Novel Hydrophobic Ultrafiltration Membranes for Treatment of Oil-Contaminated Wastewater
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Membrane Modification
2.3. FTIR Analysis
2.4. Membrane Structure Studies
2.5. Water Contact Angle Measurements
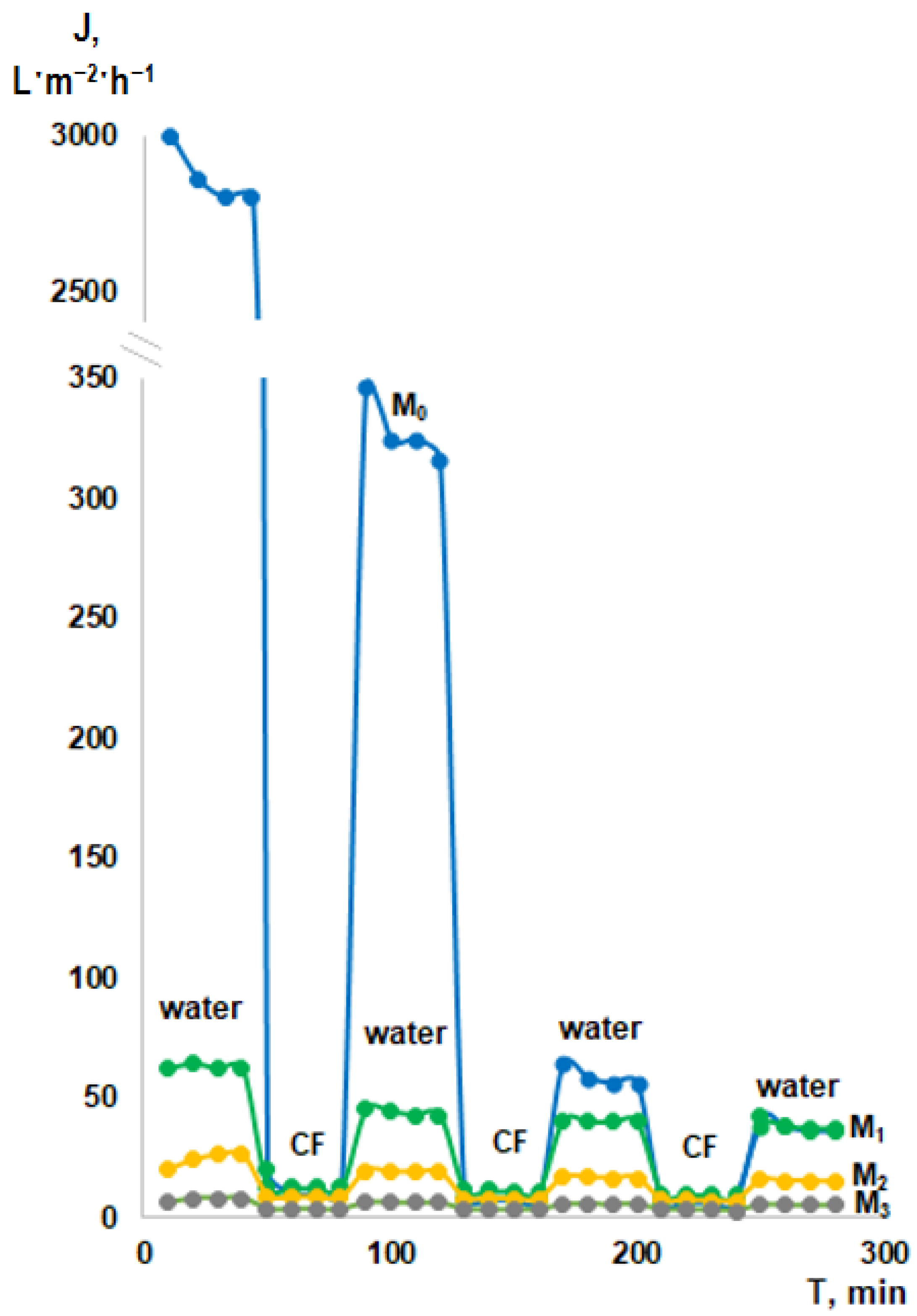
2.6. Flux, Cutting Fluid Rejection, and Antifouling Performance of the Membranes
3. Results and Discussion
3.1. Study of the Effect of PTFE-Modification on Composition of Membrane Selective Layer
3.2. Effect of PTFE-Modification on the Structure of Membranes
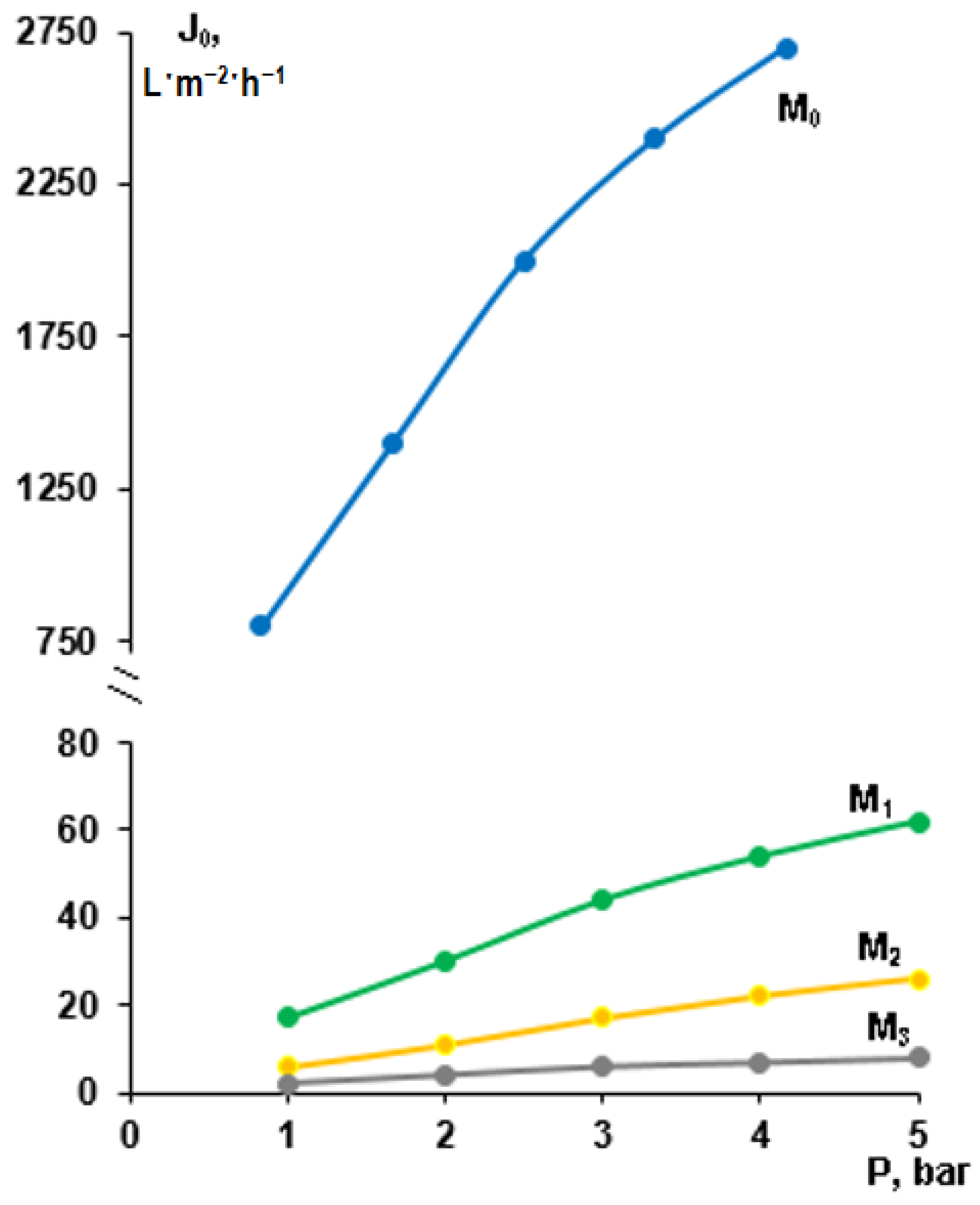
3.3. Effect of Modification by Low-Energy Electron Beam Deposition of PTFE on Membrane Permeability
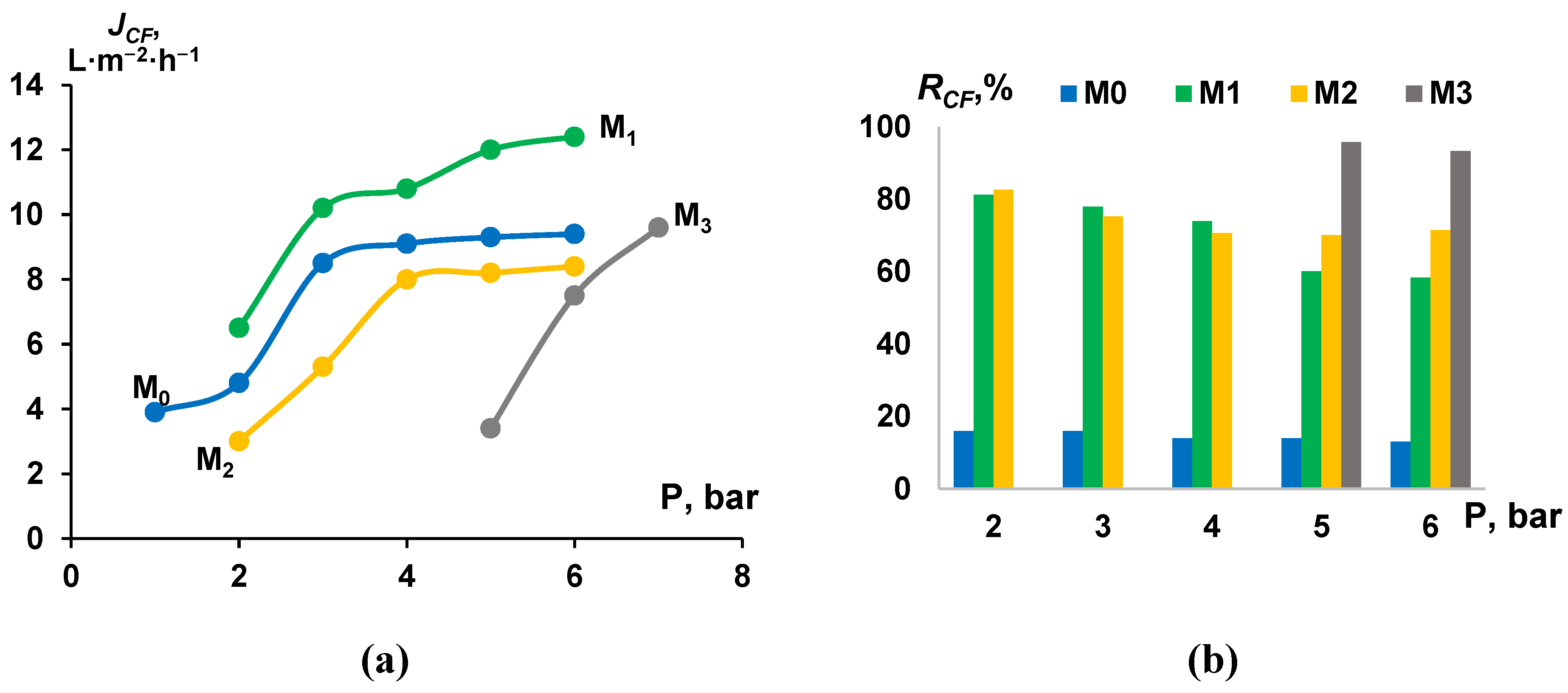
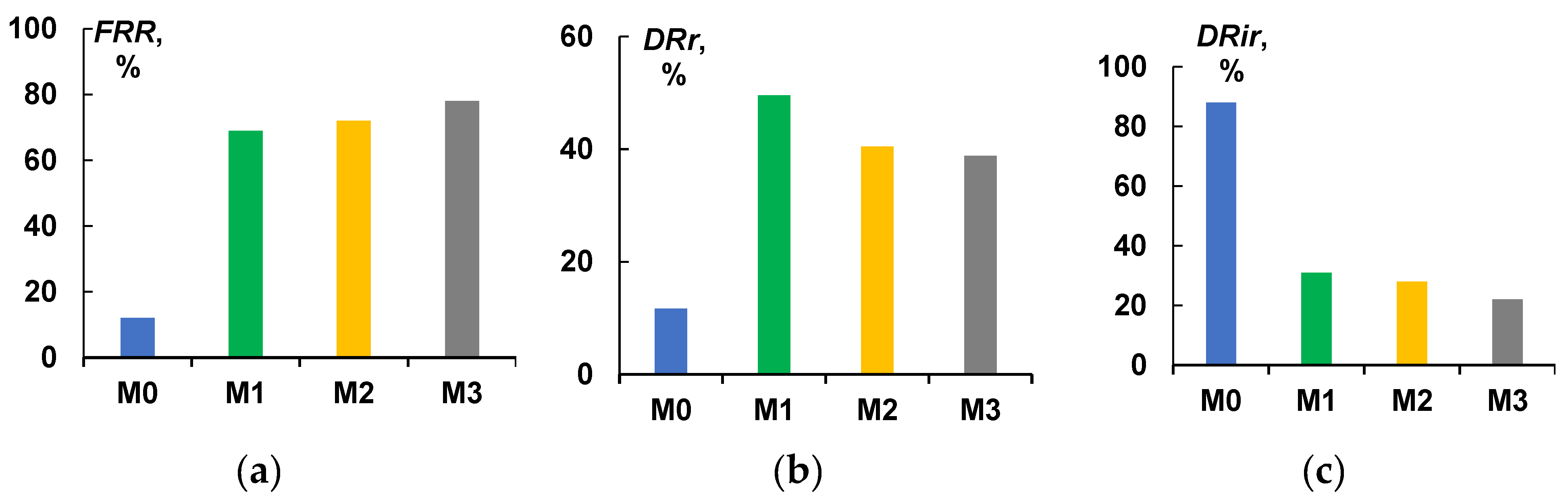
3.4. Cutting Fluid Emulsion Ultrafiltration Experiment—Fluxes and Retentions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- MacAdam, J.; Ozgencil, H.; Autin, O.; Pidou, M.; Temple, C.; Parsons, S.; Jefferson, B. Incorporating biodegradation and advanced oxidation processes in the treatment of spent metalworking fluids. Environ. Technol. 2012, 33, 2741–2750. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, T.; Tabatabai, S.A.A.; Zhang, T.; Leiknes, T. Role of surfactants in cleaning of PVDF ultrafiltration membranes fouled by emulsified cutting oil. J. Water Process Eng. 2021, 40, 101923. [Google Scholar] [CrossRef]
- Sales, W.F.; Diniz, A.E.; Machado, Á.R. Application of cutting fluids in machining processes. J. Braz. Soc. Mech. Sci. 2001, 23, 227–240. [Google Scholar] [CrossRef]
- Debnath, S.; Reddy, M.M.; Yi, Q.S. Environmental friendly cutting fluids and cooling techniques in machining: A review. J. Clean. Prod. 2014, 83, 33–47. [Google Scholar] [CrossRef]
- Amin, M.M.; Mofrad, M.M.G.; Pourzamani, H.; Sebaradar, S.M.; Ebrahim, K. Treatment of industrial wastewater contaminated with recalcitrant metal working fluids by the photo-Fenton process as post-treatment for DAF. J. Ind. Eng. Chem. 2017, 45, 412–420. [Google Scholar] [CrossRef]
- Vahid, A.; Mojtaba, F.; Abbas, S.; Reza, K. Evaluation of the Metalwork Cutting Fluid Treatment performance Using Fenton Oxidation Process in Comparison with Coagulation-Flocculation. Casp. J. Appl. Sci. Res. 2013, 2, 90–98. [Google Scholar]
- Seo, D.C.; Lee, H.J.; Hwang, H.N.; Park, M.R.; Kwak, N.W.; Cho, I.J.; Heo, J.S. Treatment of non-biodegradable cutting oil wastewater by ultrasonication-Fenton oxidation process. Water Sci. Technol. 2007, 55, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, D.; Li, A. Biodiesel production of Rhodosporidium toruloides using different carbon sources of sugar-containing wastewater: Experimental analysis and model verification. J. Clean. Prod. 2021, 323, 129112. [Google Scholar] [CrossRef]
- Rasouli, S.; Rezaei, N.; Hamedi, H.; Zendehboudi, S.; Duan, X. Superhydrophobic and superoleophilic membranes for oil-water separation application: A comprehensive review. Mater. Des. 2021, 204, 109599. [Google Scholar] [CrossRef]
- Lopez-Torres, D.; Elosua, C.; Hernaez, M.; Goicoechea, J.; Arregui, F.J. From superhydrophilic to superhydrophobic surfaces by means of polymeric layer-by-layer films. Appl. Surf. Sci. 2015, 351, 1081–1086. [Google Scholar] [CrossRef]
- Choong, L.T.S.; Lin, Y.M.; Rutledge, G.C. Separation of oil-in-water emulsions using electrospun fiber membranes and modeling of the fouling mechanism. J. Membr. Sci. 2015, 486, 229–238. [Google Scholar] [CrossRef]
- Al-Malack, M.H. Treatment of petroleum refinery wastewater using crossflow and immersed membrane processes. Desalin. Water Treat. 2013, 51, 6985–6993. [Google Scholar] [CrossRef]
- Li, Q.; Yan, Z.Q.; Wang, X.L. A poly (sulfobetaine) hollow fiber ultrafiltration membrane for the treatment of oily wastewater. Desalin. Water Treat. 2016, 57, 11048–11065. [Google Scholar] [CrossRef]
- Chen, W.; Peng, J.; Su, Y.; Zheng, L.; Wang, L.; Jiang, Z. Separation of oil/water emulsion using Pluronic F127 modified polyethersulfone ultrafiltration membranes. Sep. Purif. Technol. 2009, 66, 591–597. [Google Scholar] [CrossRef]
- Ochoa, N.A.; Masuelli, M.; Marchese, J. Effect of hydrophilicity on fouling of an emulsified oil wastewater with PVDF/PMMA membranes. J. Membr. Sci. 2003, 226, 203–211. [Google Scholar] [CrossRef]
- Wang, X.; Fang, D.; Yoon, K.; Hsiao, B.S.; Chu, B. High performance ultrafiltration composite membranes based on poly (vinyl alcohol) hydrogel coating on crosslinked nanofibrous poly (vinyl alcohol) scaffold. J. Membr. Sci. 2006, 278, 261–268. [Google Scholar] [CrossRef]
- Chakrabarty, B.; Ghoshal, A.K.; Purkait, M.K. Ultrafiltration of stable oil-in-water emulsion by polysulfone membrane. J. Membr. Sci. 2008, 325, 427–437. [Google Scholar] [CrossRef]
- Chakrabarty, B.; Ghoshal, A.K.; Purkait, M.K. Cross-flow ultrafiltration of stable oil-in-water emulsion using polysulfone membranes. Chem. Eng. J. 2010, 165, 447–456. [Google Scholar] [CrossRef]
- Jagadevan, S.; Jayamurthy, M.; Dobson, P.; Thompson, I.P. A novel hybrid nano zerovalent iron initiated oxidation–Biological degradation approach for remediation of recalcitrant waste metalworking fluids. Water Res. 2012, 46, 2395–2404. [Google Scholar] [CrossRef]
- Muszyński, A.; Załęska–Radziwiłł, M.; Łebkowska, M.; Nowak, D. Biological and electrochemical treatment of used metalworking fluids: A toxicity-reduction evaluation. Arch. Environ. Contam. Toxicol. 2007, 52, 483–488. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, Z.; Zhang, F.; Liu, X.; Jin, J.; Jiang, L. Superhydrophobic and superoleophilic PVDF membranes for effective separation of water-in-oil emulsions with high flux. Adv. Mater. 2013, 25, 2071–2076. [Google Scholar] [CrossRef] [PubMed]
- Wandera, D.; Wickramasinghe, S.R.; Husson, S.M. Modification and characterization of ultrafiltration membranes for treatment of produced water. J. Membr. Sci. 2011, 373, 178–188. [Google Scholar] [CrossRef]
- Kim, D.; Livazovic, S.; Falca, G.; Nunes, S.P. Oil–water separation using membranes manufactured from cellulose/ionic liquid solutions. ACS Sustain. Chem. Eng. 2018, 7, 5649–5659. [Google Scholar] [CrossRef]
- Usman, J.; Othman, M.H.D.; Ismail, A.F.; Rahman, M.A.; Jaafar, J.; Raji, Y.O.; Said, K.A.M. An overview of superhydrophobic ceramic membrane surface modification for oil-water separation. J. Mater. Res. Technol. 2021, 12, 643–667. [Google Scholar] [CrossRef]
- Zioui, D.; Salazar, H.; Aoudjit, L.; Martins, P.M.; Lanceros-Méndez, S. Polymer-based membranes for oily wastewater remediation. Polymers 2019, 12, 42. [Google Scholar] [CrossRef]
- Zioui, D.; Martins, P.M.; Aoudjit, L.; Salazar, H.; Lanceros-Méndez, S. Wastewater Treatment of Real Effluents by Microfiltration Using Poly (vinylidene fluoride–hexafluoropropylene) Membranes. Polymers 2023, 15, 1143. [Google Scholar] [CrossRef]
- Peer, P.; Polaskova, M.; Musilova, L. Superhydrophobic poly (vinyl butyral) nanofibrous membrane containing various silica nanoparticles. J. Text. Inst. 2019, 110, 1508–1514. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, G.; Wang, Y.; Han, W.; Zhang, Y.; Hu, W.; Xiao, C. Superhydrophobic and Breathable Polyacrylonitrile/Silica/Perfluoroalkyl Ethyl Methacrylate Nanofiber Membranes Prepared by Solution Blow Spinning. ACS Omega 2022, 7, 30333–30346. [Google Scholar] [CrossRef]
- Jiang, S.; Meng, X.; Chen, B.; Wang, N.; Chen, G. Electrospinning superhydrophobic–superoleophilic PVDF-SiO2 nanofibers membrane for oil–water separation. J. Appl. Polym. Sci. 2020, 137, 49546. [Google Scholar] [CrossRef]
- Ju, J.; Wang, T.; Wang, Q. A facile approach in fabricating superhydrophobic and superoleophilic poly (vinylidene fluoride) membranes for efficient water–oil separation. J. Appl. Polym. Sci. 2015, 132, 42077. [Google Scholar] [CrossRef]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Taurozzi, J.S.; Arul, H.; Bosak, V.Z.; Burban, A.F.; Voice, T.C.; Bruening, M.L.; Tarabara, V.V. Effect of filler incorporation route on the properties of polysulfone–silver nanocomposite membranes of different porosities. J. Membr. Sci. 2008, 325, 58–68. [Google Scholar] [CrossRef]
- Muhamad, M.S.; Salim, M.R.; Lau, W.J. Surface modification of SiO2 nanoparticles and its impact on the properties of PES-based hollow fiber membrane. RSC Adv. 2015, 5, 58644–58654. [Google Scholar] [CrossRef]
- Qing, W.; Shi, X.; Deng, Y.; Zhang, W.; Wang, J.; Tang, C.Y. Robust superhydrophobic-superoleophilic polytetrafluoroethylene nanofibrous membrane for oil/water separation. J. Membr. Sci. 2017, 540, 354–361. [Google Scholar] [CrossRef]
- Wang, S.; Li, M.; Lu, Q. Filter paper with selective absorption and separation of liquids that differ in surface tension. ACS Appl. Mater. Interfaces 2010, 2, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Wu, X.F. Electrospinning superhydrophobic–superoleophilic fibrous PVDF membranes for high-efficiency water–oil separation. Mater. Lett. 2015, 160, 423–427. [Google Scholar] [CrossRef]
- Ahmed, F.E.; Lalia, B.S.; Hashaikeh, R. A review on electrospinning for membrane fabrication: Challenges and applications. Desalination 2015, 356, 15–30. [Google Scholar] [CrossRef]
- Kravets, L.; Gainutdinov, R.; Gilman, A.; Yablokov, M.; Satulu, V.; Mitu, B.; Dinescu, G. Morphology and Contact Properties of Polytetrafluoroethylene-Like Films Deposited onto Track-Etched Membrane Surface in Vacuum. Plasma Phys. Technol. 2018, 5, 110–116. [Google Scholar] [CrossRef]
- Pierson, H.O. Handbook of Chemical Vapor Deposition: Principles, Technologies and Applications; Noyes Publications: New York, NY, USA, 1999. [Google Scholar]
- Kravets, L.I.; Yarmolenko, M.A.; Gainutdinov, R.V.; Satulu, V.; Mitu, B.; Dinescu, G. Formation of hydrophobic polymer coatings on the track-etched membrane surface. Int. J. Phys. Conf. Ser. 2021, 1954, 012022. [Google Scholar] [CrossRef]
- Kravets, L.I.; Yarmolenko, M.A.; Rogachev, A.A.; Gainutdinov, R.V.; Gilman, A.B.; Altynov, V.A.; Lizunov, N.E. Formation of Superhydrophobic Coatings on the Track-Etched Membrane Surface by the Method of Electron-Beam Deposition of Polymers in Vacuum. Inorg. Mater. Appl. Res. 2020, 11, 476–487. [Google Scholar] [CrossRef]
- Martin, I.; Bertin, M.; Domaracka, A.; Azria, R.; Illenberger, E.; Lafosse, A. Chemistry induced by low-energy electrons in condensed multilayers of pure small organic acids. Int. J. Mass Spectrom. 2008, 277, 262–268. [Google Scholar] [CrossRef]
- Sala, L.; Szymańska, I.B.; Dablemont, C.; Lafosse, A.; Amiaud, L. Response under low-energy electron irradiation of a thin film of a potential copper precursor for focused electron beam induced deposition (FEBID). Beilstein J. Nanotechnol. 2018, 9, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Thorman, R.M.; TP, R.K.; Fairbrother, D.H.; Ingólfsson, O. The role of low-energy electrons in focused electron beam induced deposition: Four case studies of representative precursors. Beilstein J. Nanotechnol. 2015, 6, 1904–1926. [Google Scholar] [CrossRef]
- Rogachev, A.A.; Yarmolenko, M.A.; Rogachev, A.V.; Xiaohong, J.; Cao, H.; Lysenko, E.N.; Surzhikov, A.P. Structure and electrical properties of polyaniline-based copper chloride or copper bromide coatings deposited via low-energy electron beam. Appl. Surf. Sci. 2019, 483, 19–25. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, X.; Rogachev, A.V.; Rogachev, A.A.; Kontsevaya, I.I.; Pyzh, A.E.; Yarmolenko, M.A. Structure and properties of microcellulose-based coatings deposited via a low-energy electron beam and their effect on the properties of onto wound dressings. Carbohydr. Polym. Technol. Appl. 2021, 2, 100146. [Google Scholar] [CrossRef]
- Hliavitskaya, T.; Plisko, T.; Bildyukevich, A.; Lipnizki, F.; Rodrigues, G.; Sjölin, M. Modification of PES ultrafiltration membranes by cationic polyelectrolyte Praestol 859: Characterization, performance and application for purification of hemicellulose. Chem. Eng. Res. Des. 2020, 162, 187–199. [Google Scholar] [CrossRef]
- Huotari, H.M.; Huisman, I.H.; Trägårdh, G. Electrically enhanced crossflow membrane filtration of oily waste water using the membrane as a cathode. J. Membr. Sci. 1999, 156, 49–60. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Kuzminova, A.; Zolotarev, A.; Markelov, D.; Komolkin, A.; Loginova, E.; Penkova, A. Modification strategies of polyacrylonitrile ultrafiltration membrane using TiO2 for enhanced antifouling performance in water treatment. Sep. Purif. Technol. 2022, 286, 120500. [Google Scholar] [CrossRef]
- Mahdavi, H.; Karami, M.; Heidari, A.A. Preparation of mixed matrix membranes made up of polysulfone and MIL-53 (Al) nanoparticles as promising membranes for separation of aqueous dye solutions. Sep. Purif. Technol. 2021, 274, 119033. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, W.; Yu, S.; Zhu, Y.; Zhang, R.; Tay, J.H. Adsorption of anion polyacrylamide from aqueous solution by polytetrafluoroethylene (PTFE) membrane as an adsorbent: Kinetic and isotherm studies. J. Colloid Interface Sci. 2019, 544, 303–311. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Abdulkarim, A.A.; Ooi, B.S.; Ismail, S. Recent development in additives modifications of polyethersulfone membrane for flux enhancement. Chem. Eng. J. 2013, 223, 246–267. [Google Scholar] [CrossRef]
- Bildyukevich, A.V.; Hliavitskaya, T.A.; Kavalenka, M.N. The Modification of polyethersulfone membranes using a Synperonic F108 block copolymer and their application for the fractionation of thermomechanical pulp mill process water. Membr. Membr. Technol. 2020, 2, 210–216. [Google Scholar] [CrossRef]
- Plisko, T.V.; Bildyukevich, A.V.; Burts, K.S.; Ermakov, S.S.; Penkova, A.V.; Kuzminova, A.I.; Ulbricht, M. One-step preparation of antifouling polysulfone ultrafiltration membranes via modification by a cationic polyelectrolyte based on polyacrylamide. Polymers 2020, 12, 1017. [Google Scholar] [CrossRef] [PubMed]
- Plisko, T.V.; Bildyukevich, A.V.; Burts, K.S.; Hliavitskaya, T.A.; Penkova, A.V.; Ermakov, S.S.; Ulbricht, M. Modification of polysulfone ultrafiltration membranes via addition of anionic polyelectrolyte based on acrylamide and sodium acrylate to the coagulation bath to improve antifouling performance in water treatment. Membranes 2020, 10, 264. [Google Scholar] [CrossRef] [PubMed]
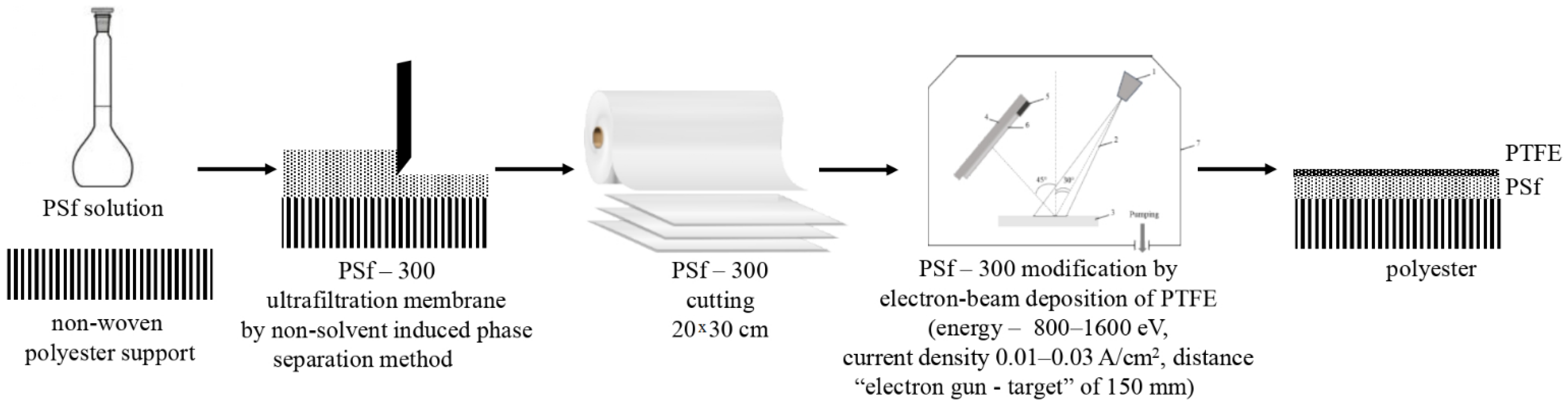
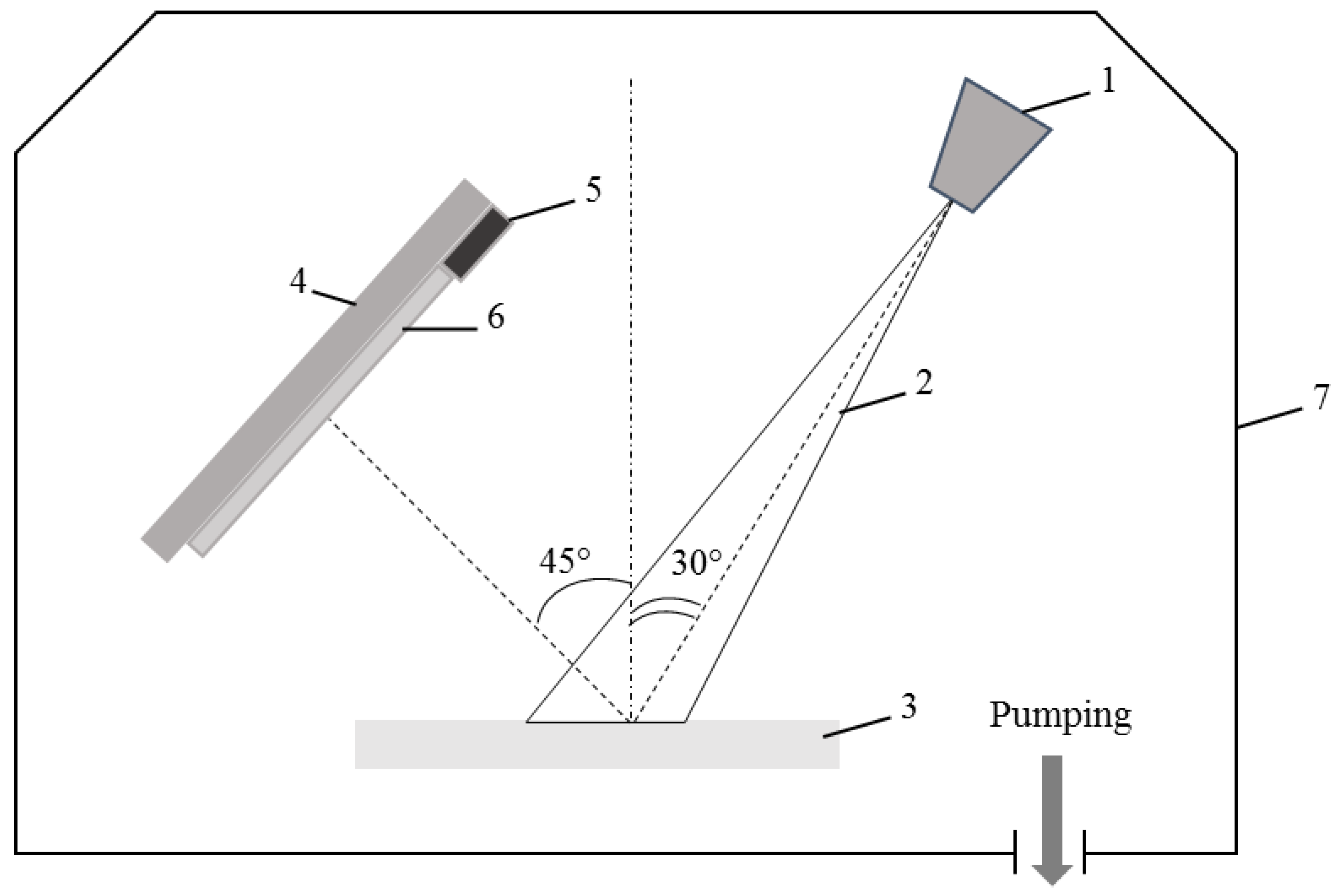
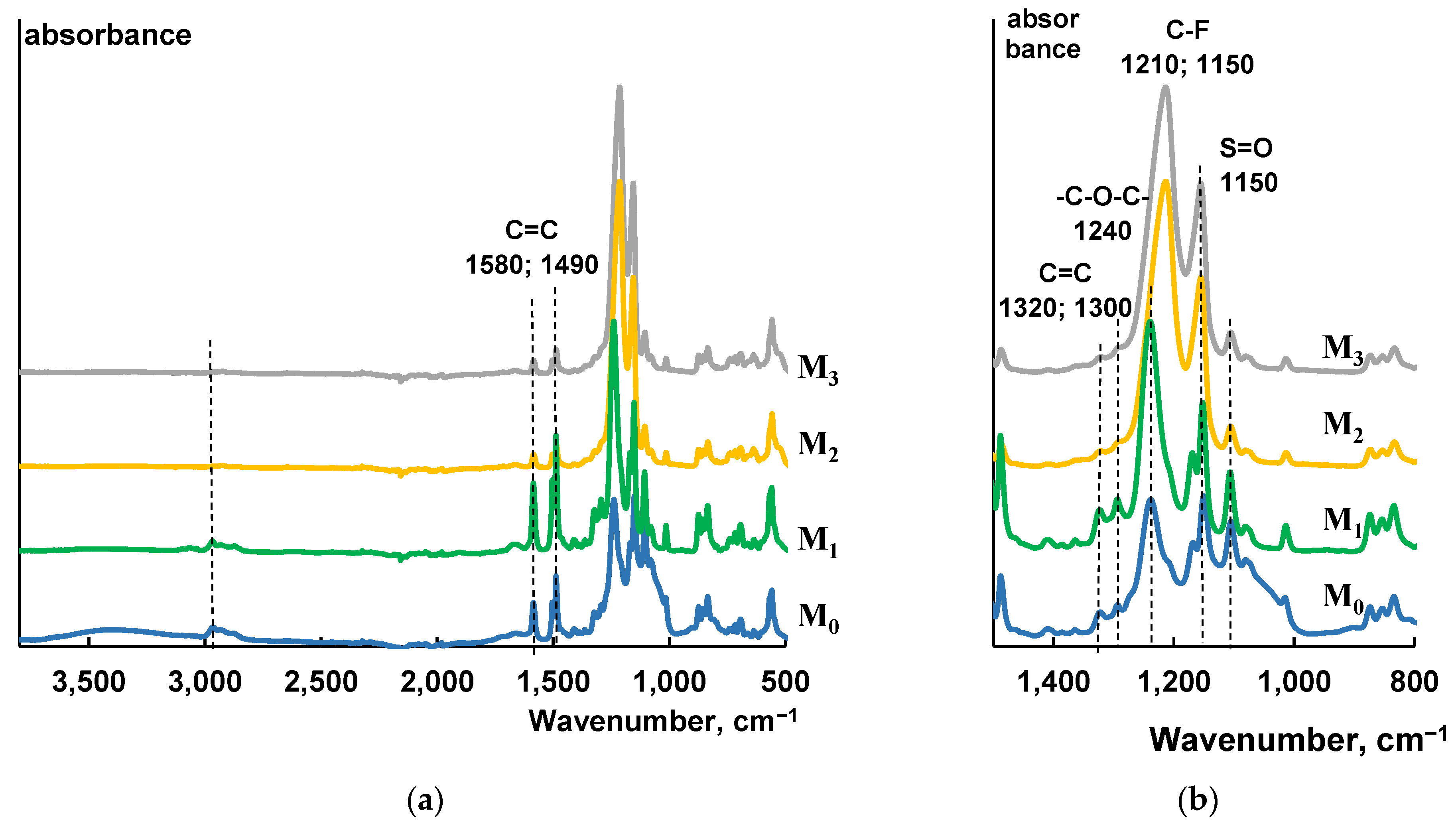

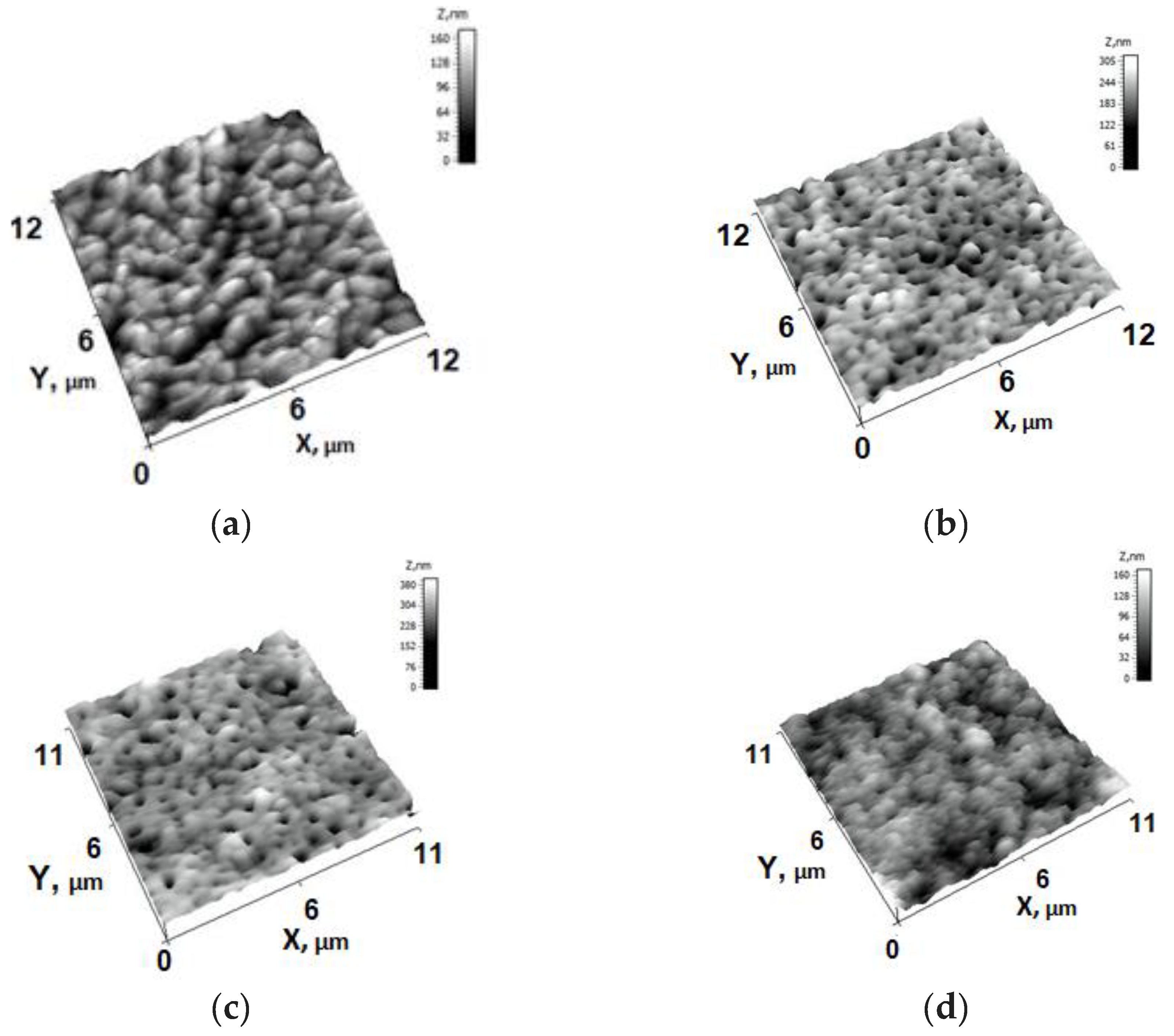
| Membrane Abbreviation | Modifying PTFE Layer Thickness (nm) |
|---|---|
| M0 | 0 |
| M1 | 45 |
| M2 | 660 |
| M3 | 1350 |
| Membrane Abbreviation | PTFE Layer Thickness, (nm) | Ra (nm) | Rq (nm) | WCA (°) |
|---|---|---|---|---|
| M0 | 0 | 39.3 | 50.8 | 56 ± 2 |
| M1 | 45 | 30.4 | 40.1 | 110 ± 2 |
| M2 | 660 | 28.8 | 36.0 | 120 ± 2 |
| M3 | 1350 | 15.7 | 19.2 | 120 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hliavitskaya, T.; Plisko, T.; Bildyukevich, A.; Liubimova, A.; Shumskaya, A.; Mikchalko, A.; Rogachev, A.A.; Melnikova, G.B.; Pratsenko, S.A. Novel Hydrophobic Ultrafiltration Membranes for Treatment of Oil-Contaminated Wastewater. Membranes 2023, 13, 402. https://doi.org/10.3390/membranes13040402
Hliavitskaya T, Plisko T, Bildyukevich A, Liubimova A, Shumskaya A, Mikchalko A, Rogachev AA, Melnikova GB, Pratsenko SA. Novel Hydrophobic Ultrafiltration Membranes for Treatment of Oil-Contaminated Wastewater. Membranes. 2023; 13(4):402. https://doi.org/10.3390/membranes13040402
Chicago/Turabian StyleHliavitskaya, Tatsiana, Tatiana Plisko, Alexandr Bildyukevich, Alena Liubimova, Alena Shumskaya, Alexey Mikchalko, Alexandr A. Rogachev, Galina B. Melnikova, and Svetlana A. Pratsenko. 2023. "Novel Hydrophobic Ultrafiltration Membranes for Treatment of Oil-Contaminated Wastewater" Membranes 13, no. 4: 402. https://doi.org/10.3390/membranes13040402
APA StyleHliavitskaya, T., Plisko, T., Bildyukevich, A., Liubimova, A., Shumskaya, A., Mikchalko, A., Rogachev, A. A., Melnikova, G. B., & Pratsenko, S. A. (2023). Novel Hydrophobic Ultrafiltration Membranes for Treatment of Oil-Contaminated Wastewater. Membranes, 13(4), 402. https://doi.org/10.3390/membranes13040402








