Adsorption Performance of Heavy Metal Ions under Multifactorial Conditions by Synthesized Organic-Inorganic Hybrid Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hybrid Membranes
2.3. Membrane Characterization
2.4. Adsorption Experiment
3. Results and Discussion
3.1. Characterization of Hybrid Membranes
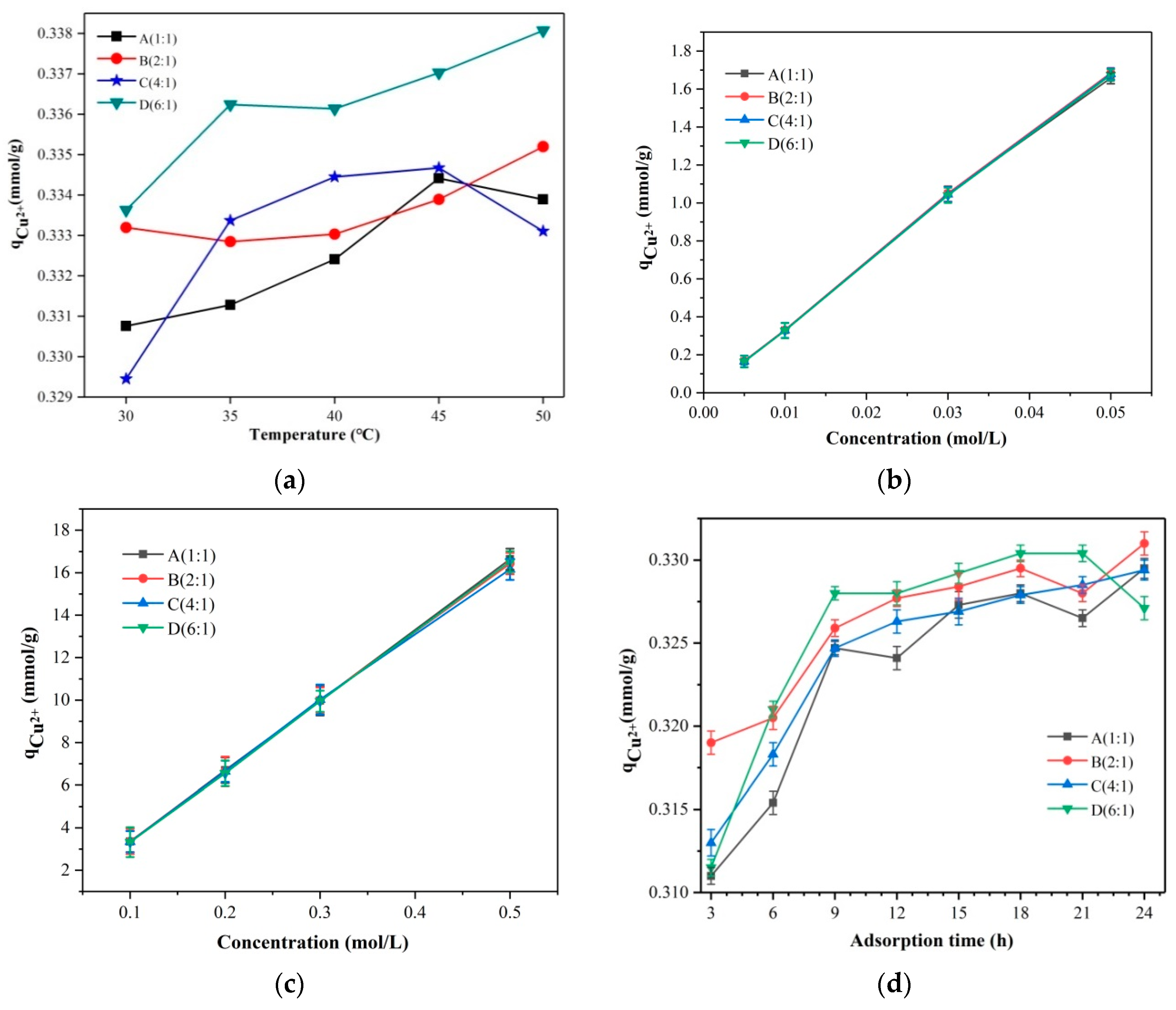
3.2. Absorption Properties of Cu2+ by Hybrid Membrane Materials
3.3. Absorption Properties of Pb2+ by Hybrid Membrane Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, L.W.; Wang, Z.B.; Li, J.X. The Effect of Urbanization on Environmental Pollution in Rapidly Developing Urban Agglomerations. J. Clean Prod. 2019, 237, 117649. [Google Scholar] [CrossRef]
- Li, K.; Fang, L.; He, L. How Population and Energy Price Affect China s Environmental Pollution? Energ. Policy 2019, 129, 386–396. [Google Scholar] [CrossRef]
- Liu, P.; Wang, J.; Sangaiah, A.K.; Xie, Y.; Yin, X.C. Analysis and Prediction of Water Quality Using LSTM Deep Neural Networks in IoT Environment. Sustainability 2019, 11, 2058. [Google Scholar] [CrossRef]
- Muhammad, Z.; Anum, R.; Saba, A. A Comprehensive Review On Polymeric Nano-composite Membranes for Water Treatment. J. Membr. Sci. Technol. 2018, 8, 1–20. [Google Scholar] [CrossRef]
- Teodor, S.; Andrei, S.; Simona, C.; Elena-Bianca, S.; Tanța-Verona, I.; Anita-Laura, C. Polymer Membranes as Innovative Means of Quality Restoring for Wastewater Bearing Heavy Metals. Membranes 2022, 12, 1179. [Google Scholar] [CrossRef]
- Caprarescu, S.; Modrogan, C.; Purcar, V.; Dancila, A.M.; Orbulet, O.D. Study of Polyvinyl Alcohol-SiO2 Nanoparticles Polymeric Membrane in Wastewater Treatment Containing Zinc Ions. Polymers 2021, 13, 1875. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.J.; Li, C.; Lai, H.; Du, Y.; Liu, H.W.; Liu, M.; Jin, L.G.; Zhang, C.G.; Zhang, N.Q.; Sun, K.N. A PH-responsive Superwetting Nanostructured Copper Mesh Membrane for Separating Both Water-in-oil and Oil-in-water Emulsions. RSC Adv. 2016, 6, 72317–72325. [Google Scholar] [CrossRef]
- Yuan, S.S.; Zhu, J.Y.; Li, J.; Volodine, A.; Yang, J.; Puyvelde, P.V.; Bruggen, B.V. Nano/microstructure Decorated Thin Membrane Composite Poly (arylene Sulfide Sulfone) Membrane Constructed By Induced Fouling in Organic Solvent Ultrafiltration. Chem. Eng. J. 2018, 348, 180–190. [Google Scholar] [CrossRef]
- Kim, D.; Park, M.S.; Choi, Y.; Lee, K.B.; Kim, J.H. Synthesis of PVA-g-POEM Graft Copolymers and Their Use in Highly Permeable Thin Membrane Composite Membranes. Chem. Eng. J. 2018, 346, 739–747. [Google Scholar] [CrossRef]
- Nichol, T.; Callaghan, J.; Townsend, R.; Stockley, I.; Hatton, P.V.; Le Maitre, C.; Smith, T.J.; Akid, R. The Antimicrobial Activity and Biocompatibility of a Controlled Gentamicin-Releasing Single-Layer Sol-Gel Coating on Hydroxyapatite-Coated Titanium. Bone Jt. J. 2021, 103-B, 522–529. [Google Scholar] [CrossRef]
- Zhang, L.K.; Gong, Y.J.; Wang, T.; Xiao, J.H.; Pang, Y.P.; Hu, Q.Z.; Yu, L. Morphological Transformation of Ultrasonically Obtained Nanofibers During Living Self-assembly. New J. Chem. 2020, 44, 10813–10818. [Google Scholar] [CrossRef]
- El-Sayed, A.; Mahdy, I.A.; Ibraheem, F.; Mahmoud, E.A.; Ortega, J.E.; Rogero, C. Harvesting Multiple Optical Energies Using ZnPc/CdS-QDs Hybrid Organic/inorganic Semiconductors. J. Mater. Sci-Mater. Electron. 2020, 31, 12735–12742. [Google Scholar] [CrossRef]
- Park, W.; Shin, H.; Choi, B.; Rhim, W.; Na, K.; Han, D.K. Advanced Hybrid Nanomaterials for Biomedical Applications. Prog. Mater. 2020, 114, 100686. [Google Scholar] [CrossRef]
- Goodman, E.D.; Zhou, C.S.; Cargnello, M. Design of Organic/Inorganic Hybrid Catalysts for Energy And Environmental Applications. ACS Cent. Sci. 2020, 6, 1916–1937. [Google Scholar] [CrossRef]
- Tsai, Y.S.; Tsai, S.C.; Kuo, C.C.; Chan, W.L.; Lin, W.H.; Wu, Y.C.S.; Lin, Y.S.; Li, M.H.; Kuo, M.Y.; Chen, H. Organic/inorganic Hybrid Nanostructures of Polycrystalline Perylene Diimide Decorated ZnO Nanorods Highly Enhanced Dual Sensing Performance of UV Light/CO Gas Sensors. Results Phys. 2021, 24, 104173. [Google Scholar] [CrossRef]
- Wei, X.F.; Li, W.Z.; Chen, H.F.; Lv, M.G.; Wang, H.W.; Qi, X.W. An Easy Preparation of BaTiO3/PAM Organic/Inorganic Hybrid Material Using Surface-Initiated Process. J. Phys. Conf. Ser. 2021, 2194, 012045. [Google Scholar] [CrossRef]
- Bachevillier, S.; Yuan, H.-K.; Tetzner, K.; Bradley, D.D.C.; Anthopoulos, T.D.; Stavrinou, P.N.; Stingelin, N. Planar Refractive Index Patterning through Microcontact Photo-Thermal Annealing of a Printable Organic/Inorganic Hybrid Material. Mater. Horiz. 2021, 9, 411–416. [Google Scholar] [CrossRef]
- Guo, H.L.; Li, L.; Wang, F.; Kim, S.W.; Sun, H.J. Mitigating The Negative Piezoelectricity in Organic/Inorganic Hybrid Materials for High-performance Piezoelectric Nanogenerators. ACS Appl. Mater. Interfaces 2022, 14, 34733–34741. [Google Scholar] [CrossRef]
- Schmidt, H. New Type of Non-Crystalline Solids Between Inorganic and Organic Materials. J. Non-Cryst. Solids 1985, 73, 681–691. [Google Scholar] [CrossRef]
- Huang, H.H.; Orler, B.; Wilkes, G.L. Structure-Property Behavior of New Hybrid Materials Incorporating Oligomeric Species into Sol-Gel Glasses. 3. Effect of Acid Content, Tetraethoxysilane Content, and Molecular Weight of Poly(dimethylsiloxane). Macromolecules 1987, 20, 1322–1330. [Google Scholar] [CrossRef]
- Baxamusa, S.H.; Im, S.G.; Gleason, K.K. Initiated And Oxidative Chemical Vapor Deposition: A Scalable Method for Conformal and Functional Polymer Membranes on Real Substrates. Phys. Chem. Chem. Phys. 2009, 11, 5227–5240. [Google Scholar] [CrossRef] [PubMed]
- Moein, M.M.; Abdel-Rehim, A.; Abdel-Rehim, M. Recent Applications of Molecularly Imprinted Sol-Gel Methodology in Sample Preparation. Molecules 2019, 24, 2889. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.Q.; Li, D.N.; Li, J.H.; Wang, C.H. Structure and Piezoelectricity Properties of V-Doped ZnO Thin Membranes Fabricated By Sol-gel Method. J. Alloys Compd. 2020, 829, 154483. [Google Scholar] [CrossRef]
- Sarjono; Riyanto, S.; Mutiara, E.; Yusnitha, E.; Yulianto, T.; Langenati, R.; Sumaryanto, A. R&D on Surrogate Kernel Fabrication in Support of Reaktor Daya Eksperimental (RDE) Project. J. Phys. Conf. Ser. 2021, 2048, 012011. [Google Scholar] [CrossRef]
- Khiterer, M.; Loy, D.A.; Cornelius, C.J.; Fujimoto, C.H.; Small, J.H.; McIntire, T.M.; Shea, K.J. Hybrid Polyelectrolyte Materials for Fuel Cell Applications: Design, Synthesis, and Evaluation of Proton-Conducting Bridged Polysilsesquioxanes. Chem. Mater. 2006, 18, 3665–3673. [Google Scholar] [CrossRef]
- Liu, J.S.; Xu, T.W.; Gong, M.; Yu, F.; Fu, Y.X. Fundamental Studies of Novel Inorganic-organic Charged Zwitterionic Hybrids 4. New Hybrid Zwitterionic Membranes Prepared From Polyethylene Glycol (PEG) And Silane Coupling Agent. J. Membr. Sci. 2006, 283, 190–200. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zheng, Z.; Wang, J.Q.; Wang, Y.F.; Xu, B.W.; Zhang, S.Q.; Hou, J.H. Fluidic Manipulating of Printable Zinc Oxide for Flexible Organic Solar Cells. Adv. Mater. 2022, 34, 2106453. [Google Scholar] [CrossRef]
- Nagarale, R.K.; Gohil, G.S.; Shahi, V.K.; Rangarajan, R. Organic−Inorganic Hybrid Membrane: Thermally Stable Cation-Exchange Membrane Prepared by the Sol−Gel Method. Macromolecules 2004, 37, 10023–10030. [Google Scholar] [CrossRef]
- Pogorelov, S.N.; Semenyak, G.S.; Kolmogorova, A. Sol-Gel Technology for the Production of High-Strength Refractory Materials Based on Binders. IOP Conf. Ser. Mater. Sci. Eng. 2020, 962, 022024. [Google Scholar] [CrossRef]
- Periyasamy, A.P.; Venkataraman, M.; Kremenakova, D.; Militky, J.; Zhou, Y. Progress in Sol-Gel Technology for the Coatings of Fabrics. Materials 2020, 13, 1838. [Google Scholar] [CrossRef]
- Nair, P.A.K.; Vasconcelos, W.L.; Paine, K.; Calabria-Holley, J. A review on applications of sol-gel science in cement. Constr. Build. Mater. 2021, 291, 123065. [Google Scholar] [CrossRef]
- Dong, Q.; Liu, J.S.; Song, L.; Shao, G.Q. Novel Zwitterionic Inorganic-Organic Hybrids: Synthesis of Hybrid Adsorbents and Their Applications for Cu2+ Removal. J. Haz. Mat. 2011, 186, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, W.X.; Liu, J.S.; Wu, L.L. Removal of Lead(II) from Aqueous Solution by Adsorption Using Zwitterionic Hy-brid Copolymers. Asian J. Chem. 2013, 25, 6575–6578. [Google Scholar] [CrossRef]
- Liu, J.S.; Wang, X. Novel Silica-Based Hybrid Adsorbents: Lead(II) Adsorption Isotherms. Sci. World J. 2013, 2013, 897159. [Google Scholar] [CrossRef]
- Liu, J.S.; Li, T.; Hu, K.Y.; Shao, G.Q. Preparation and Adsorption Performances of Novel Negatively Charged Hybrid Materials. J. Appl. Polym. Sci. 2009, 112, 2179–2184. [Google Scholar] [CrossRef]
- Liu, J.S.; Si, J.Y.; Zhang, Q.; Zheng, J.H.; Han, C.L.; Shao, G.Q. Preparation of Negatively Charged Hybrid Adsorbents and Their Applications for Pb2+ Removal. Ind. Eng. Chem. Res. 2011, 50, 8645–8657. [Google Scholar] [CrossRef]








| Sample | Ratio (x:y) | Amount of WD-60 (g) | Amount of PEG-6000 (g) | DMF Volume (mL) | Amount of Maleic Anhydride (g) |
|---|---|---|---|---|---|
| A | 1:1 | 2.36 | 6.00 | 20 | 1.00 |
| B | 2:1 | 4.72 | 6.00 | 20 | 1.00 |
| C | 4:1 | 9.44 | 6.00 | 20 | 1.00 |
| D | 6:1 | 14.14 | 6.00 | 20 | 1.00 |
| Sorbent Type | qCu2+ (mmol/g) | qPb2+ (mmol/g) | Literature |
|---|---|---|---|
| zwitterionic hybrid copolymers | 0.075 | 0.244 | [32,33] |
| silica-based hybrid adsorbents | / | 0.506 | [34] |
| pyromellitic acid dianhydride (PMDA) hybrid adsorbents | / | 6.379 | [36] |
| negatively charged siloxane hybrid membrane | 0.331 | 5.012 | this work |
| Samples | Adsorption Rate (%) | Standard Deviation (%) |
|---|---|---|
| A | 98.5 | 0.08 |
| B | 98.5 | 0.05 |
| C | 98.6 | 0.06 |
| D | 99.3 | 0.05 |
| Samples | Adsorption Rate (%) | Standard Deviation (%) |
|---|---|---|
| A | 88.5 | 0.3 |
| B | 91.1 | 0.2 |
| C | 91.3 | 0.3 |
| D | 93.0 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Zheng, J.; Han, L. Adsorption Performance of Heavy Metal Ions under Multifactorial Conditions by Synthesized Organic-Inorganic Hybrid Membranes. Membranes 2023, 13, 531. https://doi.org/10.3390/membranes13050531
Wu C, Zheng J, Han L. Adsorption Performance of Heavy Metal Ions under Multifactorial Conditions by Synthesized Organic-Inorganic Hybrid Membranes. Membranes. 2023; 13(5):531. https://doi.org/10.3390/membranes13050531
Chicago/Turabian StyleWu, Chaoqun, Jiuhan Zheng, and Limei Han. 2023. "Adsorption Performance of Heavy Metal Ions under Multifactorial Conditions by Synthesized Organic-Inorganic Hybrid Membranes" Membranes 13, no. 5: 531. https://doi.org/10.3390/membranes13050531
APA StyleWu, C., Zheng, J., & Han, L. (2023). Adsorption Performance of Heavy Metal Ions under Multifactorial Conditions by Synthesized Organic-Inorganic Hybrid Membranes. Membranes, 13(5), 531. https://doi.org/10.3390/membranes13050531









