Potential of Lipid-Based Nanocarriers against Two Major Barriers to Drug Delivery—Skin and Blood–Brain Barrier
Abstract
1. Introduction
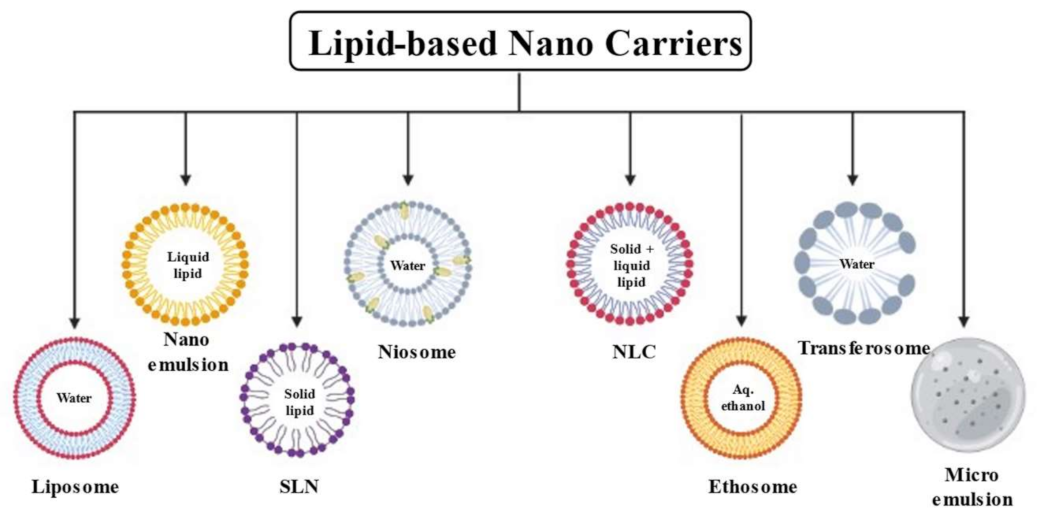
2. Lipid-Based Nanocarrier Systems
2.1. Liposome
2.2. Niosomes
2.3. Ethosome
2.4. Transethosome
2.5. Solid Lipid Nanoparticles (SLNs)
2.6. Nanostructured Lipid Carriers (NLCs)
2.7. Lipid Nanoemulsion (LNEs)
2.8. Microemulsion
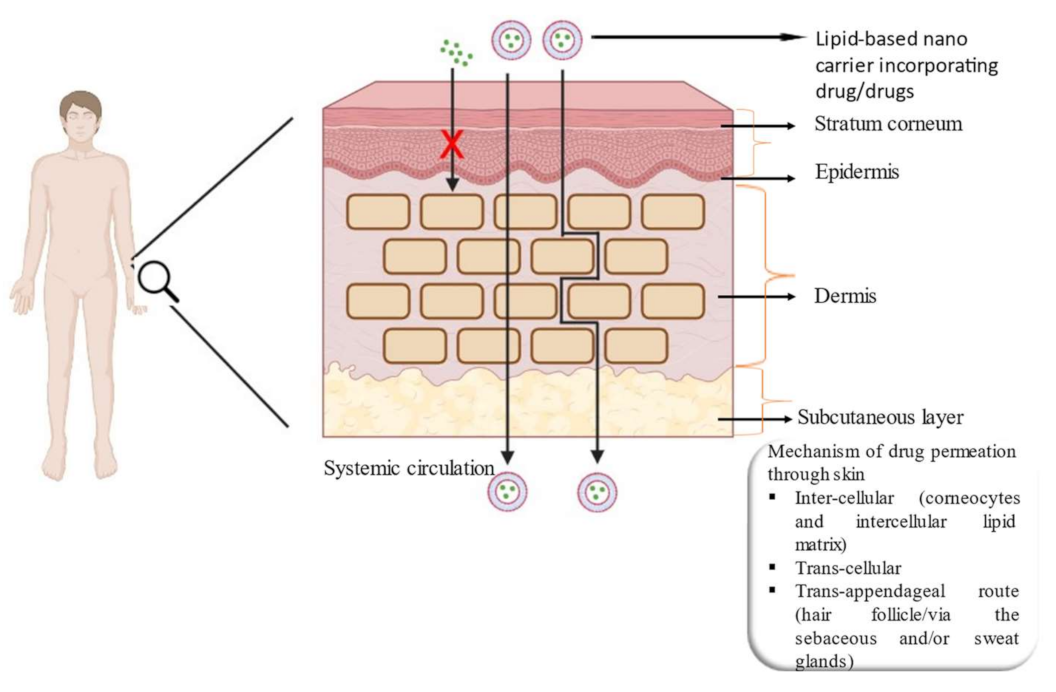
3. Lipid-Based Nanocarrier System for Skin Delivery
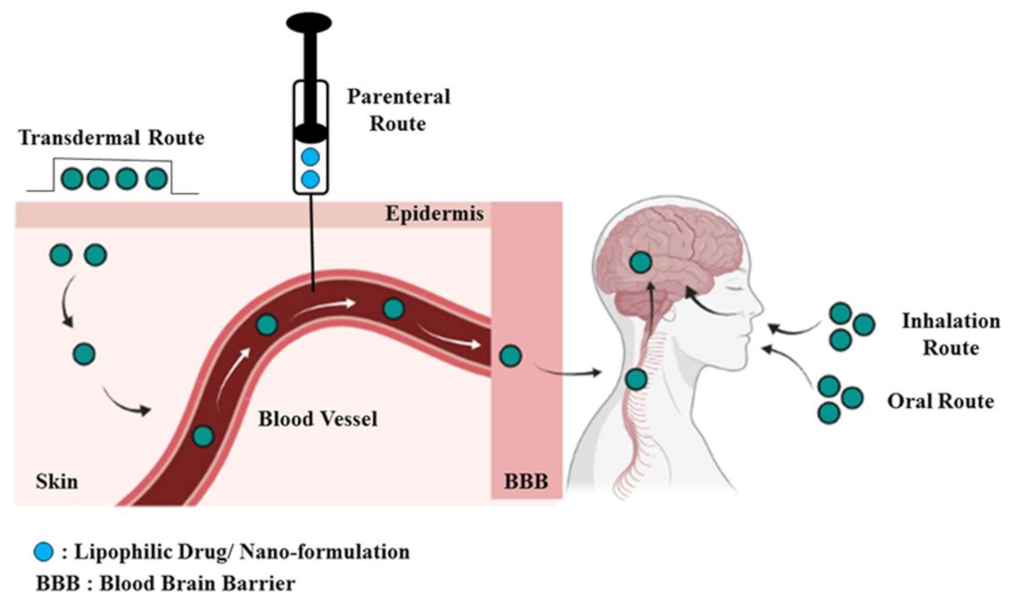
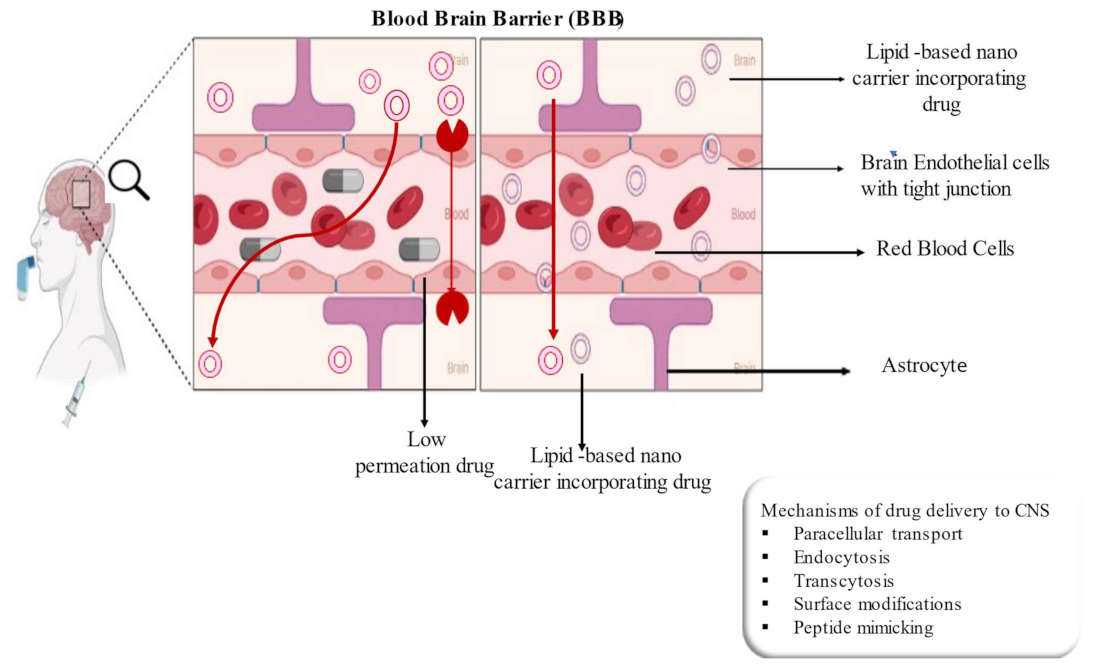
4. Lipid-Based Nanocarrier Systems for CNS Delivery
5. Conclusions and Future Prospective
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arora, A.; Prausnitz, M.R.; Mitragotri, S. Micro-scale devices for transdermal drug delivery. Int. J. Pharm. 2008, 364, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Ita, K. Dissolving microneedles for transdermal drug delivery: Advances and challenges. Biomed. Pharmacother. 2017, 93, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Dragicevic, N.; Maibach, H.I. Percutaneous Penetration Enhancers Physical Methods in Penetration Enhancement; Springer: Berlin/Heidelberg, Germany, 2017; ISBN 9783662532737. Available online: https://link.springer.com/book/10.1007/978-3-662-53273-7 (accessed on 15 July 2022).
- Mitragotri, S. Devices for overcoming biological barriers: The use of physical forces to disrupt the barriers. Adv. Drug Deliv. Rev. 2013, 65, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Touitou, E.; Dayan, N.; Bergelson, L.; Godin, B.; Eliaz, M. Ethosomes—Novel vesicular carriers for enhanced delivery: Characterization and skin penetration properties. J. Control. Release 2000, 65, 403–418. [Google Scholar] [CrossRef]
- Jung, E.C.; Maibach, H.I. Animal models for percutaneous absorption. J. Appl. Toxicol. 2015, 35, 1–10. [Google Scholar] [CrossRef]
- Christophers, E. Cellular architecture of the stratum corneum. J. Investig. Dermatol. 1971, 56, 165–169. [Google Scholar] [CrossRef]
- Hoath, S.B.; Leahy, D.G. The Organization of Human Epidermis: Functional Epidermal Units and Phi Proportionality. J. Investig. Dermatol. 2003, 121, 1440–1446. [Google Scholar] [CrossRef]
- Yu, Y.Q.; Yang, X.; Wu, X.F.; Fan, Y. Bin Enhancing Permeation of Drug Molecules Across the Skin via Delivery in Nanocarriers: Novel Strategies for Effective Transdermal Applications. Front. Bioeng. Biotechnol. 2021, 9, 646554. [Google Scholar] [CrossRef]
- Bouwstra, J.A.; Honeywell-Nguyen, P.L.; Gooris, G.S.; Ponec, M. Structure of the skin barrier and its modulation by vesicular formulations. Prog. Lipid Res. 2003, 42, 1–36. [Google Scholar] [CrossRef]
- Geusens, B.; Strobbe, T.; Bracke, S.; Dynoodt, P.; Sanders, N.; Van Gele, M.; Lambert, J. Lipid-mediated gene delivery to the skin. Eur. J. Pharm. Sci. 2011, 43, 199–211. [Google Scholar] [CrossRef]
- Baroli, B. Penetration of nanoparticles and nanomaterials in the skin: Fiction or reality? J. Pharm. Sci. 2010, 99, 21–50. [Google Scholar] [CrossRef]
- Verma, D.D.; Verma, S.; Blume, G.; Fahr, A. Particle size of liposomes influences dermal delivery of substances into skin. Int. J. Pharm. 2003, 258, 141–151. [Google Scholar] [CrossRef]
- Mohapatra, S.; Mirza, M.A.; Hilles, A.R.; Zakir, F.; Gomes, A.C.; Ansari, M.J.; Iqbal, Z.; Mahmood, S. Biomedical application, patent repository, clinical trial and regulatory updates on hydrogel: An extensive review. Gels 2021, 7, 207. [Google Scholar] [CrossRef]
- Andrews, S.N.; Jeong, E.; Prausnitz, M.R. Transdermal delivery of molecules is limited by full epidermis, not just stratum corneum. Pharm. Res. 2013, 30, 1099–1109. [Google Scholar] [CrossRef]
- Gupta, V.; Mohapatra, S.; Mishra, H.; Farooq, U.; Kumar, K.; Ansari, M.J.; Aldawsari, M.F.; Alalaiwe, A.S.; Mirza, M.A.; Iqbal, Z. Nanotechnology in Cosmetics and Cosmeceuticals—A Review of Latest Advancements. Gels 2022, 8, 173. [Google Scholar] [CrossRef]
- McMahon, D.; O’Reilly, M.A.; Hynynen, K. Therapeutic Agent Delivery across the Blood-Brain Barrier Using Focused Ultrasound. Annu. Rev. Biomed. Eng. 2021, 23, 89–113. [Google Scholar] [CrossRef]
- Tajes, M.; Ramos-Fernández, E.; Weng-Jiang, X.; Bosch-Morató, M.; Guivernau, B.; Eraso-Pichot, A.; Salvador, B.; Fernàndez-Busquets, X.; Roquer, J.; Muñoz, F.J. The blood-brain barrier: Structure, function and therapeutic approaches to cross it. Mol. Membr. Biol. 2014, 31, 152–167. [Google Scholar] [CrossRef]
- Dardet, J.P.; Serrano, N.; András, I.E.; Toborek, M. Overcoming Blood-Brain Barrier Resistance: Implications for Extracellular Vesicle-Mediated Drug Brain Delivery. Front Drug Deliv. 2022, 2, 855017. [Google Scholar] [CrossRef]
- Burgess, A.; Hynynen, K. Noninvasive and targeted drug delivery to the brain using focused ultrasound. ACS Chem. Neurosci. 2013, 4, 519–526. [Google Scholar] [CrossRef]
- Li, J.; Zheng, M.; Shimoni, O.; Banks, W.A.; Bush, A.I.; Gamble, J.R.; Shi, B. Development of Novel Therapeutics Targeting the Blood–Brain Barrier: From Barrier to Carrier. Adv. Sci. 2021, 8, e2101090. [Google Scholar] [CrossRef]
- Naseri, N.; Valizadeh, H.; Zakeri-Milani, P. Solid lipid nanoparticles and nanostructured lipid carriers: Structure preparation and application. Adv. Pharm. Bull. 2015, 5, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.; Gajbhiye, K.R.; Soni, V. Lactoferrin-appended solid lipid nanoparticles of paclitaxel for effective management of bronchogenic carcinoma. Drug Deliv. 2015, 22, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Garg, T.; Rath, G.; Goyal, A.K. Advances in nanotechnology-based carrier systems for targeted delivery of bioactive drug molecules with special emphasis on immunotherapy in drug resistant tuberculosis—A critical review. Drug Deliv. 2016, 23, 1676–1698. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Husain, S.A.; Parveen, R.; Mohapatra, S. Amalgamation of Nanotechnology for Delivery of Bioactive Constituents in Solid Tumors. Curr. Drug Deliv. 2022, 22, 457–482. [Google Scholar] [CrossRef]
- Mazayen, Z.M.; Ghoneim, A.M.; Elbatanony, R.S.; Basalious, E.B.; Bendas, E.R. Pharmaceutical nanotechnology: From the bench to the market. Future J. Pharm. Sci. 2022, 8, 12. [Google Scholar] [CrossRef]
- Halwani, A.A. Development of Pharmaceutical Nanomedicines: From the Bench to the Market. Pharmaceutics 2022, 14, 106. [Google Scholar] [CrossRef]
- Attama, A.A.; Momoh, M.A.; Builders, P.F. Chapter 5-Lipid nanoparticulate drug delivery systems: A revolution in dosage form design and development. Recent Adv. Nov. Drug Carr. Syst. 2012, 5, 107–140. [Google Scholar]
- Scioli Montoto, S.; Muraca, G.; Ruiz, M.E. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front. Mol. Biosci. 2020, 7, 587997. [Google Scholar] [CrossRef]
- Hua, S. Lipid-based nano-delivery systems for skin delivery of drugs and bioactives. Front. Pharmacol. 2015, 6, 219. [Google Scholar] [CrossRef]
- Dubey, V.; Mishra, D.; Nahar, M.; Jain, N.K. Vesicles as tools for the modulation of skin permeability. Expert Opin. Drug Deliv. 2007, 4, 579–593. [Google Scholar] [CrossRef]
- Elsayed, M.M.A.; Abdallah, O.Y.; Naggar, V.F.; Khalafallah, N.M. Lipid vesicles for skin delivery of drugs: Reviewing three decades of research. Int. J. Pharm. 2007, 332, 1–16. [Google Scholar] [CrossRef]
- Elsayed, M.M.A.; Abdallah, O.Y.; Naggar, V.F.; Khalafallah, N.M. Deformable liposomes and ethosomes: Mechanism of enhanced skin delivery. Int. J. Pharm. 2006, 322, 60–66. [Google Scholar] [CrossRef]
- Vanić, Ž. Phospholipid vesicles for enhanced drug delivery in dermatology. J. Drug Discov. Dev. Deliv. 2015, 2, 1010. [Google Scholar]
- Souto, E.B.; Fangueiro, J.F.; Fernandes, A.R.; Cano, A.; Sanchez-Lopez, E.; Garcia, M.L.; Severino, P.; Paganelli, M.O.; Chaud, M.V.; Silva, A.M. Physicochemical and biopharmaceutical aspects influencing skin permeation and role of SLN and NLC for skin drug delivery. Heliyon 2022, 2, e08938. [Google Scholar] [CrossRef]
- Wissing, S.A.; Müller, R.H. Cosmetic applications for solid lipid nanoparticles (SLN). Int. J. Pharm. 2003, 254, 65–68. [Google Scholar] [CrossRef]
- Wissing, S.A.; Müller, R.H. Solid lipid nanoparticles (SLN)—A novel carrier for UV blockers. Pharmazie 2001, 56, 783–786. [Google Scholar]
- Hooda, A.; Sradhanjali, M. Popsy Formulation and Evaluation of Novel Solid Lipid Microparticles for the Sustained Release of Ofloxacin. Pharm. Nanotechnol. 2017, 5, 329–341. [Google Scholar] [CrossRef]
- Lombardo, S.M.; Schneider, M.; Türeli, A.E.; Türeli, N.G. Key for crossing the BBB with nanoparticles: The rational design. Beilstein J. Nanotechnol. 2020, 11, 866–883. [Google Scholar] [CrossRef]
- Natarajan, J.; Baskaran, M.; Humtsoe, L.C.; Vadivelan, R.; Justin, A. Enhanced brain targeting efficacy of Olanzapine through solid lipid nanoparticles. Artif. Cells Nanomed. Biotechnol. 2017, 45, 364–371. [Google Scholar] [CrossRef]
- Haque, S.; Shadab; Alam, I.; Sahni, J.K.; Ali, J.; Baboota, S. Nanostructure-based drug delivery systems for brain targeting. Drug Dev. Ind. Pharm. 2012, 38, 387–411. [Google Scholar] [CrossRef]
- lypo-sheric-gsh. Available online: www.eastpark.com (accessed on 15 July 2022).
- California Gold Nutrition. Available online: www.californiagoldnutrition.com/collections/supplements (accessed on 15 July 2022).
- Aurora Nutrascience. Available online: in.iherb.com/pr/aurora-nutrascience-mega-liposomal-glutathione-plus-vitamin-c-organic-fruit-750-mg-16-fl-oz-480-ml/99988 (accessed on 15 July 2022).
- CLR Berlin MPC LIPOSOMES; Technical Datasheet. Available online: https://cosmetics.specialchem.com/product/i-clr-berlin-mpc-liposomes (accessed on 15 July 2022).
- sesderma-c-vit-facial-liposomal-serum. Available online: Theskinstore.in (accessed on 15 July 2022).
- NanoLipid Restore CLRTM. Available online: https://cosmetics.specialchem.com/product/i-chemishes-laboratorium-dr-kurt-richter-nanolipid-restore-clr (accessed on 15 July 2022).
- Mishra, D.K.; Shandilya, R.; Mishra, P.K. Lipid based nanocarriers: A Translational Perspective. Nanomedicine 2018, 14, 2023–2050. [Google Scholar] [CrossRef] [PubMed]
- Madaan, T.; Pandey, S.; Talegaonkar, S.; Delhi, N. Nanotechnology: A smart drug delivery tool in modern healthcare. J. Chem. Pharm. Res. 2015, 7, 257–264. [Google Scholar]
- Sable-Yong-Tests-Lancome-Advanced-Genifique-Youth-Activating-Serum. Available online: www.allure.com (accessed on 15 July 2022).
- Elmarzugi, N.A.; Eid, A.M.; Chellapa, P.; Mohamed, A.T.; Keleb, E.I.; Elmahgoubi, A.; Issa, Y.S. Nanoemulsion and Nanoemulgel as a Topical Formulation. IOSR J. Pharm. 2015, 5, 43–47. [Google Scholar]
- Coolnac gel. Available online: www.mims.com (accessed on 15 July 2022).
- Patel, D.B. Journal of Global Pharma Technology. 2009. Available online: www.jgpt.co.in (accessed on 15 July 2022).
- B019HXFNAM. Available online: www.amazon.ca (accessed on 15 July 2022).
- 2040073362313. Available online: www.digit-eyes.com (accessed on 15 July 2022).
- Sudhakar, C.K.; Upadhyay, N.; Jain, S.; Charyulu, R.N. Ethosomes as non-invasive loom for transdermal drug delivery system. In Nanomedicine and Drug Delivery; Apple Academic Press: Point Pleasant, NJ, USA, 2012; ISBN 9781466560079. [Google Scholar]
- Large, D.E.; Abdelmessih, R.G.; Fink, E.A.; Auguste, D.T. Liposome composition in drug delivery design, synthesis, characterization, and clinical application. Adv. Drug Deliv. Rev. 2021, 176, 113851. [Google Scholar] [CrossRef]
- Šturm, L.; Ulrih, N.P. Basic methods for preparation of liposomes and studying their interactions with different compounds, with the emphasis on polyphenols. Int. J. Mol. Sci. 2021, 22, 6547. [Google Scholar] [CrossRef]
- Lombardo, D.; Kiselev, M.A. Methods of Liposomes Preparation: Formation and Control Factors of Versatile Nanocarriers for Biomedical and Nanomedicine Application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Rezaei-sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N. Liposome: Classification, preparation, and applications. Nanoscale Res Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Umlauf, B.J.; Shusta, E.V. Exploiting BBB disruption for the delivery of nanocarriers to the diseased CNS. Curr. Opin. Biotechnol. 2019, 60, 146–152. [Google Scholar] [CrossRef]
- Rajan, R.; Vasudevan, D.; Biju Mukund, V.; Jose, S. Transferosomes—A vesicular transdermal delivery system for enhanced drug permeation. J. Adv. Pharm. Technol. Res. 2011, 2, 138–143. [Google Scholar] [CrossRef]
- Vora, B.; Khopade, A.J.; Jain, N.K. Proniosome based transdermal delivery of levonorgestrel for effective contraception. J. Control. Release 1998, 54, 149–165. [Google Scholar] [CrossRef]
- Karim, K.; Mandal, A.; Biswas, N.; Guha, A.; Chatterjee, S.; Behera, M.; Kuotsu, K. Niosome: A future of targeted drug delivery systems. J. Adv. Pharm. Technol. Res. 2010, 1, 374–380. [Google Scholar]
- Durak, S.; Rad, M.E.; Yetisgin, A.A.; Sutova, H.E.; Kutlu, O.; Cetinel, S.; Zarrabi, A. Niosomal drug delivery systems for ocular disease—Recent advances and future prospects. Nanomaterials 2020, 10, 1191. [Google Scholar] [CrossRef]
- Kaur, D.; Kumar, S. Niosomes: Present Scenario and Future Aspects. J. Drug Deliv. Ther. 2018, 8, 35–43. [Google Scholar] [CrossRef]
- Muzzalupo, R.; Tavano, L. RRTD-64773-niosomal-drug-delivery-for-transdermal-targeting--recent-adv. Transdermal Drug Deliv. 2015, 4, 23–33. [Google Scholar] [CrossRef]
- Gharbavi, M.; Amani, J.; Kheiri-Manjili, H.; Danafar, H.; Sharafi, A. Niosome: A Promising Nanocarrier for Natural Drug Delivery through Blood-Brain Barrier. Adv. Pharmacol. Sci. 2018, 2018, 6847971. [Google Scholar] [CrossRef]
- Touitou, E.; Ainbinde, D. 7. Ethosomes—An innovative carrier for enhanced delivery into and across the skin: Original Research Article: Ethosomes—Novel vesicular carriers for enhanced delivery: Characterization skin penetration properties, 2000. J. Control. Release 2014, 190, 44–46. [Google Scholar]
- Fang, Y.P.; Tsai, Y.H.; Wu, P.C.; Huang, Y. Bin Comparison of 5-aminolevulinic acid-encapsulated liposome versus ethosome for skin delivery for photodynamic therapy. Int. J. Pharm. 2008, 356, 144–152. [Google Scholar] [CrossRef]
- Abdulbaqi, I.M.; Darwis, Y.; Khan, N.A.K.; Assi, R.A.; Khan, A.A. Ethosomal nanocarriers: The impact of constituents and formulation techniques on ethosomal properties, in vivo studies, and clinical trials. Int. J. Nanomed. 2016, 11, 2279–2304. [Google Scholar] [CrossRef]
- Babaie, S.; Del Bakhshayesh, A.R.; Ha, J.W.; Hamishehkar, H.; Kim, K.H. Invasome: A novel nanocarrier for transdermal drug delivery. Nanomaterials 2020, 10, 341. [Google Scholar] [CrossRef]
- Mohanty, D.; Mounika, A.; Bakshi, V.; Akiful Haque, M.; Keshari Sahoo, C. Ethosomes: A Novel Approach For Transdermal Drug Delivery. Int. J. ChemTech Res. 2018, 11, 219–226. [Google Scholar] [CrossRef]
- Zahid, S.R.; Upmanyu, N.; Dangi, S.; Ray, S.K.; Jain, P.; Parkhe, G. Journal of Drug Delivery and Therapeutics Ethosome : A novel vesicular carrier for transdermal drug delivery. JDDT 2018, 8, 318–326. [Google Scholar]
- Verma, D.; Khuroo, T.; Talegaonkar, S.; Iqbal, Z. Investigation of ethosomes as surrogate carriers for bioactives. Drug Dev. Ther. 2016, 7, 125. [Google Scholar] [CrossRef]
- Mohapatra, S.; Mirza, M.; Ahmad, S.; Farooq, U.; Ansari, M.J.; Kohli, K.; Iqbal, Z. Quality by Design Assisted Optimization and Risk Assessment of Black Cohosh Loaded Ethosomal Gel for Menopause: Investigating Different Formulation and Process Variables. Pharmaceutics 2023, 15, 465. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.; Verma, D.; Khuroo, T.; Talegaonkar, S.; Iqbal, Z. Extraction of a water soluble bioactive hypoxoside and its development into an ethosomal system for deep dermal delivery. Int. J. Pharm. Pharm. Sci. 2015, 7, 211–215. [Google Scholar]
- Verma, P.; Pathak, K. Therapeutic and cosmeceutical potential of ethosomes: An overview. J. Adv. Pharm. Technol. Res. 2010, 1, 274–282. [Google Scholar] [CrossRef]
- Shaji, J.; Bajaj, R. Transethosomes: A New Prospect for Enhanced Transdermal Delivery. Int. J. Pharm. Sci. Res. 2018, 9, 2681–2685. [Google Scholar] [CrossRef]
- Bajaj, K.J.; Parab, B.S.; Shidhaye, S.S. Nano-transethosomes: A novel tool for drug delivery through skin. Indian J. Pharm. Educ. Res. 2021, 55, s1–s10. [Google Scholar] [CrossRef]
- Saupe, A.; Rades, T. Solid lipid nanoparticles. In Nanocarrier Technologies; Springer: Dordrecht, The Netherlands, 2006; pp. 41–50. [Google Scholar] [CrossRef]
- Jenning, V.; Thünemann, A.F.; Gohla, S.H. Characterisation of a novel solid lipid nanoparticle carrier system based on binary mixtures of liquid and solid lipids. Int. J. Pharm. 2000, 199, 167–177. [Google Scholar] [CrossRef]
- Solid_lipid_nanoparticle. Available online: En.wikipedia.org (accessed on 15 July 2022).
- Ashford, C.A.; Dixon, K.C. The effect of potassium on the glucolysis of brain tissue with reference to the Pasteur effect. Biochem. J. 1935, 29, 157–168. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: Applications, advantages and disadvantages. Res. Pharm. Sci. 2018, 13, 288–303. [Google Scholar] [CrossRef]
- Chauhan, I.; Yasir, M.; Verma, M.; Singh, A.P. Nanostructured lipid carriers: A groundbreaking approach for transdermal drug delivery. Adv. Pharm. Bull. 2020, 10, 150–165. [Google Scholar] [CrossRef]
- Jaiswal, P.; Gidwani, B.; Vyas, A. Nanostructured lipid carriers and their current application in targeted drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 27–40. [Google Scholar] [CrossRef]
- Contini, C.; Schneemilch, M.; Gaisford, S.; Quirke, N. Nanoparticle–membrane interactions. J. Exp. Nanosci. 2018, 13, 62–81. [Google Scholar] [CrossRef]
- Alsaad, A.A.A.; Hussien, A.A.; Gareeb, M.M. Solid lipid nanoparticles (SLN) as a novel drug delivery system: A theoretical review. Syst. Rev. Pharm. 2020, 11, 259–273. [Google Scholar]
- Desai, P.; Patlolla, R.R.; Singh, M. Interaction of nanoparticles and cell-penetrating peptides with skin for transdermal drug delivery. Mol. Membr. Biol. 2010, 27, 247–259. [Google Scholar] [CrossRef]
- Satapathy, M.K.; Yen, T.L.; Jan, J.S.; Tang, R.D.; Wang, J.Y.; Taliyan, R.; Yang, C.H. Solid lipid nanoparticles (Slns): An advanced drug delivery system targeting brain through bbb. Pharmaceutics 2021, 13, 1183. [Google Scholar] [CrossRef]
- Takino, T.; Konishi, K.; Takakura, Y.; Hashida, M. Long Circulating Emulsion Carrier Systems for Highly Lipophilic Drugs. Biol. Pharm. Bull. 1994, 17, 121–125. [Google Scholar] [CrossRef]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef]
- Deitel, M.; Friedman, K.L.; Cunnane, S.; Lea, P.J.; Chaiet, A.; Chong, J.; Almeida, B. Emulsion stability in a total nutrient admixture for total parenteral nutrition. J. Am. Coll. Nutr. 1992, 11, 5–10. [Google Scholar] [CrossRef]
- Choudhary, A.; Jain, P.; Mohapatra, S.; Mustafa, G.; Ansari, M.J.; Aldawsari, M.F.; Alalaiwe, A.S.; Mirza, M.A.; Iqbal, Z. A Novel Approach of Targeting Linezolid Nanoemulsion for the Management of Lymph Node Tuberculosis. ACS Omega 2022, 7, 15688–15694. [Google Scholar] [CrossRef]
- Gettings, S.D.; Lordo, R.A.; Feder, P.I.; Hintze, K.L. A comparison of low volume, Draize and in vitro eye irritation test data. II. Oil/water emulsions. Food Chem. Toxicol. 1998, 36, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Zakir, F.; Ahmad, A.; Farooq, U.; Mirza, M.A.; Tripathi, A.; Singh, D.; Shakeel, F.; Mohapatra, S.; Ahmad, F.J.; Kohli, K. Design and development of a commercially viable in situ nanoemulgel for the treatment of postmenopausal osteoporosis. Nanomedicine 2020, 15, 1167–1187. [Google Scholar] [CrossRef] [PubMed]
- Bonferoni, M.C.; Rossi, S.; Sandri, G.; Ferrari, F.; Gavini, E.; Rassu, G.; Giunchedi, P. Nanoemulsions for “nose-to-brain” drug delivery. Pharmaceutics 2019, 11, 84. [Google Scholar] [CrossRef] [PubMed]
- Tadros, T.; Izquierdo, P.; Esquena, J.; Solans, C. Formation and stability of nano-emulsions. Adv. Colloid Interface Sci. 2004, 108–109, 303–318. [Google Scholar] [CrossRef]
- Jaiswal, P.L.; Darekar, A.B.; Saudagar, R.B. A Recent Review on Nasal Microemulsion for Treatment of cns Disorder. Int. J. Curr. Pharm. Res. 2017, 9, 5. [Google Scholar] [CrossRef]
- Kogan, A.; Garti, N. Microemulsions as transdermal drug delivery vehicles. Adv. Colloid Interface Sci. 2006, 123–126, 369–385. [Google Scholar] [CrossRef]
- Assaf, S.; Maaroof, K.; Altaani, B.; Ghareeb, M.; Abu Alhayyal, A. Jojoba oil-based microemulsion for transdermal drug delivery. Res. Pharm. Sci. 2021, 16, 326. [Google Scholar] [CrossRef]
- Froelich, A.; Osmałek, T.; Jadach, B.; Puri, V.; Michniak-Kohn, B. Microemulsion-based media in nose-to-brain drug delivery. Pharmaceutics 2021, 13, 201. [Google Scholar] [CrossRef]
- Censi, R.; Martena, V.; Hoti, E.; Malaj, L.; Di Martino, P. Permeation and skin retention of quercetin from microemulsions containing Transcutol® P. Drug Dev. Ind. Pharm. 2012, 38, 1128–1133. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y.; Zhai, Y.; Wang, Z.; Liu, J.; Zhai, G. Ropivacaine loaded microemulsion and microemulsion-based gel for transdermal delivery: Preparation, optimization, and evaluation. Int. J. Pharm. 2014, 477, 47–56. [Google Scholar] [CrossRef]
- Kováčik, A.; Kopečná, M.; Vávrová, K. Permeation enhancers in transdermal drug delivery: Benefits and limitations. Expert Opin. Drug Deliv. 2020, 17, 145–155. [Google Scholar] [CrossRef]
- Santos, P.; Watkinson, A.C.; Hadgraft, J.; Lane, M.E. Application of microemulsions in dermal and transdermal drug delivery. Ski. Pharmacol. Physiol. 2008, 21, 246–259. [Google Scholar] [CrossRef]
- Schäfer-Korting, M.; Mehnert, W.; Korting, H.C. Lipid nanoparticles for improved topical application of drugs for skin diseases. Adv. Drug Deliv. Rev. 2007, 59, 427–443. [Google Scholar] [CrossRef]
- Waghule, T.; Rapalli, V.K.; Gorantla, S.; Saha, R.N.; Dubey, S.K.; Puri, A.; Singhvi, G. Nanostructured Lipid Carriers as Potential Drug Delivery Systems for Skin Disorders. Curr. Pharm. Des. 2020, 26, 4569–4579. [Google Scholar] [CrossRef]
- Sala, M.; Diab, R.; Elaissari, A.; Fessi, H. Lipid nanocarriers as skin drug delivery systems: Properties, mechanisms of skin interactions and medical applications. Int. J. Pharm. 2018, 535, 1–17. [Google Scholar] [CrossRef]
- Tanner, T.; Marks, R. Delivering drugs by the transdermal route: Review and comment. Ski. Res. Technol. 2008, 14, 249–260. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal drug delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Bibi, N.; Ahmed, N.; Khan, G.M. Nanostructures in Transdermal Drug Delivery Systems; 2017. Available online: https://www.sciencedirect.com/science/article/pii/B978032346143600021X (accessed on 15 July 2022).
- Singhavi, D.J.; Khan, S. Application of Nanotechnology in Transdermal Drug Delivery. Nanobiotechnol. Diagn. Drug Deliv. Treat. 2020, 113–128. [Google Scholar] [CrossRef]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Potential of nanoparticles as permeation enhancers and targeted delivery options for skin: Advantages and disadvantages. Drug Des. Dev. Ther. 2020, 14, 3271–3289. [Google Scholar] [CrossRef]
- Li, B.; Ge, Z.Q. Nanostructured lipid carriers improve skin permeation and chemical stability of idebenone. AAPS PharmSciTech 2012, 13, 276–283. [Google Scholar] [CrossRef]
- Arora, R.; Katiyar, S.S.; Kushwah, V.; Jain, S. Solid lipid nanoparticles and nanostructured lipid carrier-based nanotherapeutics in treatment of psoriasis: A comparative study. Expert Opin. Drug Deliv. 2017, 14, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Souto, E.B.; Baldim, I.; Oliveira, W.P.; Rao, R.; Yadav, N.; Gama, F.M.; Mahant, S. SLN and NLC for topical, dermal, and transdermal drug delivery. Expert Opin. Drug Deliv. 2020, 17, 357–377. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, J.C.; Baisaeng, N.; Hoppel, M.; Löw, M.; Keck, C.M.; Valenta, C. Ultra-small NLC for improved dermal delivery of coenyzme Q10. Int. J. Pharm. 2013, 447, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Dol, H.S.; Hajare, A.A.; Patil, K.S. Statistically designed novel ranolazine-loaded ethosomal transdermal gel for the treatment of angina pectoris. J. Drug Deliv. Sci. Technol. 2022, 75, 103574. [Google Scholar] [CrossRef]
- Sahoo, L.; Jena, G.K.; Patro, C.S.; Patro, C.; Satapathy, S. Box Behnken Design-Enabled Development of Nanostructured Lipid Carrier Transdermal Patch for Enhancement of Bioavailability of Olmesartan Medoxomil. J. Pharm. Innov. 2022, 17, 1405–1419. [Google Scholar] [CrossRef]
- Khotimah, H.; Ismail, D.D.L.; Widasmara, D.; Riawan, W.; Retnaningtyas, E.; Nugraheni, R.W.; Puspita, O.E.; Adianingsih, O.R.; Mardiyah, M.; Setiawan, A. Ameliorative effect of gel combination of Centella asiatica extract transfersomes and rosemary essential oil nanoemulsion against UVB-induced skin aging in Balb/c mice. F1000Research 2022, 11, 288. [Google Scholar] [CrossRef]
- Arunprasert, K.; Pornpitchanarong, C.; Piemvuthi, C.; Siraprapapornsakul, S.; Sripeangchan, S.; Lertsrimongkol, O.; Opanasopit, P.; Patrojanasophon, P. Nanostructured lipid carrier-embedded polyacrylic acid transdermal patches for improved transdermal delivery of capsaicin. Eur. J. Pharm. Sci. 2022, 173, 106169. [Google Scholar] [CrossRef]
- Kaul, S.; Jain, N.; Nagaich, U. Ultra deformable vesicles for boosting transdermal delivery of 2-arylpropionic acid class drug for management of musculoskeletal pain. J. Pharm. Investig. 2022, 52, 217–231. [Google Scholar] [CrossRef]
- Akhtar, N.; Akhtar, N. Development of stable tocopherol succinate-loaded ethosomes to enhance transdermal permeation: In vitro and in vivo characterizations. J. Cosmet. Dermatol. 2022, 21, 4942–4955. [Google Scholar] [CrossRef]
- Madan, J.R.; Khobaragade, S.; Dua, K.; Awasthi, R. Formulation, optimization, and in vitro evaluation of nanostructured lipid carriers for topical delivery of Apremilast. Dermatol. Ther. 2020, 33, e13370. [Google Scholar] [CrossRef]
- Brito Raj, S.; Chandrasekhar, K.B.; Reddy, K.B. Formulation, in-vitro and in-vivo pharmacokinetic evaluation of simvastatin nanostructured lipid carrier loaded transdermal drug delivery system. Future J. Pharm. Sci. 2019, 5, 9. [Google Scholar] [CrossRef]
- Hanna, P.A.; Ghorab, M.M.; Gad, S. Development of Betamethasone Dipropionate-Loaded Nanostructured Lipid Carriers for Topical and Transdermal Delivery. Anti-Inflamm. Anti-Allergy Agents Med. Chem. 2018, 18, 26–44. [Google Scholar] [CrossRef]
- Peng, L.H.; Wei, W.; Shan, Y.H.; Chong, Y.S.; Yu, L.; Gao, J.Q. Sustained release of piroxicam from solid lipid nanoparticle as an effective anti-inflammatory therapeutics in vivo. Drug Dev. Ind. Pharm. 2017, 43, 55–66. [Google Scholar] [CrossRef]
- Gönüllü, Ü.; Üner, M.; Yener, G.; Karaman, E.F.; Aydoʇmuş, Z. Formulation and characterization of solid lipid nanoparticles, nanostructured lipid carriers and nanoemulsion of lornoxicam for transdermal delivery. Acta Pharm. 2015, 65, 1–13. [Google Scholar] [CrossRef]
- Kumari, S.; Pandita, D.; Poonia, N.; Lather, V. Nanostructured Lipid Carriers for Topical Delivery of An Anti-Acne Drug: Characterization and ex vivo Evaluation. Pharm. Nanotechnol. 2015, 3, 122–133. [Google Scholar] [CrossRef]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Dingezweni, S. The blood–brain barrier. South. Afr. J. Anaesth. Analg. 2020, 26, S32–S34. [Google Scholar] [CrossRef]
- Kutcher, M.E.; Herman, I.M. The pericyte: Cellular regulator of microvascular blood flow. Microvasc. Res. 2009, 77, 235–246. [Google Scholar] [CrossRef]
- Eltanahy, A.M.; Koluib, Y.A.; Gonzales, A. Pericytes: Intrinsic Transportation Engineers of the CNS Microcirculation. Front. Physiol. 2021, 12, 719701. [Google Scholar] [CrossRef]
- Hellström, M.; Gerhardt, H.; Kalén, M.; Li, X.; Eriksson, U.; Wolburg, H.; Betsholtz, C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. J. Cell Biol. 2001, 153, 543–554. [Google Scholar] [CrossRef]
- On, N.; Miller, D. Transporter-Based Delivery of Anticancer Drugs to the Brain: Improving Brain Penetration by Minimizing Drug Efflux at the Blood-Brain Barrier. Curr. Pharm. Des. 2014, 20, 1499–1509. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Shen, Z.; Anraku, Y.; Kataoka, K.; Chen, X. Nanomaterial-based blood-brain-barrier (BBB) crossing strategies. Biomaterials 2019, 224, 119491. [Google Scholar] [CrossRef] [PubMed]
- Lerner, E.N.; Van Zanten, E.H.; Stewart, G.R. Enhanced delivery of octreotide to the brain via transnasal iontophoretic administration. J. Drug Target. 2004, 2, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Hu, K.; Jiang, X. From nose to brain: Understanding transport capacity and transport rate of drugs. Expert Opin. Drug Deliv. 2008, 5, 1159–1168. [Google Scholar] [CrossRef] [PubMed]
- Mistry, A.; Stolnik, S.; Illum, L. Nanoparticles for direct nose-to-brain delivery of drugs. Int. J. Pharm. 2009, 379, 146–157. [Google Scholar] [CrossRef]
- Kreuter, J. Nanoparticulate systems for brain delivery of drugs. Adv. Drug Deliv. Rev. 2012, 47, 65–81. [Google Scholar] [CrossRef]
- Koziara, J.M.; Lockman, P.R.; Allen, D.D.; Mumper, R.J. Paclitaxel nanoparticles for the potential treatment of brain tumors. J. Control. Release 2004, 99, 259–269. [Google Scholar] [CrossRef]
- Ke, W.; Shao, K.; Huang, R.; Han, L.; Liu, Y.; Li, J.; Kuang, Y.; Ye, L.; Lou, J.; Jiang, C. Gene delivery targeted to the brain using an Angiopep-conjugated polyethyleneglycol-modified polyamidoamine dendrimer. Biomaterials 2009, 30, 6976–6985. [Google Scholar] [CrossRef]
- Micheli, M.R.; Bova, R.; Magini, A.; Polidoro, M.; Emiliani, C. Lipid-based nanocarriers for CNS-targeted drug delivery. Recent Pat. CNS Drug Discov. 2012, 7, 71–86. [Google Scholar] [CrossRef]
- Blasi, P.; Giovagnoli, S.; Schoubben, A.; Puglia, C.; Bonina, F.; Rossi, C.; Ricci, M. Lipid nanoparticles for brain targeting I. Formulation optimization. Int. J. Pharm. 2011, 419, 287–295. [Google Scholar] [CrossRef]
- García-Pinel, B.; Porras-Alcalá, C.; Ortega-Rodríguez, A.; Sarabia, F.; Prados, J.; Melguizo, C.; López-Romero, J.M. Lipid-based nanoparticles: Application and recent advances in cancer treatment. Nanomaterials 2019, 9, 638. [Google Scholar] [CrossRef]
- Masserini, M. Nanoparticles for Brain Drug Delivery. ISRN Biochem. 2013, 2013, 1–18. [Google Scholar] [CrossRef]
- Krishnanand, A.; Dey, A.; Kamesh, S.; Vignesh, S.; Jawahar, N.; Senthil, A.V. Development and Evaluation of Valproic Acid Loaded Nanostructed Lipid Carrier for Enhance Brain Targeting. J. Xi’an Shiyou Univ. Nat. Sci. Ed. 2022, 18. Available online: https://www.xisdxjxsu.asia/V18I03-01.pdf (accessed on 15 July 2022).
- Khonsari, F.; Heydari, M.; Dinarvand, R.; Sharifzadeh, M.; Atyabi, F. Brain targeted delivery of rapamycin using transferrin decorated nanostructured lipid carriers. Bioimpacts 2022, 12, 21. [Google Scholar] [CrossRef]
- Hadel, A.; Abo El-Enin, E. Lipid Nanocarriers Overlaid with Chitosan for Brain Delivery of Berberine via the Nasal Route. Pharmaceutical 2022, 15, 281. [Google Scholar]
- Etal, M.I.T. Formulation, Characterization, and Cytotoxicity Evaluation of Lactoferrin Functionalized Lipid Nanoparticles for Riluzole Delivery to the Brain. Pharmaceutics 2022, 14, 185. [Google Scholar]
- Vaz, G.R.; Carrasco, M.C.F.; Batista, M.M.; Bezerra Barros, P.A.; da Conceição Oliveira, M.; Muccillo-Baisch, A.L.; Yurgel, V.C.; Buttini, F.; Soares, F.A.A.; Cordeiro, L.M.; et al. Curcumin and Quercetin-Loaded Lipid Nanocarriers: Development of Omega-3 Mucoadhesive Nanoemulsions for Intranasal Administration. Nanomaterials 2022, 12, 1073. [Google Scholar] [CrossRef]
- Qizilbash, F.F.; Ashhar, M.U.; Zafar, A.; Qamar, Z.; Ali, J.; Baboota, S.; Ghoneim, M.M.; Alshehri, S.; Ali, A. Thymoquinone-Enriched Naringenin-Loaded Nanostructured Lipid Carrier for Brain Delivery via Nasal Route: In Vitro Prospect and In Vivo Therapeutic Efficacy for the Treatment of Depression. Pharmaceutics 2022, 14, 656. [Google Scholar] [CrossRef]
- Han, Y.; Gao, C.; Wang, H.; Sun, J.; Liang, M.; Feng, Y.; Liu, Q.; Fu, S.; Cui, L.; Gao, C.; et al. Macrophage membrane-coated nanocarriers Co-Modified by RVG29 and TPP improve brain neuronal mitochondria-targeting and therapeutic efficacy in Alzheimer’s disease mice. Bioact. Mater. 2021, 6, 529–542. [Google Scholar] [CrossRef]
- Saka, R.; Chella, N.; Khan, W. Development of Imatinib Mesylate-Loaded Liposomes for Nose to Brain Delivery: In Vitro and In Vivo Evaluation. AAPS PharmSciTech 2021, 22, 1–10. [Google Scholar] [CrossRef]
- Akel, H.; Ismail, R.; Katona, G.; Sabir, F.; Ambrus, R.; Csóka, I. A comparison study of lipid and polymeric nanoparticles in the nasal delivery of meloxicam: Formulation, characterization, and in vitro evaluation. Int. J. Pharm. 2021, 604, 120724. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, R.; Ebrahimi-Hosseinzadeh, B.; Khodagholi, F.; Hatamian-Zarmi, A.; Malekpour-Galogahi, F. Preparation, characterization, and in vivo evaluation of Rose damascene extract loaded solid lipid nanoparticles for targeted brain delivery. J. Environ. Health Sci. Eng. 2021, 19, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Trapani, A.; Guerra, L.; Corbo, F.; Castellani, S.; Sanna, E.; Capobianco, L.; Monteduro, A.G.; Manno, D.E.; Mandracchia, D.; Di Gioia, S.; et al. Cyto/biocompatibility of dopamine combined with the antioxidant grape seed-derived polyphenol compounds in solid lipid nanoparticles. Molecules 2021, 26, 916. [Google Scholar] [CrossRef] [PubMed]
- Pandian, S.R.K.; Pavadai, P.; Vellaisamy, S.; Ravishankar, V.; Palanisamy, P.; Sundar, L.M.; Chandramohan, V.; Sankaranarayanan, M.; Panneerselvam, T.; Kunjiappan, S. Formulation and evaluation of rutin-loaded solid lipid nanoparticles for the treatment of brain tumor. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 735–749. [Google Scholar] [CrossRef] [PubMed]
- Madane, R.G.; Mahajan, H.S. Curcumin-loaded nanostructured lipid carriers (NLCs) for nasal administration: Design, characterization, and in vivo study. Drug Deliv. 2016, 23, 1326–1334. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Y.; Luo, G. Ligustrazine phosphate ethosomes for treatment of Alzheimer’s disease, in vitro and in animal model studies. AAPS PharmSciTech 2012, 13, 485–492. [Google Scholar] [CrossRef]
- Gunay, M.S.; Ozer, A.Y.; Erdogan, S.; Bodard, S.; Baysal, I.; Gulhan, Z.; Guilloteau, D.; Chalon, S. Development of nanosized, pramipexole-encapsulated liposomes and niosomes for the treatment of Parkinson’s disease. J. Nanosci. Nanotechnol. 2017, 17, 5155–5167. [Google Scholar] [CrossRef]
- De, A.; Venkatesh, N.; Senthil, M.; Sanapalli, B.K.R.; Shanmugham, R.; Karri, V.V.S.R. Smart niosomes of temozolomide for enhancement of brain targeting. Nanobiomedicine 2018, 5. [Google Scholar] [CrossRef]
- Sivadasu, P.; Gowda, D.V.; Subramani, N.K.; Vishweshwaraiah, B.M.; Shivanna, S.; Hatna, S. Direct brain targeted nanostructured lipid carriers for sustained release of schizophrenic drug: Formulation, characterization and pharmacokinetic studies. Indian J. Pharm. Educ. Res. 2020, 54, 73–84. [Google Scholar] [CrossRef]
- Campos, J.R.; Severino, P.; Santini, A.; Silva, A.M.; Shegokar, R.; Souto, S.B.; Souto, E.B. Solid lipid nanoparticles (SLN): Prediction of toxicity, metabolism, fate and physicochemical properties. In Nanopharmaceuticals: Volume 1: Expectations and Realities of Multifunctional Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 9780128177785. Available online: http://repositorium.sdum.uminho.pt/handle/1822/64341 (accessed on 15 July 2022).
- Marchan, R.; Reif, R.; Hengstler, J.G. Toxicology of magnetic nanoparticles: Disturbed body iron homeostasis? Arch. Toxicol. 2012, 86, 683–684. [Google Scholar] [CrossRef]

| S. No. | Type of Lipid Carriers (Delivery System) | Name of the Marketed Formulation | Active Ingredients | Advantages of Used Delivery System | Advantage/ Uniqueness | Route of Administration | Reference |
|---|---|---|---|---|---|---|---|
| 1. | Liposome | Liposomal Glutathione: Livon Labs | d-Lenolate Olive Leaf Extract, d-Lenolate Pain Serum, d-Lenolate Probiotic 10 Billion | Increases absorption of glutathione by decreasing oxidative stress biomarkers. Increases immune function markers with the liposomal delivery system | Powerful antioxidant, anti-aging property, protect liver and remove the excretory waste from the human body | Oral | [42] |
| California Gold Nutrition Liposomal Vitamin D3 | Liposomal Vitamin D3 | Increases absorption and hence increases bioavailability | Increases bone health and immunity | Oral | [43] | ||
| Aurora Nutrascience | Mega-Liposomal NAD+/Resveratrol, Organic Fruit | Increases bioavailability | Increases longevity, increases cognitive and neurological function, increases energy and healthy muscle function | Oral | [44] | ||
| MPC-Liposomes | Milk peptide complex | Liposomes are used to entrap MPC (Milk Peptide Complex) for increasing permeation | Regenerating and anti-aging agent, advised for skin regeneration and for prevention of skin damage due to the aging process | Topical | [45] | ||
| Sesderma C-VIT Facial Liposomal Serum 30 ml | Ascorbyl glucoside encapsulated in liposomes, mulberry extract, liposomed hyaluronic acid, Syncoll and Panthenol | Encapsulated vit-C in liposomes and hence increases stability. Increases penetration due to structure analogous with the biological membranes. | Offering facial luminosity and vitality | Topical | [46] | ||
| BodyBio PC liposomal phospholipid complex | Phosphatidylcholine | Liposomal system contains pure phospholipids which are not broken apart and are instantly utilized, re-building every cell in your body as compared to non-liposomal PCs, which are broken down during digestion | Increased memory, clarity, focus and cell repair | Oral | [43] | ||
| AmBisome | Amphotericin | Liposomal encapsulation can substantially affect drug’s functional properties relative to those of the unencapsulated drug, decreasese the size & increases the bioavailability | Fungal infection in febrile, neutropenic patients | Parenteral | [47] | ||
| DEPOCYT- Enzon Pharmaceuticals Inc., North Bridgewater, NJ, USA | Antimetabolite cytarabine | Sustained-release formulation | Symptoms of Acute Nonlymphocytic Leukemia, Meningeal Leukemia, Refractory Leukemia, and Lymphomatous Meningitis | Parenteral | [47] | ||
| Estrasorb- Novavax, Rockville, MD, USA | Estradiol | Increased efficacy with a lesser dose | Hot flashes and symptoms of Vulvar and Vaginal Atrophy related to menopause | Topical | [48] | ||
| 2. | Niosome | LEP-ETU: Neopharma | Paclitaxel | Decreases toxicity, while increasing the efficacy | Anti-cancerous, used in advanced cancer treatment | Parenteral | [49] |
| Lancome®—Loreal, Paris, France | 10% Bifidus Prebiotic Grand Rose Extracts, Vitamin E, Pro-Xylane, and a gentle Exfoliating Acid Complex | Increases stability and increases penetrating power through the skin | Anti-aging agent; increases epidermal cell renewal to obtain younger-looking skin | Topical | [50] | ||
| 3. | Nano emulsion | Restasis®: Allergan | Cyclosporine A | Decreases size, increases ocular penetration, provide sustain release action and decreases side effects | Lubricate eyes | Ophthalmic | [49] |
| Hanacure: Nano Emulsion Multi-Peptide Moisturizer | Peptides, squalane, sodium hyaluronate and mushroom extract | Decreases size, increases penetration | The moisturizer intensifies the anti-aging effects and increases the appearance of the skin’s tone and texture | Topical | [43] | ||
| Coolnac Gel Emulgel 1%—Chumchon | Diclofenac diethylammonium | Decreases size, increases penetration | Anti-inflammatory gel | Topical | [51,52] | ||
| Ketopatch 30mg transdermal patch | Ketoprofen [30mg] | Decreases size, increases penetration | Anti-inflammatory action | Topical | [47] | ||
| 4. | Solid lipid nanoparticles | Under clinical trial | Rifampicin + isoniazide | Decreases dosing frequency and increases patient compliance for better management | Anti-TB agent | Inhalation | [49] |
| Cutanova Cream Nano Repair Q10—Dr. Rimpler | Q10, polypeptide, hibiscus extract, ginger extract, keto sugar | Increases moisturizing properties, increases consistency and spreadability | Anti-aging agent | Topical | [53,54] | ||
| 5. | Nanostructured lipid carriers | NLC Deep Effect Eye Serum: Beate Johnen | Coenzyme Q10, a highly active oligosaccharide | Deeper penetration | Remove dark circles, anti-aging, rejuvenate skin | Topical | [55] |
| Dr. Rimpler Cutanova Cleanser | Soluble Collagen, Tocopherol, Helianthus Annuus Seed Oil | Prevent oxidation and permit controlled release | Skin cleanser without removing valuable skin lipids and maintains the moisture balance of the skin | Topical | [43] | ||
| NanoLipid Restore CLR™ by CLR Berlin | The liquid suspension containing 27% black current seed oil | Prevent oxidation and permit controlled release | Forms a film on the skin due to its physical properties, thus providing an excellent skin feeling. It is designed for regenerative care of dry, rough, scaly and aged skin | Topical | [47] | ||
| 6. | Ethosome | Nanominox—Sinere, Germany | 4% minoxidil, adenosine, sophora flavescens extract, creatine ethyl ester, cepharanthine, B12, ethanol | Increases skin delivery | Baldness | Topical | [56] |
| Transethosomes | Ketoprofen transdermal—IDEA AG | Ketoprofen | Decreases size and increases skin permeation | Musculoskeletal pain | Topical | [43] |
| S. No. | Study Title | NCT No. | Volunteer/Actual Enrolment | Recruitment Status | The Phase of the Clinal Trial | Treatment Intervention | Clinical Trial Sponsor | Country |
|---|---|---|---|---|---|---|---|---|
| 1. | Formulation and Clinical Evaluation of Ethosomal and Liposomal Preparations of Anthralin in Psoriasis | NCT03348462 | 20 | Completed | 4 | Psoriasis Vulgaris | Assiut University | Egypt |
| 2. | Proof-of-Concept Study of Topical 3%-Diclofenac-Nano-Emulsion Cream for Knee OA Pain | NCT00484120 | 123 | Completed | 2 | Osteoarthritis of the Knee | Pharmos | Israel |
| 3. | Clinical Assessment of Oxiconazole Nitrate Solid Lipid Nanoparticles Loaded Gel | NCT03823040 | 28 | Completed | 1 | Tinea | Minia University | Egypt |
| 4. | The Study of Efficacy and Safety of a 60-day Use of the Magnox Comfort Compared to the Placebo in Subjects With NLC | NCT03807219 | 216 | Completed | NA | Nocturnal Leg Cramps | Naveh Pharma LTD | Ukraine |
| 5. | Liposomal Lidocaine Gel for Oral Topical Anesthesia | NCT01425840 | 40 | Completed | 1 | Anesthesia | University of Campinas, Brazil | Brazil |
| 6. | Transdermal Testosterone Nanoemulsion in Women Libido (Biolipid/B2) | NCT02445716 | 70 | Unknown | 2 | Menopause | University Potiguar | Brazil |
| 7. | NB-001 Treatment of Recurrent Herpes LabialisNanoemulsion | NCT01695187 | 362 | Unknown | 3 | Herpes Labialis | NanoBio Corporation | USA |
| 8 | Efficacy of Topical Liposomal Form of Drugs in Cutaneous Leishmaniasis | NCT01050777 | 30 | Completed | Early Phase 1 | Cutaneous Leishmaniasis | Tehran University of Medical Sciences | Iran, Islamic Republic of |
| S. No. | Study Title | NCT No | Volunteer/Actual Enrolment | Recruitment Status | Phases of Clinical Trial | Treatment Intervention | Clinical Trial Sponsor | Country |
|---|---|---|---|---|---|---|---|---|
| 1. | Efficacy of Transdermal Nicotine, on Motor Symptoms in Advanced Parkinson’s Disease (NICOPARK2) | NCT00873392 | 40 | Completed | 2 | Idiopathic Parkinson’s Disease | Assistance Publique—Hôpitaux de Paris | France |
| 2. | Efficacy and Safety of Rivastigmine Transdermal Patch in Patients with Mild to Moderate Alzheimer’s Disease | NCT00423085 | 859 | Completed | 3 | Alzheimer’s Disease | Novartis Pharmaceuticals Ono Pharmaceutical Co. Ltd. | Japan |
| 3. | The Efficacy and Tolerability of NP101 Patch in the Treatment of Acute Migraine (NP101-007) | NCT00724815 | 530 | Completed | 3 | Migraine Disorders | NuPathe Inc. | USA |
| 4. | Study to Evaluate Safety & Efficacy of d-Amphetamine transdermal System Compared to Placebo in Children & Adolescents With ADHD | NCT01711021 | 106 | Completed | 2 | ADHD | Noven Pharmaceuticals, Inc. | USA |
| 5. | Phase I Open Label Single-Dose Study to Compare the Pharmacokinetics of NP101 Healthy Volunteers (NP101-006) | NCT00720018 | 4 | Completed | 1 | Migraine Disorders | NuPathe Inc. | USA |
| 6. | Treatment of Parkinson’s Disease with a Transdermal Skin Patch | NCT00001931 | 20 | Completed | 2 | Parkinson Disease | National Institute of Neurological Disorders and Stroke (NINDS) | USA |
| 7. | Does Topical Steroid Treatment Impair the Adrenal Function? | NCT00476489 | 50 | Unknown | NA | Hypothalamus–Pituitary–Adrenal Axis Assessement | HaEmek Medical Center, Israel | Israel |
| 8. | Efficacy of Euminz® for Tension-Type Headache (CAS/B/016611) | NCT01770080 | 211 | Completed | 4 | Episodic Tension-Type Headache | Charite University, Berlin, Germany Cassella-med GmbH & Co. KG | Germany |
| 9. | Study of Intranasal Clonazepam in Adult Subjects with Epileptic Seizures | NCT00594945 | 45 | Completed | 2 | Epilepsy | Jazz Pharmaceuticals | USA |
| 10. | Effect of Intranasal Oxytocin on Headache in Chronic Daily Headache | NCT00963040 | 40 | Completed | NA | Chronic Daily Headache | MedVadis Research Corporation | USA |
| 11. | TI-001 (Intranasal Oxytocin) for Treatment of High-Frequency Episodic Migraine and Chronic Migraine | NCT01839149 | 240 participants | Completed | 2 | High-Frequency Episodic Migraine and Chronic Migraine | Trigemina, Inca | Australia |
| 12. | Intranasal Civamide for Episodic Cluster Headache | NCT00069082 | 2 | Completed | 3 | Episodic Cluster Headache | Winston Laboratories | USA |
| 13. | Study of Nasal Insulin to Fight Forgetfulness—Long-acting Insulin Detemir—21 Days (SNIFF-LONG 21) | NCT01547169 | 60 | Completed | 2 | Alzheimer’s Disease and mild Cognitive Impairment | University of Washington National Institute on Aging (NIA) | USA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.S.; Mohapatra, S.; Gupta, V.; Ali, A.; Naseef, P.P.; Kurunian, M.S.; Alshadidi, A.A.F.; Alam, M.S.; Mirza, M.A.; Iqbal, Z. Potential of Lipid-Based Nanocarriers against Two Major Barriers to Drug Delivery—Skin and Blood–Brain Barrier. Membranes 2023, 13, 343. https://doi.org/10.3390/membranes13030343
Khan MS, Mohapatra S, Gupta V, Ali A, Naseef PP, Kurunian MS, Alshadidi AAF, Alam MS, Mirza MA, Iqbal Z. Potential of Lipid-Based Nanocarriers against Two Major Barriers to Drug Delivery—Skin and Blood–Brain Barrier. Membranes. 2023; 13(3):343. https://doi.org/10.3390/membranes13030343
Chicago/Turabian StyleKhan, Mohammad Sameer, Sradhanjali Mohapatra, Vaibhav Gupta, Ahsan Ali, Punnoth Poonkuzhi Naseef, Mohamed Saheer Kurunian, Abdulkhaliq Ali F. Alshadidi, Md Shamsher Alam, Mohd. Aamir Mirza, and Zeenat Iqbal. 2023. "Potential of Lipid-Based Nanocarriers against Two Major Barriers to Drug Delivery—Skin and Blood–Brain Barrier" Membranes 13, no. 3: 343. https://doi.org/10.3390/membranes13030343
APA StyleKhan, M. S., Mohapatra, S., Gupta, V., Ali, A., Naseef, P. P., Kurunian, M. S., Alshadidi, A. A. F., Alam, M. S., Mirza, M. A., & Iqbal, Z. (2023). Potential of Lipid-Based Nanocarriers against Two Major Barriers to Drug Delivery—Skin and Blood–Brain Barrier. Membranes, 13(3), 343. https://doi.org/10.3390/membranes13030343






