Proton-Conducting Polymer-Coated Carbon Nanofiber Mats for Pt-Anodes of High-Temperature Polymer-Electrolyte Membrane Fuel Cell
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrocatalyst Preparation
2.1.1. Electrospinning
2.1.2. Stabilization, Zinc Deposition and Pyrolysis
2.1.3. Polymer Deposition
2.1.4. Platinum Deposition
2.1.5. Inverted Platinum and Polymer Deposition
2.1.6. Elemental Analysis and Electrical Conductivity
2.2. Morphological Characterization
2.3. HT-PEM Fuel Cell Operation
2.3.1. Electrochemical Characterization
2.3.2. Hydrogen Crossover Measurements
2.4. Adsorption Studies
2.4.1. N2 Adsorption
2.4.2. CO2 Adsorption
3. Results
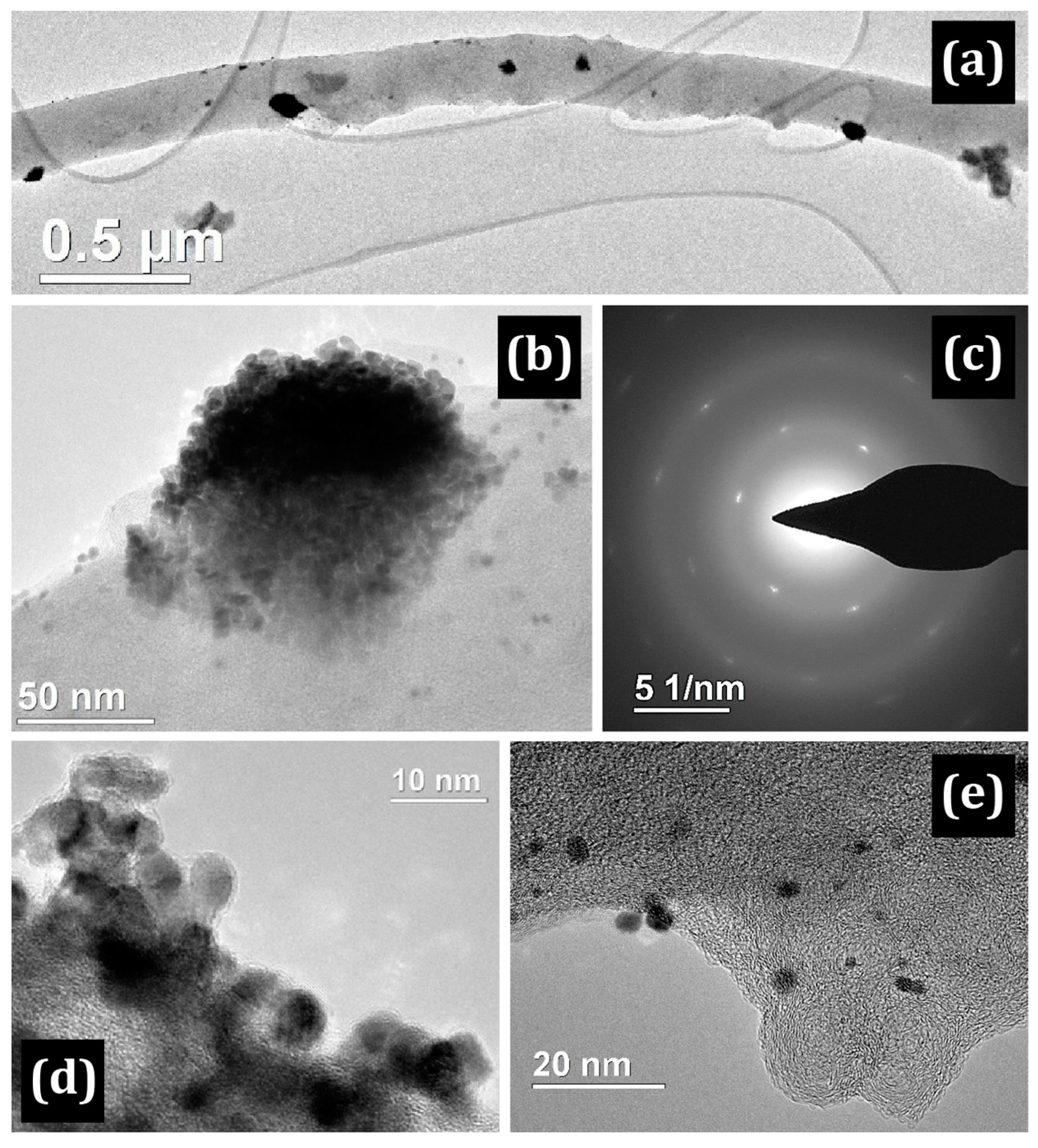
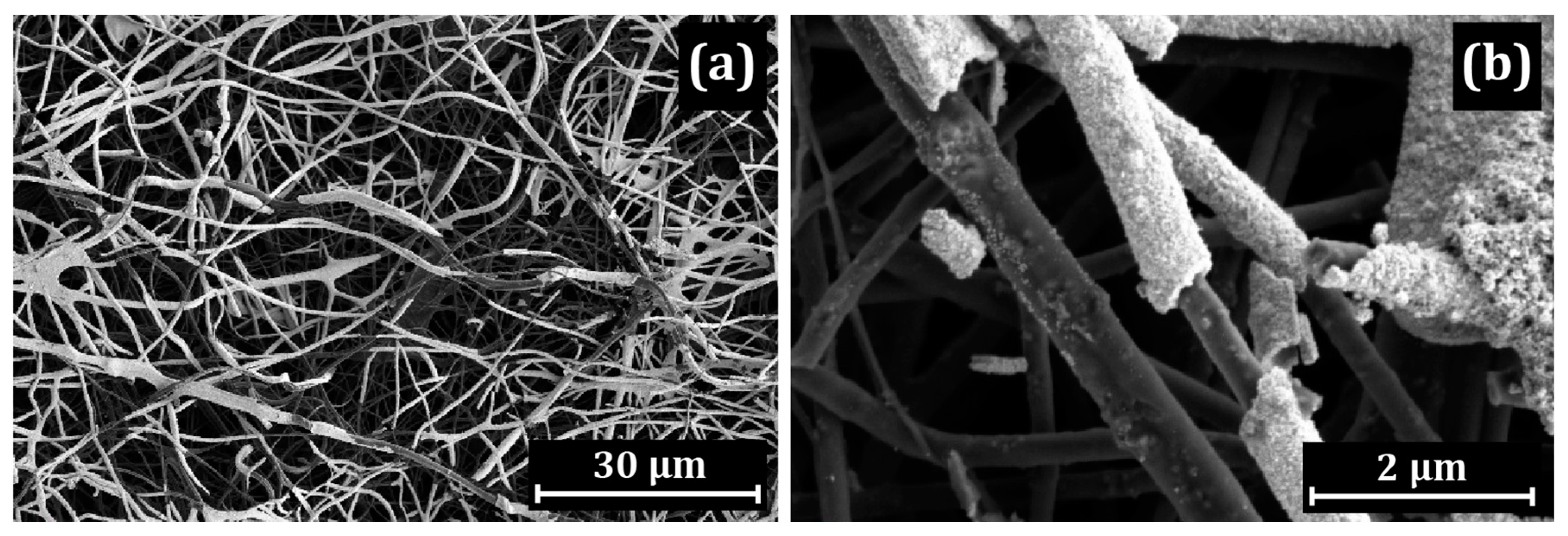
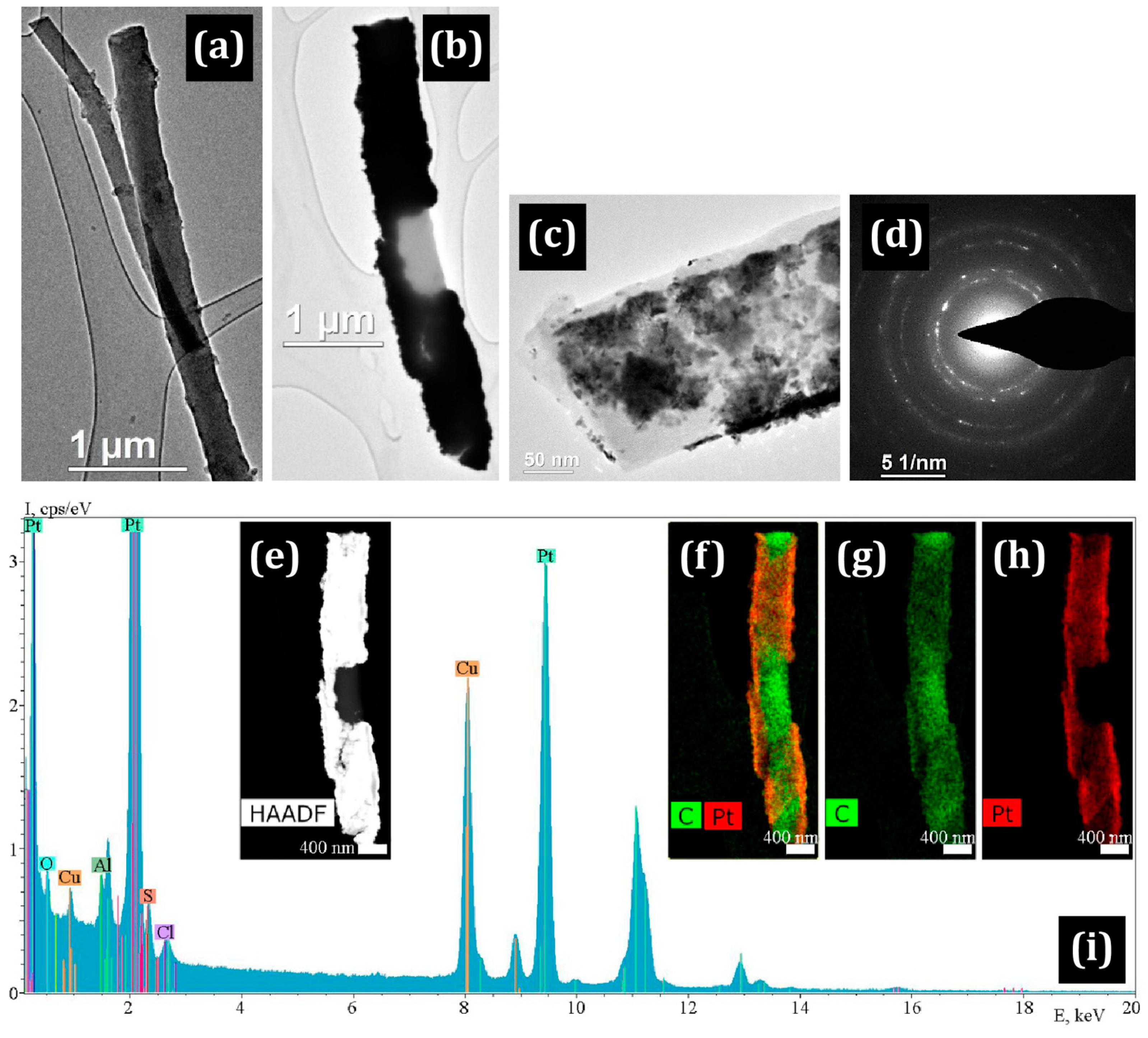
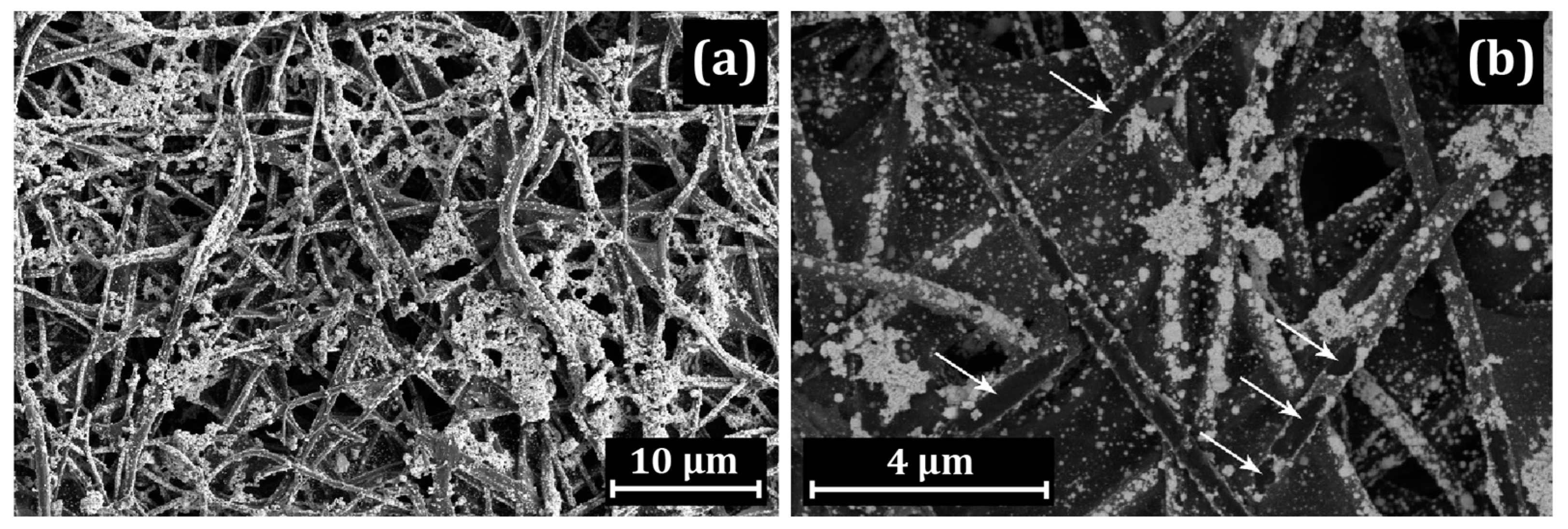
3.1. Electron Microscopy
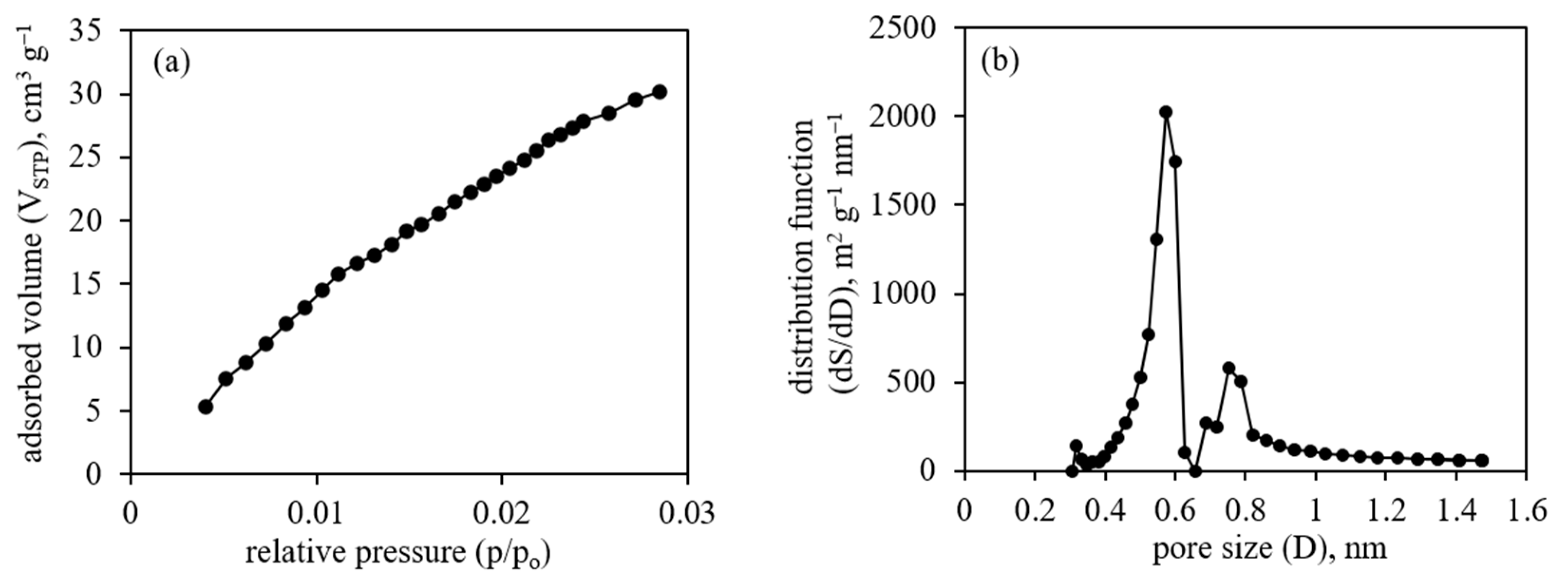
3.2. Adsorption Studies
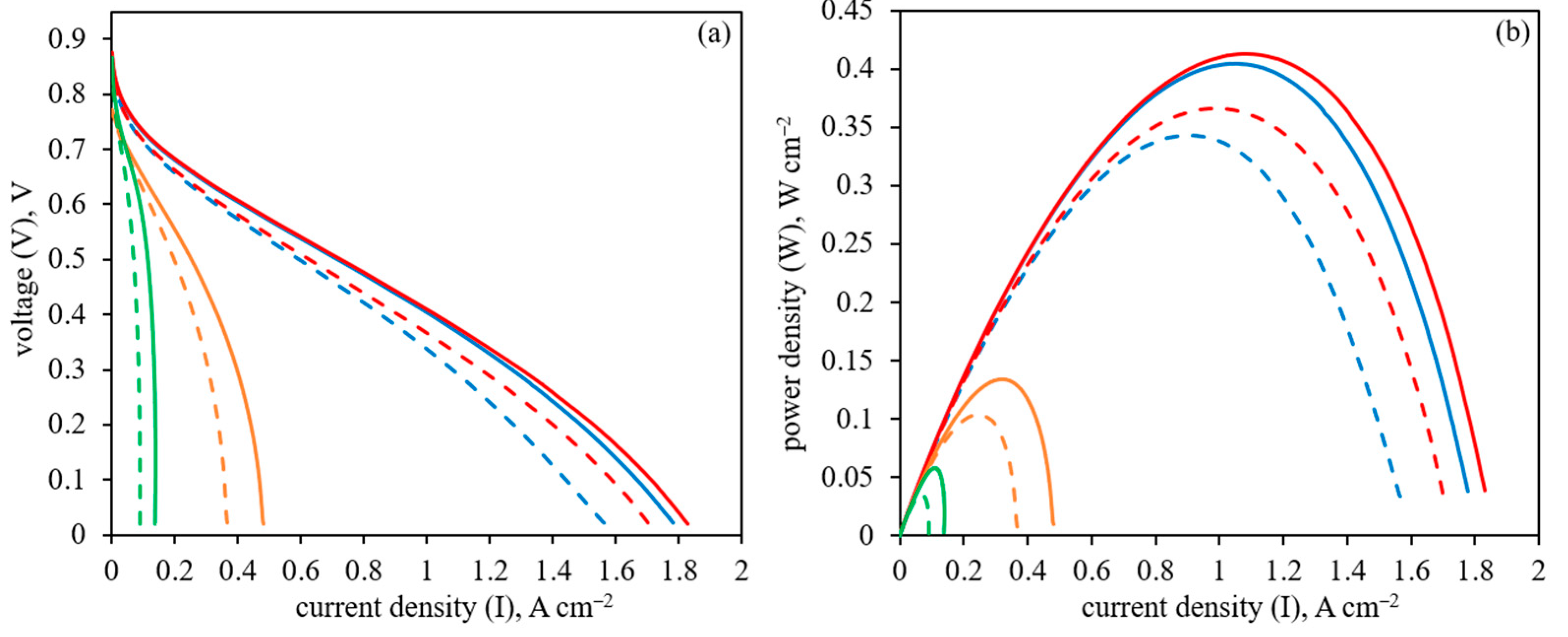
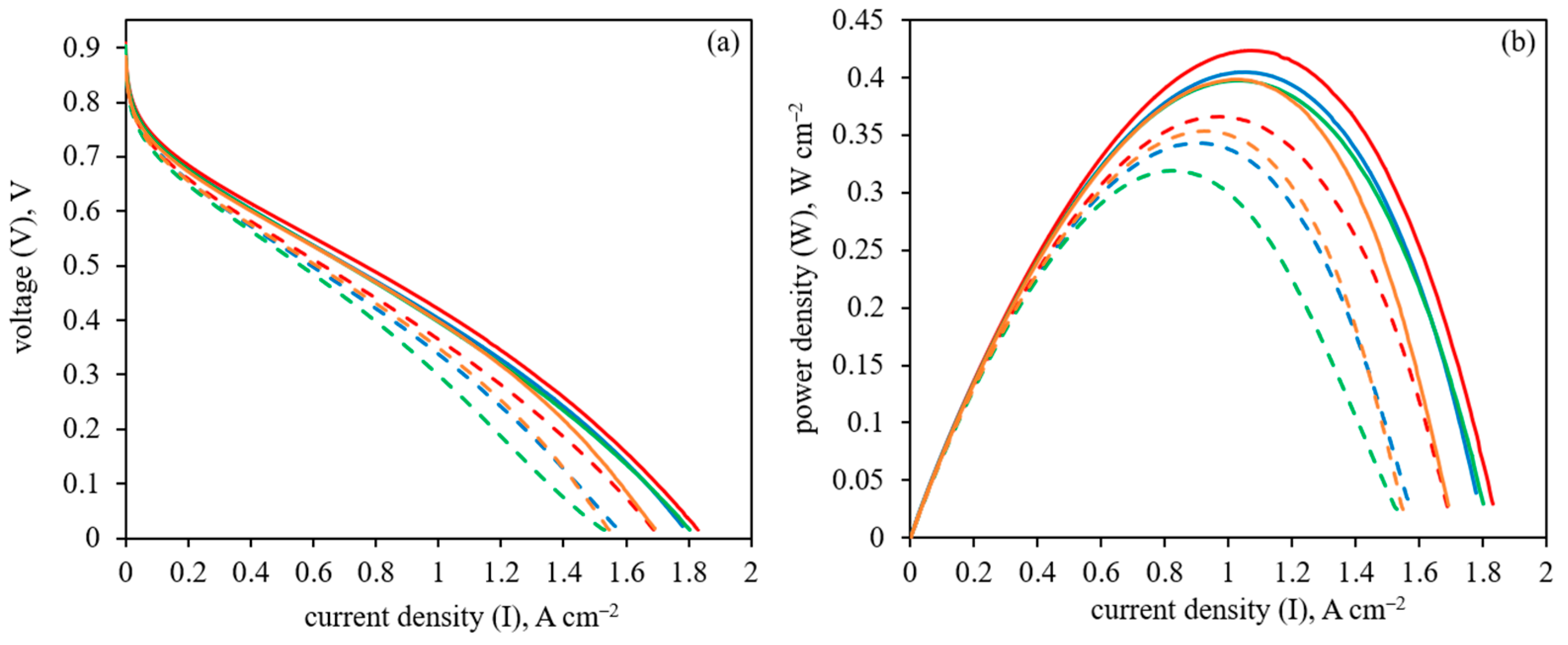
3.3. HT-PEM Fuel Cell Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Q.; Aili, D.; Hjuler, H.A.; Jensen, J.O. High Temperature Polymer Electrolyte Membrane Fuel Cells, Approaches, Status and Perspectives; Springer: London, UK, 2016. [Google Scholar] [CrossRef]
- Zhang, J. PEM Fuel Cell Electrocatalysts and Catalyst Layers, Fundamentals and Applications; Springer: London, UK, 2008. [Google Scholar] [CrossRef]
- Rosli, R.E.; Sulong, A.B.; Daud, W.R.W.; Zulkifley, M.A.; Husaini, T.; Rosli, M.I.; Majlan, E.H.; Haque, M.A. A review of high-temperature proton exchange membrane fuel cell (HT-PEMFC) system. Int. J. Hydrogen Energy 2017, 42, 9293–9314. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, C.; Ge, M. Review of the Research Status of Cost-Effective Zinc–Iron Redox Flow Batteries. Batteries 2022, 8, 202. [Google Scholar] [CrossRef]
- Kalathil, A.; Raghavan, A.; Kandasubramanian, B. Polymer Fuel Cell Based on Polybenzimidazole Membrane: A Review. Polym. Plast. Technol. Mater. 2019, 58, 465–497. [Google Scholar] [CrossRef]
- Chandan, A.; Hattenberger, M.; El-Kharouf, A.; Du, S.; Dhir, A.; Self, V.; Pollet, B.G.; Ingram, A.; Bujalski, W. High temperature (HT) polymer electrolyte membrane fuel cells (PEMFC)—A review. J. Power Sources 2013, 231, 264–278. [Google Scholar] [CrossRef]
- Araya, S.S.; Zhou, F.; Liso, V.; Sahlin, S.L.; Vang, J.R.; Thomas, S.; Gao, X.; Jeppesen, C.; Kær, S.K. A comprehensive review of PBI-based high temperature PEM fuel cells. Int. J. Hydrogen Energy 2016, 41, 21310–21344. [Google Scholar] [CrossRef]
- Pingitore, A.T.; Molleo, M.; Schmidt, T.J.; Benicewicz, B.C. Polybenzimidazole Fuel Cell Technology: Theory, Performance, and Applications. In Fuel Cells and Hydrogen Production. Encyclopedia of Sustainability Science and Technology Series; Lipman, T., Weber, A., Eds.; Springer: New York, NY, USA, 2019; pp. 477–515. [Google Scholar] [CrossRef]
- Zeis, R. Materials and characterization techniques for high-temperature polymer electrolyte membrane fuel cells. Beilstein J. Nanotechnol. 2015, 6, 68–83. [Google Scholar] [CrossRef]
- Authayanum, S.; Im-Orb, K.; Arpornwichnop, A. A review of the development of high temperature proton exchange membrane fuel cells. Chin. J. Catal. 2015, 36, 473–483. [Google Scholar] [CrossRef]
- Quartarone, E.; Angioni, S.; Mustarelli, P. Polymer and Composite Membranes for Proton-Conducting, High-Temperature Fuel Cells: A Critical Review. Materials 2017, 10, 687. [Google Scholar] [CrossRef] [PubMed]
- Escorihuela, J.; Olvera-Mancilla, J.; Alexandrova, L.; del Castillo, L.F.; Compañ, V. Recent Progress in the Development of Composite Membranes Based on Polybenzimidazole for High Temperature Proton Exchange Membrane (PEM) Fuel Cell Applications. Polymers 2020, 12, 1861. [Google Scholar] [CrossRef]
- Myles, T.; Bonville, L.; Maric, R. Catalyst, Membrane, Free Electrolyte Challenges, and Pathways to Resolutions in High Temperature Polymer Electrolyte Membrane Fuel Cells. Catalysts 2017, 7, 16. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, Z.; Zhang, J.; Tang, Y.; Song, C.; Navessin, T.; Shi, Z.; Song, D.; Wang, H.; Wilkinson, D.P.; et al. High temperature PEM fuel cells. J. Power Sources 2006, 160, 872–891. [Google Scholar] [CrossRef]
- Delikaya, O.; Bevilacqua, N.; Eifert, L.; Kunz, U.; Zeis, R.; Roth, C. Porous electrospun carbon nanofibers network as an integrated electrode@gas diffusion layer for high temperature polymer electrolyte membrane fuel cells. Electrochim. Acta 2020, 345, 136192. [Google Scholar] [CrossRef]
- Wang, P.; Li, X.; Liu, Z.; Peng, J.; Shi, C.; Li, T.; Yang, J.; Shan, C.; Hu, W.; Liu, B. Construction of highly conductive PBI-based alloy membranes by incorporating PIMs with optimized molecular weights for high-temperature proton exchange membrane fuel cells. J. Membr. Sci. 2022, 659, 120790. [Google Scholar] [CrossRef]
- Guo, Z.; Perez-Page, M.; Chen, J.; Ji, Z.; Holmes, S.M. Recent advances in phosphoric acid–based membranes for high–temperature proton exchange membrane fuel cells. J. Energy Chem. 2021, 63, 393–429. [Google Scholar] [CrossRef]
- Skupov, K.M.; Vtyurina, E.S.; Ponomarev, I.I.; Ponomarev, I.I.; Aysin, R.R. Prospective carbon nanofibers based on polymer of intrinsic microporosity (PIM-1): Pore structure regulation for higher carbon sequestration and renewable energy source applications. Polymer 2023, 264, 125546. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Razorenov, D.Y.; Ponomarev, I.I.; Volkova, Y.A.; Skupov, K.M.; Lysova, A.A.; Yaroslavtsev, A.B.; Modestov, A.D.; Buzin, M.I.; Klemenkova, Z.S. Polybenzimidazoles via polyamidation: A more environmentally safe process to proton conducting membrane for hydrogen HT-PEM fuel cell. Eur. Polym. J. 2021, 156, 110613. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Skupov, K.M.; Zhigalina, O.M.; Naumkin, A.V.; Modestov, A.D.; Basu, V.G.; Sufiyanova, A.E.; Razorenov, D.Y.; Ponomarev, I.I. New Carbon Nanofiber Composite Materials Containing Lanthanides and Transition Metals Based on Electrospun Polyacrylonitrile for High Temperature Polymer Electrolyte Membrane Fuel Cell Cathodes. Polymers 2020, 12, 1340. [Google Scholar] [CrossRef]
- Zhigalina, V.G.; Zhigalina, O.M.; Ponomarev, I.I.; Skupov, K.M.; Razorenov, D.Y.; Ponomarev, I.I.; Kiselev, N.A.; Leitinger, G. Electron microscopy study of new composite materials based on electrospun carbon nanofibers. Crystengcomm 2017, 19, 3792–3800. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Skupov, K.M.; Naumkin, A.V.; Basu, V.G.; Zhigalina, O.M.; Razorenov, D.Y.; Ponomarev, I.I.; Volkova, Y.A. Probing of complex carbon nanofiber paper as gas-diffusion electrode for high temperature polymer electrolyte membrane fuel cell. RSC Adv. 2019, 9, 257–267. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Zhigalina, O.M.; Skupov, K.M.; Modestov, A.D.; Basu, V.G.; Sufiyanova, A.E.; Ponomarev, I.I.; Razorenov, D.Y. Preparation and thermal treatment influence on Pt-decorated electrospun carbon nanofiber electrocatalysts. RSC Adv. 2019, 9, 27406–27418. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Skupov, K.M.; Zhigalina, O.M.; Khmelenin, D.N.; Ponomarev, I.I.; Vtyurina, E.S.; Cherkovskiy, E.N.; Basu, V.G.; Modestov, A.D. Deposition of Pt Nanoparticles by Ascorbic Acid on Composite Electrospun Polyacrylonitrile-Based Carbon Nanofiber for HT-PEM Fuel Cell Cathodes. Catalysts 2022, 12, 891. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Ponomarev, I.I.; Filatov, I.Y.; Filatov, Y.N.; Razorenov, D.Y.; Volkova, Y.A.; Zhigalina, O.M.; Zhigalina, V.G.; Grebenev, V.V.; Kiselev, N.A. Design of electrodes based on a carbon nanofiber nonwoven material for the membrane electrode assembly of a polybenzimidazole-membrane fuel cell. Dokl. Phys. Chem. 2013, 448, 23–27. [Google Scholar] [CrossRef]
- Vol’fkovich, Y.M.; Ponomarev, I.I.; Sosenkin, V.E.; Ponomarev, I.I.; Skupov, K.M.; Razorenov, D.Y. A Porous Structure of Nanofiber Electrospun Polyacrylonitrile-Based Materials: A Standard Contact Porosimetry Study. Prot. Met. Phys. Chem. Surf. 2019, 55, 195–202. [Google Scholar] [CrossRef]
- Ponomarev, I.; Skupov, K.M.; Razorenov, D.; Zhigalina, V.G.; Zhigalina, O.M.; Ponomarev, I.I.; Volkova, Y.A.; Kondratenko, M.; Bukalov, S.; Davydova, E.S. Electrospun nanofiber pyropolymer electrodes for fuel cells on polybenzimidazole membranes. Russ. J. Electrochem. 2016, 52, 735–739. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Filatov, Y.N.; Ponomarev, I.I.; Filatov, I.Y.; Razorenov, D.Y.; Skupov, K.M.; Zhigalina, O.M.; Zhigalina, V.G. Electroforming of Nitrogen-Containing Polymers and Derived Nonfabric Nanofibre Carbon Materials. Fibre Chem. 2017, 49, 183–187. [Google Scholar] [CrossRef]
- Skupov, K.M.; Ponomarev, I.; Razorenov, D.Y.; Zhigalina, V.G.; Zhigalina, O.M.; Ponomarev, I.I.; Volkova, Y.A.; Volfkovich, Y.M.; Sosenkin, V.E. Carbon nanofiber paper cathode modification for higher performance of phosphoric acid fuel cells on polybenzimidazole membrane. Russ. J. Electrochem. 2017, 53, 728–733. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Skupov, K.M.; Ponomarev, I.I.; Razorenov, D.Y.; Volkova, Y.A.; Basu, V.G.; Zhigalina, O.M.; Bukalov, S.S.; Volfkovich, Y.M.; Sosenkin, V.E. New Gas-Diffusion Electrode Based on Heterocyclic Microporous Polymer PIM-1 for High-Temperature Polymer Electrolyte Membrane Fuel Cell. Russ. J. Electrochem. 2019, 55, 552–557. [Google Scholar] [CrossRef]
- Skupov, K.M.; Ponomarev, I.I.; Vol’fkovich, Y.M.; Modestov, A.D.; Ponomarev, I.I.; Volkova, Y.A.; Razorenov, D.Y.; Sosenkin, V.E. The Effect of the Stabilization and Carbonization Temperatures on the Properties of Microporous Carbon Nanofiber Cathodes for Fuel Cells on Polybenzimidazole Membrane. Polym. Sci. Ser. C 2020, 62, 231–237. [Google Scholar] [CrossRef]
- Skupov, K.M.; Ponomarev, I.I.; Razorenov, D.Y.; Zhigalina, V.G.; Zhigalina, O.M.; Ponomarev, I.I.; Volkova, Y.A.; Volfkovich, Y.M.; Sosenkin, V.E. Carbon nanofiber paper electrodes based on heterocyclic polymers for high temperature polymer electrolyte membrane fuel cell. Macromol. Symp. 2017, 375, 1600188. [Google Scholar] [CrossRef]
- Skupov, K.M.; Ponomarev, I.I.; Volfkovich, Y.M.; Sosenkin, V.E.; Ponomarev, I.I.; Volkova, Y.A.; Razorenov, D.Y.; Buyanovskaya, A.G.; Talanova, V.N. Porous structure optimization of electrospun carbon materials. Russ. Chem. Bull. 2020, 69, 1106–1113. [Google Scholar] [CrossRef]
- Inagaki, M.; Yang, Y.; Kang, F. Carbon Nanofibers Prepared via Electrospinning. Adv. Mater. 2012, 24, 2547–2566. [Google Scholar] [CrossRef] [PubMed]
- Tenchurin, T.K.; Krasheninnikov, S.N.; Orekhov, A.S.; Chvalun, S.N.; Shepelev, A.D.; Belousov, S.L.; Gulyaev, A.I. Rheological Features of Fiber Spinning from Polyacrylonitrile Solutions in an Electric Field. Structure and Properties. Fibre Chem. 2014, 46, 151–160. [Google Scholar] [CrossRef]
- Dong, Z.; Kennedy, S.J.; Wu, Y. Electrospinning materials for energy-related applications and devices. J. Power Sources 2011, 196, 4886–4904. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Sun, C. Characterization on oxidative stabilization of polyacrylonitrile nanofibers prepared by electrospinning. J. Polym. Res. 2007, 14, 467–474. [Google Scholar] [CrossRef]
- Dubal, S.; Chavan, S.; Jadhav, P. Oxidative Stabilization and Characterization of Electrospun Polyacrylonitrile Nanofiber Web on Different Substrates. J. Inst. Eng. India Ser. C 2022, 103, 1415–1422. [Google Scholar] [CrossRef]
- Yusof, N.; Ismail, A.F. Post spinning and pyrolysis processes of polyacrylonitrile (PAN)-based carbon fiber and activated carbon fiber: A review. J. Anal. Appl. Pyrol. 2012, 93, 1–13. [Google Scholar] [CrossRef]
- Zhang, L.; Aboagye, A.; Kelkar, A.; Lai, C.; Fong, H. A review: Carbon nanofibers from electrospun polyacrylonitrile and their applications. J. Mater. Sci. 2014, 49, 463–480. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, Y.; Luo, X.-D.; Sun, C.Y.; Chen, N. Polarization Effects of a Rayon and Polyacrylonitrile Based Graphite Felt for Iron-Chromium Redox Flow Batteries. ChemElectroChem 2019, 6, 3175–3188. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, N.; Sun, C.; Luo, X. Investigations on physicochemical properties and electrochemical performance of graphite felt and carbon felt for iron-chromium redox flow battery. Int. J. Energy Res. 2020, 44, 3839–3853. [Google Scholar] [CrossRef]
- Low, Z.-X.; Budd, P.M.; McKeown, N.B.; Patterson, D.A. Gas Permeation Properties, Physical Aging, and Its Mitigation in High Free Volume Glassy Polymers. Chem. Rev. 2018, 118, 5871–5911. [Google Scholar] [CrossRef]
- Budd, P.M.; Ghanem, B.S.; Makhseed, S.; McKeown, N.B.; Msayib, K.J.; Tattershall, C.E. Polymers of intrinsic microporosity (PIMs): Robust, solution-processable, organic nanoporous materials. Chem. Commun. 2004, 230–231. [Google Scholar] [CrossRef]
- Budd, P.M.; Msayib, K.J.; Tattershall, C.E.; Ghanem, B.S.; Reynolds, K.J.; McKeown, N.B.; Fritsch, D. Gas separation membranes from polymers of intrinsic microporosity. J. Membr. Sci. 2005, 251, 263–269. [Google Scholar] [CrossRef]
- Budd, P.M.; McKeown, N.B.; Ghanem, B.S.; Msayib, K.J.; Fritsch, D.; Starannikova, L.; Belov, N.; Sanfirova, O.; Yampolskii, Y.; Shantarovich, V. Gas permeation parameters and other physicochemical properties of a polymer of intrinsic microporosity: Polybenzodioxane PIM-1. J. Membr. Sci. 2008, 325, 851–860. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Y.; Fan, B.; Carta, M.; Malpass-Evans, R.; McKeown, N.B.; Marken, F. Polymer of intrinsic microporosity (PIM) films and membranes in electrochemical energy storage and conversion: A mini-review. Electrochem. Commun. 2020, 118, 106798. [Google Scholar] [CrossRef]
- Thomas, S.; Pinnau, I.; Du, N.; Guiver, M.D. Pure- and mixed-gas permeation properties of a microporous spirobisindane-based ladder polymer (PIM-1). J. Membr. Sci. 2009, 333, 125–131. [Google Scholar] [CrossRef]
- Foster, A.B.; Beal, J.L.; Tamaddondar, M.; Luque-Alled, J.M.; Robertson, B.; Mathias, M.; Gorgojo, P.; Budd, P.M. Importance of small loops within PIM-1 topology on gas separation selectivity in thin film composite membranes. J. Mater. Chem. A 2021, 9, 21807–21823. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Tan, X. High Performance of PIM-1/ZIF-8 Composite Membranes for O2/N2 Separation. ACS Omega 2019, 4, 16572–16577. [Google Scholar] [CrossRef]
- Staiger, C.L.; Pas, S.J.; Hill, A.J.; Cornelius, C.J. Gas Separation, Free Volume Distribution, and Physical Aging of a Highly Microporous Spirobisindane Polymer. Chem. Mater. 2008, 20, 2606–2608. [Google Scholar] [CrossRef]
- Larsen, G.S.; Lin, P.; Hart, K.E.; Colina, C.M. Molecular Simulations of PIM-1-like Polymers of Intrinsic Microporosity. Macromolecules 2011, 44, 6944–6951. [Google Scholar] [CrossRef]
- Song, J.; Du, N.; Dai, Y.; Robertson, G.P.; Guiver, M.D.; Thomas, S.; Pinnau, I. Linear High Molecular Weight Ladder Polymers by Optimized Polycondensation of Tetrahydroxytetramethylspirobisindane and 1,4-Dicyanotetrafluorobenzene. Macromolecules 2008, 41, 7411–7417. [Google Scholar] [CrossRef]
- McKeown, N.B. Polymers of Intrinsic Microporosity (PIMs). Polymer 2020, 202, 122736. [Google Scholar] [CrossRef]
- Sizov, V.E.; Zefirov, V.V.; Volkova, Y.A.; Gusak, D.I.; Kharitonova, E.P.; Ponomarev, I.I.; Gallyamov, M.O. Celgard/PIM-1 proton conducting composite membrane with reduced vanadium permeability. J. Appl. Polym. Sci. 2022, 139, 51985. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Moore, R.B. State of Understanding of Nafion. Chem. Rev. 2004, 104, 4535–4585. [Google Scholar] [CrossRef] [PubMed]
- Karimi, M.B.; Mohammadi, F.; Hooshyari, K. Recent approaches to improve Nafion performance for fuel cell applications: A review. Int. J. Hydrogen Energy 2019, 44, 28919–28938. [Google Scholar] [CrossRef]
- Peron, J.; Mani, A.; Zhao, X.; Edwards, D.; Adachi, M.; Soboleva, T.; Shi, Z.; Xie, Z.; Navessin, T.; Holdcroft, S. Properties of Nafion® NR-211 membranes for PEMFCs. J. Membr. Sci. 2010, 356, 44–51. [Google Scholar] [CrossRef]
- De Almeida, S.H.; Kawano, Y. Thermal Behavior of Nafion Membranes. J. Therm. Anal. Calorim. 1999, 58, 569–577. [Google Scholar] [CrossRef]
- Schalenbach, M.; Hoefner, T.; Paciok, P.; Carmo, M.; Wiebke, W.; Stolten, D. Gas Permeation through Nafion. Part 1: Measurements. J. Phys. Chem. C 2015, 119, 25145–25155. [Google Scholar] [CrossRef]
- Wainright, J.S.; Wang, J.-T.; Weng, D.; Savinell, R.F.; Litt, M. Acid-Doped Polybenzimidazoles: A New Polymer Electrolyte. J. Electrochem. Soc. 1995, 142, L121. [Google Scholar] [CrossRef]
- Bouchet, R.; Siebert, E. Proton conduction in acid doped polybenzimidazole. Solid State Ionics 1999, 118, 287–299. [Google Scholar] [CrossRef]
- Mader, J.; Xiao, L.; Schmidt, T.J.; Benicewicz, B.C. Polybenzimidazole/Acid Complexes as High-Temperature Membranes. In Fuel Cells II. Advances in Polymer Science; Scherer, G.G., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 216, pp. 63–124. [Google Scholar] [CrossRef]
- Pu, H.; Meyer, W.H.; Wegner, G. Proton transport in polybenzimidazole blended with H3PO4 or H2SO4. J. Polym. Sci. B Polym. Phys. 2002, 40, 663–669. [Google Scholar] [CrossRef]
- Lobato, J.; Cañizares, P.; Rodrigo, M.A.; Linares, J.J.; Aguilar, J.A. Improved polybenzimidazole films for H3PO4-doped PBI-based high temperature PEMFC. J. Membr. Sci. 2007, 306, 47–55. [Google Scholar] [CrossRef]
- Bitter, J.H.; Tashvigh, A.A. Recent Advances in Polybenzimidazole Membranes for Hydrogen Purification. Ind. Eng. Chem. Res. 2022, 61, 6125–6134. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Ponomarev, I.I.; Petrovskii, P.V.; Volkova, Y.A.; Razorenov, D.Y.; Goryunova, I.B.; Starikova, Z.A.; Fomenkov, A.I.; Khokhlov, A.R. Synthesis of N-phosphonoethylated cardo poly(benzimidazole) and testing of proton-conducting membranes made of it. Dokl. Chem. 2010, 432, 168–174. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Blagodatskikh, I.V.; Muranov, A.V.; Volkova, Y.A.; Razorenov, D.Y.; Ponomarev, I.I.; Skupov, K.M. Dimethyl sulfoxide as a green solvent for successful precipitative polyheterocyclization based on nucleophilic aromatic substitution, resulting in high molecular weight PIM-1. Mendeleev Commun. 2016, 26, 362–364. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Blagodatskikh, I.V.; Muranov, A.V.; Volkova, Y.A.; Razorenov, D.Y.; Ponomarev, I.I.; Skupov, K.M. Ultrasonic Activation of PIM-1 Synthesis and Properties of Polymers Obtained by Precipitation Polyheterocyclization in Dimethyl Sulfoxide. Polym. Sci. Ser. C 2020, 62, 259–265. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Baurmeister, J. Properties of high-temperature PEFC Celtec®-P 1000 MEAs in start/stop operation mode. J. Power Sources 2008, 176, 428–434. [Google Scholar] [CrossRef]
- Kondratenko, M.S.; Ponomarev, I.I.; Gallyamov, M.O.; Razorenov, D.Y.; Volkova, Y.A.; Kharitonova, E.P.; Khokhlov, A.R. Novel composite Zr/PBI-O-PhT membranes for HT-PEFC applications. Beilstein J. Nanotechnol. 2013, 4, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Ponomarev, I.I.; Skupov, K.M.; Modestov, A.D.; Lysova, A.A.; Ponomarev, I.I.; Vtyurina, E.S. Cardo polybenzimidazole (PBI-O-PhT) based membrane rein-forced with m-polybenzimidazole electrospun nanofiber mat for HT-PEM fuel cell applications. Membranes 2022, 12, 956. [Google Scholar] [CrossRef]
- Lysova, A.A.; Ponomarev, I.I.; Skupov, K.M.; Vtyurina, E.S.; Lysov, K.A.; Yaroslavtsev, A.B. Effect of Organo-Silanes Structure on the Properties of Silane-Crosslinked Membranes Based on Cardo Polybenzimidazole PBI-O-PhT. Membranes 2022, 12, 1078. [Google Scholar] [CrossRef]
- Rouquerol, J.; Rouquerol, F.; Sing, K.S.W.; Llewellyn, P.; Maurin, G. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications, 2nd ed.; Academic Press: Amsterdam, The Netherlands, 2012. [Google Scholar] [CrossRef]
- Linares-Solano, A.; Stoeckli, F. Commentary on the paper “On the adsorption affinity coefficient of carbon dioxide in microporous carbons” by E.S. Bickford et al. (Carbon 2004; 42: 1867–71). Carbon 2005, 43, 658–660. [Google Scholar] [CrossRef]
- Lysova, A.A.; Ponomarev, I.I.; Volkova, Y.A.; Ponomarev, I.I.; Yaroslavtsev, A.B. Effect of Phosphorylation of Polybenzimidazole on Its Conductive Properties. Pet. Chem. 2018, 58, 958–964. [Google Scholar] [CrossRef]
- Lozano-Castelló, D.; Cazorla-Amorós, D.; Linares-Solano, A. Usefulness of CO2 adsorption at 273 K for the characterization of porous carbons. Carbon 2004, 42, 1233–1242. [Google Scholar] [CrossRef]


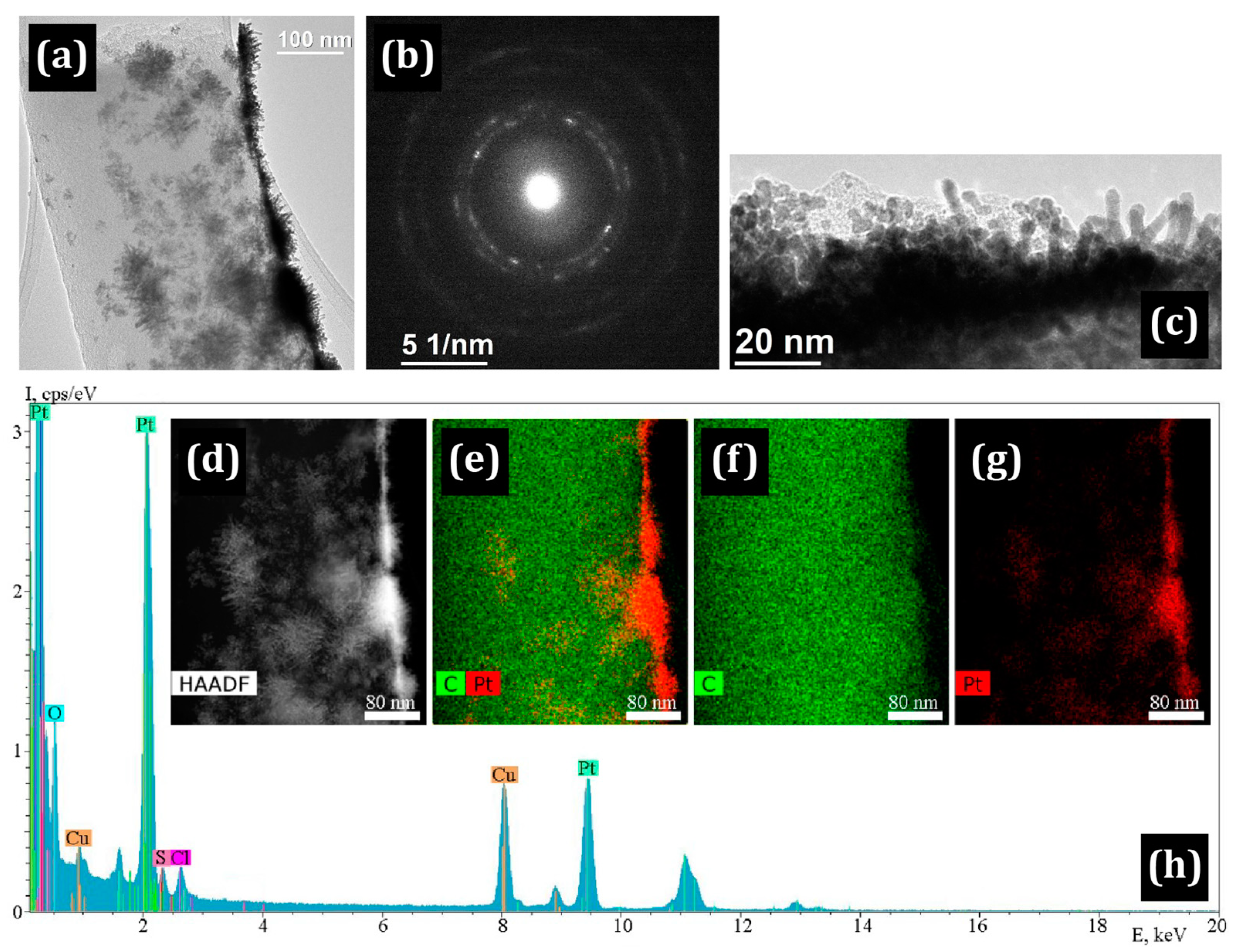

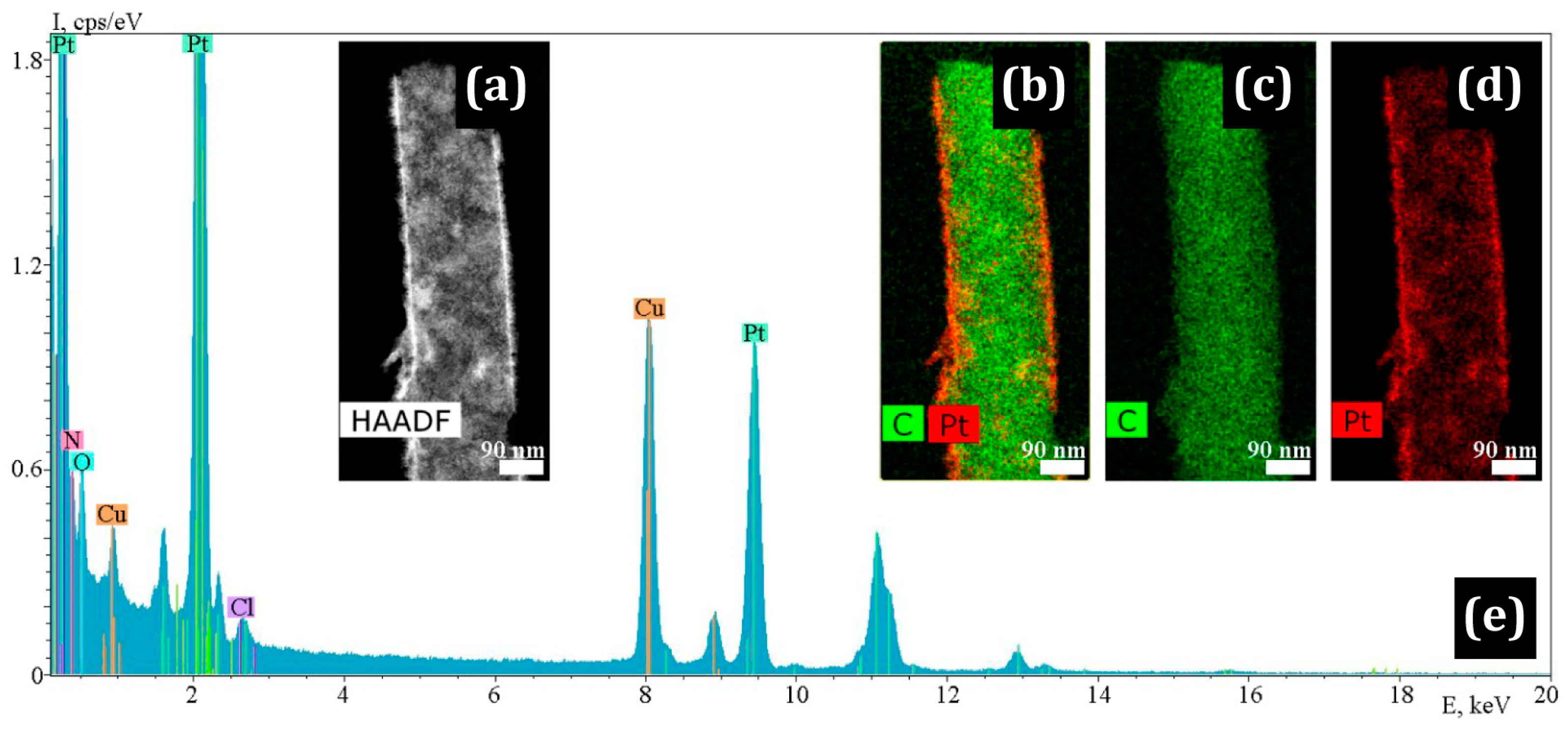
| Sample | σ, S/cm | %C | %N | %H | %Zr |
|---|---|---|---|---|---|
| CNF | 24.7 | 82.9 | 5.24 | 1.47 | 0.2 |
| Sample | DR | NL-DFT | GCMC | ||||
|---|---|---|---|---|---|---|---|
| V, cm3 g−1 | E, kJ mol−1 | D, nm | S, m2 g−1 | V, cm3 g−1 | S, m2 g−1 | V, cm3 g−1 | |
| CNF | 0.194 | 20.99 | 1.24 | 310 | 0.107 | 341 | 0.135 |
| Sample | Rmemb (160 °C), mΩ cm2 | Rmemb (180 °C), mΩ cm2 | IH2 crossover (160 °C), mA cm−2 | IH2 crossover (180 °C), mA cm−2 |
|---|---|---|---|---|
| Pt/CNF | 59.0 ± 0.5 | 65.0 ± 0.5 | 4.8 ± 0.1 | 5.4 ± 0.1 |
| Pt/PBI-O-PhT-P/CNF | 61.0 ± 0.5 | 69.0 ± 0.5 | 1.1 ± 0.1 | 1.3 ± 0.1 |
| Pt/PIM-1/CNF | n/d | n/d | n/d | n/d |
| Pt/Nafion/CNF | 91.2 ± 0.5 | 90.5 ± 0.5 | n/d | n/d |
| PBI-O-PhT-P/Pt/CNF | 78.5 ± 0.5 | 76.0 ± 0.5 | 0.2 ± 0.1 | 0.3 ± 0.1 |
| PIM-1/Pt/CNF | 71.1 ± 0.5 | 68.5 ± 0.5 | n/d | n/d |
| Nafion/Pt/CNF | 82.0 | 80.0 | 0.2 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skupov, K.M.; Ponomarev, I.I.; Vtyurina, E.S.; Volkova, Y.A.; Ponomarev, I.I.; Zhigalina, O.M.; Khmelenin, D.N.; Cherkovskiy, E.N.; Modestov, A.D. Proton-Conducting Polymer-Coated Carbon Nanofiber Mats for Pt-Anodes of High-Temperature Polymer-Electrolyte Membrane Fuel Cell. Membranes 2023, 13, 479. https://doi.org/10.3390/membranes13050479
Skupov KM, Ponomarev II, Vtyurina ES, Volkova YA, Ponomarev II, Zhigalina OM, Khmelenin DN, Cherkovskiy EN, Modestov AD. Proton-Conducting Polymer-Coated Carbon Nanofiber Mats for Pt-Anodes of High-Temperature Polymer-Electrolyte Membrane Fuel Cell. Membranes. 2023; 13(5):479. https://doi.org/10.3390/membranes13050479
Chicago/Turabian StyleSkupov, Kirill M., Igor I. Ponomarev, Elizaveta S. Vtyurina, Yulia A. Volkova, Ivan I. Ponomarev, Olga M. Zhigalina, Dmitry N. Khmelenin, Evgeny N. Cherkovskiy, and Alexander D. Modestov. 2023. "Proton-Conducting Polymer-Coated Carbon Nanofiber Mats for Pt-Anodes of High-Temperature Polymer-Electrolyte Membrane Fuel Cell" Membranes 13, no. 5: 479. https://doi.org/10.3390/membranes13050479
APA StyleSkupov, K. M., Ponomarev, I. I., Vtyurina, E. S., Volkova, Y. A., Ponomarev, I. I., Zhigalina, O. M., Khmelenin, D. N., Cherkovskiy, E. N., & Modestov, A. D. (2023). Proton-Conducting Polymer-Coated Carbon Nanofiber Mats for Pt-Anodes of High-Temperature Polymer-Electrolyte Membrane Fuel Cell. Membranes, 13(5), 479. https://doi.org/10.3390/membranes13050479









