Occurrence and Treatment of Antibiotic-Resistant Bacteria Present in Surface Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Surface Water Matrix
2.1.1. Total coliforms and Escherichia coli
2.1.2. Enterococci
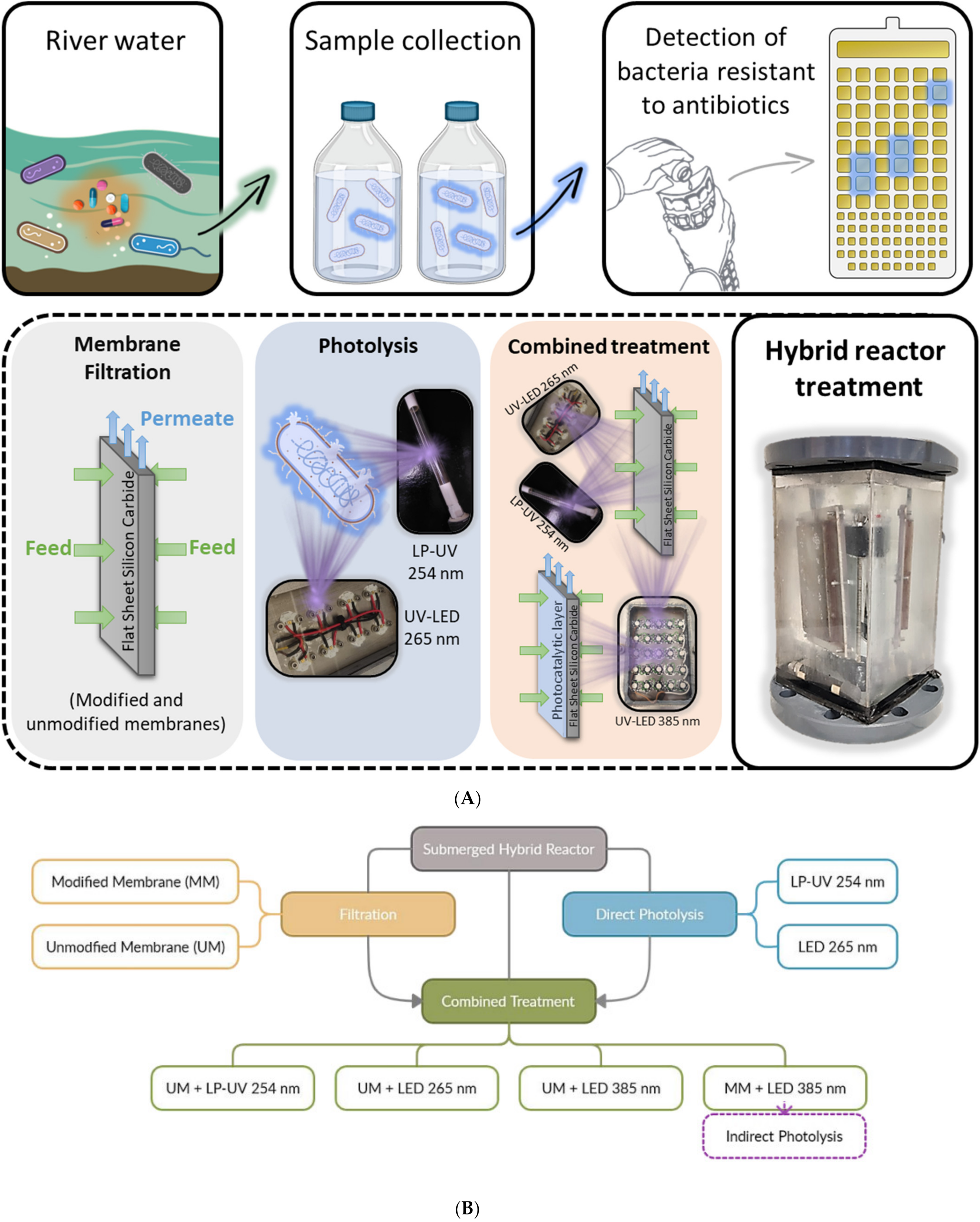
2.2. Surface Water Treatment Experiments
- (1)
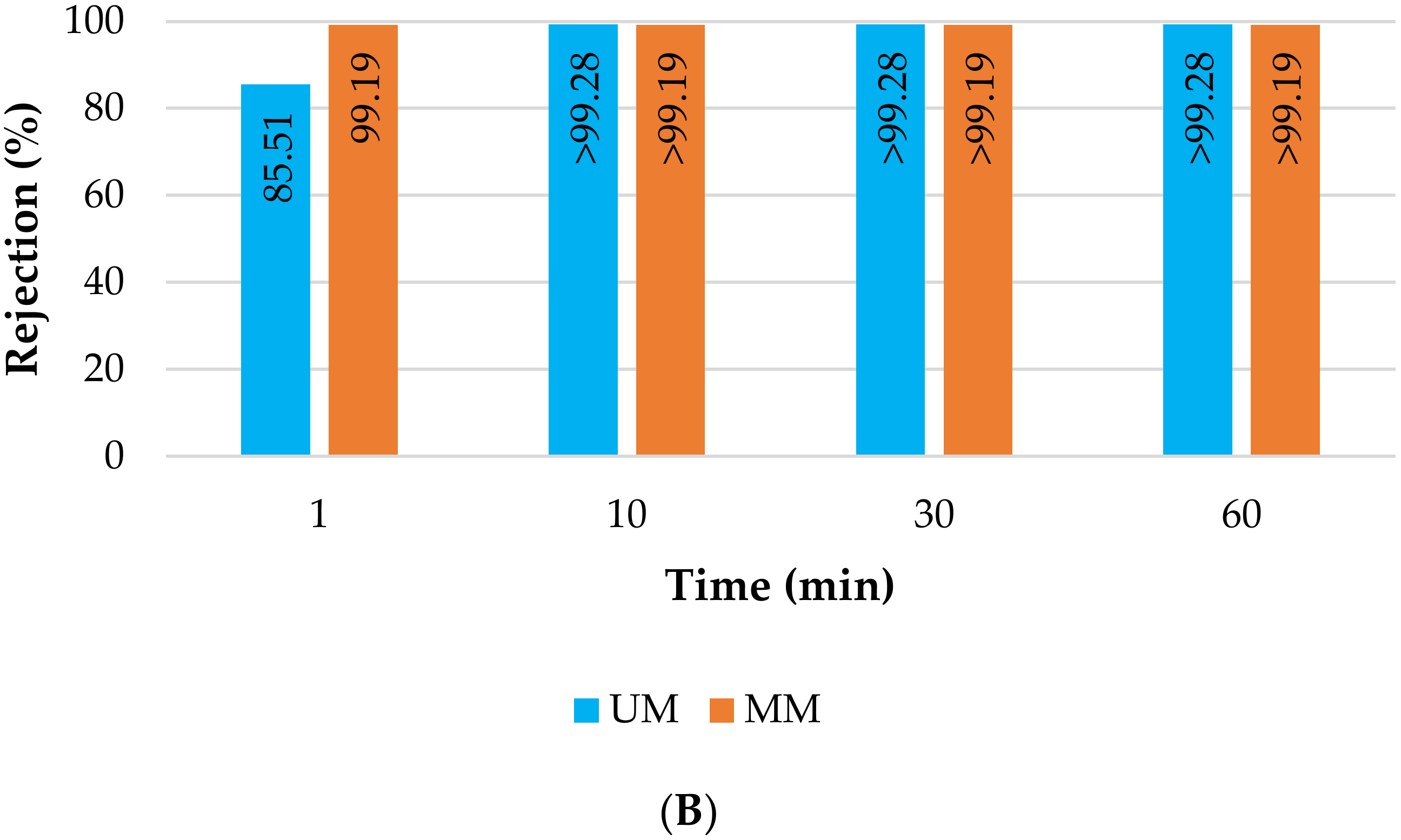
- Membrane filtration tests without the light sources using silicon carbide ceramic unmodified (UM) and modified membranes (MM) with titanium dioxide and copper previously described by Marques et al. [22]. The deposition of photocatalytic layers did not significantly affect the estimated porous properties of the modified membranes (for the unmodified and modified membrane the mean pore areas were 0.025 and 0.033 µm2, the average Feret diameters were 0.14 and 0.17 μm, and the average pore density values were 2.62 and 1.98 µm−2, respectively); The membranes used were highly hydrophilic, since a stable water contact angle was impossible to measure.
- (2)
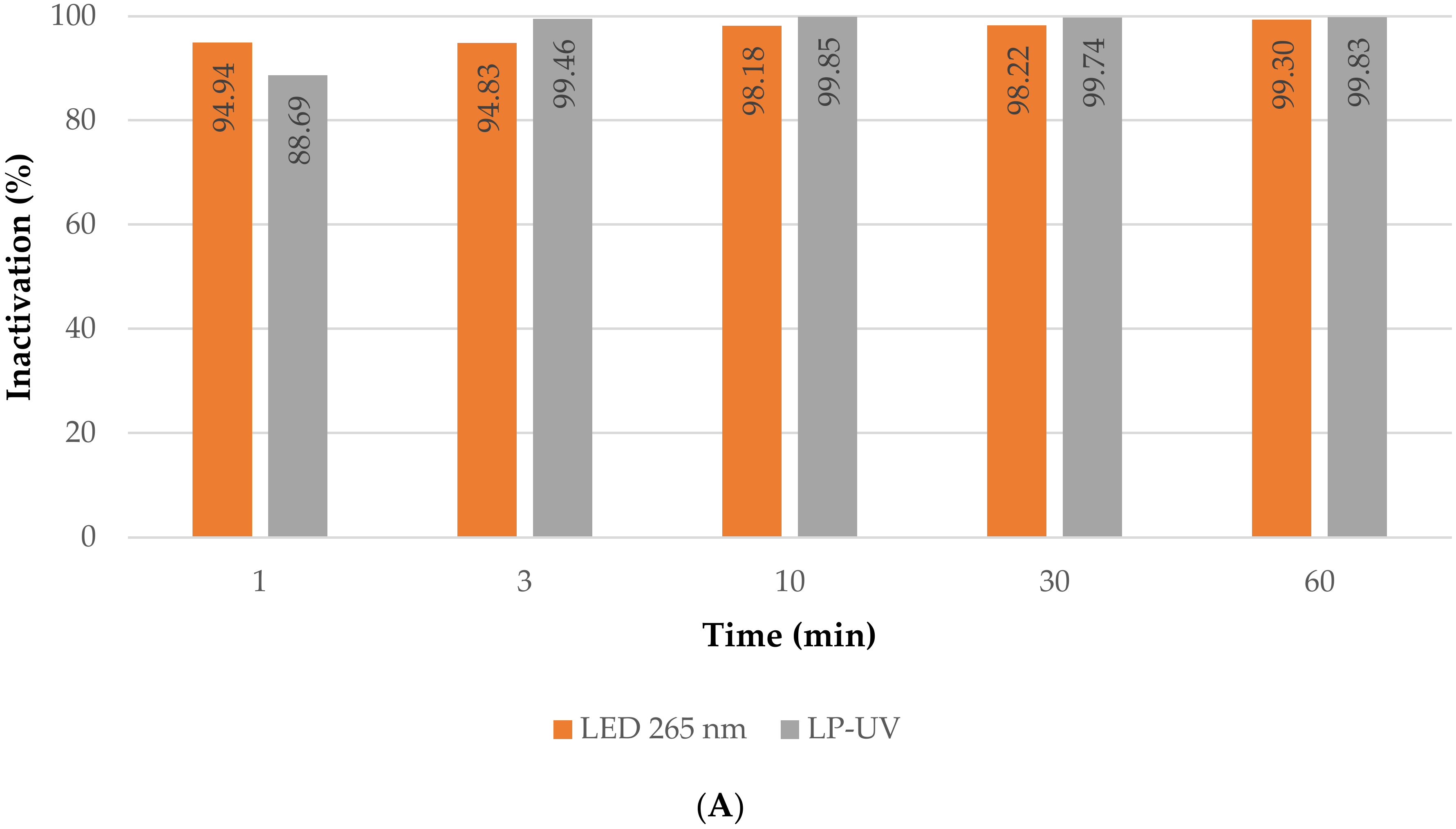
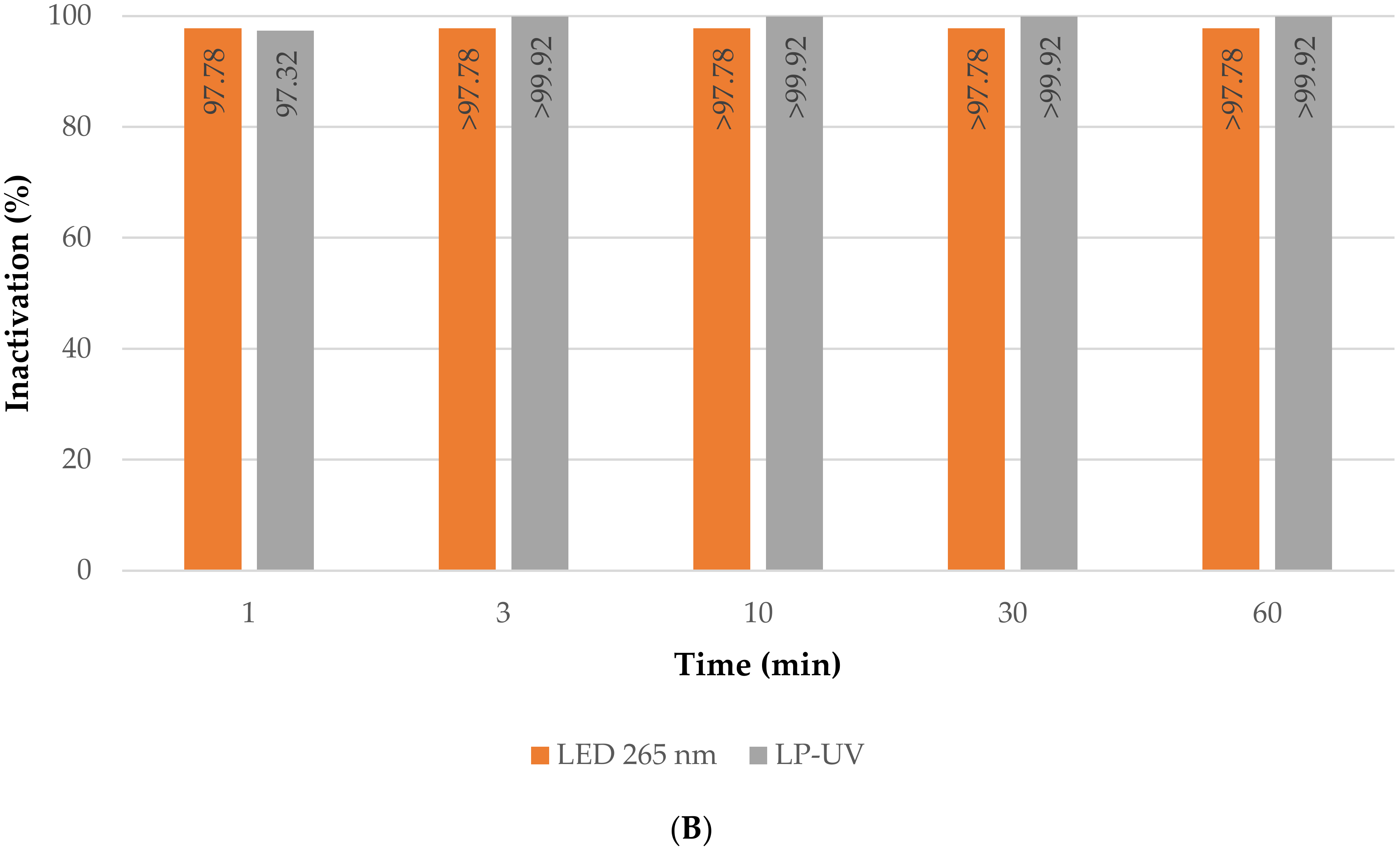
- Direct photolysis inactivation tests, without membrane filtration, using the different light sources. Two commercial low-pressure mercury lamps were tested (Puro TAP, UVC, 11 W, type GPH212T5L, Christchurch, New Zealand), cylindrical in shape, with a diameter of 15 mm and a length of 212 mm. Two custom-built LED panels were also tested: two panels (to place on each side of the membrane) with 8 LEDs each that emit light at 265 nm with an average irradiance of 15.33 µW/cm2.The panels emitting at 265 nm were custom-built for inactivation by direct photolysis.
- (3)
- Combined treatment tests with membrane filtration (using the unmodified and modified membranes) and the different light sources (low-pressure mercury lamps, LEDs that emit light at 265 nm and LED panels that emit light at 385 nm) to evaluate direct and indirect photolysis. The two panels with 25 LEDs each that emit light at 385 nm with an average irradiance of 313.18 µW/cm2 were built to test the activation of photocatalytic coatings (indirect photolysis).
3. Results and Discussion
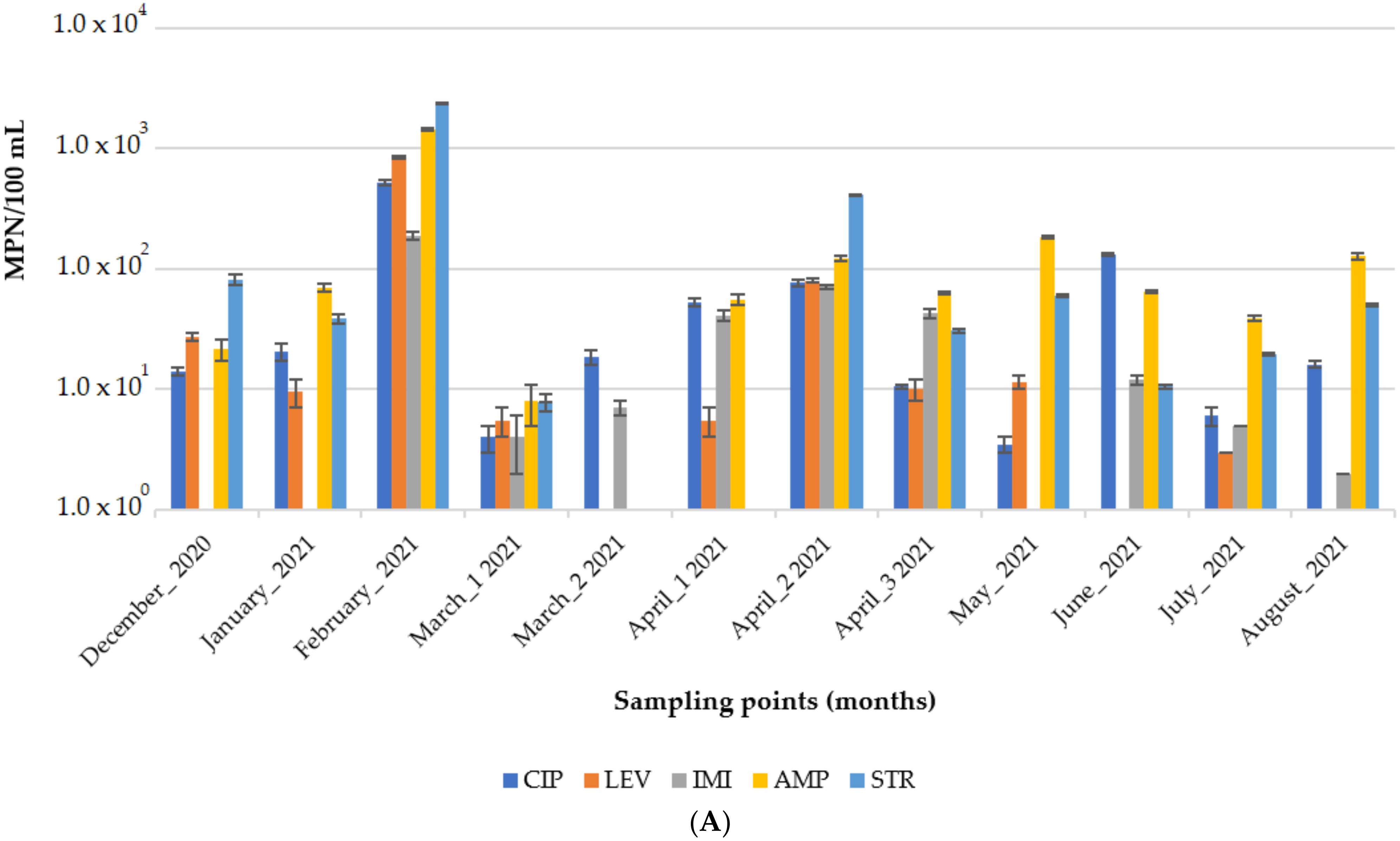
3.1. Characterization of the Water Matrix
Occurrence of Water Quality Indicators and Antibiotic-Resistant Bacteria
3.2. Water Treatment of Antibiotic-Resistant Bacteria
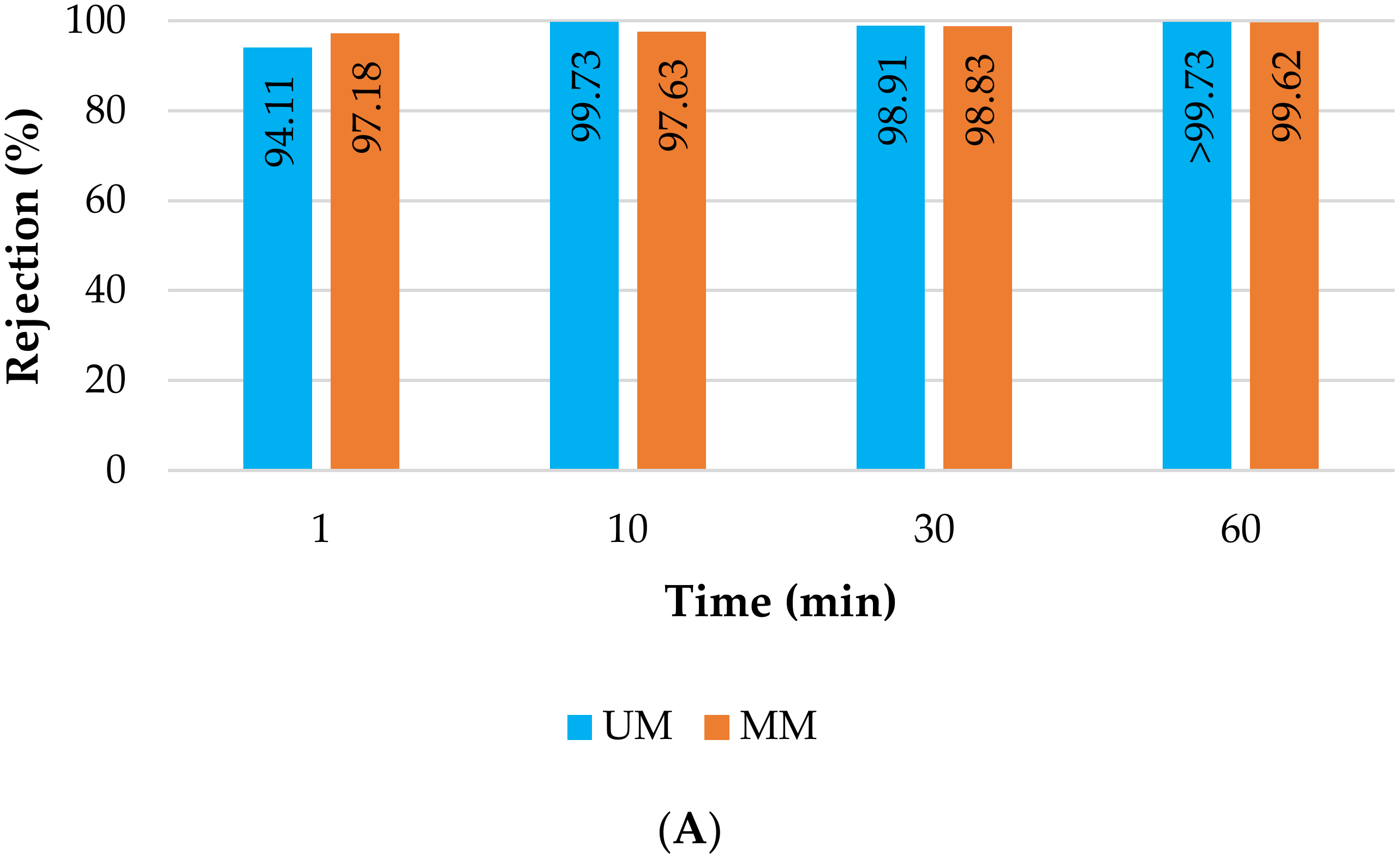
3.2.1. Membrane Filtration Treatment
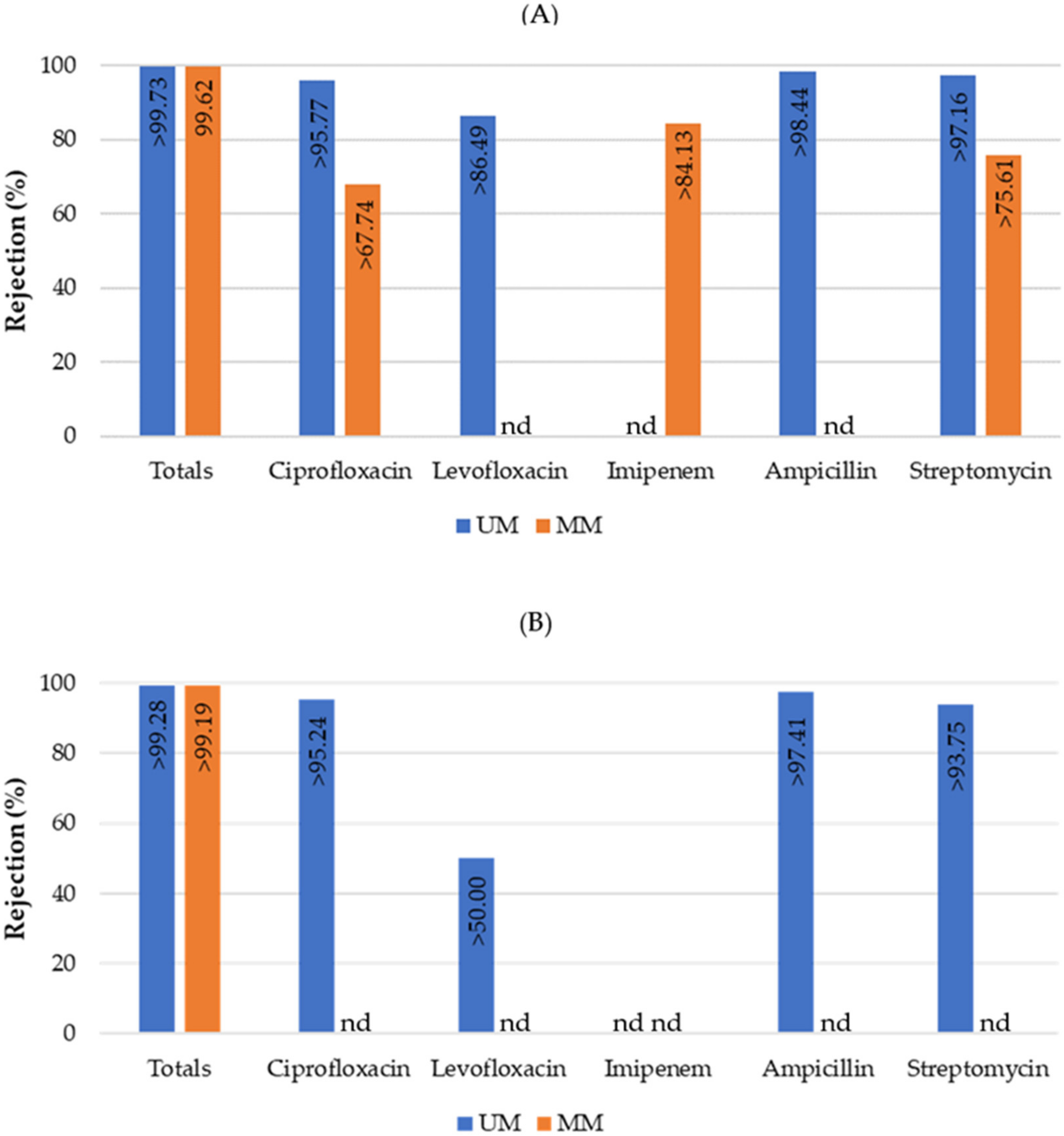
3.2.2. Direct Photolysis Treatment

3.2.3. Combined Treatment


4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A

| Total Coliforms (MPN/100 mL) | Escherichia coli (MPN/100 mL) | Total Solids (g/L) | Total Suspended Solids (g/L) | Total Dissolvel Solids (g/L) | |
|---|---|---|---|---|---|
| Unmodified membrane | 376 | 138 | 32 | 1 | 31 |
| Modified membrane | 266 | 124 | 41 | 1 | 40 |
References
- Gonçalves, V.D.; Meirelles-Pereira, F.; Cataldo, M.; Fonseca, B.D.O.; Nogueira, B.A.; Olivella, J.G.B.; Esteves, F.D.A.; Mattos-Guaraldi, A.L.; de Andrade, A.F.B.; Bello, A.R.; et al. Detection of multidrug-resistant Enterobacteriaceae isolated from river waters flowing to the Guanabara Bay and from clinical samples of hospitals in Rio de Janeiro, Brazil. Biomedica 2019, 39, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Mittal, P.; Prasoodanan, P.K.V.; Dhakan, D.B.; Kumar, S.; Sharma, V.K. Metagenome of a Polluted River Reveals a Reservoir of Metabolic and Antibiotic Resistance Genes. Environ. Microbiome 2019, 14, 5. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, Z.; Shen, C.; Xu, Y. Inactivation of Antibiotic-Resistant Bacteria and Antibiotic Resistance Genes by Electro-chemical Oxidation/Electro-Fenton Process. Water Sci. Technol. 2020, 81, 2221–2231. [Google Scholar] [CrossRef] [PubMed]
- Mills, M.C.; Lee, J. The Threat of Carbapenem-Resistant Bacteria in the Environment: Evidence of Widespread Contamination of Reservoirs at a Global Scale. Environ. Pollut. 2019, 255, 113143. [Google Scholar] [CrossRef]
- WHO Report. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 1 March 2023).
- Tagliabue, A.; Rappuoli, R. Changing Priorities in Vaccinology: Antibiotic Resistance Moving to the Top. Front. Immunol. 2018, 9, 1068. [Google Scholar] [CrossRef] [PubMed]
- Lucien, M.A.B.; Canarie, M.F.; Kilgore, P.E.; Jean-Denis, G.; Fénélon, N.; Pierre, M.; Cerpa, M.; Joseph, G.A.; Maki, G.; Zervos, M.J.; et al. Antibiotics and antimicrobial resistance in the COVID-19 era: Perspective from resource-limited settings. Int. J. Infect. Dis. 2021, 104, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Bornman, C.; Zafer, M.M. Antimicrobial Resistance Threats in the Emerging COVID-19 Pandemic: Where Do We Stand? J. Infect. Public Health 2021, 14, 555–560. [Google Scholar] [CrossRef]
- Azuma, T.; Usui, M.; Hayashi, T. Inactivation of Antibiotic-Resistant Bacteria in Wastewater by Ozone-Based Advanced Water Treatment Processes. Antibiotics 2022, 11, 210. [Google Scholar] [CrossRef]
- Teixeira, P.; Tacão, M.; Pureza, L.; Gonçalves, J.; Silva, A.; Cruz-Schneider, M.P.; Henriques, I. Occurrence of Carbapenemase-Producing Enterobacteriaceae in a Portuguese River: BlaNDM, BlaKPC and BlaGES among the Detected Genes. Environ. Pollut. 2020, 260, 113913. [Google Scholar] [CrossRef]
- Cohen, R.; Paikin, S.; Rokney, A.; Rubin-Blum, M.; Astrahan, P. Multidrug-resistant enterobacteriaceae in coastal water: An emerging threat. Antimicrob. Resist. Infect. Control. 2020, 9, 1–9. [Google Scholar] [CrossRef]
- Uluseker, C.; Kaster, K.M.; Thorsen, K.; Basiry, D.; Shobana, S.; Jain, M.; Kumar, G.; Kommedal, R.; Pala-Ozkok, I. A Review on Occurrence and Spread of Antibiotic Resistance in Wastewaters and in Wastewater Treatment Plants: Mechanisms and Perspectives. Front. Microbiol. 2021, 12, 717809. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, G.; Ng, W.J.; Tan, S.K. A review on removing pharmaceutical contaminants from wastewater by constructed wetlands: Design, performance and mechanism. Sci. Total. Environ. 2014, 468, 908–932. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, S.; Boopathy, R.; Nathaniel, R.; Corbin, A.; LaFleur, G. Presence of Antibiotic Resistant Bacteria and Antibiotic Resistance Genes in Raw Source Water and Treated Drinking Water. Int. Biodeterior. Biodegrad. 2015, 102, 370–374. [Google Scholar] [CrossRef]
- Pal, A.; Gin, K.Y.-H.; Lin, A.Y.-C.; Reinhard, M. Impacts of Emerging Organic Contaminants on Freshwater Resources: Re-view of Recent Occurrences, Sources, Fate and Effects. Sci. Total Environ. 2010, 408, 6062–6069. [Google Scholar] [CrossRef]
- Byrne, C.; Subramanian, G.; Pillai, S.C. Recent advances in photocatalysis for environmental applications. J. Environ. Chem. Eng. 2018, 6, 3531–3555. [Google Scholar] [CrossRef]
- Oliveira, B.R.; Sanches, S.; Huertas, R.M.; Barreto Crespo, M.T.B.; Pereira, V.J. Treatment of a real water matrix inoculated with Aspergillus fumigatus using a photocatalytic membrane reactor. J. Membr. Sci. 2020, 598, 117788. [Google Scholar] [CrossRef]
- Chahal, C.; Akker, B.V.D.; Young, F.; Franco, C.; Blackbeard, J.; Monis, P. Pathogen and Particle Associations in Wastewater. Adv. Appl. Microbiol. 2016, 97, 63–119. [Google Scholar] [CrossRef] [PubMed]
- Eray, E.; Boffa, V.; Jørgensen, M.K.; Magnacca, G.; Candelario, V.M. Enhanced fabrication of silicon carbide membranes for wastewater treatment: From laboratory to industrial scale. J. Membr. Sci. 2020, 606, 118080. [Google Scholar] [CrossRef]
- Fraga, M.; Sanches, S.; Crespo, J.; Pereira, V. Assessment of a New Silicon Carbide Tubular Honeycomb Membrane for Treatment of Olive Mill Wastewaters. Membranes 2017, 7, 12. [Google Scholar] [CrossRef]
- Bernardo, J.; Sério, J.; Oliveira, B.; Marques, A.P.; Huertas, R.; Crespo, J.G.; Pereira, V.J. Towards a Novel Combined Treatment Approach Using Light-Emitting Diodes and Photocatalytic Ceramic Membranes. Water 2022, 14, 292. [Google Scholar] [CrossRef]
- Marques, A.P.; Huertas, R.; Bernardo, J.; Oliveira, B.; Crespo, J.G.; Pereira, V.J. Retention and Inactivation of Quality Indica-tor Bacteria Using a Photocatalytic Membrane Reactor. Catalysts 2022, 12, 680. [Google Scholar] [CrossRef]
- Huertas, R.M.; Fraga, M.C.; Crespo, J.G.; Pereira, V.J. Solvent Free Process for the Development of Photocatalytic Membranes. Molecules. 2019, 24, 4481. [Google Scholar] [CrossRef]
- Chang, S.-M.; Liu, W.-S. The roles of surface-doped metal ions (V, Mn, Fe, Cu, Ce, and W) in the interfacial behavior of TiO2 photocatalysts. Appl. Catal. B Environ. 2014, 156, 466–475. [Google Scholar] [CrossRef]
- Sosa, S.M.; Huertas, R.; Pereira, V.J. Combination of Zinc Oxide Photocatalysis with Membrane Filtration for Surface Water Disinfection. Membranes 2023, 13, 56. [Google Scholar] [CrossRef]
- Perarasan, T.; John Peter, I.; Muthu Kumar, A.; Rajamanickam, N.; Ramachandran, K.; Raja Mohan, C. Copper Doped Titanium Dioxide for Enhancing the Photovoltaic Behavior in Solar Cell. Mater. Today Proc. 2021, 35, 66–68. [Google Scholar] [CrossRef]
- Mahmud, M.A.; Chin, L.Y.; Khusaimi, Z.; Zainal, Z. Influence of Deposition Time on the Surface Morphology and Photoelectrochemical Properties of Copper Doped Titania Nanotubes Prepared by Electrodeposition; AIP Publishing LLC: Selangor, Malaysia, 2018; p. 20030. [Google Scholar]
- Pongwan, P.; Wetchakun, K.; Phanichphant, S.; Wetchakun, N. Enhancement of visible-light photocatalytic activity of Cu-doped TiO2 nanoparticles. Res. Chem. Intermed. 2015, 42, 2815–2830. [Google Scholar] [CrossRef]
- Dunlop, P.; Sheeran, C.; Byrne, J.; McMahon, M.; Boyle, M.; McGuigan, K. Inactivation of clinically relevant pathogens by photocatalytic coatings. J. Photochem. Photobiol. A Chem. 2010, 216, 303–310. [Google Scholar] [CrossRef]
- Hijnen, W.A.M.; Beerendonk, E.F.; Medema, G.J. Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cysts in water: A review. Water Res. 2006, 40, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Mohseni, M.; Taghipour, F. Application of ultraviolet light-emitting diodes (UV-LEDs) for water disinfection: A review. Water Res. 2016, 94, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Loeb, S.; Kim, J.-H. LED revolution: Fundamentals and prospects for UV disinfection applications. Environ. Sci. Water Res. Technol. 2017, 3, 188–202. [Google Scholar] [CrossRef]
- Oliveira, B.; Crespo, M.B.; Pereira, V. Small but powerful: Light-emitting diodes for inactivation of Aspergillus species in real water matrices. Water Res. 2020, 168, 115108. [Google Scholar] [CrossRef]
- Oliveira, B.R.; Marques, A.P.; Asif, M.; Crespo, M.T.B.; Pereira, V.J. Light-emitting diodes effect on Aspergillus species in filtered surface water: DNA damage, proteome response and potential reactivation. Environ. Pollut. 2021, 287, 117553. [Google Scholar] [CrossRef]
- Gonzalez-Perez, A.; Persson, K.M.; Samuelson, L. Semiconductor Eco-Materials for Water Treatment. In Handbook of Eco-materials; Martínez, L.M.T., Kharissova, O.V., Kharisov, B.I., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 413–439. [Google Scholar]
- Chatterley, C.; Linden, K. Demonstration and evaluation of germicidal UV-LEDs for point-of-use water disinfection. J. Water Health 2010, 8, 479–486. [Google Scholar] [CrossRef]
- Xiong, P.; Hu, J. Inactivation/reactivation of antibiotic-resistant bacteria by a novel UVA/LED/TiO2 system. Water Res. 2013, 47, 4547–4555. [Google Scholar] [CrossRef] [PubMed]
- Claro, E.M.T.; Bidoia, E.D.; de Moraes, P.B. A high-performance doped photocatalysts for inactivation of total coliforms in superficial waters using different sources of radiation. J. Environ. Manag. 2016, 177, 264–270. [Google Scholar] [CrossRef]
- Biancullo, F.; Moreira, N.F.F.; Ribeiro, A.R.; Manaia, C.M.; Faria, J.L.; Nunes, O.C.; Silva, A.M.T. Heterogeneous photocatalysis using UVA-LEDs for the removal of antibiotics and antibiotic resistant bacteria from urban wastewater treatment plant effluents. Chem. Eng. J. 2019, 367, 304–313. [Google Scholar] [CrossRef]
- Fraga, M.C.; Huertas, R.M.; Crespo, J.G.; Pereira, V.J. Novel Submerged Photocatalytic Membrane Reactor for Treatment of Olive Mill Wastewaters. Catalysts 2019, 9, 769. [Google Scholar] [CrossRef]
- SOLIDS. In Standard Methods For the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2017.
- Fewtrell, L.; Bartram, J. (Eds.) Water Quality: Guidelines, Standards and Health: Assessment of Risk and Risk Management for Water-Related Infectious Diseases; IWA: London, UK, 2001. [Google Scholar]
- Collivignarelli, M.C.; Abbà, A.; Miino, M.C.; Caccamo, F.M.; Torretta, V.; Rada, E.C.; Sorlini, S. Disinfection of Wastewater by UV-Based Treatment for Reuse in a Circular Economy Perspective. Where Are We at? Int. J. Environ. Res. Public Health 2020, 18, 77. [Google Scholar] [CrossRef] [PubMed]
- Warden, P.S.; DeSarno, M.S.; Volk, S.E.; Eldred, B.J. Evaluation of Colilert-18® for Detection and Enumeration of Fecal Coliform Bacteria in Wastewater Using the U.S. Environmental Protection Agency Alternative Test Procedure Protocol. J. AOAC Int. 2011, 94, 1573–1580. [Google Scholar] [CrossRef]
- Yakub, G.P.; Castric, D.A.; Stadterman-Knauer, K.L.; Tobin, M.J.; Blazina, M.; Heineman, T.N.; Yee, G.Y.; Frazier, L. Evaluation of Colilert and Enterolert Defined Substrate Methodology for Wastewater Applications. Water Environ. Res. 2002, 74, 131–135. [Google Scholar] [CrossRef]
- ISO 9308-2; Water Quality—Enumeration of Escherichia coli and Coliform Bacteria—Part 2: Most Probable Number Method. ISO: Geneva, Switzerland, 2012.
- Galvin, S.; Boyle, F.; Hickey, P.; Vellinga, A.; Morris, D.; Cormican, M. Enumeration and Characterization of Antimicrobial-Resistant Escherichia coli Bacteria in Effluent from Municipal, Hospital, and Secondary Treatment Facility Sources. Appl. Env. Microbiol. 2010, 76, 4772–4779. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Surveillance of Antimicrobial Resistance in Europe: Annual Report of the European Antimicrobial Resistance Surveillance Network (EARS Net) 2018; Publications Office: Luxembourg, Luxembourg, 2019. [Google Scholar]
- Weinstein, M.P. Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Coleman, B.L.; Louie, M.; Salvadori, M.I.; McEwen, S.A.; Neumann, N.; Sibley, K.; Rebecca, J.I.; Jamieson, F.B.; Daignault, D.; Majury, A.; et al. Contamination of Canadian private drinking water sources with antimicrobial resistant Escherichia coli. Water Res. 2013, 47, 3026–3036. [Google Scholar] [CrossRef]
- Fecal Enterococci. In Standard Methods For the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2017.
- ISO 7899-1:1998/COR 1; Water Quality—Detection and Enumeration of Intestinal Enterococci—Part 1: Miniaturized Method (Most Probable Number) for Surface and Wastewater—Technical Corrigendum 1. ISO: Geneva, Switzerland, 2000.
- Kamel, A.; Fuentes, M.; Palacios, A.M.; Rodrigo, M.J.; Vivar, M. Deactivating environmental strains of Escherichia coli, Entero-coccus faecalis and Clostridium perfringens from a real wastewater effluent using UV-LEDs. Heliyon 2022, 23, e12628. [Google Scholar] [CrossRef]
- Pereira, A.; Santos, A.; Tacão, M.; Alves, A.; Henriques, I.; Correia, A. Genetic diversity and antimicrobial resistance of Escherichia coli from Tagus estuary (Portugal). Sci. Total. Environ. 2013, 461–462, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Henriques, I.S.; Araújo, S.; Azevedo, J.S.; Alves, M.S.; Chouchani, C.; Pereira, A.; Correia, A. Prevalence and Diversity of Carbapenem-Resistant Bacteria in Untreated Drinking Water in Portugal. Microb. Drug Resist. 2012, 18, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Tornevi, A.; Bergstedt, O.; Forsberg, B. Precipitation Effects on Microbial Pollution in a River: Lag Structures and Seasonal Effect Modification. PLoS ONE 2014, 9, e98546. [Google Scholar] [CrossRef]
- Gekenidis, M.T.; Qi, W.; Hummerjohann, J.; Zbiden, R.; Walsh, F.; Drissner, D. Antibiotic-resistant indicator bacteria in irrigation water: High prevalence of extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli. PLoS ONE 2018, 11, 207857. [Google Scholar] [CrossRef] [PubMed]
- Harmon, D.E.; Miranda, O.A.; McCarley, A.; Eshaghian, M.; Carlson, N.; Ruiz, C. Prevalence and characterization of carbapenem-resistant bacteria in water bodies in the Los Angeles–Southern California area. Microbiologyopen 2019, 8, e00692. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety (EFSA). Report from the Task Force on Zoonoses Data Collection including guidance for harmonized monitoring and reporting of antimicrobial resistance in commensal Escherichia coli and Enterococcus spp. from food animals. EFSA J. 2008, 141, 1–44. [Google Scholar]
- Scheutz, F.; Strockbine, N.A. Escherichia. In Bergey’s Manual of Systematics of Archaea and Bacteria, 1st ed.; Whitman, W.B., Rainey, F., Kämpfer, P., Trujillo, M., Chun, J., DeVos, P., Hedlund, B., Dedysh, S., Eds.; Wiley: New York, NY, USA, 2015; pp. 1–49. [Google Scholar]
- Shen, C.-H. Detection and Analysis of Nucleic Acids. In Diagnostic Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 167–185. [Google Scholar]
- Vilhunen, S.; Särkkä, H.; Sillanpää, M. Ultraviolet light-emitting diodes in water disinfection. Environ. Sci. Pollut. Res. 2009, 16, 439–442. [Google Scholar] [CrossRef]
- Oguma, K.; Kita, R.; Sakai, H.; Murakami, M.; Takizawa, S. Application of UV light emitting diodes to batch and flow-through water disinfection systems. Desalination 2013, 328, 24–30. [Google Scholar] [CrossRef]
- Sholtes, K.A.; Lowe, K.; Walters, G.W.; Sobsey, M.D.; Linden, K.G.; Casanova, L.M. Comparison of ultraviolet light-emitting diodes and low-pressure mercury-arc lamps for disinfection of water. Environ. Technol. 2016, 37, 2183–2188. [Google Scholar] [CrossRef]
- Li, G.-Q.; Wang, W.-L.; Huo, Z.-Y.; Lu, Y.; Hu, H.-Y. Comparison of UV-LED and low pressure UV for water disinfection: Photoreactivation and dark repair of Escherichia coli. Water Res. 2017, 126, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Song, K.; Taghipour, F.; Mohseni, M. Microorganisms inactivation by continuous and pulsed irradiation of ultraviolet light-emitting diodes (UV-LEDs). Chem. Eng. J. 2018, 343, 362–370. [Google Scholar] [CrossRef]
- Jarvis, P.; Autin, O.; Goslan, E.H.; Hassard, F. Application of Ultraviolet Light-Emitting Diodes (UV-LED) to Full-Scale Drinking-Water Disinfection. Water 2019, 11, 1894. [Google Scholar] [CrossRef]
- Meckes, M.C. Effect of UV light disinfection on antibiotic-resistant coliforms in wastewater effluents. Appl. Environ. Microbiol. 1982, 43, 371–377. [Google Scholar] [CrossRef]
- Zhang, C.M.; Xu, L.M.; Wang, X.C.; Zhuang, K.; Liu, Q.Q. Effects of ultraviolet disinfection on antibiotic-resistant Escherichia coli from wastewater: Inactivation, antibiotic resistance profiles and antibiotic resistance genes. J. Appl. Microbiol. 2017, 123, 295–306. [Google Scholar] [CrossRef]
- Guo, M.-T.; Kong, C. Antibiotic resistant bacteria survived from UV disinfection: Safety concerns on genes dissemination. Chemosphere 2019, 224, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hou, A.; Chen, T.; Yang, D.; Chen, Z.; Shen, Z.; Qiu, Z.; Yin, J.; Yang, Z.; Shi, D.; et al. Decreased Antibiotic Susceptibility in Pseudomonas aeruginosa Surviving UV Irradition. Front. Microbiol. 2021, 12, 604245. [Google Scholar] [CrossRef]


| Parameters | Average and Standard Deviation |
|---|---|
| pH | 7.2 ± 0.6 |
| Total solids (g/L) | 34.6 ± 3.6 |
| Total suspended solids (g/L) | 1.0 ± 0.3 |
| Total dissolved solids (g/L) | 33.6 ± 3.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sério, J.; Marques, A.P.; Huertas, R.; Crespo, J.G.; Pereira, V.J. Occurrence and Treatment of Antibiotic-Resistant Bacteria Present in Surface Water. Membranes 2023, 13, 425. https://doi.org/10.3390/membranes13040425
Sério J, Marques AP, Huertas R, Crespo JG, Pereira VJ. Occurrence and Treatment of Antibiotic-Resistant Bacteria Present in Surface Water. Membranes. 2023; 13(4):425. https://doi.org/10.3390/membranes13040425
Chicago/Turabian StyleSério, João, Ana Paula Marques, Rosa Huertas, João Goulão Crespo, and Vanessa Jorge Pereira. 2023. "Occurrence and Treatment of Antibiotic-Resistant Bacteria Present in Surface Water" Membranes 13, no. 4: 425. https://doi.org/10.3390/membranes13040425
APA StyleSério, J., Marques, A. P., Huertas, R., Crespo, J. G., & Pereira, V. J. (2023). Occurrence and Treatment of Antibiotic-Resistant Bacteria Present in Surface Water. Membranes, 13(4), 425. https://doi.org/10.3390/membranes13040425







