Polymer-Infiltrated Metal–Organic Frameworks for Thin-Film Composite Mixed-Matrix Membranes with High Gas Separation Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of MOF-808
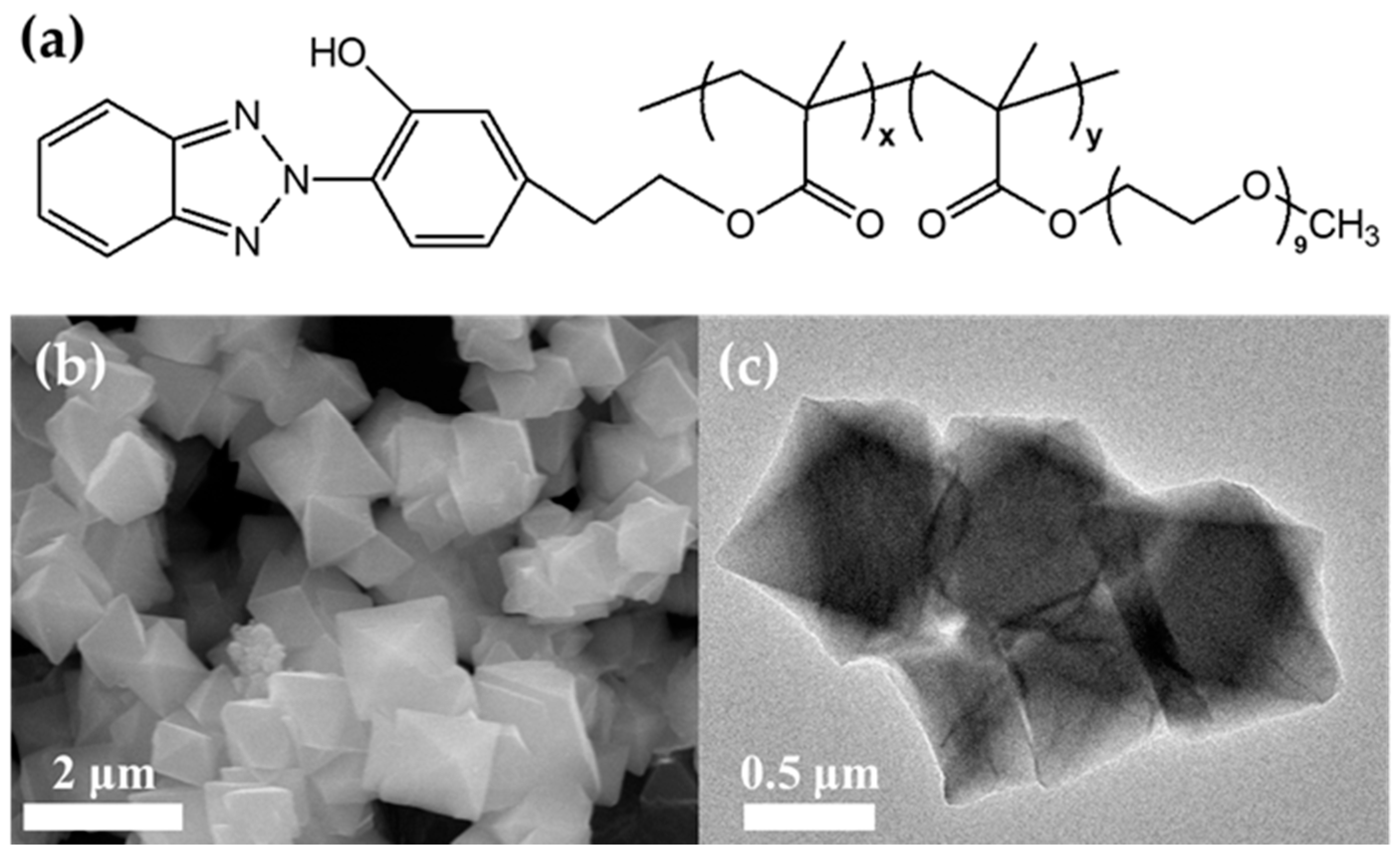
2.3. Synthesis of the PBE Comb Copolymer
2.4. Preparation of TFC-MMMs
2.5. Characterization
2.6. Gas Permeation Measurements
3. Results
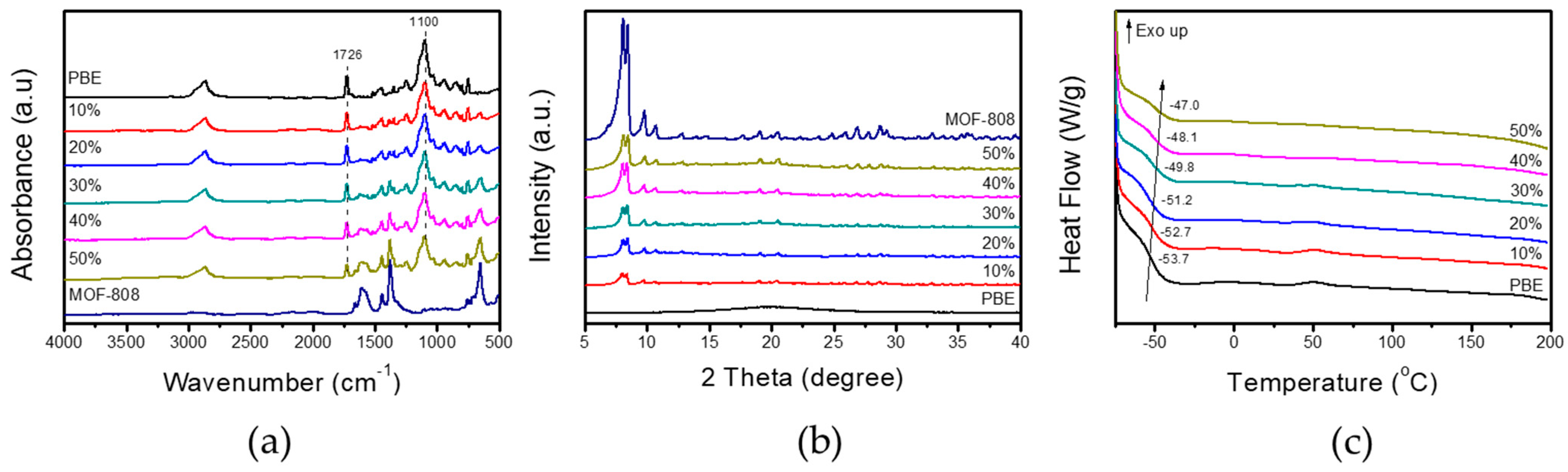
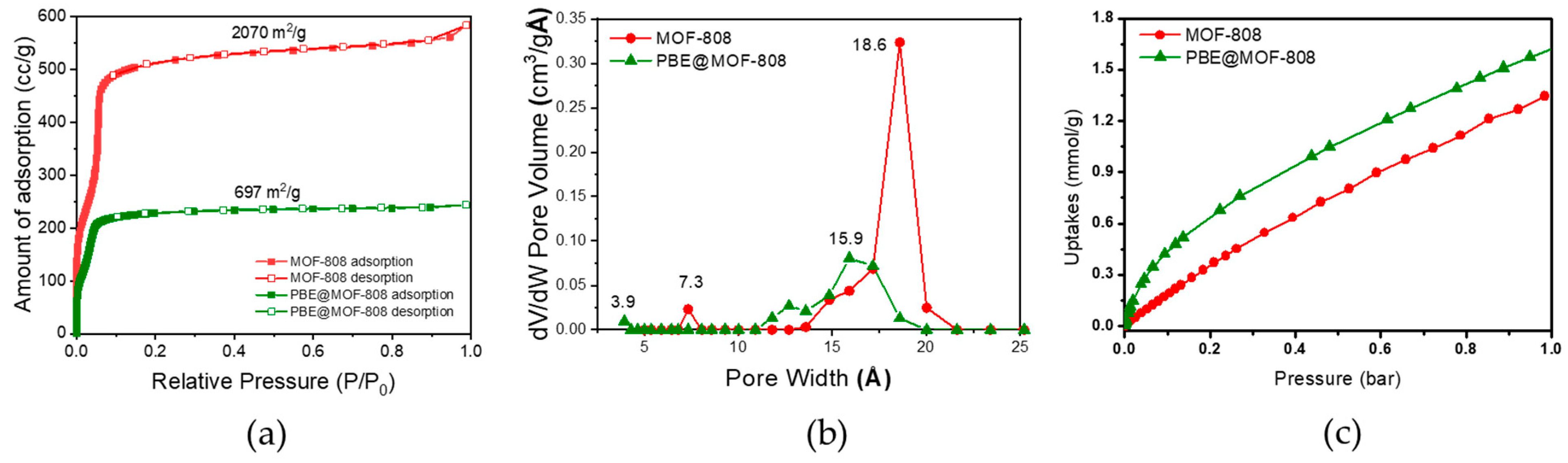
3.1. Characterization of MOF-808 Nanoparticles and PBE/MOF-808 MMMs
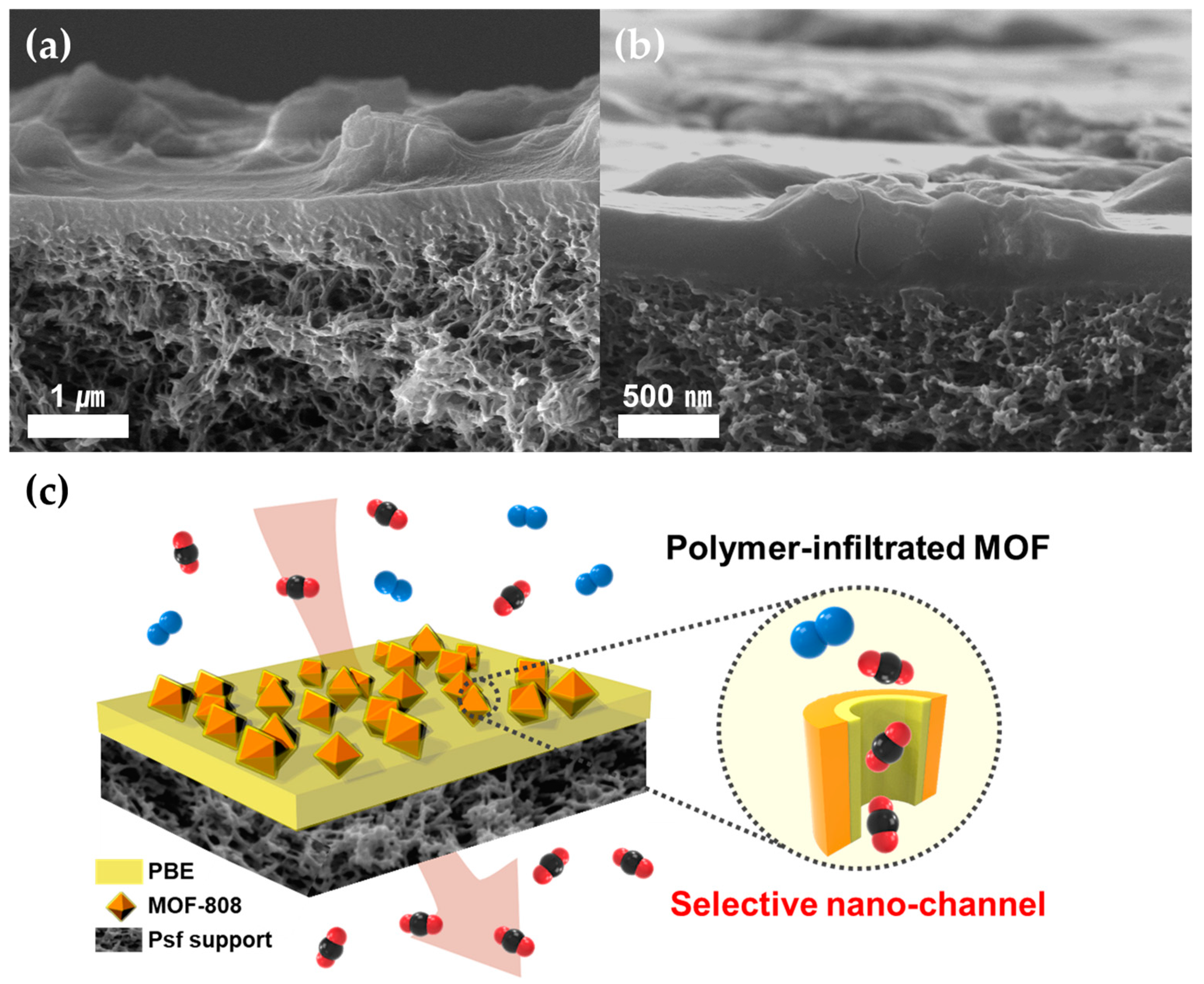
3.2. Preparation and Gas Separation Performance of TFC-MMMs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Keith, D.W. Why capture CO2 from the atmosphere? Science 2009, 325, 1654–1655. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Feron, P.H.; Deng, L.; Favre, E.; Chabanon, E.; Yan, S.; Hou, J.; Chen, V.; Qi, H. Status and progress of membrane contactors in post-combustion carbon capture: A state-of-the-art review of new developments. J. Membr. Sci. 2016, 511, 180–206. [Google Scholar] [CrossRef]
- Min, H.J.; Kang, M.; Lee, C.S.; Kim, J.H. Dual-functional interconnected pebble-like structures in highly crystalline poly (ethylene oxide) membranes for CO2 separation. Sep. Purif. Technol. 2021, 263, 118363. [Google Scholar] [CrossRef]
- Lv, X.; Li, X.; Huang, L.; Ding, S.; Lv, Y.; Zhang, J. Tailoring physical and chemical microenvironments by polyether-amine in blended membranes for efficient CO2 separation. Korean J. Chem. Eng. 2022, 39, 475–483. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, J.; Wang, X.; Liu, A.; Gleason, K.K. Recent progress on submicron gas-selective polymeric membranes. J. Mater. Chem. A 2017, 5, 8860–8886. [Google Scholar] [CrossRef]
- Aroon, M.; Ismail, A.; Matsuura, T.; Montazer-Rahmati, M. Performance studies of mixed matrix membranes for gas separation: A review. Sep. Purif. Technol. 2010, 75, 229–242. [Google Scholar] [CrossRef]
- Galizia, M.; Chi, W.S.; Smith, Z.P.; Merkel, T.C.; Baker, R.W.; Freeman, B.D. 50th anniversary perspective: Polymers and mixed matrix membranes for gas and vapor separation: A review and prospective opportunities. Macromolecules 2017, 50, 7809–7843. [Google Scholar] [CrossRef]
- Kim, B.J.; Kang, S.W. Composites of poly (vinyl pyrrolidone) and polarized Ag nanoparticles for CO2 separation. Korean J. Chem. Eng. 2022, 39, 2542–2547. [Google Scholar] [CrossRef]
- Ji, Y.; Song, Y.; Huang, Y.; Zhu, H.; Yue, C.; Liu, F.; Zhao, J. One-Step Synthesis of Ultrathin Zeolitic Imidazole Framework-8 (ZIF-8) Membrane on Unmodified Porous Support via Electrophoretic Deposition. Membranes 2022, 12, 1062. [Google Scholar] [CrossRef]
- Usman, M.; Khan, M.Y.; Anjum, T.; Khan, A.L.; Hoque, B.; Helal, A.; Hakeem, A.S.; Al-Maythalony, B.A. Controlled Covalent Functionalization of ZIF-90 for Selective CO2 Capture & Separation. Membranes 2022, 12, 1055. [Google Scholar] [CrossRef]
- Sun, J.; Li, Q.; Chen, G.; Duan, J.; Liu, G.; Jin, W. MOF-801 incorporated PEBA mixed-matrix composite membranes for CO2 capture. Sep. Purif. Technol. 2019, 217, 229–239. [Google Scholar] [CrossRef]
- Kim, N.U.; Park, B.J.; Lee, J.H.; Kim, J.H. High-performance ultrathin mixed-matrix membranes based on an adhesive PGMA-co-POEM comb-like copolymer for CO2 capture. J. Mater. Chem. A 2019, 7, 14723–14731. [Google Scholar] [CrossRef]
- Venna, S.R.; Lartey, M.; Li, T.; Spore, A.; Kumar, S.; Nulwala, H.B.; Luebke, D.R.; Rosi, N.L.; Albenze, E. Fabrication of MMMs with improved gas separation properties using externally-functionalized MOF particles. J. Mater. Chem. A 2015, 3, 5014–5022. [Google Scholar] [CrossRef]
- Mannan, H.A.; Idris, A.; Nasir, R.; Mukhtar, H.; Qadir, D.; Suleman, H.; Basit, A. Interfacial Tailoring of Polyether Sulfone-Modified Silica Mixed Matrix Membranes for CO2 Separation. Membranes 2022, 12, 1129. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Hernandez, B.V.; Ge, L.; Zhu, Z. Metal organic framework based mixed matrix membranes: An overview on filler/polymer interfaces. J. Mater. Chem. A 2018, 6, 293–312. [Google Scholar] [CrossRef]
- Ban, Y.; Li, Z.; Li, Y.; Peng, Y.; Jin, H.; Jiao, W.; Guo, A.; Wang, P.; Yang, Q.; Zhong, C. Confinement of ionic liquids in nanocages: Tailoring the molecular sieving properties of ZIF-8 for membrane-based CO2 capture. Angew. Chem. Int. Ed. 2015, 54, 15483–15487. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Moreton, J.C.; Tavares, S.R.; Semino, R.; Maurin, G.; Cohen, S.M.; Schmidt-Rohr, K. Polymer infiltration into metal–organic frameworks in mixed-matrix membranes detected in situ by NMR. J. Am. Chem. Soc. 2019, 141, 7589–7595. [Google Scholar] [CrossRef]
- Cheng, Y.; Ying, Y.; Japip, S.; Jiang, S.D.; Chung, T.S.; Zhang, S.; Zhao, D. Advanced porous materials in mixed matrix membranes. Adv. Mater. 2018, 30, 1802401. [Google Scholar] [CrossRef]
- Baker, R.W.; Low, B.T. Gas separation membrane materials: A perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356, eaab0530. [Google Scholar] [CrossRef]
- Xie, K.; Fu, Q.; Qiao, G.G.; Webley, P.A. Recent progress on fabrication methods of polymeric thin film gas separation membranes for CO2 capture. J. Membr. Sci. 2019, 572, 38–60. [Google Scholar] [CrossRef]
- Lee, C.S.; Kang, M.; Kim, K.C.; Kim, J.H. In-situ formation of asymmetric thin-film, mixed-matrix membranes with ZIF-8 in dual-functional imidazole-based comb copolymer for high-performance CO2 capture. J. Membr. Sci. 2022, 642, 119913. [Google Scholar] [CrossRef]
- Plonka, A.M.; Grissom, T.G.; Musaev, D.G.; Balboa, A.; Gordon, W.O.; Collins-Wildman, D.L.; Ghose, S.K.; Tian, Y.; Ebrahim, A.M.; Mitchell, M.B. Effect of carbon dioxide on the degradation of chemical warfare agent simulant in the presence of Zr metal organic framework MOF-808. Chem. Mater. 2019, 31, 9904–9914. [Google Scholar] [CrossRef]
- Kim, Y.K.; Cha, S.I.; Hong, S.H.; Jeong, Y.J. A new hybrid architecture consisting of highly mesoporous CNT/carbon nanofibers from starch. J. Mater. Chem. 2012, 22, 20554. [Google Scholar] [CrossRef]
- Furukawa, H.; Gandara, F.; Zhang, Y.-B.; Jiang, J.; Queen, W.L.; Hudson, M.R.; Yaghi, O.M. Water adsorption in porous metal–organic frameworks and related materials. J. Am. Chem. Soc. 2014, 136, 4369–4381. [Google Scholar] [CrossRef] [PubMed]
- Kulak, H.; Thür, R.; Vankelecom, I.F. MOF/Polymer Mixed-Matrix Membranes Preparation: Effect of Main Synthesis Parameters on CO2/CH4 Separation Performance. Membranes 2022, 12, 425. [Google Scholar] [CrossRef] [PubMed]
- Thür, R.; Van Havere, D.; Van Velthoven, N.; Smolders, S.; Lamaire, A.; Wieme, J.; Van Speybroeck, V.; De Vos, D.; Vankelecom, I.F. Correlating MOF-808 parameters with mixed-matrix membrane (MMM) CO2 permeation for a more rational MMM development. J. Mater. Chem. A 2021, 9, 12782–12796. [Google Scholar] [CrossRef]
- Thür, R.; Van Velthoven, N.; Lemmens, V.; Bastin, M.; Smolders, S.; De Vos, D.; Vankelecom, I.F. Modulator-mediated functionalization of MOF-808 as a platform tool to create high-performance mixed-matrix membranes. ACS Appl. Mater. Interfaces 2019, 11, 44792–44801. [Google Scholar] [CrossRef]
- Wang, L.; Chen, P.; Dong, X.; Zhang, W.; Zhao, S.; Xiao, S.; Ouyang, Y. Porous MOF-808@ PVDF beads for removal of iodine from gas streams. RSC Adv. 2020, 10, 44679–44687. [Google Scholar] [CrossRef]
- Lee, J.H.; Jung, J.P.; Jang, E.; Lee, K.B.; Kang, Y.S.; Kim, J.H. CO2-philic PBEM-g-POEM comb copolymer membranes: Synthesis, characterization and CO2/N2 separation. J. Membr. Sci. 2016, 502, 191–201. [Google Scholar] [CrossRef]
- Lee, C.S.; Kim, N.U.; Park, J.T.; Kim, J.H. Imidazole-functionalized hydrophilic rubbery comb copolymers: Microphase-separation and good gas separation properties. Sep. Purif. Technol. 2020, 242, 116780. [Google Scholar] [CrossRef]
- Rojas-Buzo, S.; García-García, P.; Corma, A. Zr-MOF-808@ MCM-41 catalyzed phosgene-free synthesis of polyurethane precursors. Catal. Sci. Technol. 2019, 9, 146–156. [Google Scholar] [CrossRef]
- Hardian, R.; Dissegna, S.; Ullrich, A.; Llewellyn, P.L.; Coulet, M.V.; Fischer, R.A. Tuning the properties of MOF-808 via defect engineering and metal nanoparticle encapsulation. Chem. Eur. J. 2021, 27, 6804–6814. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.U.; Park, B.J.; Choi, Y.; Lee, K.B.; Kim, J.H. High-performance self-cross-linked PGP–POEM comb copolymer membranes for CO2 capture. Macromolecules 2017, 50, 8938–8947. [Google Scholar] [CrossRef]
- Kim, M.-B.; Yoon, T.-U.; Hong, D.-Y.; Kim, S.-Y.; Lee, S.-J.; Kim, S.-I.; Lee, S.-K.; Chang, J.-S.; Bae, Y.-S. High SF6/N2 selectivity in a hydrothermally stable zirconium-based metal–organic framework. Chem. Eng. J. 2015, 276, 315–321. [Google Scholar] [CrossRef]
- Qian, J.; Song, E.; Lian, H.; Jiang, J.; Wang, C.; Pan, Y. High-performance ZIF-302 mixed-matrix membranes for efficient CO2 capture. Korean J. Chem. Eng. 2022, 39, 1020–1027. [Google Scholar] [CrossRef]
- Kárászová, M.; Zach, B.; Petrusová, Z.; Červenka, V.; Bobák, M.; Šyc, M.; Izák, P. Post-combustion carbon capture by membrane separation, Review. Sep. Purif. Technol. 2020, 238, 116448. [Google Scholar] [CrossRef]
- Choi, O.; Hossain, I.; Jeong, I.; Park, C.-H.; Kim, Y.; Kim, T.-H. Modified Graphene Oxide-Incorporated Thin-Film Composite Hollow Fiber Membranes through Interface Polymerization on Hydrophilic Substrate for CO2 Separation. Membranes 2021, 11, 650. [Google Scholar] [CrossRef]
- Min, H.J.; Kang, M.; Bae, Y.-S.; Blom, R.; Grande, C.A.; Kim, J.H. Thin-film composite mixed-matrix membrane with irregular micron-sized UTSA-16 for outstanding gas separation performance. J. Membr. Sci. 2022, 669, 121295. [Google Scholar] [CrossRef]
- Martínez-Izquierdo, L.; Téllez, C.; Coronas, J. Highly stable Pebax® Renew® thin-film nanocomposite membranes with metal organic framework ZIF-94 and ionic liquid [Bmim][BF4] for CO2 capture. J. Mater. Chem. A 2022, 10, 18822–18833. [Google Scholar] [CrossRef]
- Xie, K.; Fu, Q.; Kim, J.; Lu, H.; He, Y.; Zhao, Q.; Scofield, J.; Webley, P.A.; Qiao, G.G. Increasing both selectivity and permeability of mixed-matrix membranes: Sealing the external surface of porous MOF nanoparticles. J. Membr. Sci. 2017, 535, 350–356. [Google Scholar] [CrossRef]
- Kang, M.; Kim, T.-H.; Han, H.H.; Min, H.J.; Bae, Y.-S.; Kim, J.H. Submicron-thick, mixed-matrix membranes with metal-organic frameworks for CO2 separation: MIL-140C vs. UiO-67. J. Membr. Sci. 2022, 659, 120788. [Google Scholar] [CrossRef]
- Sutrisna, P.D.; Hou, J.; Zulkifli, M.Y.; Li, H.; Zhang, Y.; Liang, W.; D’Alessandro, D.M.; Chen, V. Surface functionalized UiO-66/Pebax-based ultrathin composite hollow fiber gas separation membranes. J. Mater. Chem. A 2018, 6, 918–931. [Google Scholar] [CrossRef]
- Merkel, T.C.; Lin, H.; Wei, X.; Baker, R. Power plant post-combustion carbon dioxide capture: An opportunity for membranes. J. Membr. Sci. 2010, 359, 126–139. [Google Scholar] [CrossRef]

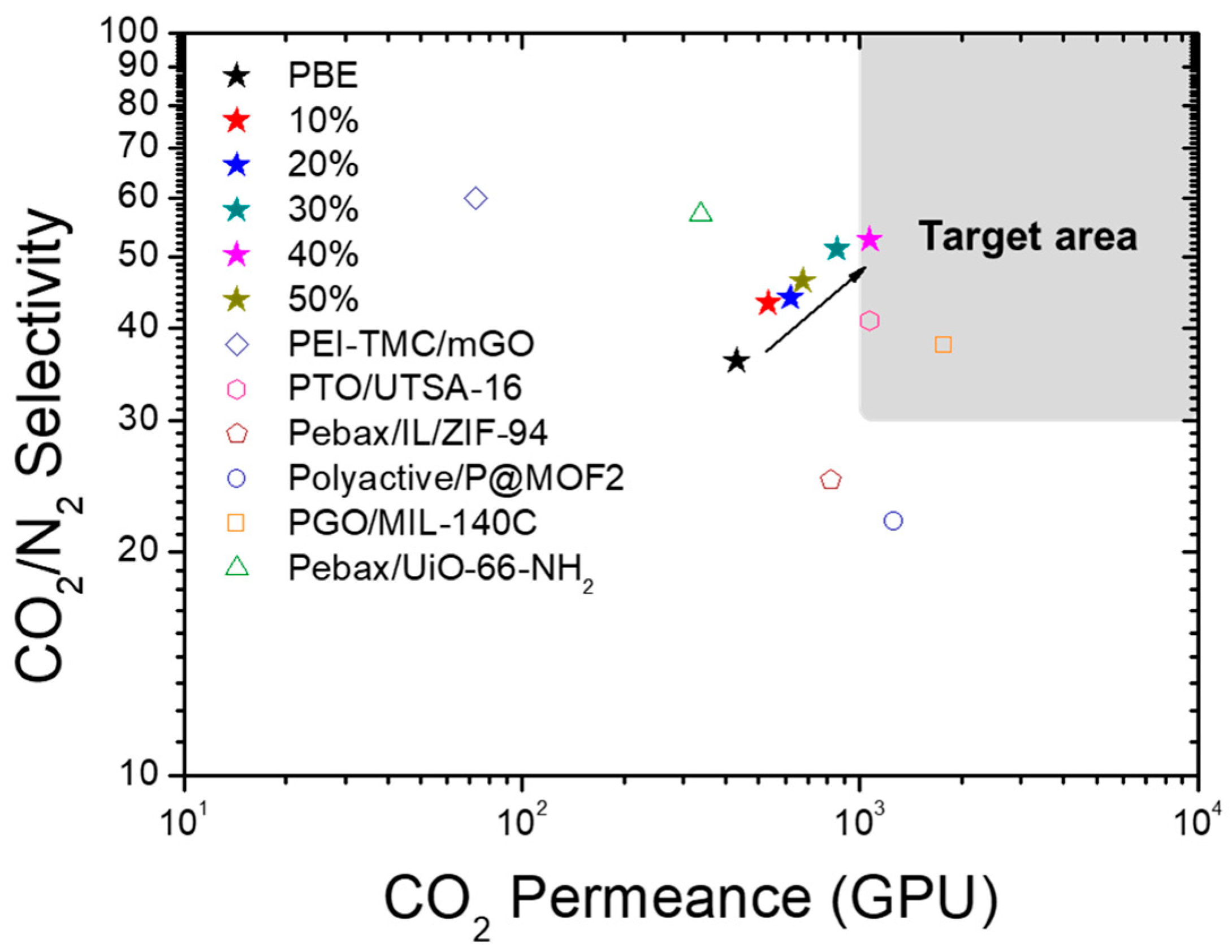
| Sample | CO2 Permeance (GPU) | CO2/N2 Selectivity | Condition (T (°C)/P (bar)) | Reference |
|---|---|---|---|---|
| PBE | 431 | 36.2 | 30 °C, 1 bar | This study |
| MOF-808 10% | 535 | 43.3 | ||
| MOF-808 20% | 623 | 44.0 | ||
| MOF-808 30% | 853 | 51.2 | ||
| MOF-808 40% | 1069.0 | 52.7 | ||
| MOF-808 50% | 677 | 46.4 | ||
| PEI-TMC/mGO | 73 | 60 | 25 °C, 0.25 bar | [38] |
| PTO/UTSA-16 | 1070 | 41.0 | 30 °C, 1 bar | [39] |
| Pebax/IL/ZIF-94 | 819 | 25 | 35 °C, 3 bar | [40] |
| Polyactive/P@MOF2 | 1260 | 22 | 35 °C, 3 bar | [41] |
| PGO/MIL-140C | 1768 | 38 | 30 °C, 1 bar | [42] |
| Pebax/UiO-66-NH2 | 338 | 57 | 25 °C, 2 bar | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, H.J.; Kim, M.-B.; Bae, Y.-S.; Thallapally, P.K.; Lee, J.H.; Kim, J.H. Polymer-Infiltrated Metal–Organic Frameworks for Thin-Film Composite Mixed-Matrix Membranes with High Gas Separation Properties. Membranes 2023, 13, 287. https://doi.org/10.3390/membranes13030287
Min HJ, Kim M-B, Bae Y-S, Thallapally PK, Lee JH, Kim JH. Polymer-Infiltrated Metal–Organic Frameworks for Thin-Film Composite Mixed-Matrix Membranes with High Gas Separation Properties. Membranes. 2023; 13(3):287. https://doi.org/10.3390/membranes13030287
Chicago/Turabian StyleMin, Hyo Jun, Min-Bum Kim, Youn-Sang Bae, Praveen K. Thallapally, Jae Hun Lee, and Jong Hak Kim. 2023. "Polymer-Infiltrated Metal–Organic Frameworks for Thin-Film Composite Mixed-Matrix Membranes with High Gas Separation Properties" Membranes 13, no. 3: 287. https://doi.org/10.3390/membranes13030287
APA StyleMin, H. J., Kim, M.-B., Bae, Y.-S., Thallapally, P. K., Lee, J. H., & Kim, J. H. (2023). Polymer-Infiltrated Metal–Organic Frameworks for Thin-Film Composite Mixed-Matrix Membranes with High Gas Separation Properties. Membranes, 13(3), 287. https://doi.org/10.3390/membranes13030287








