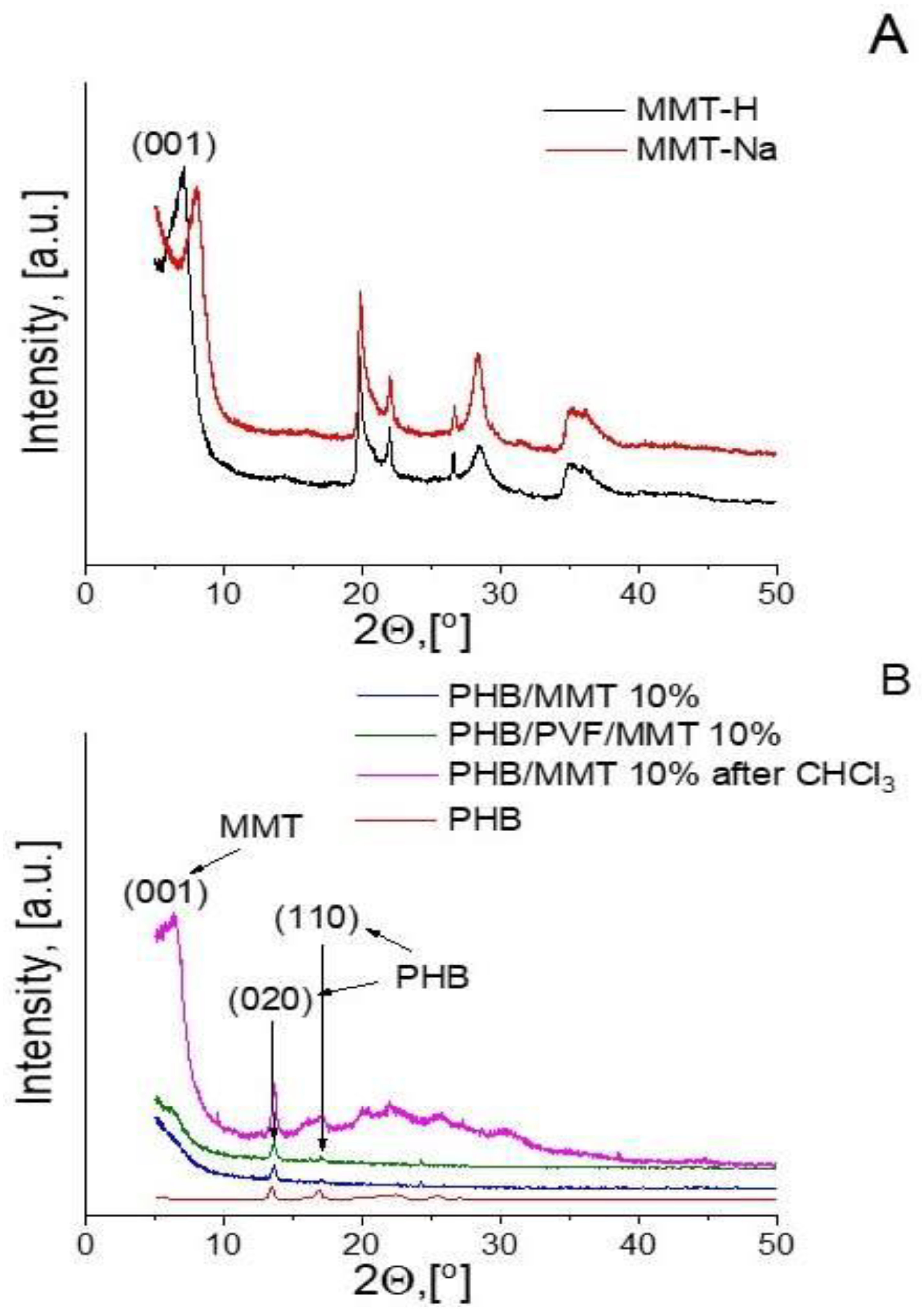
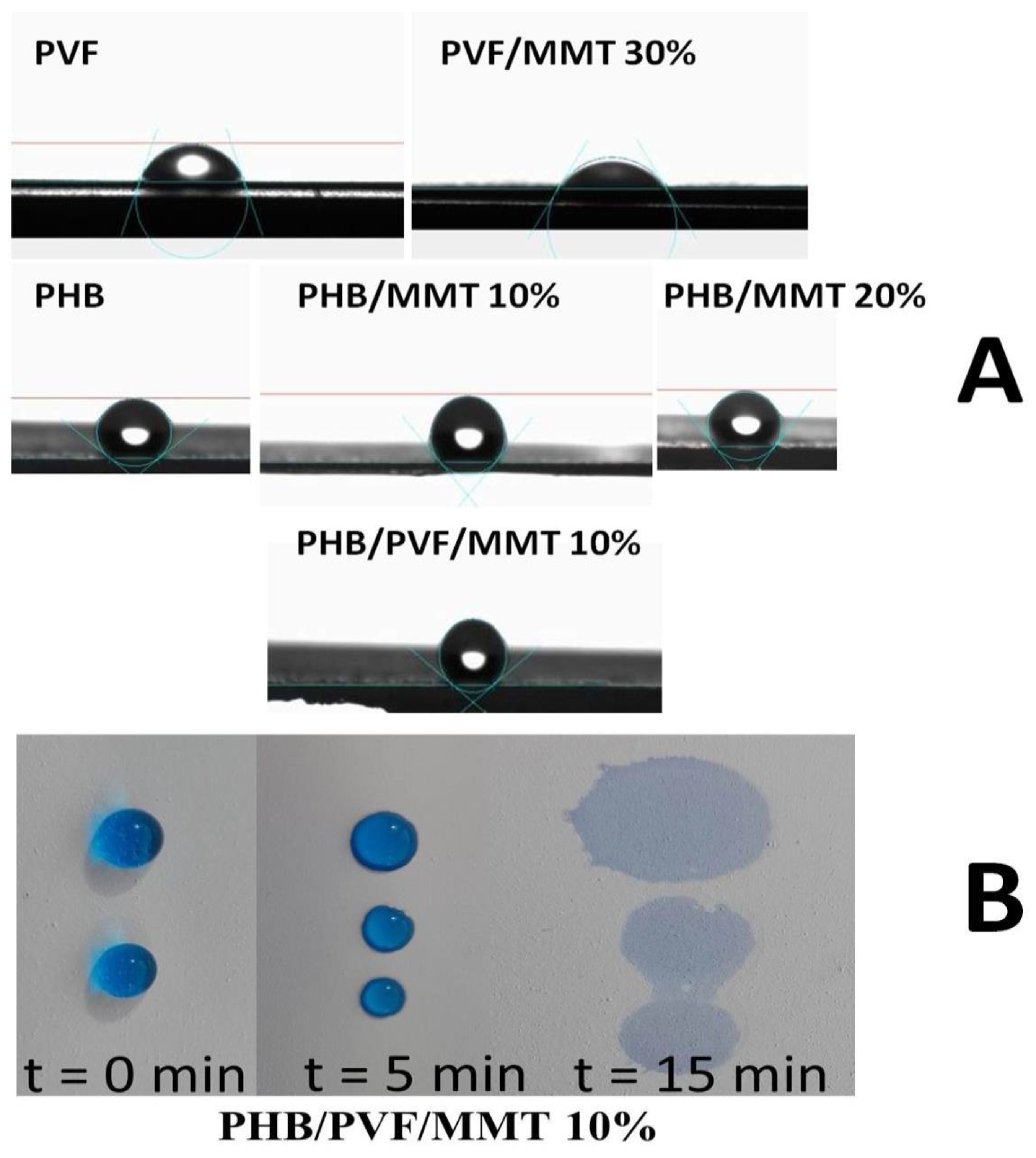
3.1. Characterization of the Electrospun Membranes
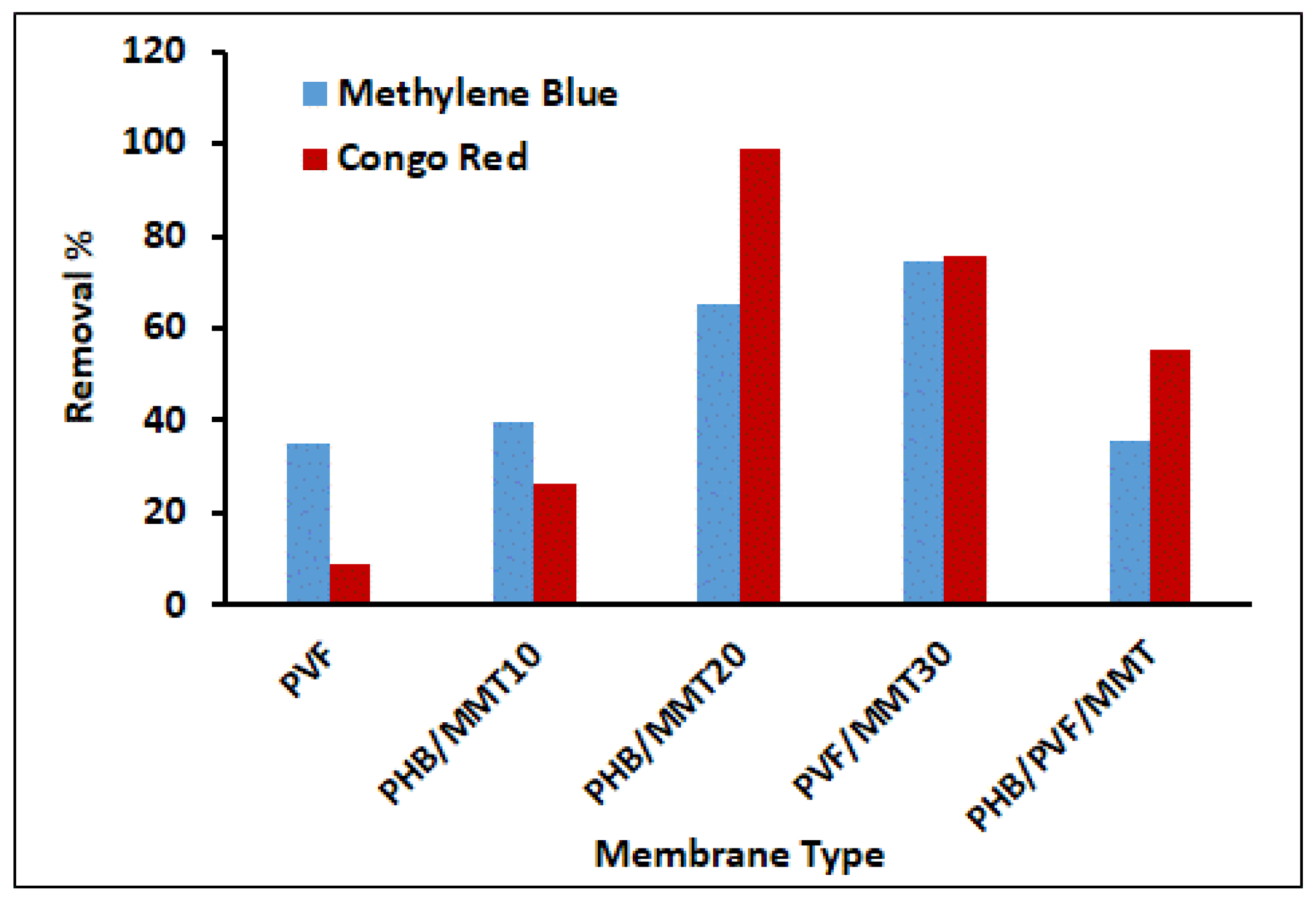
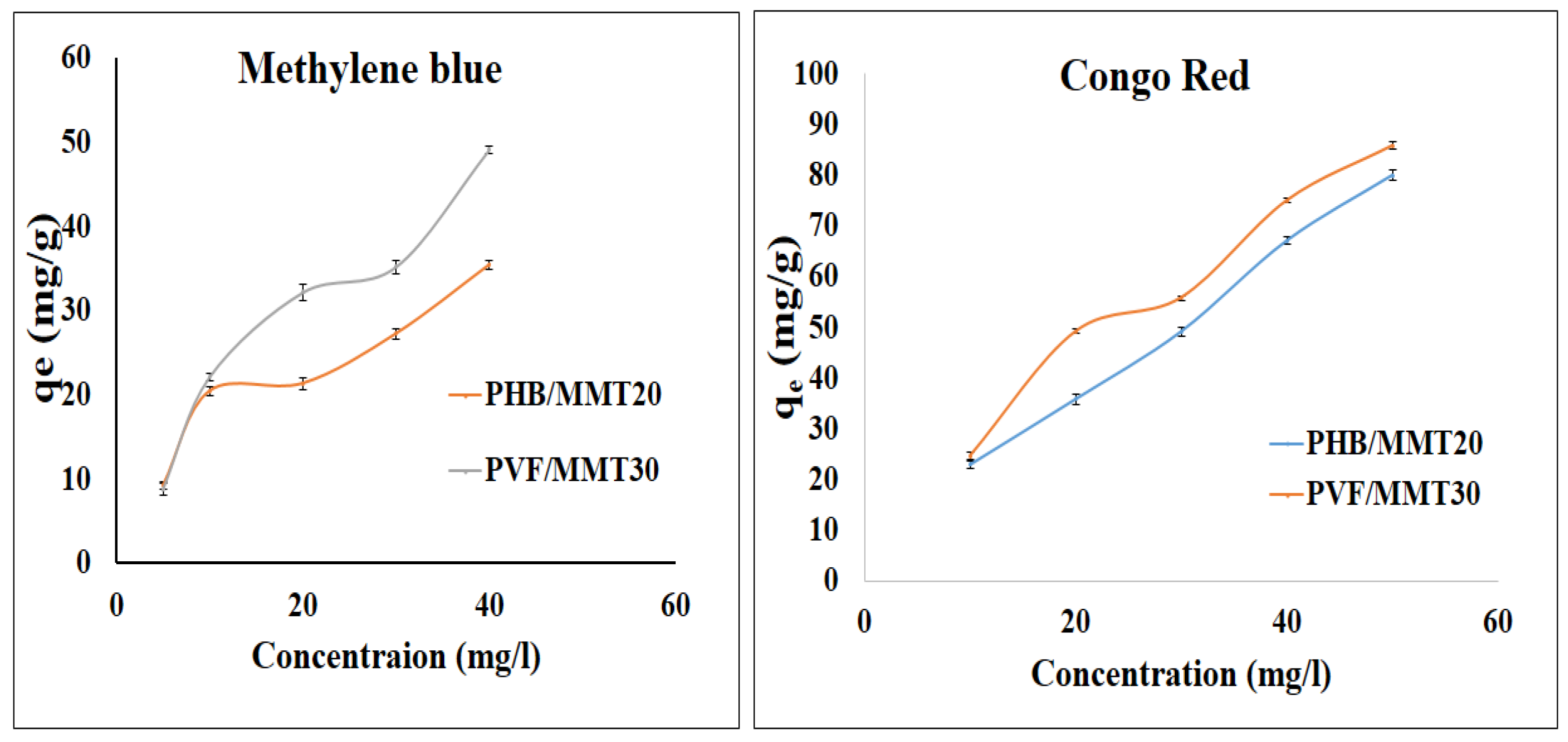
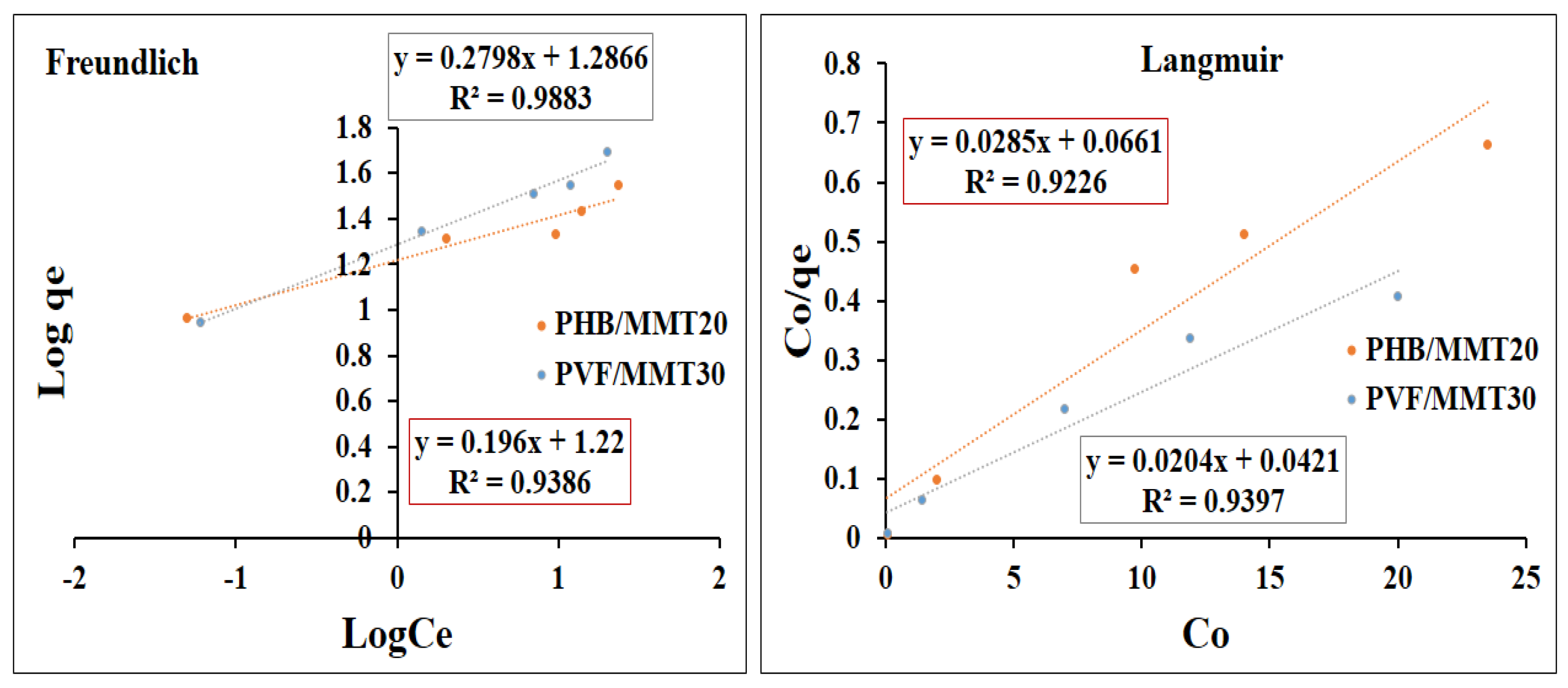
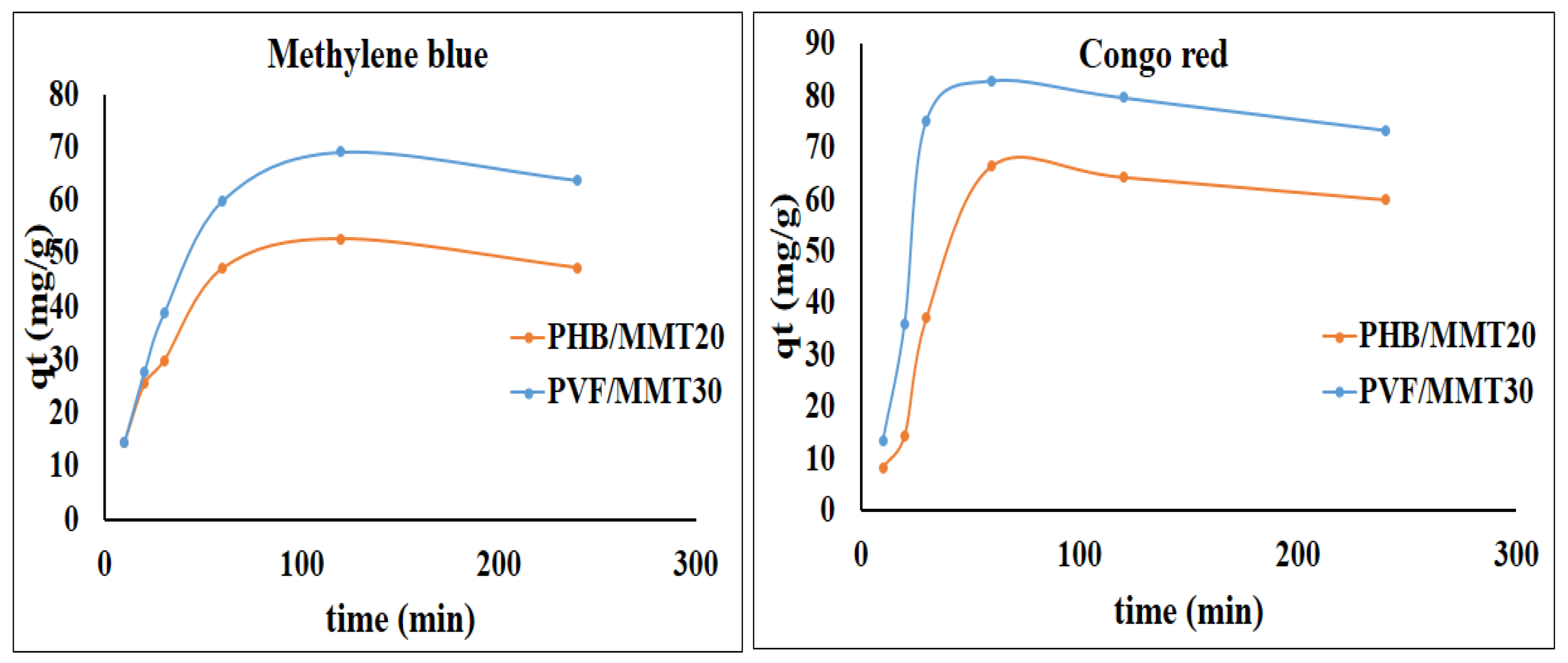
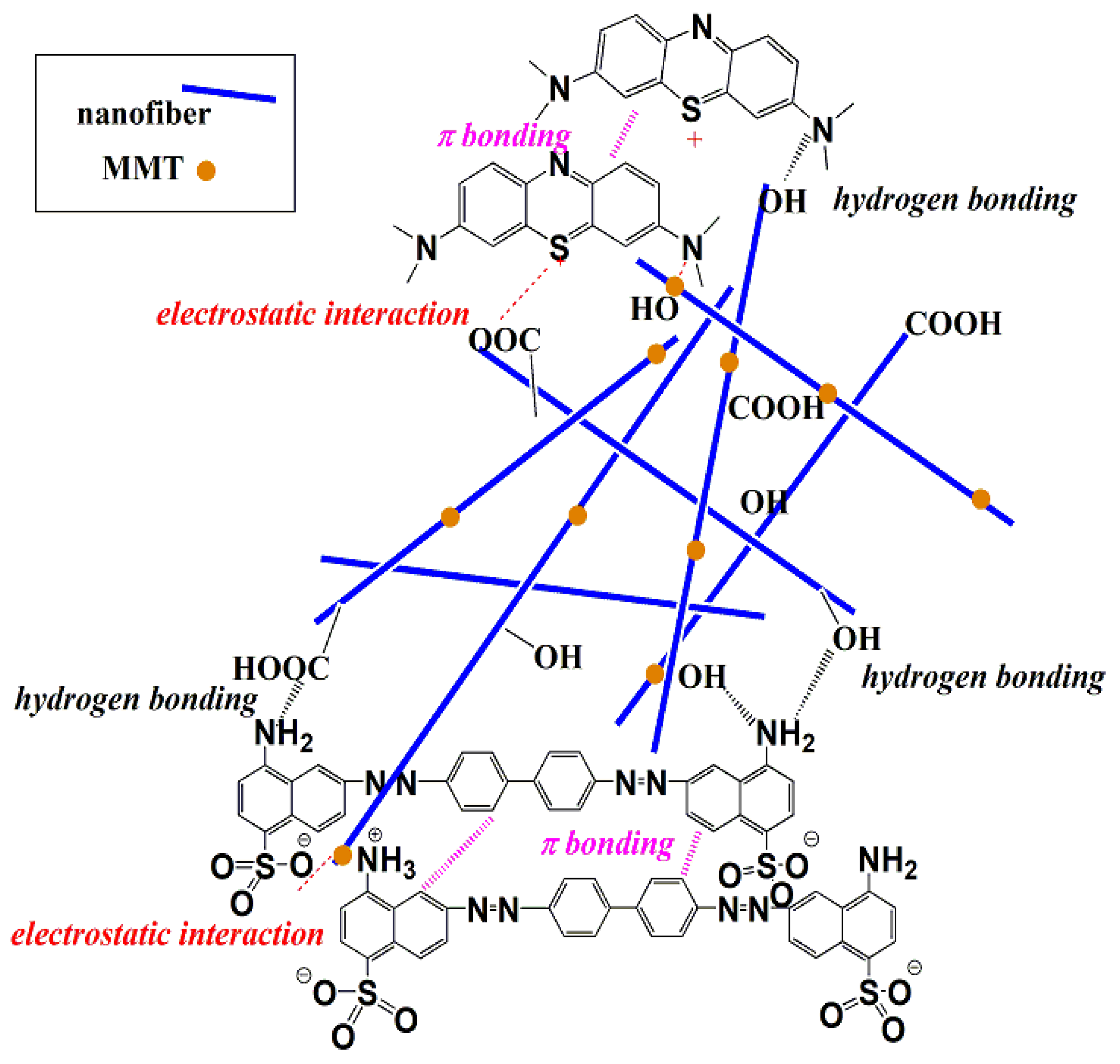
3.2. Adsorption Isotherms and Kinetics Investigations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McAdam, B.; Brennan Fournet, M.; McDonald, P.; Mojicevic, M. Production of polyhydroxybutyrate (PHB) and factors impacting its chemical and mechanical characteristics. Polymers 2020, 12, 2908. [Google Scholar] [CrossRef]
- Vroman, I.; Tighzert, L. Biodegradable Polymers. Mater. Mol. Divers. Preserv. Int. 2009, 2, 307–344. [Google Scholar]
- Utsunomia, C.; Ren, Q.; Zinn, M. Poly(4-Hydroxybutyrate): Current State and Perspectives. Front. Bioeng. Biotechnol. 2020, 8, 257. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, J.-C.; Ma, Y.; Chen, G.-Q. Engineering Biosynthesis of Polyhydroxyalkanoates (PHA) for Diversity and Cost Reduction. Metab. Eng. 2019, 58, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Avella, M.; Martuscelli, E. Poly-d-(−)(3-hydroxybutyrate)/poly (ethylene oxide) blends: Phase diagram, thermal and crystallization behaviour. Polymer 1988, 29, 1731–1737. [Google Scholar] [CrossRef]
- Ol’khov, A.A.; Iordanskii, A.L.; Shatalova, O.V. Blends based on polyvinyl alcohol and polyhydroxybutyrate. Int. Polym. Sci. Technol. 2003, 30, 47–50. [Google Scholar] [CrossRef]
- Khlyustova, A.; Sirotkin, N.; Kraev, A.; Agafonov, A.; Titov, V. Effect of metal oxides added onto polyvinyl alcohol via pulsed underwater plasma on their thermal, electrical and dielectric properties. J. Appl. Polym. Sci. 2021, 138, 51174. [Google Scholar] [CrossRef]
- Asran, A.S.; Razghandi, K.; Aggarwal, N.; Michler, G.H.; Groth, T. Nanofibers from blends of polyvinyl alcohol and polyhydroxy butyrate as potential scaffold material for tissue engineering of skin. Biomacromolecules 2010, 11, 3413–3421. [Google Scholar] [CrossRef]
- Budurova, D.; Ublekov, F.; Penchev, H. The use of formic acid as a common solvent for electrospinning of hybrid PHB/Soy protein fibers. Mater. Lett. 2021, 301, 130313. [Google Scholar] [CrossRef]
- Herrmann, W.O.; Deisenhofen; Wolfram, H. Process for the Preparation of Polyvinyl Formate. U.S. Patent 2,278,783A, 17 January 1940. [Google Scholar]
- Ushakov, S.N.; Kononova, T.A. Synthesis of esters of polyvinyl alcohol. Bull. Acad. Sci. USSR Div. Chem. Sci. 1955, 4, 103–108. [Google Scholar] [CrossRef]
- Essawy, H.A.; Tawfik, M.E.; Khalil, A.M.; El-Sabbagh, S.H. Systematic organophilization of montmorillonite: The impact thereof on the rheometric and mechanical characteristics of NBR and SBR based nanocomposites. Polym. Eng. Sci. 2014, 54, 942–948. [Google Scholar] [CrossRef]
- Bee, S.L.; Abdullah, M.A.A.; Bee, S.T.; Sin, L.T.; Rahmat, A.R. Polymer nanocomposites based on silylated-montmorillonite: A review. Prog. Polym. Sci. 2018, 85, 57–82. [Google Scholar] [CrossRef]
- Chan, M.L.; Lau, K.T.; Wong, T.T.; Ho, M.P.; Hui, D. Mechanism of reinforcement in a nanoclay/polymer composite. Compos. B Eng. 2011, 42, 1708–1712. [Google Scholar] [CrossRef]
- Trigueiro, P.; Pereira, F.A.; Guillermin, D.; Rigaud, B.; Balme, S.; Janot, J.M.; Jaber, M. When anthraquinone dyes meet pillared montmorillonite: Stability or fading upon exposure to light? Dyes Pigm. 2018, 159, 384–394. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, Y.; Wang, J.; Zhang, X. Ultrasonic-assisted fabrication of montmorillonite-lignin hybrid hydrogel: Highly efficient swelling behaviors and super-sorbent for dye removal from wastewater. Colloids Surf. 2017, 520, 903–913. [Google Scholar] [CrossRef]
- Yotsuji, K.; Tachi, Y.; Sakuma, H.; Kawamura, K. Effect of interlayer cations on montmorillonite swelling: Comparison between molecular dynamic simulations and experiments. Appl. Clay Sci. 2021, 204, 106034. [Google Scholar] [CrossRef]
- Liu, J.; Boo, W.J.; Clearfield, A.; Sue, H.J. Intercalation and exfoliation: A review on morphology of polymer nanocomposites reinforced by inorganic layer structures. Mater. Manuf. 2006, 21, 143–151. [Google Scholar] [CrossRef]
- Sohrabnezhad, S.; Pourahmad, A. Comparison absorption of new methylene blue dye in zeolite and nanocrystal zeolite. Desalination 2010, 256, 84–89. [Google Scholar] [CrossRef]
- Al Kausor, M.; Gupta, S.S.; Bhattacharyya, K.G.; Chakrabortty, D. Montmorillonite and modified montmorillonite as adsorbents for removal of water soluble organic dyes: A review on current status of the art. Inorg. Chem. Commun. 2022, 143, 109686. [Google Scholar] [CrossRef]
- Khalil, A.M.; Kenawy, S.H. Hybrid membranes based on clay-polymer for removing methylene blue from water. Acta Chim. Slov. 2020, 67, 96–104. [Google Scholar] [CrossRef]
- Patel, H.A.; Somani, R.S.; Bajaj, H.C.; Jasra, R.V. Nanoclays for polymer nanocomposites, paints, inks, greases and cosmetics formulations, drug delivery vehicle and waste water treatment. Bull. Mater. Sci. 2006, 29, 133–145. [Google Scholar] [CrossRef]
- Gao, T.; Guan, G.; Wang, X.; Lou, T. Electrospun molecularly imprinted sodium alginate/polyethylene oxide nanofibrous membranes for selective adsorption of methylene blue. Int. J. Biol. Macromol. 2022, 207, 62–71. [Google Scholar] [CrossRef]
- Snoussi, Y.; Sifaoui, I.; El Garah, M.; Khalil, A.M.; Barroso, J.E.; Jouini, M.; Chehimi, M.M. Green, zero-waste pathway to fabricate supported nanocatalysts and anti-kinetoplastid agents from sugarcane bagasse. Waste Manag. 2023, 155, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.A.P.; Debacher, N.A.; Downs, A.J.; Cottet, L.; Mello, C.A.D. Removal of methylene blue from colored effluents by adsorption on montmorillonite clay. J. Colloid Interface Sci. 2009, 332, 46–53. [Google Scholar] [CrossRef]
- Fil, B.A.; Özmetin, C.; Korkmaz, M. Cationic dye (methylene blue) removal from aqueous solution by montmorillonite. Bull. Korean Chem. Soc. 2012, 33, 10. [Google Scholar] [CrossRef]
- Khaniabadi, Y.O.; Basiri, H.; Nourmoradi, H.; Mohammadi, M.J.; Yari, A.R.; Sadeghi, S.; Amrane, A. Adsorption of congo red dye from aqueous solutions by montmorillonite as a low-cost adsorbent. Int. J. Chem. React. Eng. 2017, 16, 20160203. [Google Scholar] [CrossRef]
- Özcan, A.S.; Özcan, A. Adsorption of acid dyes from aqueous solutions onto acid-activated bentonite. J. Colloid Interface Sci. 2004, 276, 39–46. [Google Scholar] [CrossRef]
- Youssef, H.F.; Nasr, R.A.; Abou El-Anwar, E.A.; Mekky, H.S.; Abd El Rahim, S.A. Preparation and characterization of different zeolites from andesite rock: Product evaluation for efficient dye removal. Microporous Mesoporous Mater. 2021, 328, 111485. [Google Scholar] [CrossRef]
- Shahid, M.K.; Kim, Y.; Choi, Y.G. Adsorption of phosphate on magnetite-enriched particles (MEP) separated from the mill scale. Front. Environ. Sci. Eng. 2019, 13, 1–12. [Google Scholar] [CrossRef]
- Shahid, M.K.; Kim, J.Y.; Shin, G.; Choi, Y. Effect of pyrolysis conditions on characteristics and fluoride adsorptive performance of bone char derived from bone residue. J. Water Process. Eng. 2020, 37, 101499. [Google Scholar] [CrossRef]
- Moussout, H.; Ahlafi, H.; Aazza, M.; Maghat, H. Critical of linear and nonlinear equations of pseudo-first order and pseudo-second order kinetic models. Karbala Int. J. Mod. Sci. 2018, 4, 244–254. [Google Scholar] [CrossRef]
- Ublekov, F.; Budurova, D.; Staneva, M.; Natova, M.; Penchev, H. Self-supporting electrospun PHB and PHBV/organoclay nanocomposite fibrous scaffolds. Mater. Lett. 2018, 218, 353–356. [Google Scholar] [CrossRef]
- Olkhov, A.A.; Staroverova, O.V.; Kuherenko, E.L.; Iordanskii, A.L. Effect of electrospinning solution parameters on the properties of nonvolven fibrous material based on polyhydroxibutyrate. J. Phys. Conf. Ser. 2020, 1431, 012029. [Google Scholar] [CrossRef]
- Du, H.; Zhou, T.; Zhang, J.; Liu, X. Moving-window two-dimensional correlation infrared spectroscopy study on structural variations of partially hydrolyzed poly (vinyl alcohol). Anal. Bioanal. Chem. 2010, 397, 3127–3132. [Google Scholar] [CrossRef]
- Sadeghi, M. Synthesis of a biocopolymer carrageenan-g-poly (AAm-co-IA)/montmorilonite superabsorbent hydrogel composite. Braz. J. Chem. Eng. 2012, 29, 295–305. [Google Scholar] [CrossRef]
- Ublekov, F.; Baldrian, J.; Nedkov, E. Crystalline β-structure of PHBV grown epitaxially on silicate layers of MMT. J. Polym. Sci. B Polym. Phys. 2009, 47, 751–755. [Google Scholar] [CrossRef]
- Sindhu, R.; Binod, P.; Pandey, A. Microbial poly-3-hydroxybutyrate and related copolymers. In Industrial Biorefineries & White Biotechnology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 575–605. [Google Scholar]
- Haken, J.K.; Werner, R.L. The infrared spectrum of polyvinyl formate. Spectrochim. Acta A Mol. Biomol. 1971, 27, 343–351. [Google Scholar] [CrossRef]
- Sharma, P.; Borah, D.J.; Das, P.; Das, M.R. Cationic and anionic dye removal from aqueous solution using montmorillonite clay: Evaluation of adsorption parameters and mechanism. Desalin. Water Treat. 2016, 57, 8372–8388. [Google Scholar] [CrossRef]
- Reddy, C.R.; Nagendrappa, G.; Prakash, B.J. Surface acidity study of Mn+-montmorillonite clay catalysts by FT-IR spectroscopy: Correlation with esterification activity. Catal. Commun. 2007, 8, 241–246. [Google Scholar] [CrossRef]
- Selim, S.E.; Meligi, G.A.; Abdelhamid, A.E.; Mabrouk, M.A.; Hussain, A.I. Novel Composite Films Based on Acrylic Fibers Waste/Nano-chitosan for Congo Red Adsorption. J. Polym. Environ. 2022, 30, 2642–2657. [Google Scholar] [CrossRef]
- Ali, E.A.; Ismail, M.N.; Elsabee, M. Chitosan based polyelectrolyte complexes development for anionic and cationic dyes adsorption. Egypt. J. Chem. 2020, 63, 537–554. [Google Scholar] [CrossRef]
- Abdelhamid, A.E.; Kandil, H. Facile approach to synthesis super-adsorptive hydrogel based on hyperbranched polymer for water remediation from methylene blue. React. Funct. Polym. 2022, 177, 105312. [Google Scholar] [CrossRef]


| Composition (wt.%) | Electrospinning Parameters | |||||
|---|---|---|---|---|---|---|
| Sample Name | PHB | PVF | MMT | Flow Rate (mL·h−1) | Voltage (kV) | Distance to Collector (cm) |
| PVF | 0 | 100 | 0 | 0.2 | 20 | 15 |
| PVF/MMT 30% | 0 | 70 | 30 | 0.5 | 20 | 15 |
| PHB | 100 | 0 | 0 | 0.5 | 20 | 20 |
| PHB/MMT 10% | 90 | 0 | 10 | 0.7 | 20 | 20 |
| PHB/MMT 20% | 80 | 0 | 20 | 0.5 | 20 | 20 |
| PHB/PVF/MMT 10% | 63 | 27 | 10 | 1.5 | 20 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penchev, H.; Abdelhamid, A.E.; Ali, E.A.; Budurova, D.; Grancharov, G.; Ublekov, F.; Koseva, N.; Zaharieva, K.; El-Sayed, A.A.; Khalil, A.M. Novel Electrospun Composite Membranes Based on Polyhydroxybutyrate and Poly(vinyl formate) Loaded with Protonated Montmorillonite for Organic Dye Removal: Kinetic and Isotherm Studies. Membranes 2023, 13, 582. https://doi.org/10.3390/membranes13060582
Penchev H, Abdelhamid AE, Ali EA, Budurova D, Grancharov G, Ublekov F, Koseva N, Zaharieva K, El-Sayed AA, Khalil AM. Novel Electrospun Composite Membranes Based on Polyhydroxybutyrate and Poly(vinyl formate) Loaded with Protonated Montmorillonite for Organic Dye Removal: Kinetic and Isotherm Studies. Membranes. 2023; 13(6):582. https://doi.org/10.3390/membranes13060582
Chicago/Turabian StylePenchev, Hristo, Ahmed E. Abdelhamid, Eman A. Ali, Dessislava Budurova, Georgy Grancharov, Filip Ublekov, Neli Koseva, Katerina Zaharieva, Ahmed A. El-Sayed, and Ahmed M. Khalil. 2023. "Novel Electrospun Composite Membranes Based on Polyhydroxybutyrate and Poly(vinyl formate) Loaded with Protonated Montmorillonite for Organic Dye Removal: Kinetic and Isotherm Studies" Membranes 13, no. 6: 582. https://doi.org/10.3390/membranes13060582
APA StylePenchev, H., Abdelhamid, A. E., Ali, E. A., Budurova, D., Grancharov, G., Ublekov, F., Koseva, N., Zaharieva, K., El-Sayed, A. A., & Khalil, A. M. (2023). Novel Electrospun Composite Membranes Based on Polyhydroxybutyrate and Poly(vinyl formate) Loaded with Protonated Montmorillonite for Organic Dye Removal: Kinetic and Isotherm Studies. Membranes, 13(6), 582. https://doi.org/10.3390/membranes13060582









