Decoration of β-Cyclodextrin and Tuning Active Layer Chemistry Leading to Nanofiltration Membranes for Desalination and Wastewater Decontamination
Abstract
1. Introduction
2. Materials and Methods
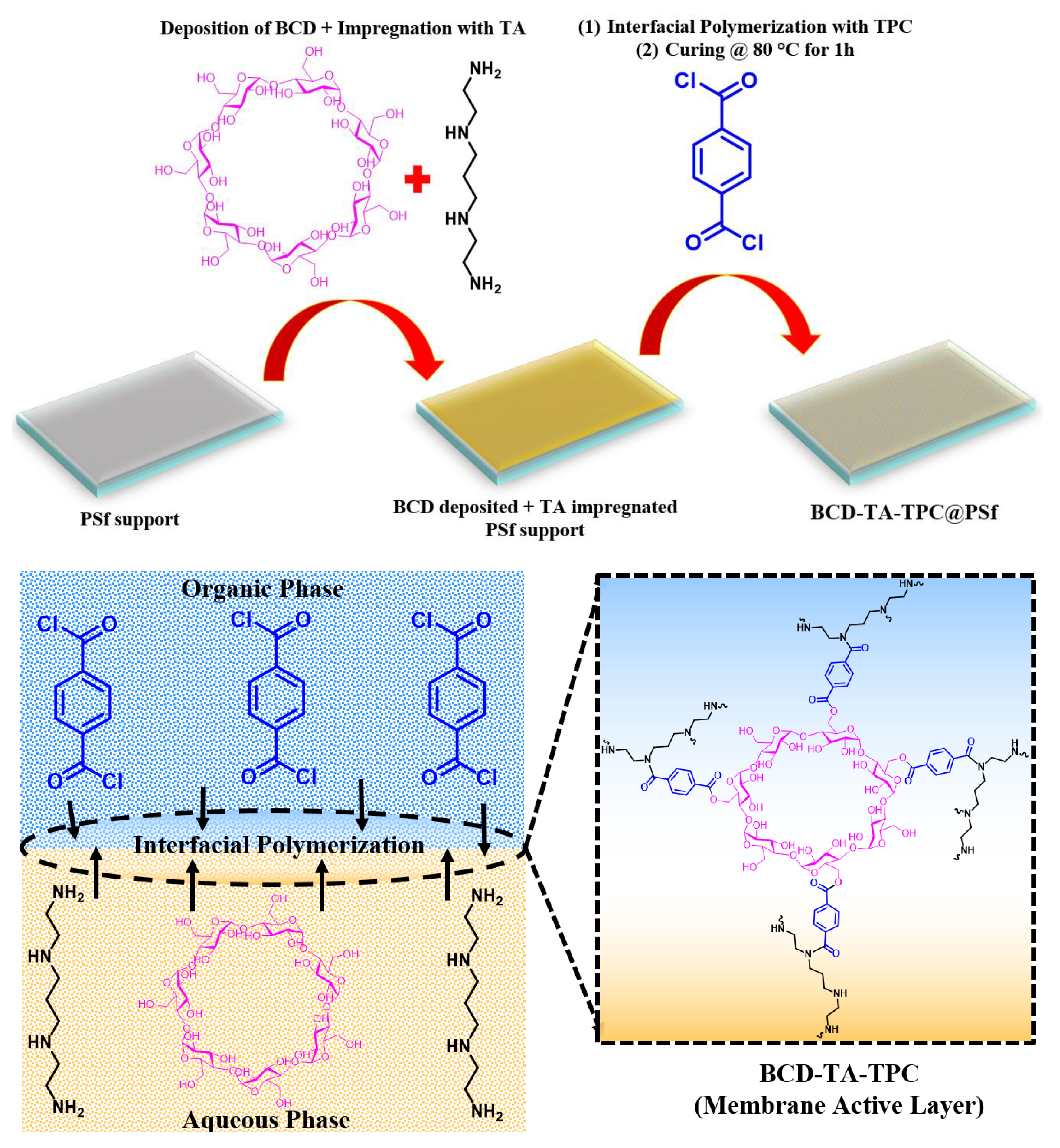
Membrane Fabrication
3. Results and Discussion
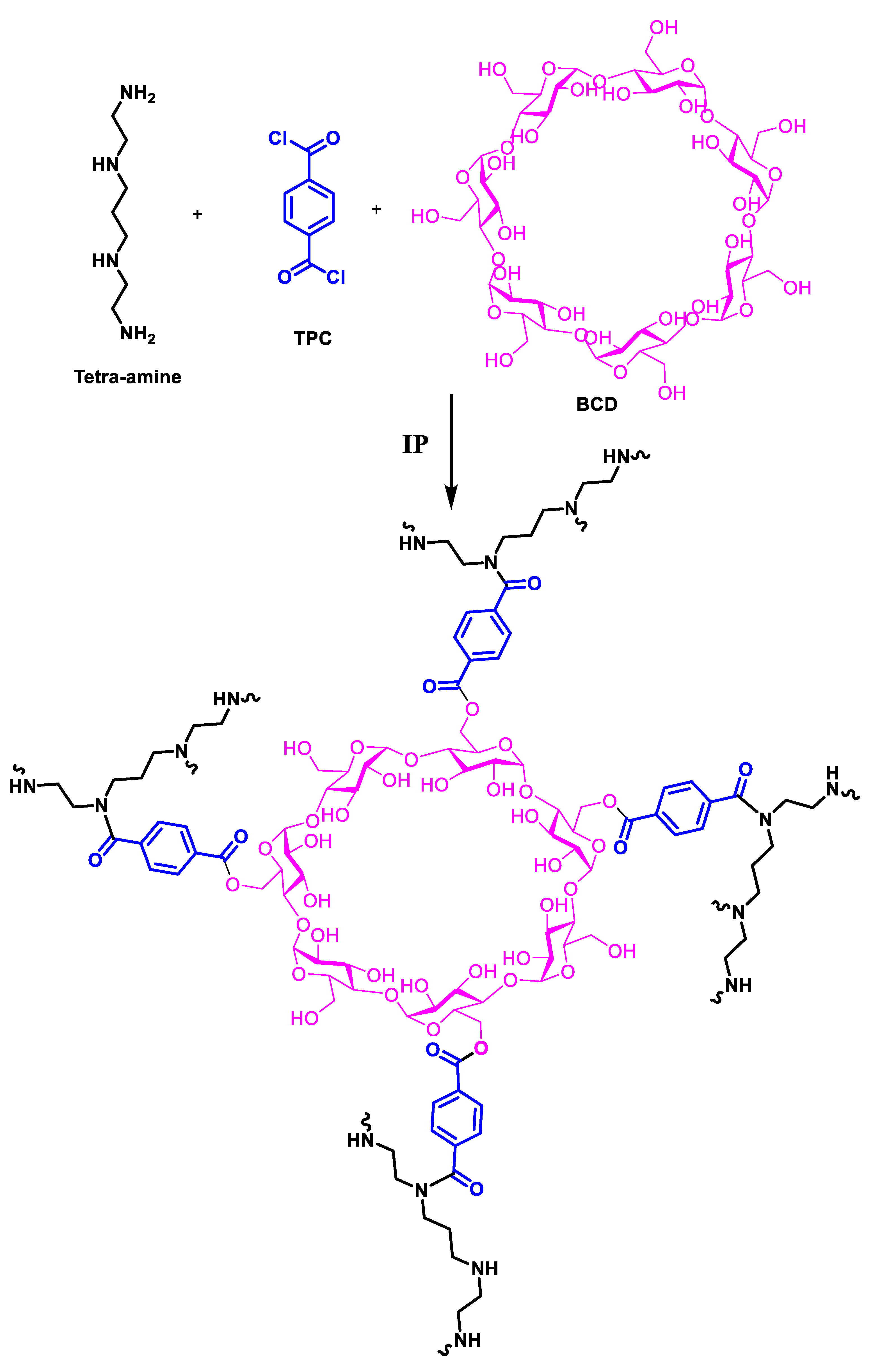
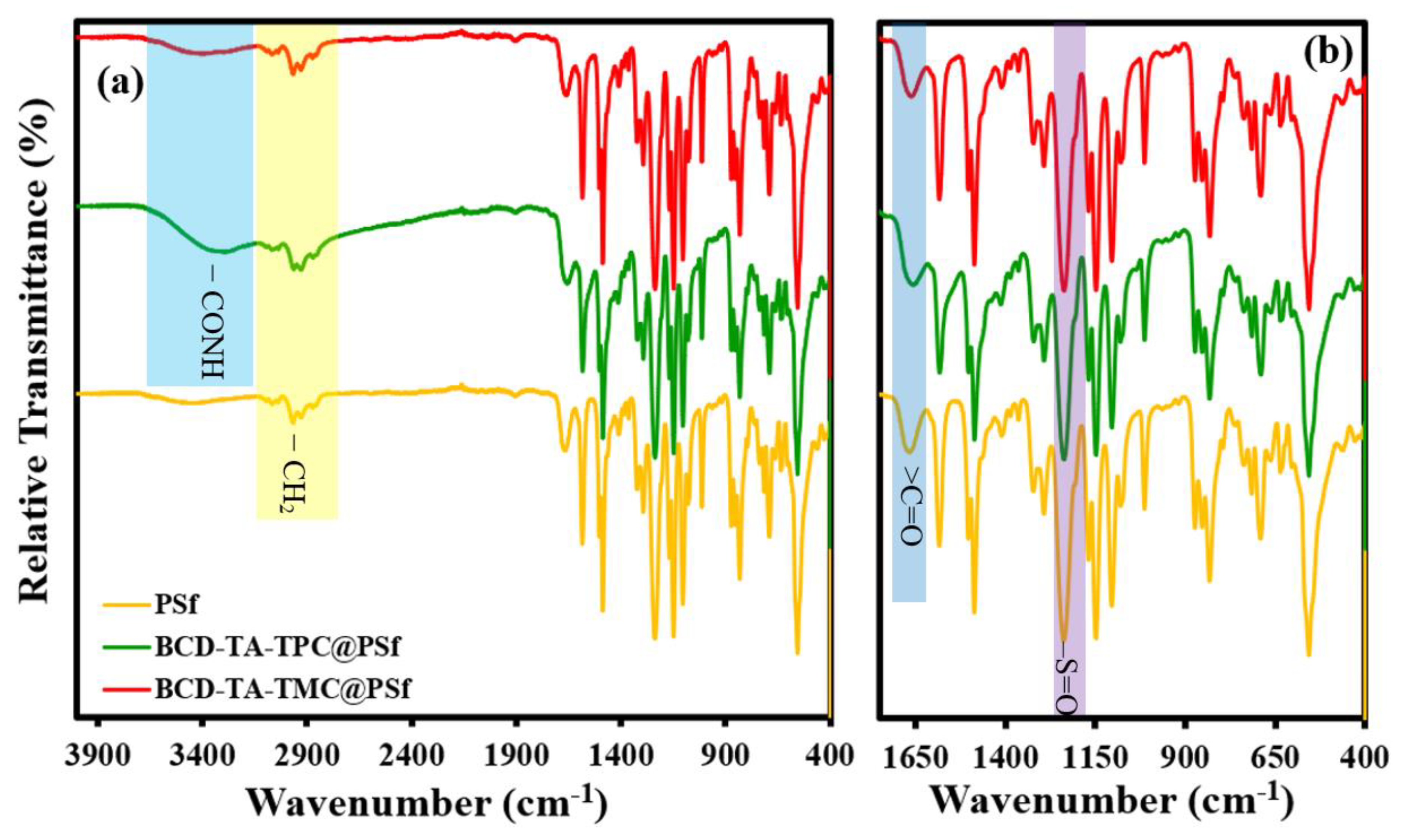
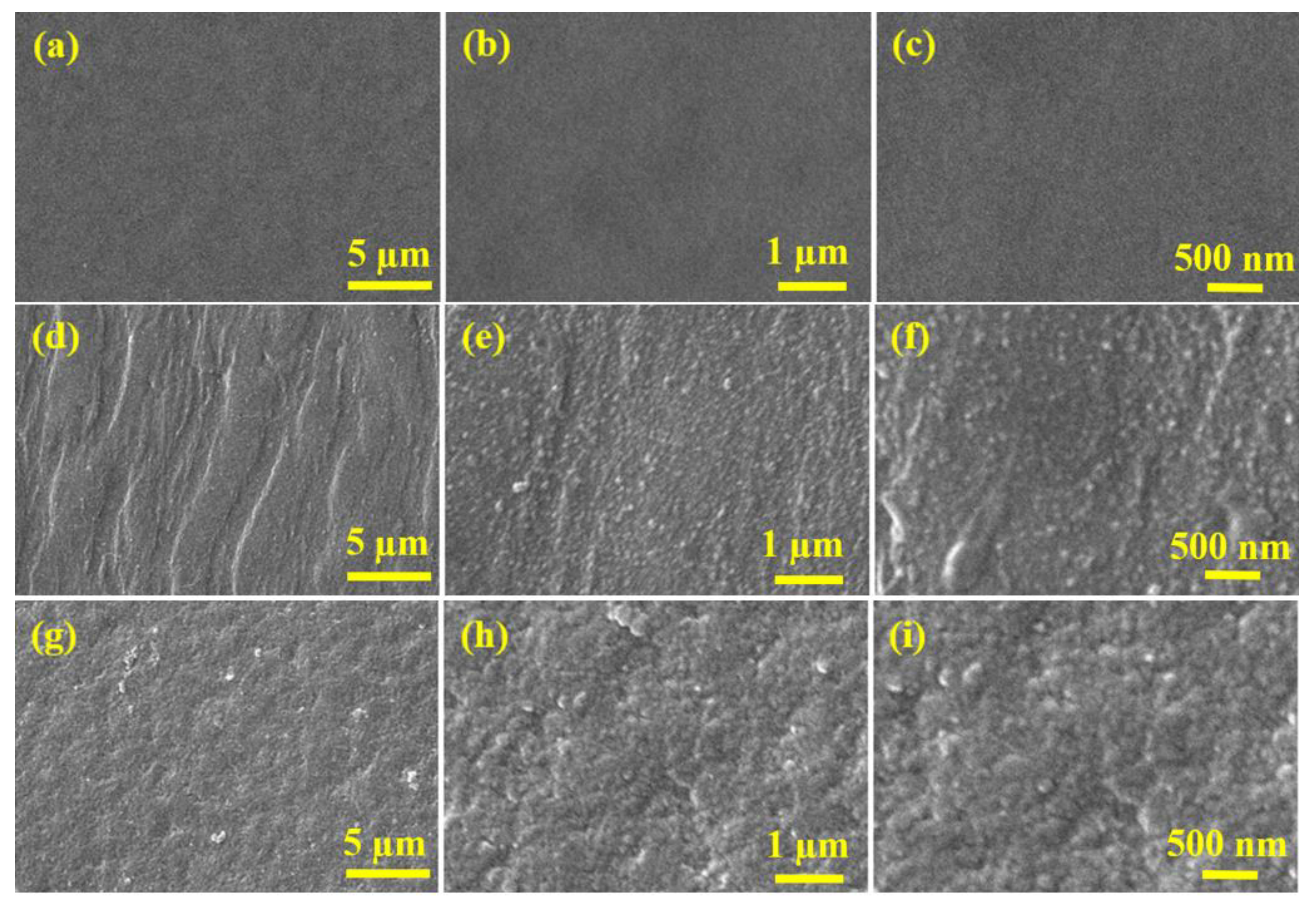
3.1. Membrane Fabrication and Characterization
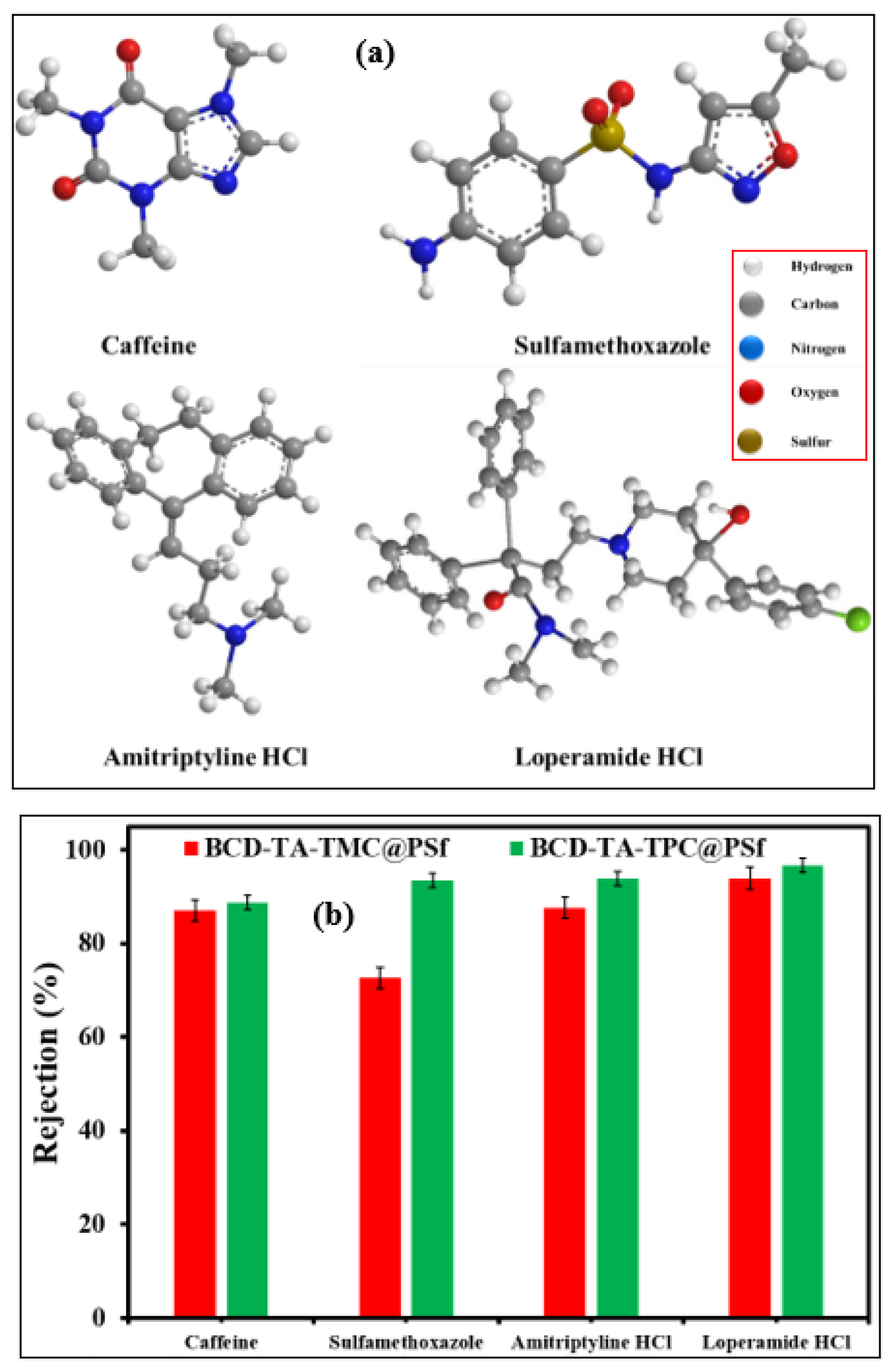
3.2. Nanofiltration Performance of Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McGinnis, R.L.; Elimelech, M. Global Challenges in Energy and Water Supply: The Promise of Engineered Osmosis. Environ. Sci. Technol. 2008, 42, 8625–8629. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Liu, J.; Zhang, X.; Chen, L.; Zhang, Q.; Ma, L. Recent advances in membrane-based materials for desalination and gas separation. J. Clean. Prod. 2023, 387, 135845. [Google Scholar] [CrossRef]
- Sathyanath, R.; Aarthi, A.; Kalpathy, S.K. Features of colloidal silica deposits dip coated onto porous alumina membranes from aqueous suspensions. Colloid Interface Sci. Commun. 2021, 45, 100526. [Google Scholar] [CrossRef]
- Kamali, M.; Suhas, D.P.; Costa, M.E.; Capela, I.; Aminabhavi, T.M. Sustainability considerations in membrane-based technologies for industrial effluents treatment. Chem. Eng. J. 2019, 368, 474–494. [Google Scholar] [CrossRef]
- Bo, Z.; Huang, Z.; Xu, C.; Chen, Y.; Wu, E.; Yan, J.; Cen, K.; Yang, H.; Ostrikov, K. Anion-kinetics-selective graphene anode and cation-energy-selective MXene cathode for high-performance capacitive deionization. Energy Storage Mater. 2022, 50, 395–406. [Google Scholar] [CrossRef]
- Bao, W.; Tang, X.; Guo, X.; Choi, S.; Wang, C.; Gogotsi, Y.; Wang, G. Porous Cryo-Dried MXene for Efficient Capacitive Deionization. Joule 2018, 2, 778–787. [Google Scholar] [CrossRef]
- Waheed, A.; Baig, U.; Jillani, S.M.S. Optimization of amine functionalization of MCM-41 for its covalent decoration in nanofiltration membranes for purification of saline- and micropollutant-contaminated feeds. Environ. Sci. Water Res. Technol. 2023, 9, 1371–1384. [Google Scholar] [CrossRef]
- Pham, M.-X.; Le, T.M.; Tran, T.T.; Ha, H.K.P.; Phong, M.T.; Nguyen, V.-H.; Tran, L.-H. Fabrication and characterization of polyamide thin-film composite membrane via interfacial polycondensation for pervaporation separation of salt and arsenic from water. RSC Adv. 2021, 11, 39657–39665. [Google Scholar] [CrossRef]
- Waheed, A.; Baig, U.; Matin, A.; Jillani, S.M.S.; Qasem, N.A.A.; Aljundi, I.H. Synthesis of co-polyamide reverse osmosis membrane constituting a linear aliphatic triamine and m-phenylenediamine for enhanced desalination performance. Desalination 2023, 549, 116311. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, T.; Dai, Z.; Su, F.; Xia, X.; Dong, P.; Zhang, J. A novel positively charged nanofiltration membrane stimulated by amino-functionalized MXene Ti3C2T for high rejection of water hardness ions. J. Membr. Sci. 2023, 671, 121385. [Google Scholar] [CrossRef]
- Hao, Y.; Li, Q.; He, B.; Liao, B.; Li, X.; Hu, M.; Ji, Y.; Cui, Z.; Younas, M.; Li, J. An ultrahighly permeable-selective nanofiltration membrane mediated by an in situ formed interlayer. J. Mater. Chem. A 2020, 8, 5275–5283. [Google Scholar] [CrossRef]
- Kang, Y.; Jang, J.; Kim, S.; Lim, J.; Lee, Y.; Kim, I.S. PIP/TMC Interfacial Polymerization with Electrospray: Novel Loose Nanofiltration Membrane for Dye Wastewater Treatment. ACS Appl. Mater. Interfaces 2020, 12, 36148–36158. [Google Scholar] [CrossRef]
- Yuan, M.; Wang, J.; Li, Y.; Zhao, M.; Li, Y.-Q.; Li, W.; Qu, Y. Metal-organic frameworks for high performance desalination through thickness control and structural fine-tuning. Water Res. 2023, 230, 119576. [Google Scholar] [CrossRef]
- Guan, M.; Yang, D.; Li, Q.; Zhang, H.; Xu, J.; Cai, M.; Lin, W.; Ma, S.; Liu, Q. Desalination behavior of composite membrane with petal shaped pore—Formed by superimposition of covalent organic framework with large aperture difference. Appl. Surf. Sci. 2023, 616, 156441. [Google Scholar] [CrossRef]
- Jillani, S.M.S.; Baig, U.; Waheed, A.; Ansari, M.A. NH2-CuO-MCM-41 covalently cross-linked multipurpose membrane for applications in water treatment: Removal of hazardous pollutants from water, water desalination and anti-biofouling performance. Chemosphere 2022, 307, 135592. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Kim, I.S.; Dai, S.; Sarwar, M.N.; Yang, X. Heterogeneous Ag@ZnO nanorods decorated on polyacrylonitrile fiber membrane for enhancing the photocatalytic and antibacterial properties. Colloid Interface Sci. Commun. 2021, 45, 100543. [Google Scholar] [CrossRef]
- Kar, S.; Bindal, R.C.; Tewari, P.K. Carbon nanotube membranes for desalination and water purification: Challenges and opportunities. Nano Today 2012, 7, 385–389. [Google Scholar] [CrossRef]
- Zhu, B.; Myat, D.T.; Shin, J.-W.; Na, Y.-H.; Moon, I.-S.; Connor, G.; Maeda, S.; Morris, G.; Gray, S.; Duke, M. Application of robust MFI-type zeolite membrane for desalination of saline wastewater. J. Memb. Sci. 2015, 475, 167–174. [Google Scholar] [CrossRef]
- You, Y.; Sahajwalla, V.; Yoshimura, M.; Joshi, R.K. Graphene and graphene oxide for desalination. Nanoscale 2016, 8, 117–119. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Ooi, B.S.; Mohammad, A.W.; Choudhury, J.P. Development of a highly hydrophilic nanofiltration membrane for desalination and water treatment. Desalination 2004, 168, 215–221. [Google Scholar] [CrossRef]
- Ahmad, N.N.R.; Mohammad, A.W.; Mahmoudi, E.; Ang, W.L.; Leo, C.P.; Teow, Y.H. An Overview of the Modification Strategies in Developing Antifouling Nanofiltration Membranes. Membranes 2022, 12, 1276. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Li, T.; Ren, X.-K.; Wang, J.; Wang, Z.; Zhao, S. Nanofiltration membrane with crown ether as exclusive Li+ transport channels achieving efficient extraction of lithium from salt lake brine. Chem. Eng. J. 2022, 438, 135658. [Google Scholar] [CrossRef]
- Huang, T.; Puspasari, T.; Nunes, S.P.; Peinemann, K. Ultrathin 2D-Layered Cyclodextrin Membranes for High- Performance Organic Solvent Nanofiltration. Adv. Funct. Mater. 2020, 30, 1906797. [Google Scholar] [CrossRef]
- Chung, T.-S.; Lai, J.-Y. The potential of calixarenes for membrane separation. Chem. Eng. Res. Des. 2022, 183, 538–545. [Google Scholar] [CrossRef]
- Tang, M.-J.; Liu, M.-L.; Wang, D.-A.; Shao, D.-D.; Wang, H.-J.; Cui, Z.; Cao, X.-L.; Sun, S.-P. Precisely Patterned Nanostrand Surface of Cucurbituril[n]-Based Nanofiltration Membranes for Effective Alcohol–Water Condensation. Nano Lett. 2020, 20, 2717–2723. [Google Scholar] [CrossRef]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Juvancz, Z.; Szejtli, J. The role of cyclodextrins in chiral selective chromatography. TrAC Trends Anal. Chem. 2002, 21, 379–388. [Google Scholar] [CrossRef]
- Crini, G. Review: A History of Cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef] [PubMed]
- van de Manakker, F.; Vermonden, T.; van Nostrum, C.F.; Hennink, W.E. Cyclodextrin-Based Polymeric Materials: Synthesis, Properties, and Pharmaceutical/Biomedical Applications. Biomacromolecules 2009, 10, 3157–3175. [Google Scholar] [CrossRef]
- Alsbaiee, A.; Smith, B.J.; Xiao, L.; Ling, Y.; Helbling, D.E.; Dichtel, W.R. Rapid removal of organic micropollutants from water by a porous β-cyclodextrin polymer. Nature 2016, 529, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Hemine, K.; Łukasik, N.; Gazda, M.; Nowak, I. β-cyclodextrin-containing polymer based on renewable cellulose resources for effective removal of ionic and non-ionic toxic organic pollutants from water. J. Hazard. Mater. 2021, 418, 126286. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, M.; Fei, Z.; Li, J.; Ren, Z.; Hou, Y. Synergistic regulation of macrocyclic polyamine-based polyamide nanofiltration membranes by the interlayer and surfactant for divalent ions rejection and mono-/di-ions sieving. Desalination 2022, 544, 116131. [Google Scholar] [CrossRef]
- Jimenez-Solomon, M.F.; Song, Q.; Jelfs, K.E.; Munoz-Ibanez, M.; Livingston, A.G. Polymer nanofilms with enhanced microporosity by interfacial polymerization. Nat. Mater. 2016, 15, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Tian, L.; Hou, Y.; Niu, Q.J. Nanofiltration membranes with enhanced microporosity and inner-pore interconnectivity for water treatment: Excellent balance between permeability and selectivity. J. Memb. Sci. 2019, 586, 192–201. [Google Scholar] [CrossRef]
- Wang, K.; Fu, W.; Wang, X.; Xu, C.; Gao, Y.; Liu, Y.; Zhang, X.; Huang, X. Molecular Design of the Polyamide Layer Structure of Nanofiltration Membranes by Sacrificing Hydrolyzable Groups toward Enhanced Separation Performance. Environ. Sci. Technol. 2022, 56, 17955–17964. [Google Scholar] [CrossRef] [PubMed]
- Waheed, A.; Baig, U.; Aljundi, I.H. Fabrication of molecularly porous hyper-cross-linked thin film composite nanofiltration membrane using cyclic amine and linear cross-linker for highly selective organic solvent nanofiltration. Colloid Interface Sci. Commun. 2021, 45, 100530. [Google Scholar] [CrossRef]
- Barger, J.P.; Dillon, P.F. Near-membrane electric field calcium ion dehydration. Cell Calcium 2016, 60, 415–422. [Google Scholar] [CrossRef]
- Wang, L.; Cao, T.; Dykstra, J.E.; Porada, S.; Biesheuvel, P.M.; Elimelech, M. Salt and Water Transport in Reverse Osmosis Membranes: Beyond the Solution-Diffusion Model. Environ. Sci. Technol. 2021, 55, 16665–16675. [Google Scholar] [CrossRef]
- Radjenović, J.; Petrović, M.; Ventura, F.; Barceló, D. Rejection of pharmaceuticals in nanofiltration and reverse osmosis membrane drinking water treatment. Water Res. 2008, 42, 3601–3610. [Google Scholar] [CrossRef] [PubMed]
- Koyuncu, I.; Topacik, D. Effects of operating conditions on the salt rejection of nanofiltration membranes in reactive dye/salt mixtures. Sep. Purif. Technol. 2003, 33, 283–294. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Yang, H.; Xie, Y.F.; Huang, X. Preparation of nanofiltration membranes for high rejection of organic micropollutants and low rejection of divalent cations. J. Memb. Sci. 2019, 572, 152–160. [Google Scholar] [CrossRef]
- Lai, G.S.; Lau, W.J.; Goh, P.S.; Ismail, A.F.; Yusof, N.; Tan, Y.H. Graphene oxide incorporated thin film nanocomposite nanofiltration membrane for enhanced salt removal performance. Desalination 2016, 387, 14–24. [Google Scholar] [CrossRef]
- Dong, L.; Huang, X.; Wang, Z.; Yang, Z.; Wang, X.; Tang, C.Y. A thin-film nanocomposite nanofiltration membrane prepared on a support with in situ embedded zeolite nanoparticles. Sep. Purif. Technol. 2016, 166, 230–239. [Google Scholar] [CrossRef]
- Maheswari, P.; Prasannadevi, D.; Mohan, D. Preparation and performance of silver nanoparticle incorporated polyetherethersulfone nanofiltration membranes. High Perform. Polym. 2013, 25, 174–187. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Wang, X.; Wen, X.; Huang, X.; Xie, Y.F. Effect of varying piperazine concentration and post-modification on prepared nanofiltration membranes in selectively rejecting organic micropollutants and salts. J. Memb. Sci. 2019, 582, 274–283. [Google Scholar] [CrossRef]


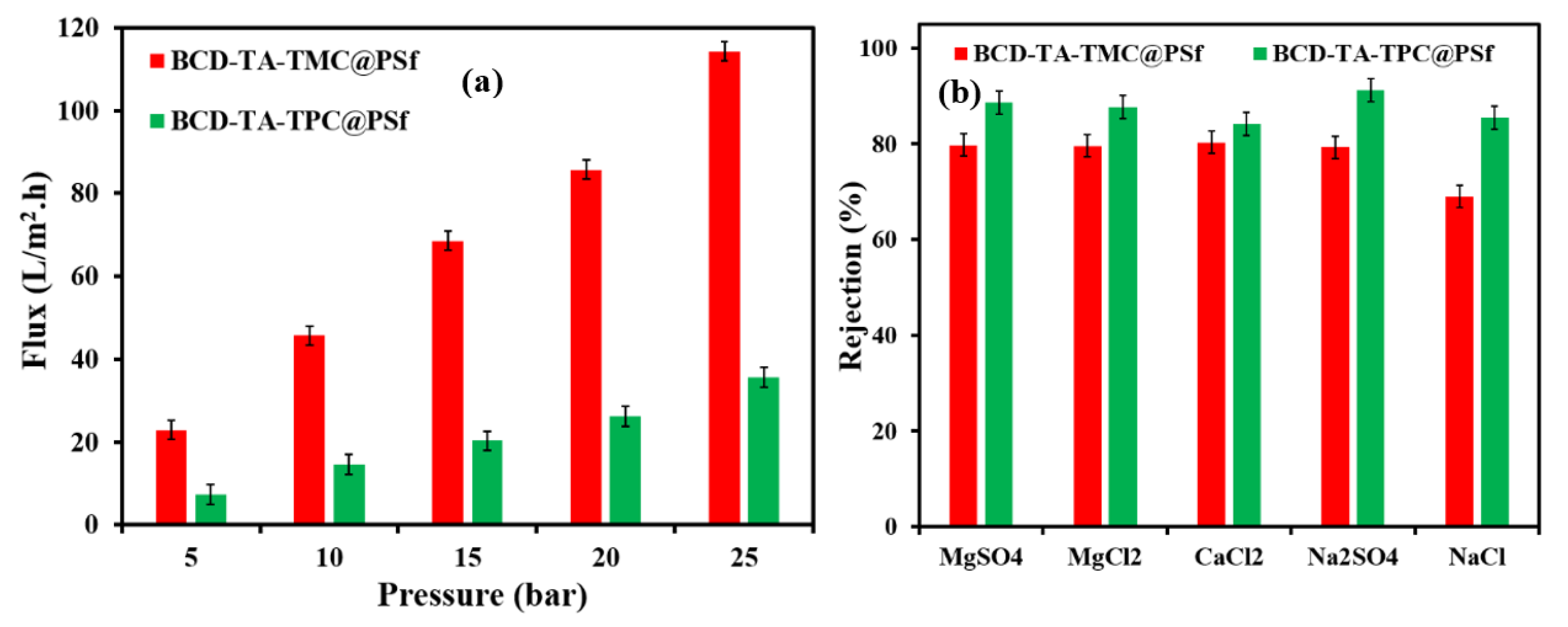
| Feeds | Membranes | |
|---|---|---|
| BCD-TA-TMC@PSf | BCD-TA-TPC@PSf | |
| Salts | Salt Rejections (%) | Salt Rejections (%) |
| Na2SO4 | 80 | 93 |
| MgSO4 | 80 | 92 |
| MgCl2 | 80 | 91 |
| CaCl2 | 80 | 84 |
| Pharmaceuticals | Pharmaceuticals Rejection (%) | Pharmaceuticals Rejection (%) |
| Loperamide | 92 | 94 |
| Amitryptiline HCl | 85 | 92 |
| Sulfamethoxazole | 70 | 90 |
| Caffeine | 86 | 88 |
| Water | Permeate Flux (LMH) | Permeate Flux (LMH) |
| Pure water | 115 | 36 |
| Sr. No. | Membrane Structure | Salt Rejection | Pharmaceutical Rejection | Ref. |
|---|---|---|---|---|
| 1 | Commercial BW30LE-440 and nf90-400 | - | 85% | [39] |
| 2 | Commercial DS5 DK membrane | 17–65% | - | [40] |
| 3 | 3,5-diamino benzoic acid /PA@PS | 25.8–60% | 80% | [41] |
| 4 | PA@Graphene oxide/PS | 60–95.2% | - | [42] |
| 5 | PA@zeolites/PS | 27.7–93.6% | 90% | [43] |
| 6 | PEES/Ag-NP | 31.3–79.4% | - | [44] |
| 7 | Diethanolamine/ monoethanolamine/ PA@PS | 33.1% | 90.8% | [45] |
| 8 | BCD-TA-TPC@PSf | 93% | 94% | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baig, U.; Jillani, S.M.S.; Waheed, A. Decoration of β-Cyclodextrin and Tuning Active Layer Chemistry Leading to Nanofiltration Membranes for Desalination and Wastewater Decontamination. Membranes 2023, 13, 528. https://doi.org/10.3390/membranes13050528
Baig U, Jillani SMS, Waheed A. Decoration of β-Cyclodextrin and Tuning Active Layer Chemistry Leading to Nanofiltration Membranes for Desalination and Wastewater Decontamination. Membranes. 2023; 13(5):528. https://doi.org/10.3390/membranes13050528
Chicago/Turabian StyleBaig, Umair, Shehzada Muhammad Sajid Jillani, and Abdul Waheed. 2023. "Decoration of β-Cyclodextrin and Tuning Active Layer Chemistry Leading to Nanofiltration Membranes for Desalination and Wastewater Decontamination" Membranes 13, no. 5: 528. https://doi.org/10.3390/membranes13050528
APA StyleBaig, U., Jillani, S. M. S., & Waheed, A. (2023). Decoration of β-Cyclodextrin and Tuning Active Layer Chemistry Leading to Nanofiltration Membranes for Desalination and Wastewater Decontamination. Membranes, 13(5), 528. https://doi.org/10.3390/membranes13050528






