Aliquots of MIL-140 and Graphene in Smart PNIPAM Mixed Hydrogels: A Nanoenvironment for a More Eco-Friendly Treatment of NaCl and Humic Acid Mixtures by Membrane Distillation
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Membrane Preparation
2.3. Methods
2.4. Membrane Distillation Tests
3. Results and Discussion
3.1. Membrane Fabrication
3.2. Characterization of LAN Complexes
3.3. Membrane Distillation Testing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muluneh, M.G. Impact of climate change on biodiversity and food security: A global perspective. Agric. Food Secur. 2021, 10, 36. [Google Scholar] [CrossRef]
- Adejumoke, A.I.; Babatunde, O.A.; Abimbola, P.O.; Tabitha, A.A.-A.; Adewumi, O.D.; Toyin, A.O. Water Pollution: Effects, Prevention, and Climatic Impact. In Water Challenges of an Urbanizing World; Glavan, M., Ed.; Intechopen: London, UK, 2019. [Google Scholar]
- Qu, X.; Shi, L.; Qu, X.; Qiu, M.; Gao, W.; Wang, J. Evaluation of Groundwater Resources and Exploitation Potential: A Case from Weifang City of Shandong Province in China. ACS Omega 2021, 6, 10592–10606. [Google Scholar] [CrossRef]
- Macknick, J.; Newmark, R.; Heath, G.; Hallett, K.C. A Review of Operational Water Consumption and Withdrawal Factors for Electricity Generating Technologies; Technical Report NREL/TP-6A20-50900; IOPscience: Bristol, UK, 2011. [Google Scholar]
- Boretti, A.; Rosa, L. Reassessing the projections of the World Water Development Report. Npj Clean Water 2019, 2, 15. [Google Scholar] [CrossRef][Green Version]
- Fu, G.; Jin, Y.; Sun, S.; Yuan, Z.; Butler, D. The role of deep learning in urban water management: A critical review. Water Res. 2022, 223, 118973. [Google Scholar] [CrossRef] [PubMed]
- Gugliuzza, A.; Basile, A. Membranes for Clean and Renewable Power Applications; Gugliuzza, A., Basile, A., Eds.; Woodhead Publishing: Cambridge, UK, 2014; pp. 1–410. [Google Scholar]
- Cosgrove, W.J.; Loucks, D.P. Water management: Current and future challenges and research directions. Water Resour. Res. 2015, 51, 4823–4839. [Google Scholar] [CrossRef][Green Version]
- González, D.; Amigo, J.; Suáre, F. Membrane distillation: Perspectives for sustainable and improved desalination. Renew. Sustain. Energy Rev. 2017, 80, 238–259. [Google Scholar] [CrossRef]
- Khayet, M. Solar desalination by membrane distillation: Dispersion in energy consumption analysis and water production costs (a review). Desalination 2013, 308, 89–101. [Google Scholar] [CrossRef]
- Ashoor, B.B.; Mansour, S.; Giwa, A.; Dufour, V.; Hasan, S.W. Principles and applications of direct contact membrane distillation (DCMD): A comprehensive review. Desalination 2016, 398, 222–246. [Google Scholar] [CrossRef]
- Speranza, V.; Trotta, F.; Drioli, E.; Gugliuzza, A. High-definition polymeric membranes: Construction of 3d lithographed channel arrays through control of natural building blocks dynamics. ACS Appl. Mater. Interf. 2010, 2, 459–466. [Google Scholar] [CrossRef]
- Perrotta, M.L.; Saielli, G.; Casella, G.; Macedonio, F.; Giorno, L.; Drioli, E.; Gugliuzza, A. An ultrathin suspended hydrophobic porous membrane for high-efficiency water desalination. Appl. Mater. Today 2017, 9, 1–9. [Google Scholar] [CrossRef]
- Rasool, M.; Banat, Q.F. Desalination by solar powered membrane distillation systems. Desalination 2013, 308, 186–197. [Google Scholar]
- Usman, H.S.; Touati, K.; Rahaman, M.S. An economic evaluation of renewable energy-powered membrane distillation for desalination of brackish water. Renew. Energy 2021, 169, 1294–1304. [Google Scholar] [CrossRef]
- Olatunji, S.O.; Camacho, L.M. Heat and Mass Transport in Modeling Membrane Distillation Configurations: A Review. Front. Energy Res. 2018, 6, 130. [Google Scholar] [CrossRef]
- Abdallah, S.B.; Frikha, N.; Gabsi, S. Design of an autonomous solar desalination plant using vacuum membrane distillation, the MEDINA project. Chem. Eng. Res. Des. 2013, 91, 2782–2788. [Google Scholar] [CrossRef]
- Khayet, M.; Matsuura, T. Preparation and Characterization of Polyvinylidene Fluoride Membranes for Membrane Distillation. Ind. Eng. Chem. Res. 2001, 40, 5710–5718. [Google Scholar] [CrossRef]
- Francis, L.; Ejaz, F.; Nidal Hilal, A. Electrospun membranes for membrane distillation: The state of play and recent advances. Desalination 2022, 526, 115511. [Google Scholar] [CrossRef]
- Yeszhanov, A.B.; Korolkov, I.V.; Dosmagambetova, S.S.; Zdorovets, M.V.; Güven, O. Recent Progress in the Membrane Distillation and Impact of Track-Etched Membranes. Polymers 2021, 13, 2520. [Google Scholar] [CrossRef]
- Gugliuzza, A.; Basile, A. Membrane contactors: Fundamentals, membrane materials and key operations. In Handbook of Membrane Reactors; Basile, A., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands; Woodhead Publishing Limited: Cambridge, UK, 2013; Volume 2, p. 54. [Google Scholar]
- García-Fernández, L.; Khayet, M.; García-Payo, M.C. Membranes used in membrane distillation: Preparation and characterization. In Pervaporation, Vapour Permeation and Membrane Distillation Principles and Applications; Basile, A., Figoli, A., Khayet, M., Eds.; Woodhead Publishing Series in Energy; Woodhead Publishing Limited: Cambridge, UK, 2015; p. 317. [Google Scholar]
- Frappa, M.; Castillo del Rio, A.E.; Macedonio, F.; Di Luca, G.; Drioli, E.; Gugliuzza, A. Exfoliated Bi2Te3-enabled membranes for new concept water desalination: Freshwater production meets new routes. Water Res. 2021, 203, 117503. [Google Scholar] [CrossRef]
- Liu, L.; Xiao, Z.; Liu, Y.; Li, X.; Yin, H.; Volkov, A.; He, T. Understanding the fouling/scaling resistance of superhydrophobic/omniphobic membranes in membrane distillation. Desalination 2021, 499, 114864. [Google Scholar] [CrossRef]
- Bilad, M.R.; Al Marzooqi, F.A.; Arafat, H.A. New Concept for Dual-Layer Hydrophilic/Hydrophobic Composite Membrane for Membrane Distillation. J. Membr. Separ. Technol. 2015, 4, 122–133. [Google Scholar]
- Frappa, M.; Del Rio Castillo, A.E.; Macedonio, F.; Politano, A.; Drioli, E.; Bonaccorso, F.; Pellegrini, V.; Gugliuzza, A. Few-layer graphene for advanced composite PVDF membranes dedicated to water distillation: A comparative study. Nanoscale Adv. 2020, 2, 4728–4739. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.; Yang, H.; Wu, S.; Xiong, G.; Yan, J.; Cen, K.; Bo, Z.; Ostrikov, K. Graphene Array-Based Anti-fouling Solar Vapour Gap Membrane Distillation with High Energy Efficiency. Nano-Micro Lett. 2019, 11, 51. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mao, Y.; Huang, Q.; Meng, B.; Zhou, K.; Liu, G.; Gugliuzza, A.; Drioli, E.; Jin, W. Roughness-enhanced hydrophobic graphene oxide membrane for water desalination via membrane distillation. J. Membr. Sci. 2020, 611, 118364. [Google Scholar] [CrossRef]
- Liao, X.; Goh, K.; Liao, Y.; Wang, R.; Razaqpur, A.G. Bio-inspired super liquid-repellent membranes for membrane distillation: Mechanisms, fabrications and applications. Adv. Coll. Interf. Sci. 2021, 297, 102547. [Google Scholar] [CrossRef]
- Gugliuzza, A.; Drioli, E. Role of additives in the water vapor transport through block co-poly(amide/ether) membranes: Effects on surface and bulk polymer properties. Eur. Polym. J. 2004, 40, 2381–2389. [Google Scholar] [CrossRef]
- Wang, W.; Du, X.; Vahabi, H.; Zhao, S.; Yin, Y.; Kota, A.K.; Tong, T. Trade-off in membrane distillation with monolithic omniphobic membranes. Nat. Comm. 2019, 10, 3220. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.; Shena, F.; Cao, W.; Wan, Y. YHydrophilic/hydrophobic Janus membranes with a dual-function surface coating for rapid and robust membrane distillation desalination. Desalination 2020, 491, 114561. [Google Scholar] [CrossRef]
- Öjemyr, L.N.; Lee, H.J.; Gennis, R.B.; Brzezinski, P. Functional interactions between membrane-bound transporters and membranes. Proc. Natl. Acad. Sci. USA 2010, 107, 15763. [Google Scholar] [CrossRef][Green Version]
- Georgouvelas, D.; Abdelhamida, H.N.; Li, J.; Edlund, U.; Mathew, A.P. All-cellulose functional membranes for water treatment: Adsorption of metal ions and catalytic decolorization of dyes. Carbohydr. Polym. 2021, 264, 118044. [Google Scholar] [CrossRef]
- Gontarek-Castro, E.; Di Luca, G.; Lieder, M.; Gugliuzza, A. Graphene-Coated PVDF Membranes: Effects of Multi-Scale Rough Structure on Membrane Distillation Performance. Membranes 2022, 12, 511. [Google Scholar] [CrossRef]
- De Luca, G.; Gugliuzza, A.; Drioli, E. Competitive hydrogen-bonding interactions in modified polymer membranes: A density functional theory investigation. J. Phys. Chem. 2009, 113, 5473–5477. [Google Scholar] [CrossRef]
- Teng, J.; Shen, L.; He, Y.; Liao, B.-Q.; Wu, G.; Lin, H. Novel insights into membrane fouling in a membrane bioreactor: Elucidating interfacial interactions with real membrane surface. Chemosphere 2018, 210, 769–778. [Google Scholar] [CrossRef]
- Pingitore, V.; Miriello, D.; Drioli, E.; Gugliuzza, A. Integrated carboxylic carbon nanotubes pathway with membranes for voltage-activated humidity detection and microclimate regulation. Soft Matter 2015, 11, 4461–4468. [Google Scholar] [CrossRef]
- Zielinska, D.; Radecka, H.; Radecki, J. Contribution of membrane surface charge in the interaction of lead and tin derivatives with model lipid membrane. Chemosphere 2000, 40, 327–330. [Google Scholar] [CrossRef]
- Perrotta, M.L.; Macedonio, F.; Tocci, E.; Giorno, L.; Drioli, E.; Gugliuzza, A. Graphene stimulates the nucleation and growth rate of NaCl crystals from hypersaline solution via membrane crystallization. Environ. Sci. Water Res. Technol. 2020, 6, 1723–1736. [Google Scholar] [CrossRef]
- Hoek, E.M.V.; Bhattacharjee, S.; Elimelech, M. Effect of Membrane Surface Roughness on Colloid-Membrane DLVO Interactions. Langmuir 2003, 19, 4836–4847. [Google Scholar] [CrossRef]
- Curcio, S.; Petrosino, F.; Morrone, M.; De Luca, G. Interactions between Proteins and the Membrane Surface in Multiscale Modeling of Organic Fouling. J. Chem. Inf. Model. 2018, 58, 1815–1827. [Google Scholar] [CrossRef]
- Gugliuzza, A.; Fabiano, R.; Garavaglia, M.G.; Spisso, A.; Drioli, E. Study of the surface character as responsible for controlling interfacial forces at membrane-feed interface. J. Coll. Interf. Sci. 2006, 303, 388–403. [Google Scholar] [CrossRef]
- Tanaka, M. Interplays of Interfacial Forces Modulate Structure and Function of Soft and Biological Matters in Aquatic Environments. Front. Chem. 2020, 8, 165. [Google Scholar] [CrossRef][Green Version]
- Titus, A.R.; Ferreira, L.A.; Belgovskiy, A.I.; Kooijman, E.E.; Mann, E.K.; Mann Jr, J.A.; Meyer, W.V.; Smart, A.E.V.; Uverskygh, N.; Zaslavsky, B.Y. Interfacial tension and mechanism of liquid–liquid phase separation in aqueous media. Phys. Chem. Chem. Phys. 2020, 22, 4574–4580. [Google Scholar] [CrossRef]
- Fengler, C.; Arens, L.; Horn, H.; Wilhelm, M. Desalination of Seawater Using Cationic Poly(acrylamide) Hydrogels and Mechanical Forces for Separation. Macromol. Mater. Eng. 2020, 305, 2000383. [Google Scholar] [CrossRef]
- Saravia, F.; Zwiener, C.; Frimmel, F.H. Interactions between membrane surface, dissolved organic substances and ions in submerged membrane filtration. Desalination 2006, 192, 280–287. [Google Scholar] [CrossRef]
- Rana, D.; Matsuura, T. Surface Modifications for Antifouling Membranes. Chem. Rev. 2010, 110, 2448–2471. [Google Scholar] [CrossRef] [PubMed]
- Dakheel, A.; Darwish, N.A.; Hilal, N. A Review of Colloidal Interactions in Membrane Separation. Iran. J. Energy Environ. 2010, 1, 144–159. [Google Scholar]
- Decher, G.; Eckle, M.; Schmitt, J.; Struth, B. Layer-by-layer assembly multicomposite films. Curr. Opin. Coll. Interf. Sci. 1998, 3, 32–39. [Google Scholar] [CrossRef]
- Pingitore, V.; Gugliuzza, A. Fabrication of porous semiconductor interfaces by pH-driven assembly of carbon nanotubes on honeycomb structured membranes. J. Phys. Chem. 2013, 117, 26562–26572. [Google Scholar] [CrossRef]
- Yilong, H.; Giorno, L.; Gugliuzza, A. Photoactive gel for assisted cleaning during olive mill wastewater membrane microfiltration. Membranes 2017, 7, 66. [Google Scholar]
- Cho, K.L.; Lomas, H.A.J.; Hill, F.; Caruso, S.E. Kentish, Spray Assembled, Cross-Linked Polyelectrolyte Multilayer Membranes for Salt Removal. Langmuir 2014, 29, 8784–8790. [Google Scholar] [CrossRef]
- Arslan, M.; Dönmez, G.; Ergün, A.; Okutan, M.; Arı, G.A.; Deligöz, H. Preparation, Characterization, and Separation Performances of Novel Surface Modified LbL Composite Membranes from Polyelectrolyte Blends and MWCNT. Polym. Eng. Sci. 2020, 60, 346–351. [Google Scholar] [CrossRef]
- Xu, G.R.; Wang, S.H.; Zhao, H.L.; Wu, S.B.; Xu, J.L.; Liu, X.Y. Layer-by-layer (LBL) assembly technology as promising strategy for tailoring pressure-driven desalination membranes. J. Membr. Sci. 2015, 493, 428–443. [Google Scholar] [CrossRef]
- Qi, S.; Tang, C.Y. Cross-Linked Layer-by-Layer Membranes. In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Krizak, D.; Abbaszadeh, M.; Kundu, S. Desalination membranes by deposition of polyamide on polyvinylidene fluoride supports using the automated layer-by-layer technique. Sep. Sci. Technnol. 2022, 57, 1119–1127. [Google Scholar] [CrossRef]
- Starr, B.J.; Tarabara, V.V.; Herrera-Robledo, M.; Zhou, M.; Roualdes, S.A.; Ayral, A. Coating porous membranes with a photocatalyst: Comparison of LbL self-assembly and plasma-enhanced CVD techniques. J. Membr. Sci. 2016, 514, 340–349. [Google Scholar] [CrossRef][Green Version]
- Gu, L.; Xie, M.-Y.; Jin, Y.; He, M.; Xing, X.-Y.; Yu, Y.; Wu, Q.-Y. Construction of Antifouling Membrane Surfaces through Layer-by-Layer Self-Assembly of Lignosulfonate and Polyethyleneimine. Polymers 2019, 11, 1782. [Google Scholar] [CrossRef][Green Version]
- Wågberg, L.; Erlandsson, J. The Use of Layer-by-Layer Self-Assembly and Nanocellulose to Prepare Advanced Functional Materials. Adv. Mater. 2021, 33, 2001474. [Google Scholar] [CrossRef]
- Gontarek, E.; Macedonio, F.; Militano, F.; Giorno, L.; Lieder, M.; Politano, A.; Drioli, E.; Gugliuzza, A. Adsorption-assisted transport of water vapour in super-hydrophobic membranes filled with multilayer graphene platelets. Nanoscale 2019, 11, 11521–11529. [Google Scholar] [CrossRef]
- Safaei, J.; Xiong, P.; Wang, G. Progress and prospects of two-dimensional materials for membrane-based water desalination. Mater. Today Adv. 2020, 8, 10010. [Google Scholar] [CrossRef]
- Seo, D.H.; Pineda, S.; Woo, Y.C.; Xie, M.; Murdock, A.T.; Ang, E.Y.M.; Jiao, Y.; Park, M.J.; Lim, S.I.; Lawn, M.; et al. Anti-fouling graphene-based membranes for effective water desalination. Nat. Comm. 2018, 9, 683. [Google Scholar] [CrossRef][Green Version]
- Yang, J.; Li, Z.; Wang, Z.; Yuan, S.; Li, Y.; Zhao, W.; Zhan, X. 2D Material Based Thin-Film Nanocomposite Membranes for Water Treatment. Adv. Mater. Technol. 2021, 6, 2000862. [Google Scholar] [CrossRef]
- Fatima, J.; Noor Shah, A.; Bilal Tahir, M.; Mehmood, T.; Ali Shah, A.; Tanveer, M.; Nazir, R.; Jan, B.L.; Alansi, S. Tunable 2D Nanomaterials; Their Key Roles and Mechanisms in Water Purification and Monitoring. Front. Environ. Sci. 2022, 10, 766743. [Google Scholar] [CrossRef]
- Aqra, M.W.; Ramanathan, A.A. Graphene and related 2D materials for desalination: A review of recent patents. Jordan J. Phys. 2020, 13, 233–242. [Google Scholar] [CrossRef]
- Cao, Z.; Liu, V.; Farimani, A.B. Water Desalination with Two-Dimensional Metal−Organic Framework Membranes. Nano Lett. 2019, 19, 8638–8643. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, A.; Ismadji, S.; Soetaredjo, F.E.; Santoso, S.P.; Yuliansa, M.; Anggorowati, A.A. Metal-organic framework-based processes for water desalination: Current development and future prospects. In Aquananotechnology Applications of Nanomaterials for Water Purification; Abd-Elsalam, K.A., Zahid, M., Eds.; Micro Nano Technol Series; Elsevier: Amsterdam, The Netherlands, 2021; p. 491. [Google Scholar]
- Wan, L.; Zhou, C.; Xu, K.; Feng, B.; Huang, A. Synthesis of highly stable UiO-66-NH2 membranes with high ions rejection for seawater desalination. Microporous Mesoporous Mater. 2017, 252, 207–213. [Google Scholar] [CrossRef]
- Zhu, J.; Qin, L.; Uliana, A.; Hou, J.; Wang, J.; Zhang, Y.; Li, X.; Yuan, S.; Li, J.; Tian, M.; et al. Elevated performance of thin film nanocomposite membranes enabled by modified hydrophilic MOFs for nanofiltration. ACS Appl. Mater. Interf. 2017, 9, 1975–1986. [Google Scholar] [CrossRef] [PubMed]
- Werber, J.R.; Osuji, C.O.; Elimelech, M. Materials for next-generation desalination and water purification membranes. Nat. Rev. Mater. 2016, 1, 16018. [Google Scholar] [CrossRef]
- Yang, F.; Efome, J.E.; Rana, D.; Matsuura, T.; Lan, C. Metal−Organic Frameworks Supported on Nanofiber for Desalination by Direct Contact Membrane Distillation. ACS Appl. Mater. Interf. 2018, 10, 11251–11260. [Google Scholar] [CrossRef]
- Efome, J.E.; Rana, D.; Matsuura, T.; Yang, F.; Cong, Y.; Lan, C.Q. Triple-Layered Nanofibrous Metal−Organic Framework-Based Membranes for Desalination by Direct Contact Membrane Distillation. ACS Sustain. Chem. Eng. 2020, 8, 6601–6610. [Google Scholar] [CrossRef]
- Cheng, D.; Zhao, L.; Li, N.; Smith, S.J.D.D.; Wu, J.; Zhang, J.; Ng, D.; Wu, C.; Martinez, M.R.; Batten, M.P.; et al. Aluminum fumarate MOF/PVDF hollow fiber membrane for enhancement of water flux and thermal efficiency in direct contact membrane distillation. J. Membr. Sci. 2019, 588, 117204. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Zhang, J.; Tang, L.; Luo, L.; Zeng, G. Iron containing metal-organic frameworks: Structure, synthesis, and applications in environmental remediation. ACS Appl. Mater. Interf. 2017, 9, 20255–20275. [Google Scholar] [CrossRef]
- Duan, J.; Pan, Y.; Liu, G.; Jin, W. Metal-organic framework adsorbents and membranes for separation applications. Curr. Opin. Chem. Eng. 2018, 20, 122–131. [Google Scholar] [CrossRef]
- Yang, D.; Gates, B.C. Catalysis by metal organic frameworks: Perspective and suggestions for future research. ACS Catal. 2019, 9, 1779–1798. [Google Scholar] [CrossRef]
- Xia, W.; Mahmood, A.; Zou, R.; Xu, Q. Metal organic frameworks and their derived nanostructures for electrochemical energy storage and conversion. Energy Environ. Sci. 2015, 8, 1837–1866. [Google Scholar] [CrossRef]
- Chen, X.; Tong, R.; Shi, Z.; Yang, B.; Liu, H.; Ding, S.; Wang, X.; Lei, Q.; Wu, J.; Fang, W. MOF nanoparticles with encapsulated autophagy inhibitor in controlled drug delivery system for antitumor. ACS Appl. Mater. Inter. 2018, 10, 2328–2337. [Google Scholar] [CrossRef]
- Zuo, J.; Chung, T.-S. Metal–Organic Framework-Functionalized Alumina Membranes for Vacuum Membrane Distillation. Water 2016, 8, 586. [Google Scholar] [CrossRef][Green Version]
- Dedecker, K.; Pillai, R.S.; Nouar, F.; Pires, J.; Steunou, N.; Dumas, E.; Maurin, G.; Serre, C.; Pinto, M.L. Metal-organic frameworks for cultural heritage preservation: The case of acetic acid removal. J. ACS Appl. Mater. Interf. 2018, 10, 13886–13894. [Google Scholar] [CrossRef]
- Nakayama, M.; Okano, T.; Winnik, F.M. Poly(N-isopropylacrylamide)-based smart surfaces for cell sheet tissue engineering. Mater. Matters 2010, 5, 56. [Google Scholar]
- Jain, K.; Vedarajan, R.; Watanabe, M.; Ishikiriyama, M.; Matsumi, N. Tunable LCST behavior of poly(N-isopropylacrylamide/ionic liquid) copolymers. Polym. Chem. 2015, 6, 6819–6825. [Google Scholar] [CrossRef]
- Sarkar, N. Kinetics of thermal gelation of methylcellulose and hydroxypropylmethylcellulose in aqueous solutions. Carbohydr. Polym. 1995, 26, 195–203. [Google Scholar] [CrossRef]
- Costa, M.C.M.; Silva, S.M.C.; Antunes, F.E. Adjusting the low critical solution temperature of poly(N-isopropylacrylamide) solutions by salts, ionic surfactants and solvents: A rheological study. J. Mol. Liq. 2015, 210, 113–118. [Google Scholar] [CrossRef]
- Tauer, K.; Gau, D.; Schulze, S.; Völkel, A.; Dimova, R. Thermal property changes of poly(N-isopropylacrylamide) microgel particles and block copolymers. Colloid Polym. Sci. 2009, 287, 299–312. [Google Scholar] [CrossRef][Green Version]
- Gao, X.; Cao, Y.; Song, X.; Zhang, Z.; Xiao, C.; He, C.; Chena, X. pH- and thermo-responsive poly(N-isopropylacrylamideco-acrylic acid derivative) copolymers and hydrogels with LCST dependent on pH and alkyl side groups. J. Mater. Chem. B 2013, 1, 5578–5587. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus Chemistry; John Wiley & Sons: Hoboken, NY, USA, 1982; pp. 1–443. [Google Scholar]
- Hou, J.; Wang, H.; Zhang, H. Zirconium Metal–Organic Framework Materials for Efficient Ion Adsorption and Sieving. Ind. Eng. Chem. Res. 2020, 59, 12907–12923. [Google Scholar] [CrossRef]
- Furukawa, H.; Gándara, F.; Zhang, Y.B.; Jiang, J.; Queen, W.L. Water Adsorption in Porous Metal−Organic Frameworks and Related Materials. J. Am. Chem. Soc. 2014, 136, 4369–4381. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Q.; Mirza, N.R.; Huang, Z.; Zhang, J.; Zheng, Y.M.; Xiang, J.; Xi, Z. Enhanced desalination performance of aluminium fumarate MOF-incorporated electrospun nanofiber membrane with bead-on-string structure for membrane distillation. Desalination 2021, 520, 115338. [Google Scholar] [CrossRef]
- Grasso, G.; Galiano, F.; Yoo, M.J.; Mancuso, R.; Park, H.B.; Gabriele, B.; Figoli, A.; Drioli, E. Development of graphene-PVDF composite membranes for membrane distillation. J. Membr. Sci. 2020, 604, 118017. [Google Scholar] [CrossRef]
- Jafari, A.; Reza, M.; Kebria, S.; Rahimpour, A.; Bakeri, G. Graphene quantum dots modified polyvinylidenefluride (PVDF) nanofibrous membranes with enhanced performance for air Gap membrane distillation. Chem. Eng. Process.-Process Intensif. 2018, 126, 222. [Google Scholar] [CrossRef]

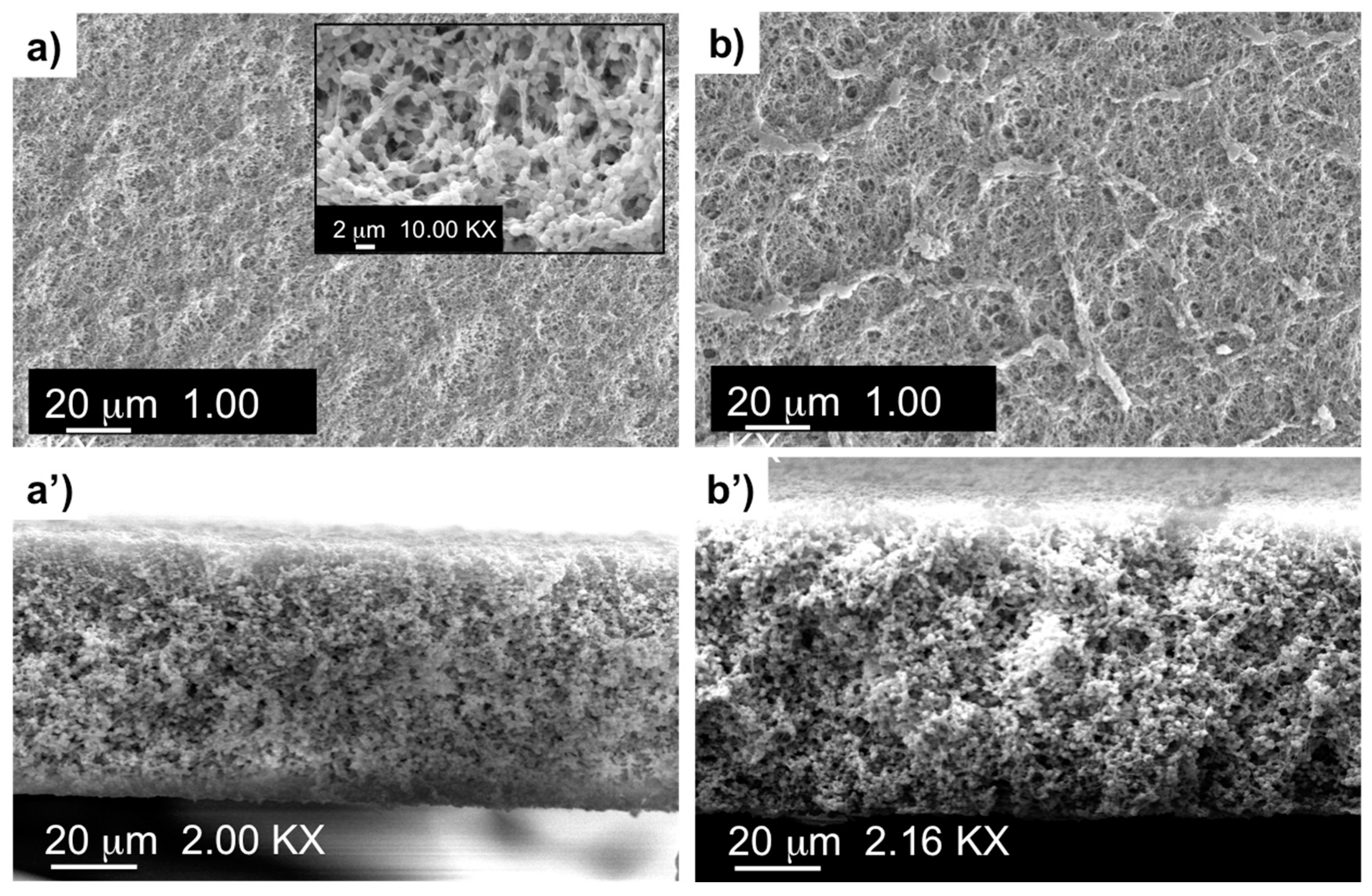
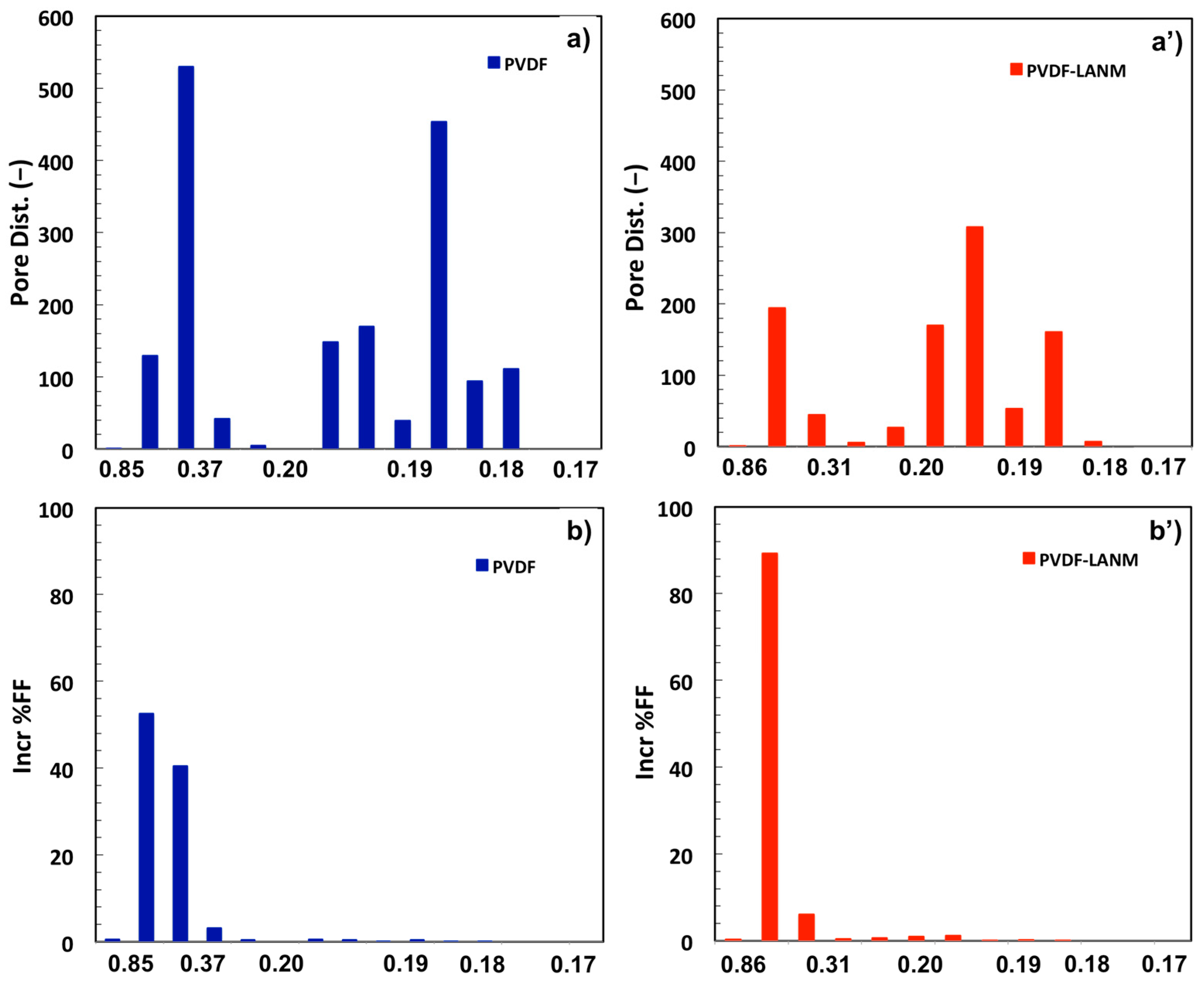

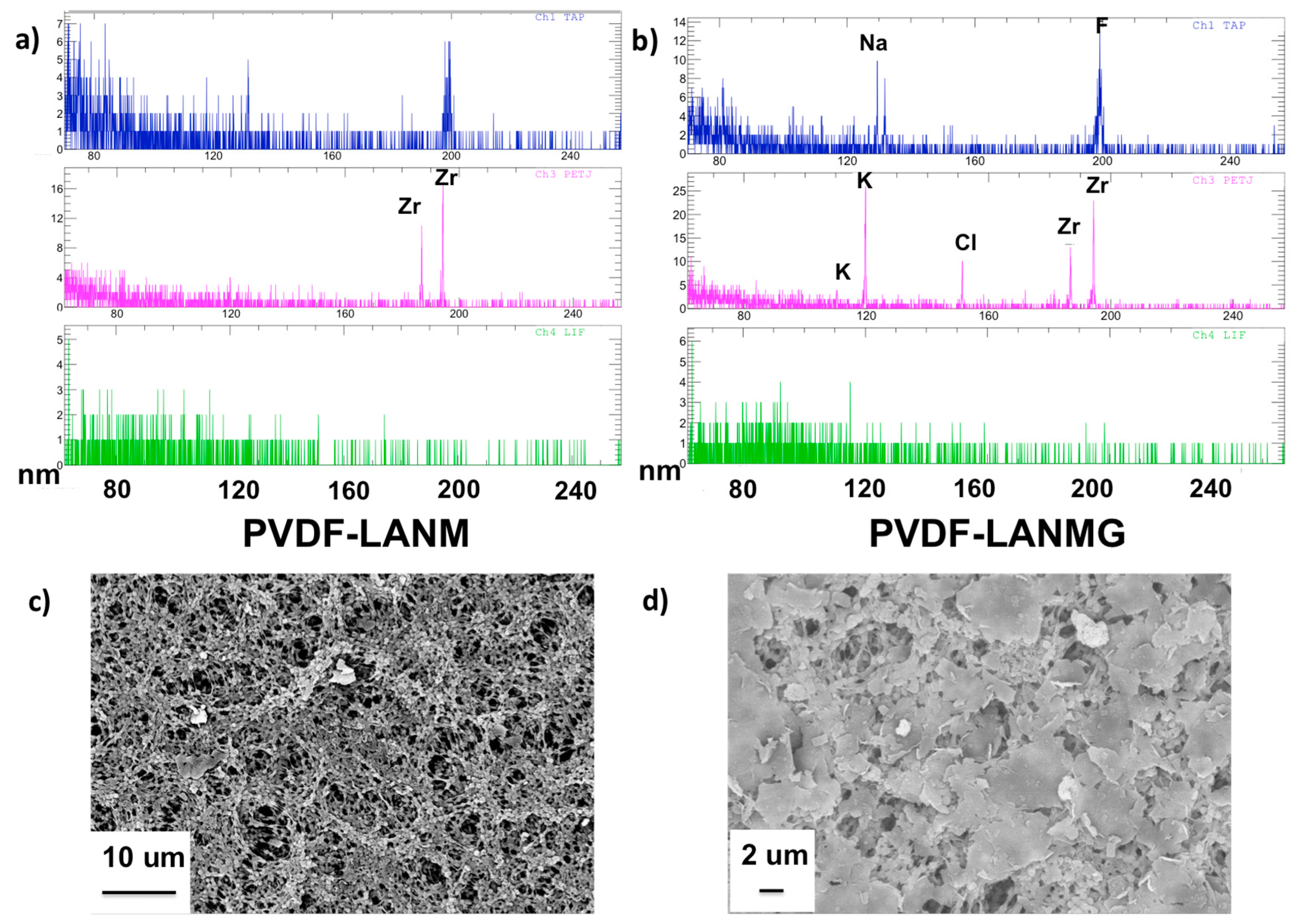
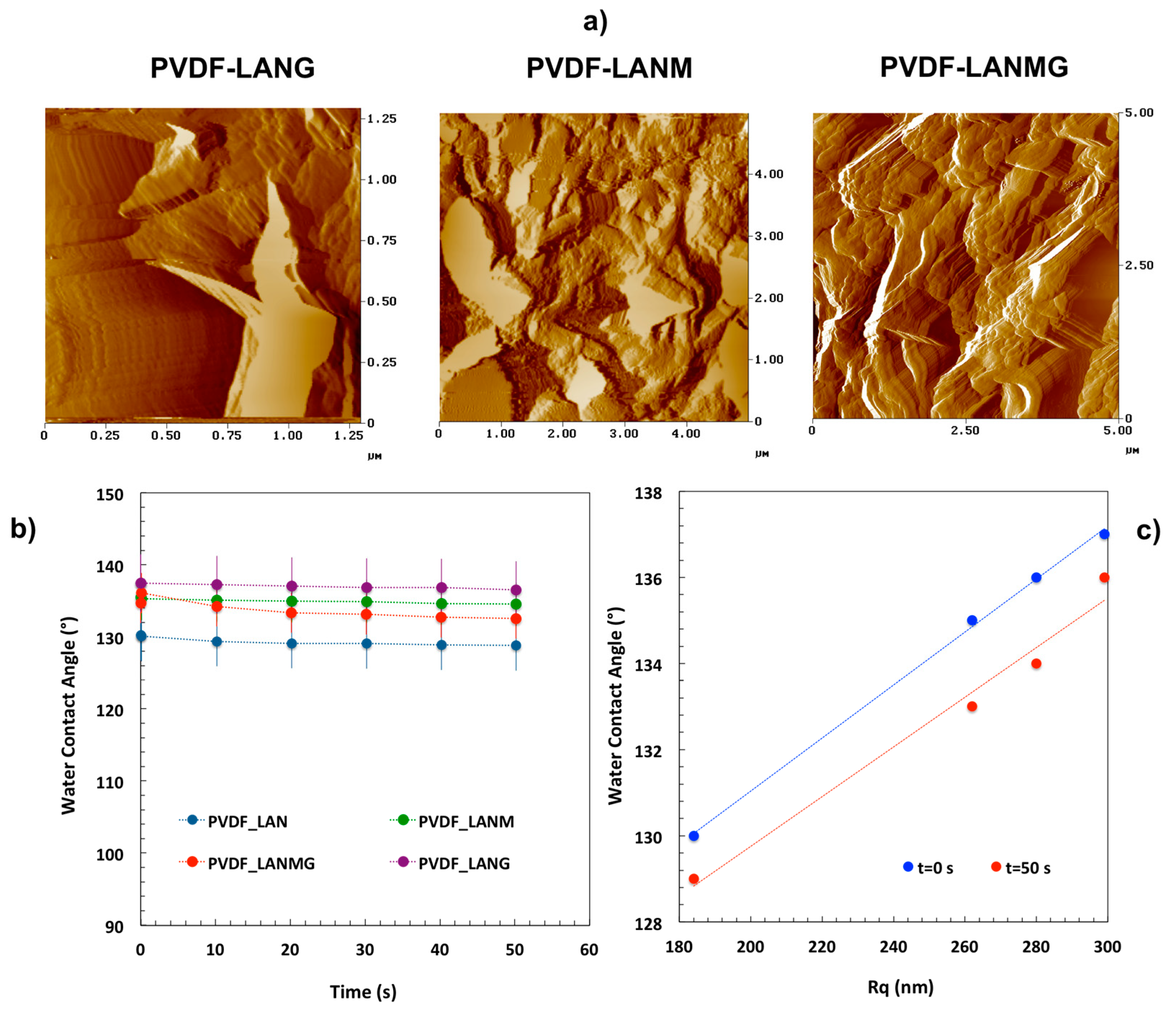
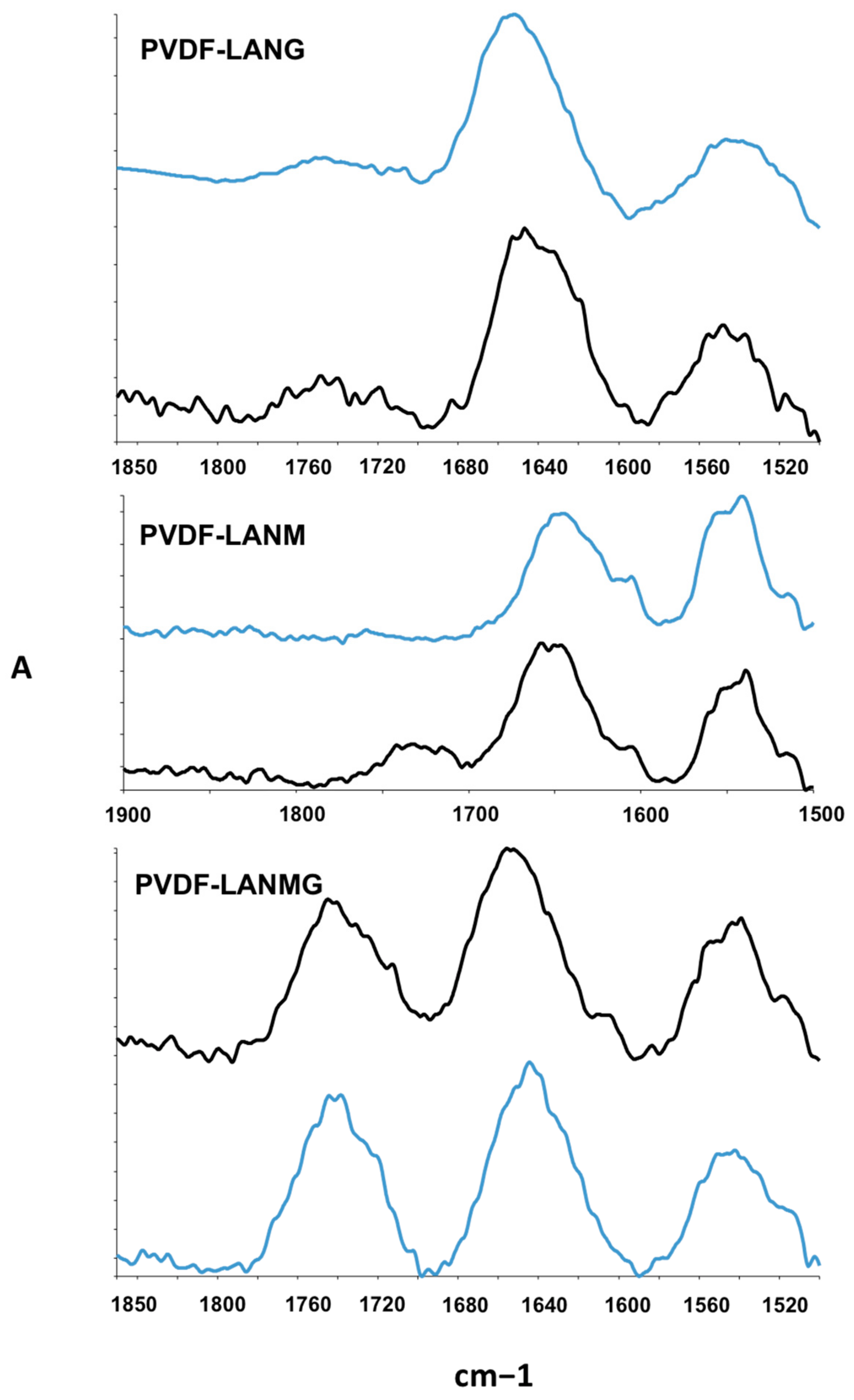
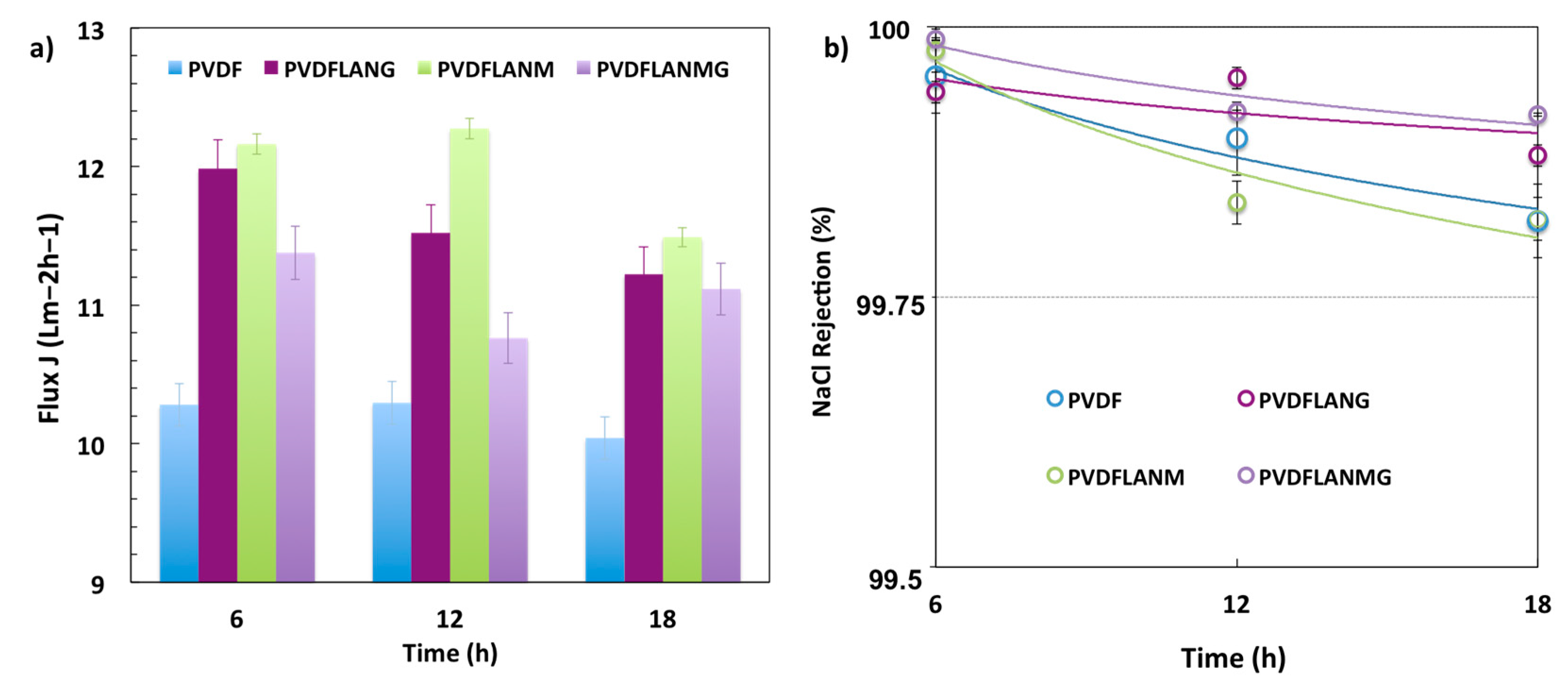
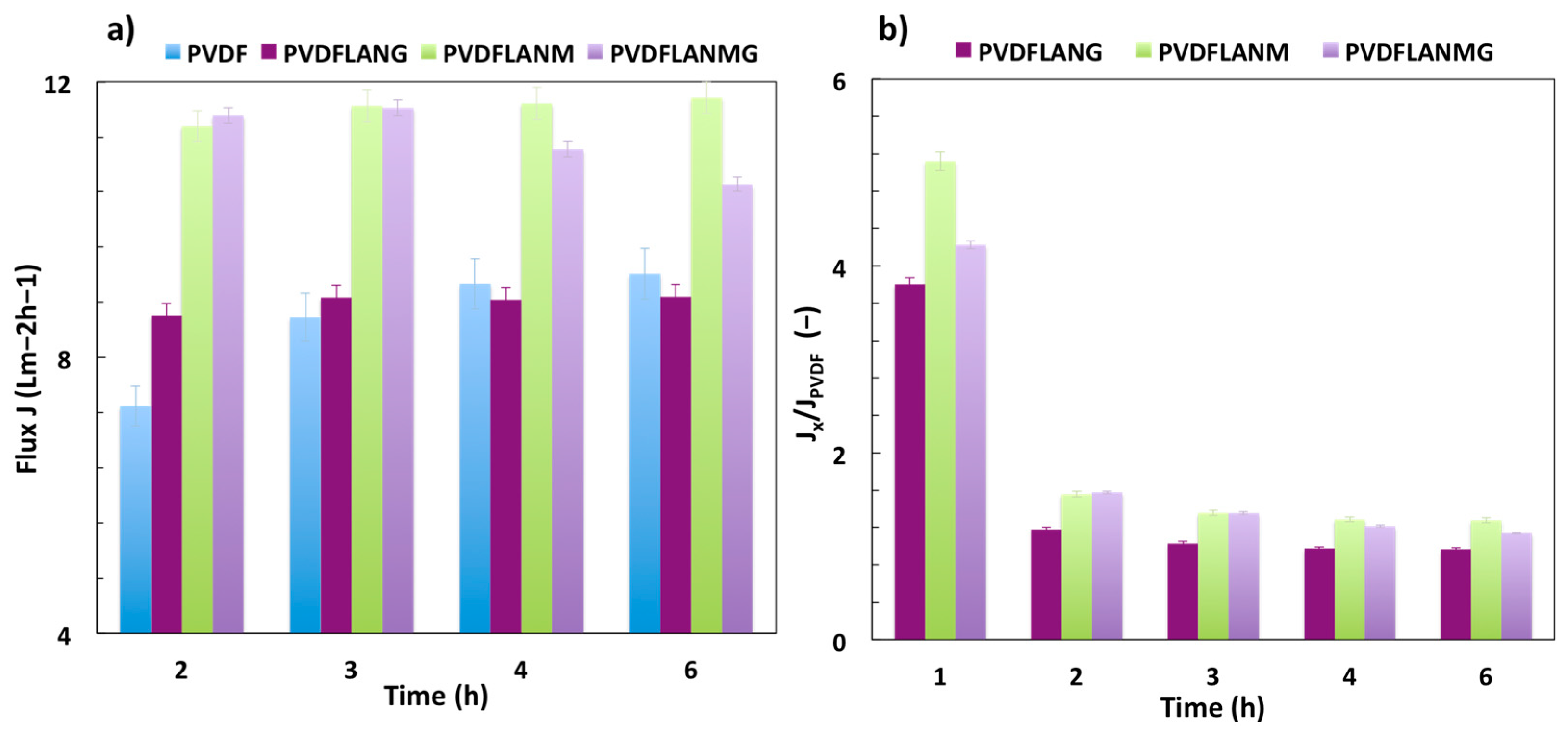
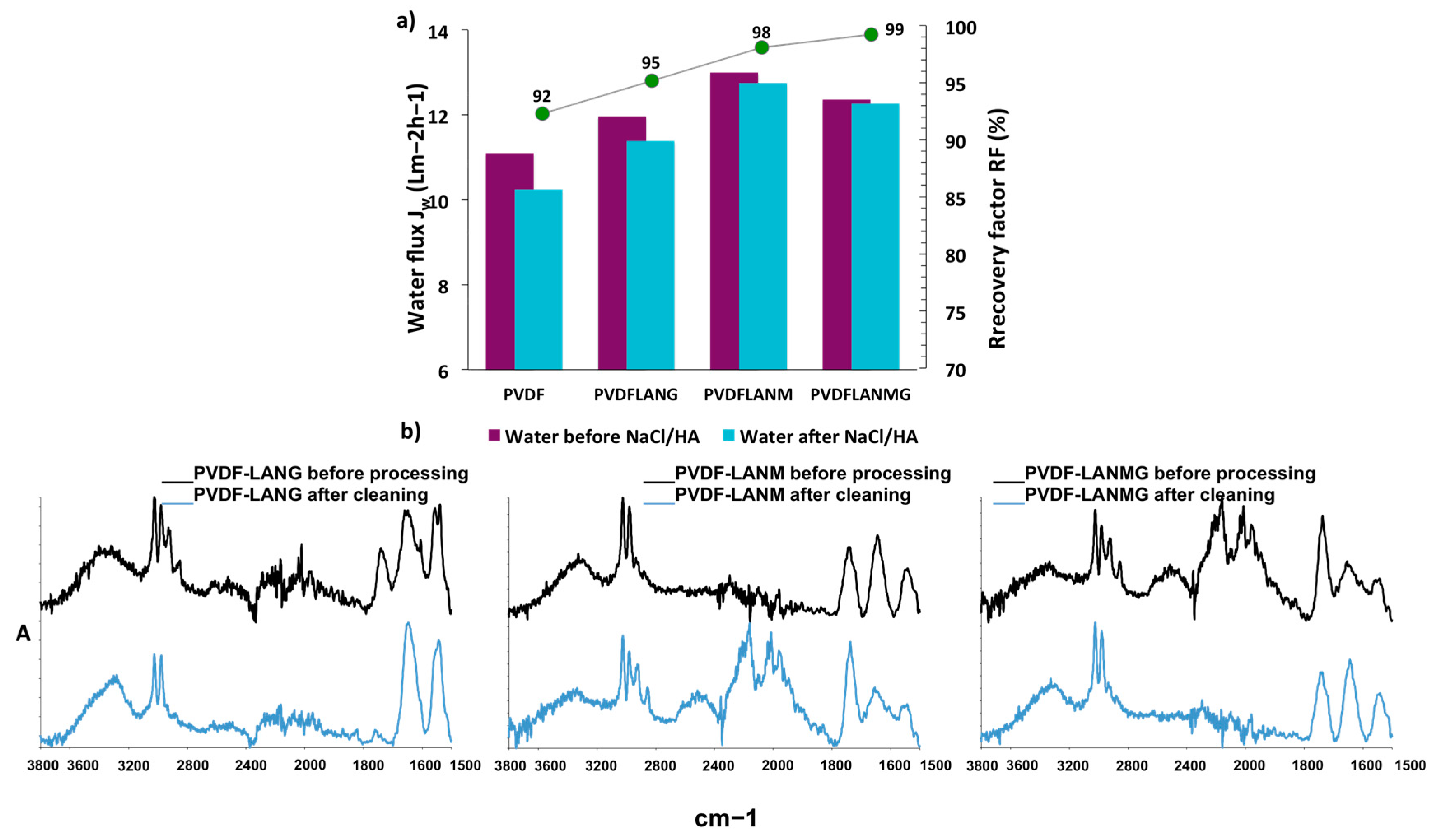
| Complex | Overall Porosity (%) | Largest Pore Size (μm) | Mean Pore Size (μm) | Smallest Pore Size (μm) |
|---|---|---|---|---|
| PVDF | 68 ± 2 | 0.88 ± 0.02 | 0.47 ± 0.01 | 0.197 ± 0.002 |
| PVDF-LAN | 67 ± 3 | 0.85 ± 0.03 | 0.40 ± 0.02 | 0.193 ± 0.007 |
| PVDF-LANG | 62 ± 1 | 0.85 ± 0.04 | 0.42 ± 0.03 | 0.198 ± 0.003 |
| PVDF-LANM | 62 ± 1 | 0.90 ± 0.03 | 0.40 ± 0.01 | 0.195 ± 0.002 |
| PVDF-LANMG | 68 ± 1 | 0.86 ± 0.04 | 0.42 ± 0.05 | 0.197 ± 0.005 |
| Complex | CAt = 0 (°) | CAeq (°) | Ra (nm) | Rq (nm) |
|---|---|---|---|---|
| PVDF-LAN | 130 ± 4 | 129 ± 4 | 141 ± 66 | 184 ± 83 |
| PVDF-LANMG | 135 ± 3 | 133 ± 3 | 215 ± 72 | 262 ± 64 |
| PVDF-LANM | 136 ± 3 | 134 ± 3 | 223 ± 65 | 280 ± 77 |
| PVDF-LANG | 137 ± 4 | 136 ± 4 | 236 ± 59 | 299 ± 73 |
| Membrane | Nanofiller | Amount | ΔT | Feed/Permeate Flow Rate | Prist./Func. Membr. Flux | Reference |
|---|---|---|---|---|---|---|
| [%]/Mode | [°C] | [mLmin−1] | [Lm−2h−1] | |||
| PVDF nanofibrous membranes | Iron 1,3,5-benzenetricarboxylate MOF | 5 (bulk) | 32 | 1500/1500 | 2.1/3.3 | [72] |
| Triple-layer PVDF/PAN/ PVDF ENMs | Hydrophobic SiO2/ MOF/hydrophilic SiO2 | 5/1.5/1 (bulk) | 30 | 1500/1500 | 2.68/4.4 | [73] |
| PVDF hollow fiber membrane | AlFu MOF | 1 (bulk) | 30 | 450/450 | 6.1/8.0 | [74] |
| PVDF-HFP ENMs * | AlFu MOF | 0.1 (bulk) | 40 | 500/500 | 10.6/17.0 | [91] |
| PVDF-f-G | Graphene | CVD (surface) | 50 | 1000/1000 | ~0 **/3 | [92] |
| PVDF/G0.005 | Graphene coating | WF *** (surface) | 24 | 100/80 | 5.4/7.5 | [61] |
| PVDF-LANM | [ZrO(O2C-C10H6-CO2)] | LBL (surface) | 30 | 100/80 | 10.2/12.3 | In this work |
| PVDF-LANG | Graphene | LBL (surface) | 30 | 100/80 | 10.2/11.5 | In this work |
| PVDF-LANMG | [ZrO(O2C-C10H6-CO2)] Graphene | LBL (surface) | 30 | 100/80 | 10.2/11.0 | In this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Luca, G.; Chen, G.; Jin, W.; Gugliuzza, A. Aliquots of MIL-140 and Graphene in Smart PNIPAM Mixed Hydrogels: A Nanoenvironment for a More Eco-Friendly Treatment of NaCl and Humic Acid Mixtures by Membrane Distillation. Membranes 2023, 13, 437. https://doi.org/10.3390/membranes13040437
Di Luca G, Chen G, Jin W, Gugliuzza A. Aliquots of MIL-140 and Graphene in Smart PNIPAM Mixed Hydrogels: A Nanoenvironment for a More Eco-Friendly Treatment of NaCl and Humic Acid Mixtures by Membrane Distillation. Membranes. 2023; 13(4):437. https://doi.org/10.3390/membranes13040437
Chicago/Turabian StyleDi Luca, Giuseppe, Guining Chen, Wanqin Jin, and Annarosa Gugliuzza. 2023. "Aliquots of MIL-140 and Graphene in Smart PNIPAM Mixed Hydrogels: A Nanoenvironment for a More Eco-Friendly Treatment of NaCl and Humic Acid Mixtures by Membrane Distillation" Membranes 13, no. 4: 437. https://doi.org/10.3390/membranes13040437
APA StyleDi Luca, G., Chen, G., Jin, W., & Gugliuzza, A. (2023). Aliquots of MIL-140 and Graphene in Smart PNIPAM Mixed Hydrogels: A Nanoenvironment for a More Eco-Friendly Treatment of NaCl and Humic Acid Mixtures by Membrane Distillation. Membranes, 13(4), 437. https://doi.org/10.3390/membranes13040437







