Development of High-Performance Hydrogen-Air Fuel Cell with Flourine-Free Sulfonated Co-Polynaphthoyleneimide Membrane †
Abstract
1. Introduction
2. Materials and Methods
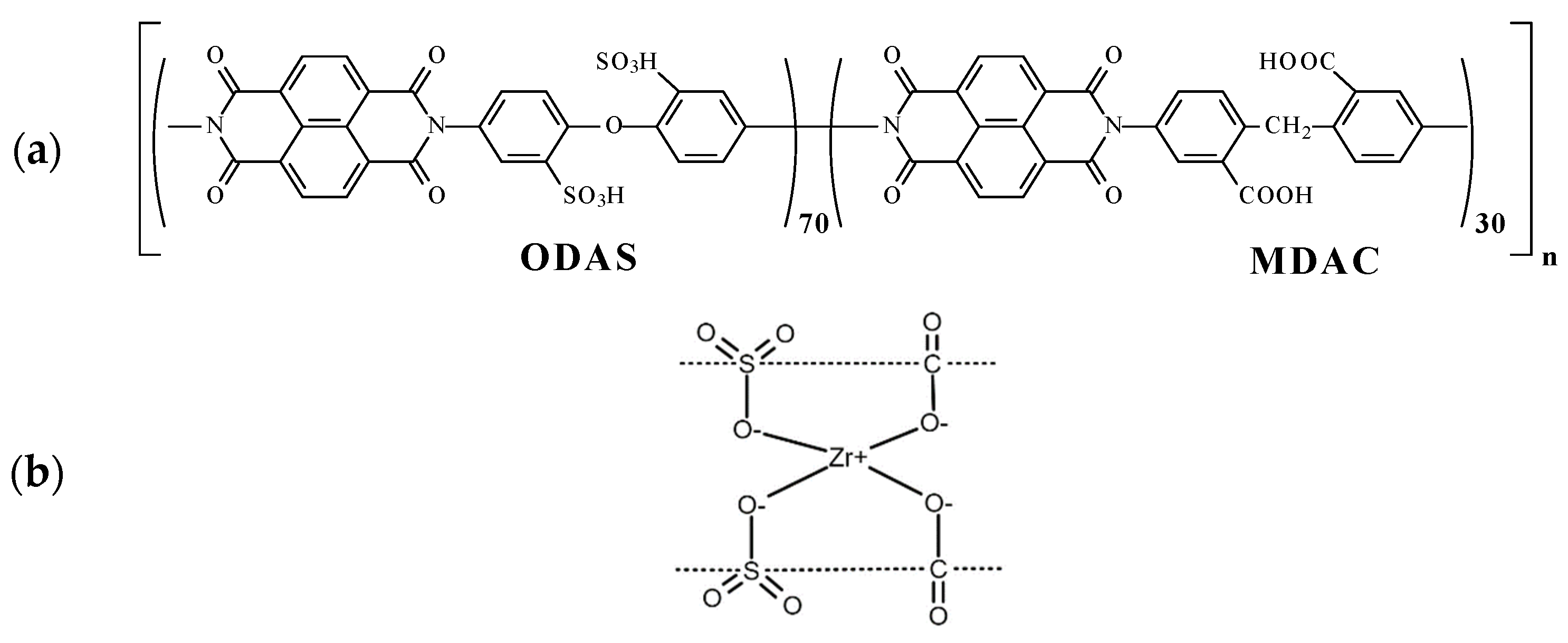
2.1. Co-PNIS with ODAS/MDAC = 70/30 Synthesis
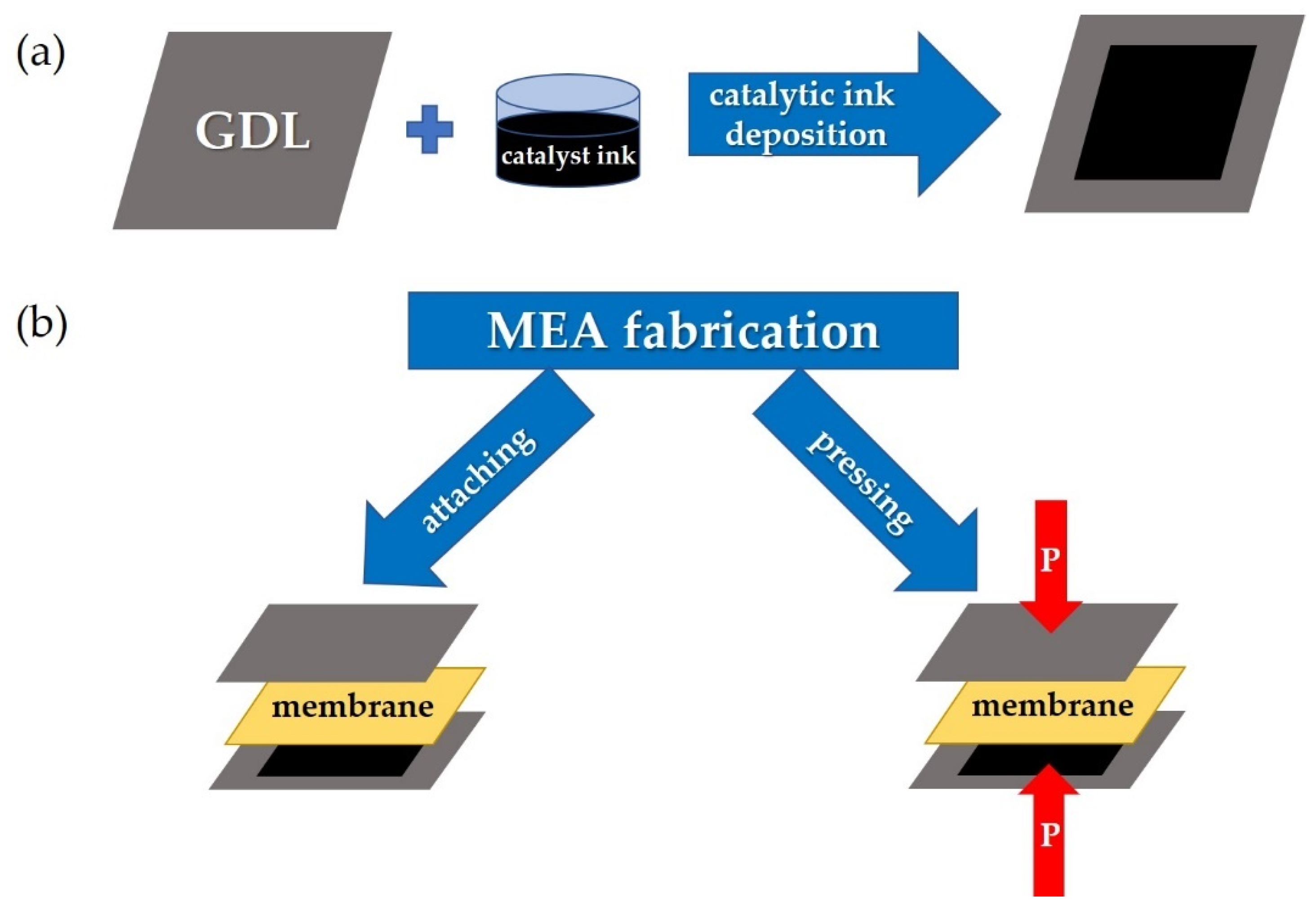
2.2. Fabrication of Membrane-Electrode Assemblies and Investigations of Their Current–Voltage Characteristics
3. Results and Discussion
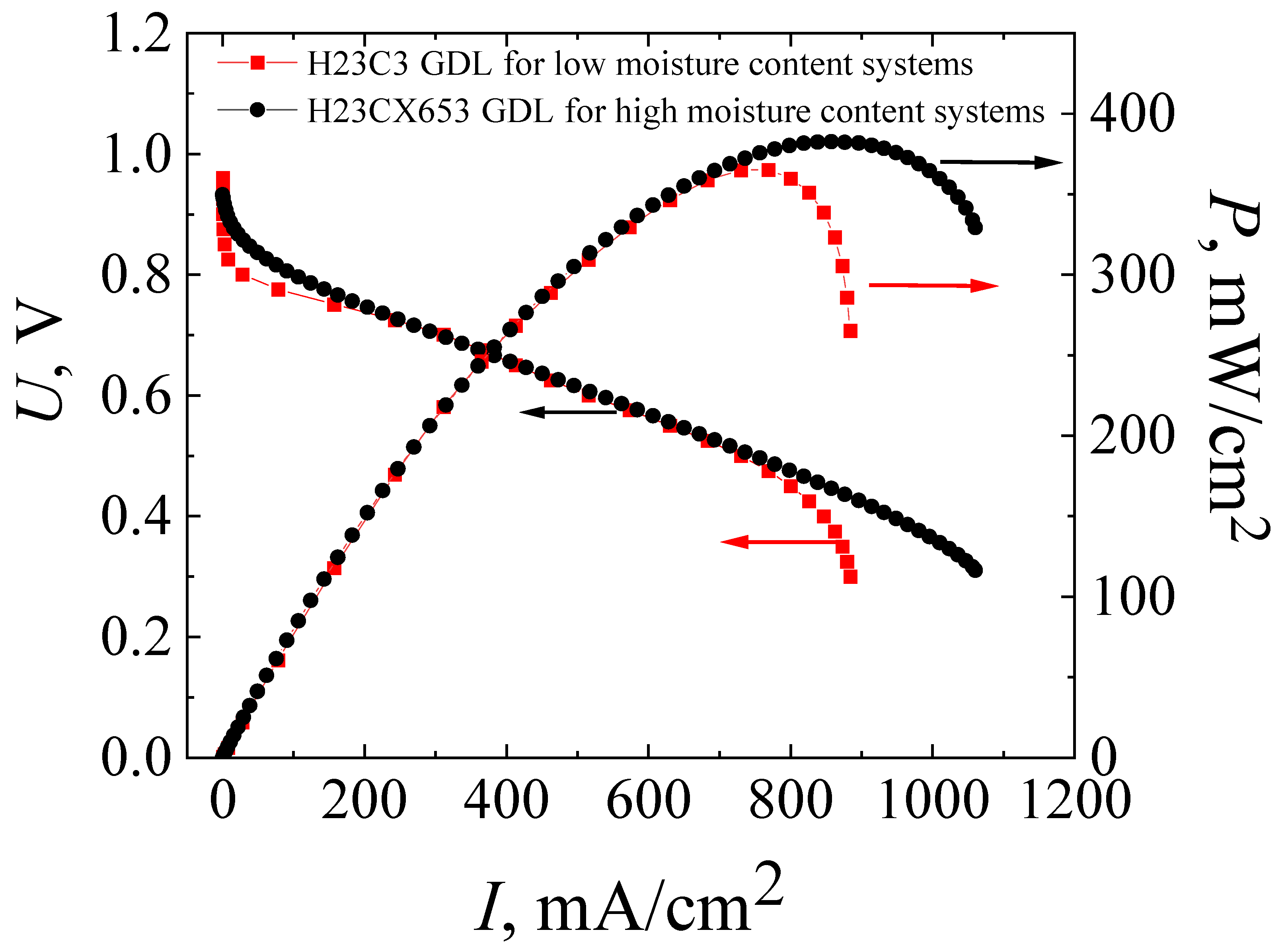
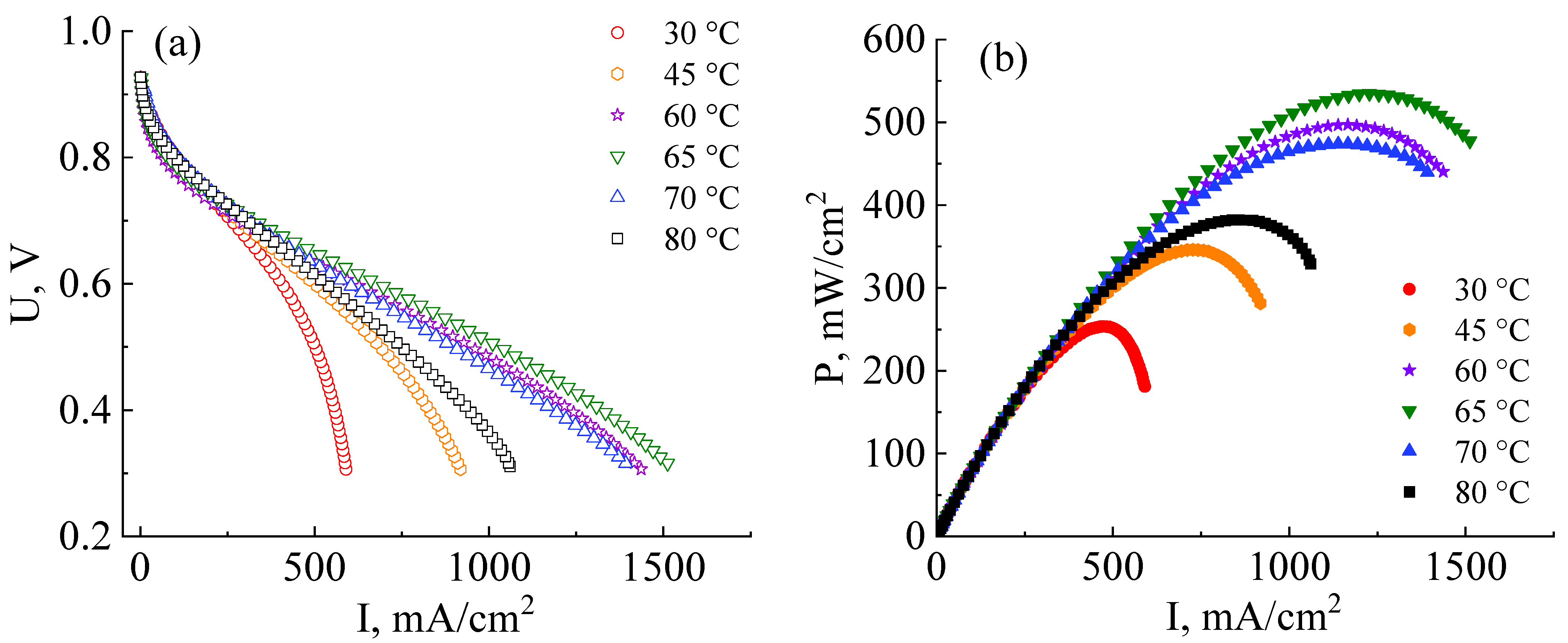
3.1. Characteristics of MEAs Fabricated by Standard Technology with the Co-Polynaphthoyleneimide Membrane
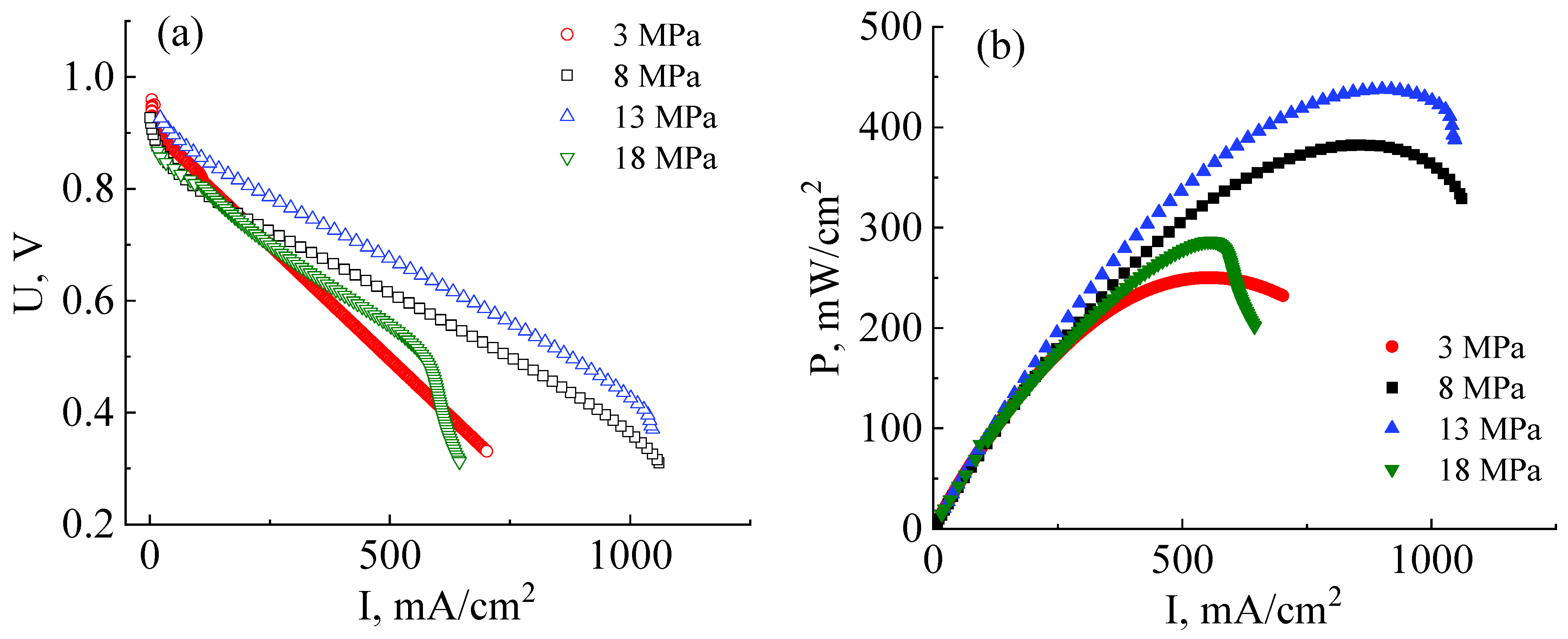
3.2. Development of Technology for the Fabrication of a High-Performance MEA with a Co-Polynaphthoyleneimide Membrane
- At temperatures of T > 70–80 °C, fuel cell ohmic resistance increases due to a decrease in the stability of the Zr cross-link;
- At temperatures of T < 55–60 °C, an increase in the fuel cell ohmic resistance is mainly associated with an increase in the interface resistance between the catalytic layer deposited onto the GDL and the membrane.
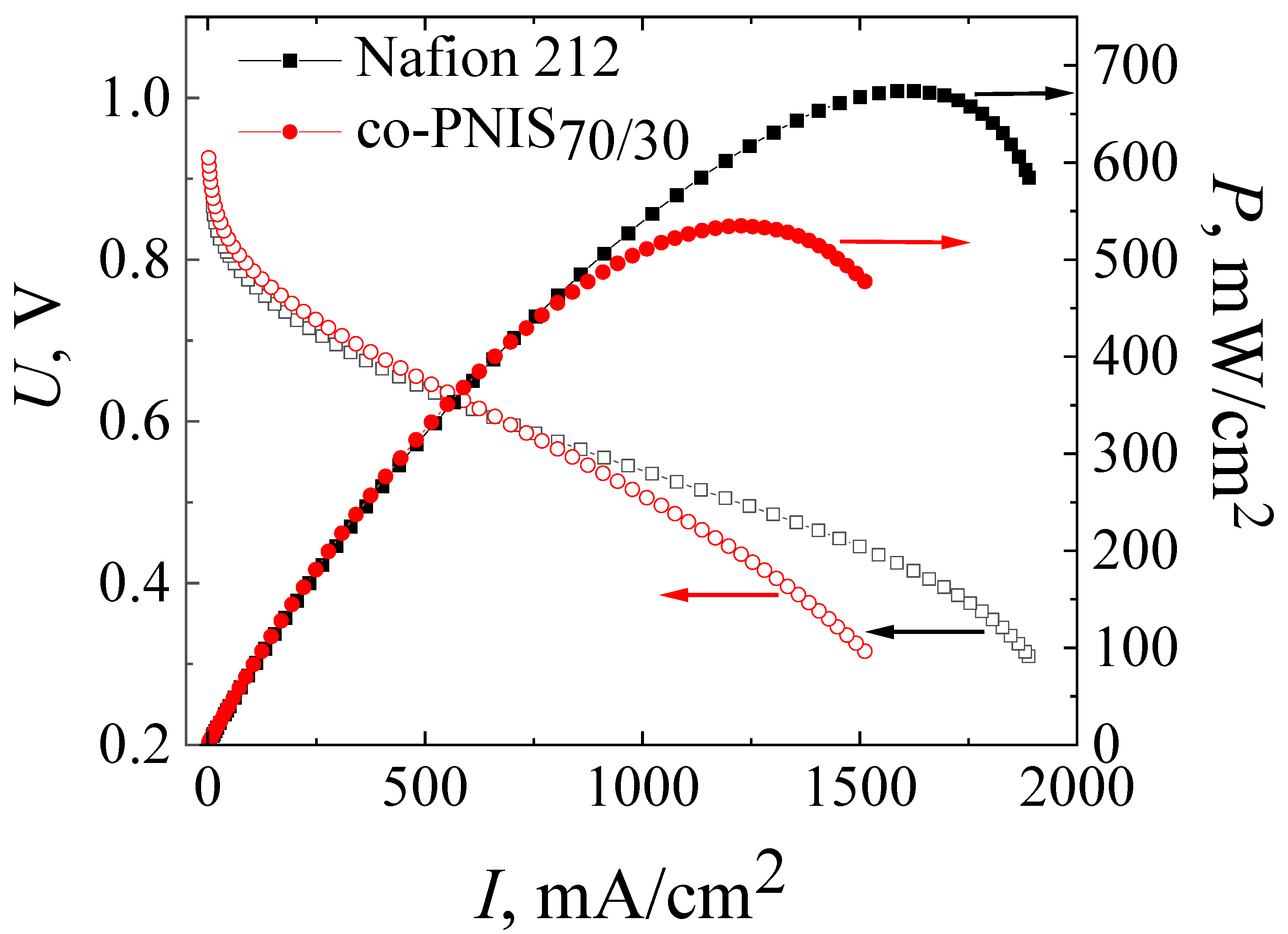
3.3. Comparison of the Performance of an MEA Based on a Co-Polynaphthoyleneimide Membrane Fabricated by a Modified Technology and an MEA Based on a Nafion Membrane Fabricated by a Standard Technology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Najafi, B.; Mamaghani, A.H.; Baricci, A.; Rinaldi, F.; Casalegno, A. Mathematical modelling and parametric study on a 30 kWel high temperature PEM fuel cell based residential micro cogeneration plant. Int. J. Hydrogen Energy 2015, 40, 1569–1583. [Google Scholar] [CrossRef]
- Wang, Y.; Pang, Y.; Xu, H.; Martinez, A.; Chen, K.S. PEM Fuel cell and electrolysis cell technologies and hydrogen infrastructure development—A review. Energy Environ. Sci. 2022, 15, 2288–2328. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, C.; Ge, M. Review of the research status of cost-effective zinc–iron redox flow batteries. Batteries 2022, 8, 202. [Google Scholar] [CrossRef]
- Wee, J.-H. Contribution of fuel cell systems to CO2 emission reduction in their application fields. Renew. Sustain. Energy Rev. 2010, 14, 735–744. [Google Scholar] [CrossRef]
- De Bruijn, F. The current status of fuel cell technology for mobile and stationary applications. Green Chem. 2005, 7, 132–150. [Google Scholar] [CrossRef]
- Peighambardoust, S.J.; Rowshanzamir, S.; Amjadi, M. Review of the proton exchange membranes for fuel cell applications. Int. J. Hydrogen Energy 2010, 35, 9349–9384. [Google Scholar] [CrossRef]
- Bose, S.; Kuila, T.; Nguyen, T.X.H.; Kim, N.H.; Lau, K.T.; Lee, J.H. Polymer membranes for high temperature proton exchange membrane fuel cell: Recent advances and challenges. Prog. Polym. Sci. 2011, 36, 813–843. [Google Scholar] [CrossRef]
- Scofield, M.E.; Liu, H.; Wong, S.S. A concise guide to sustainable PEFCs: Recent advances in improving both oxygen reduction catalysts and proton exchange membranes. Chem. Soc. Rev. 2015, 44, 5836–5860. [Google Scholar] [CrossRef]
- Chandan, A.; Hattenberger, M.; El-kharouf, A.; Du, S.; Dhir, A.; Self, V.; Pollet, B.G.; Ingram, A.; Bujalski, W. High temperature (HT) polymer electrolyte membrane fuel cells (PEMFC)—A review. J. Power Sour. 2013, 231, 264–278. [Google Scholar] [CrossRef]
- Connolly, D.J.; Gresham, W.F. Fluorocarbon Vinyl Ether Polymers. U.S. Patent 3,282,875, 1 November 1966. [Google Scholar]
- Kreuer, K.-D. Fuel Cells: Selected Entries from the Encyclopedia of Sustainability Science and Technology; Springer: New York, NY, USA, 2013. [Google Scholar]
- Mugtasimova, R.; Melnikov, A.P.; Galitskaya, E.A.; Kashin, A.M.; Dobrovolskiy, Y.A.; Don, G.M.; Likhomanov, V.S.; Sivak, A.V.; Sinitsyn, V.V. Fabrication of Aquivion-type membranes and optimization of their elastic and transport characteristics. Ionics 2018, 24, 3897–3903. [Google Scholar] [CrossRef]
- Peron, J.; Edwards, D.; Besson, A.; Shi, Z.; Holdcroft, S. Microstructure–performance relationships of sPEEK-based catalyst layers. J. Electrochem. Soc. 2010, 157, 1230–1236. [Google Scholar] [CrossRef]
- Holdcroft, S. Fuel cell catalyst layers: A polymer science perspective. Chem. Mater. 2013, 26, 381–393. [Google Scholar] [CrossRef]
- Goto, K.; Rozhanskii, I.; Yamakawa, Y.; Otsuki, T.; Naito, Y. Development of aromatic polymer electrolyte membrane with high conductivity and durability for fuel cell. Polym. J. 2009, 41, 95–104. [Google Scholar] [CrossRef]
- Miyatake, K. Membrane electrolytes from perfluorosulfonic acid (PFSA) to hydrocarbon ionomers. In Fuel Cells: Selected Entries from the Encyclopedia of Sustainability Science and Technology; Kreuer, K.-D., Ed.; Springer: New York, NY, USA, 2013. [Google Scholar] [CrossRef]
- Chen, C.; Levitin, G.; Hess, D.W.; Fuller, T.F. XPS investigation of Nafion membrane degradation. J. Power Sour. 2007, 169, 288–295. [Google Scholar] [CrossRef]
- Yu, T.H.; Liu, W.-G.; Sha, Y.; Merinov, B.V. The effect of different environments on Nafion degradation: Quantum mechanics study. J. Membr. Sci. 2013, 437, 276–285. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Moore, R.B. State of understanding of Nafion. Chem. Rev. 2004, 104, 4535–4586. [Google Scholar] [CrossRef]
- Houchins, C.; Kleen, G.J.; Spendelow, J.S.; Kopasz, J. US DOE progress towards developing low-cost, high performance, durable polymer electrolyte membranes for fuel cell applications. Membranes 2012, 2, 855–878. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, Z.; Zhang, J.; Tang, Y.; Song, C.; Navessin, T.; Shi, Z.; Song, D.; Wang, H.; Wilkinson, D.P.; et al. High temperature PEM fuel cells. J. Power Sources 2006, 160, 872–891. [Google Scholar] [CrossRef]
- Inaba, M.; Kinumoto, T.; Kiriake, M.; Umebayashi, R. Gas crossover and membrane degradation in polymer electrolyte fuel cells. Electrochim. Acta 2006, 51, 5746–5753. [Google Scholar] [CrossRef]
- Kreuer, K.D. On the development of proton conducting polymer membranes for hydrogen and methanol fuel cells. J. Membr. Sci. 2001, 185, 29–39. [Google Scholar] [CrossRef]
- Curtin, D.E.; Lousenberg, R.D.; Henry, T.J.; Tangeman, P.C.; Tisack, M.E. Advanced materials for improved PEMFC performance and life. J. Power Sour. 2004, 131, 41–48. [Google Scholar] [CrossRef]
- Kima, K.; Baea, J.; Lima, M.-Y.; Heob, P. Enhanced physical stability and chemical durability of sulfonated poly(arylene ether sulfone) composite membranes having antioxidant grafted graphene oxide for polymer electrolyte membrane fuel cell applications. J. Membr. Sci. 2017, 525, 125–134. [Google Scholar] [CrossRef]
- Adjemian, K.; Lee, S.; Srinivasan, S.; Benziger, J.; Bocarsly, A. Silicon oxide Nafion composite membranes for proton-exchange membrane fuel cell operation at 80–140 °C. J. Electrochem. Soc. 2002, 149, 256–261. [Google Scholar] [CrossRef]
- Roziere, J.; Jones, D.J. Non-fluorinated polymer materials for proton exchange membrane fuel cells. Annu. Rev. Mater. Res. 2003, 33, 503–555. [Google Scholar] [CrossRef]
- Kim, K.; Choi, S.-W.; Park, J.O.; Kim, S.-K.; Lim, M.-Y.; Kim, K.-H.; Ko, T.; Lee, J.-C. Proton conductive cross-linked benzoxazine-benzimidazole copolymers as novel porous substrates for reinforced pore-filling membranes in fuel cells operating at high temperatures. J. Membr. Sci. 2017, 536, 76–85. [Google Scholar] [CrossRef]
- Kim, J.; Kim, K.; Han, J.; Lee, H.; Kim, H.; Kim, S.; Sung, Y.-E.; Lee, J.-C. End group cross-linked membranes based on highly sulfonated poly(arylene ether sulfone) with vinyl functionalized graphene oxide as a cross-linker and a filler for proton exchange membrane fuel cell application. J. Polym. Sci. Part A Polym. Chem. 2020, 58, 3456–3466. [Google Scholar] [CrossRef]
- Adamski, M.; Skalski, T.J.G.; Britton, B.; Peckham, T.J.; Metzler, L.; Holdcroft, S. Highly stable, low gas crossover, proton-conducting phenylated polyphenylenes. Angew. Chem. Int. Ed. 2017, 56, 9058–9061. [Google Scholar] [CrossRef]
- Skalski, T.J.G.; Adamski, M.; Britton, B.; Schibli, E.M.; Peckham, T.J.; Weissbach, T.; Moshisuki, T.; Lyonnard, S.; Frisken, B.J.; Holdcroft, S. Sulfophenylated terphenylene copolymer membranes and ionomers. ChemSusChem 2018, 11, 4033–4043. [Google Scholar] [CrossRef]
- Zavorotnaya, U.M.; Ponomarev, I.I.; Volkova, Y.A.; Modestov, A.D.; Andreev, V.N.; Privalov, A.F.; Vogel, M.; Sinitsyn, V.V. Preparation and study of sulfonated co-polynaphthoyleneimide proton-exchange membrane for a H2/Air fuel cell. Materials 2020, 13, 5297. [Google Scholar] [CrossRef]
- Zavorotnaya, U.M.; Privalov, A.F.; Kresse, B.; Vogel, M.; Ponomarev, I.I.; Volkova, Y.A.; Sinitsyn, V.V. Diffusion in sulfonated co-polynaphthoyleneimide proton exchange membranes with different ratios of hydrophylic to hydrophobic groups studied using SFG NMR. Macromolecules 2022, 55, 8823–8833. [Google Scholar] [CrossRef]
- Maier, G.; Meier-Haack, J. Sulfonated aromatic polymers for fuel cell membranes. In Fuel Cells II—Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2008; Volume 216, pp. 1–62. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Grinberg, V.A.; Emets, V.V.; Maiorova, N.A.; Zharinova, M.Y.; Volkova, Y.A.; Razorenov, D.Y.; Skupov, K.M.; Nizhnikovskii, E.A. Development of methanol–air fuel cells with membrane materials based on new sulfonated polyheteroarylenes. Russ. J. Electrochem. 2016, 52, 525–532. [Google Scholar] [CrossRef]
- Emets, V.V.; Ponomarev, I.I.; Grinberg, V.A.; Mayorova, N.A.; Zharinova, M.Y.; Volkova, Y.A.; Nizhnikovskii, E.A.; Skupov, K.M.; Razorenov, D.Y.; Andreev, V.N.; et al. Development of hydrogen-air fuel cells with membranes based on sulfonated polyheteroarylenes. Russ. J. Electrochem. 2017, 53, 86–91. [Google Scholar] [CrossRef]
- Fang, J.; Guo, X.; Harada, S.; Watari, T.; Tanaka, K.; Kita, H.; Okamoto, K. Novel sulfonated polyimides as polyelectrolytes for fuel cell Application. Synthesis, proton conductivity, and water stability of polyimides from 4,4′-diaminodiphenyl ether-2,2′-disulfonic acid. Macromolecules 2002, 35, 9022–9028. [Google Scholar] [CrossRef]
- Hickner, M.A.; Pivovar, B.S. The chemical and structural nature of proton exchange membrane fuel cell properties. Fuel Cells 2005, 2, 213–229. [Google Scholar] [CrossRef]
- Peckham, T.; Schmeisser, J.; Rodgers, M.; Holdcroft, S. Main-chain, statistically sulfonated proton exchange membranes: The relationships of acid concentration and proton mobility to water content and their effect upon proton conductivity. J. Mater. Chem. 2007, 17, 3255–3268. [Google Scholar] [CrossRef]
- Vona, M.L.D.; Knauth, P. Sulfonated aromatic polymers as proton-conducting solid electrolytes for fuel cells: A short review. Z. Phys. Chem. 2013, 227, 595–614. [Google Scholar] [CrossRef]
- Galitskaya, E.A.; Gerasimova, E.V.; Dobrovol’skii, Y.A.; Don, G.M.; Afanas’ev, A.S.; Levchenko, A.V.; Sivak, A.V.; Sinitsyn, V.V. Pulsed activation of a fuel cell on the basis of a proton-conducting polymer membrane. Tech. Phys. Lett. 2018, 44, 570–573. [Google Scholar] [CrossRef]
- Lim, T.W.; Kim, S.H.; Ahn, S.Y.; Hong, B.K.; Ahn, B.K. Systemand Method for Activating Fuel Cell. US Patent 8,455,121 B2, 4 June 2013. [Google Scholar]
- Yuan, X.-Z.; Sun, J.C.; Wang, H.; Li, H. Accelerated conditioning for a proton exchange membrane fuel cell. J. Power Sources 2012, 205, 340–344. [Google Scholar] [CrossRef]
- Nguyen, T.; Mack, W. Knobbe A liquid water management strategy for PEM fuel cell stacks. J. Power Sources 2003, 114, 70–79. [Google Scholar] [CrossRef]
- Vielstich, W.; Lamm, A.; Gasteiger, H.A. Handbook of Fuel Cells, 1st ed.; Wiley, J., Ed.; Sons Ltd.: Chichester, UK, 2009; Volume 5, pp. 24–31. [Google Scholar] [CrossRef]
- Kim, Y.S.; Dong, L.; Hickner, M.A.; Glass, T.E.; Webb, V.; McGrath, J.E. State of water in disulfonated poly (arylene ether sulfone) copolymers and a perfluorosulfonic acid copolymer (Nafion) and its effect on physical and electrochemical properties. Macromolecules 2003, 36, 6281–6285. [Google Scholar] [CrossRef]
- Kidena, K.; Ohkubo, T.; Takimoto, N.; Ohira, A. PFG-NMR approach to determining the water transport mechanism in polymer electrolyte membranes conditioned at different temperatures. Eur. Polym. J. 2010, 46, 450–455. [Google Scholar] [CrossRef]
- Gasa, J.V.; Weiss, R.A.; Shaw, M.T. Structured polymer electrolyte blends based on sulfonated polyetherketoneketone (SPEKK) and a poly(ether imide) (PEI). J. Membrane Sci. 2008, 320, 215–223. [Google Scholar] [CrossRef]
- Pivovar, B. To appear symposium on proton conducting membrane fuel cells III. In Proceedings of the 202nd Meeting of the ECS, Salt Lake City, Utah, 20–24 October 2002. [Google Scholar]
- Kim, Y.S.; Sumner, M.J.; Harrison, W.L.; Riffle, J.S.; McGrath, J.E.; Pivovar, B.S. Direct methanol fuel cell performance of disulfonated poly(arylene ether benzonitrile) copolymers. J. Electrochem. Soc. 2004, 151, 2150–2156. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zavorotnaya, U.M.; Ponomarev, I.I.; Volkova, Y.A.; Sinitsyn, V.V. Development of High-Performance Hydrogen-Air Fuel Cell with Flourine-Free Sulfonated Co-Polynaphthoyleneimide Membrane. Membranes 2023, 13, 485. https://doi.org/10.3390/membranes13050485
Zavorotnaya UM, Ponomarev II, Volkova YA, Sinitsyn VV. Development of High-Performance Hydrogen-Air Fuel Cell with Flourine-Free Sulfonated Co-Polynaphthoyleneimide Membrane. Membranes. 2023; 13(5):485. https://doi.org/10.3390/membranes13050485
Chicago/Turabian StyleZavorotnaya, Ulyana M., Igor I. Ponomarev, Yulia A. Volkova, and Vitaly V. Sinitsyn. 2023. "Development of High-Performance Hydrogen-Air Fuel Cell with Flourine-Free Sulfonated Co-Polynaphthoyleneimide Membrane" Membranes 13, no. 5: 485. https://doi.org/10.3390/membranes13050485
APA StyleZavorotnaya, U. M., Ponomarev, I. I., Volkova, Y. A., & Sinitsyn, V. V. (2023). Development of High-Performance Hydrogen-Air Fuel Cell with Flourine-Free Sulfonated Co-Polynaphthoyleneimide Membrane. Membranes, 13(5), 485. https://doi.org/10.3390/membranes13050485






