Nylon 6,6 Waste Nanofiber Membrane for Produced Water Filtration: Experimental, Performance Modelling, Optimization and Techno-Economic Analysis
Abstract
1. Introduction
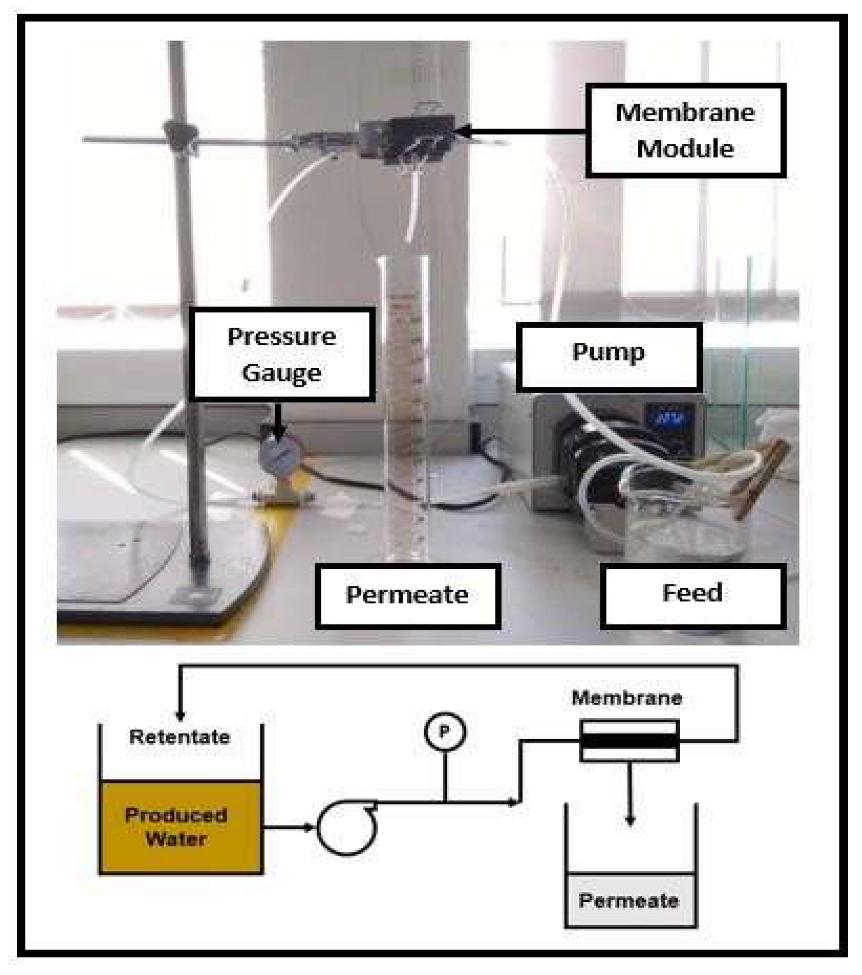
2. Experiments Methodology
2.1. Preparation of Nylon 6,6 Waste Solution
2.2. Electrospinning of Nylon 6,6 Waste NFM
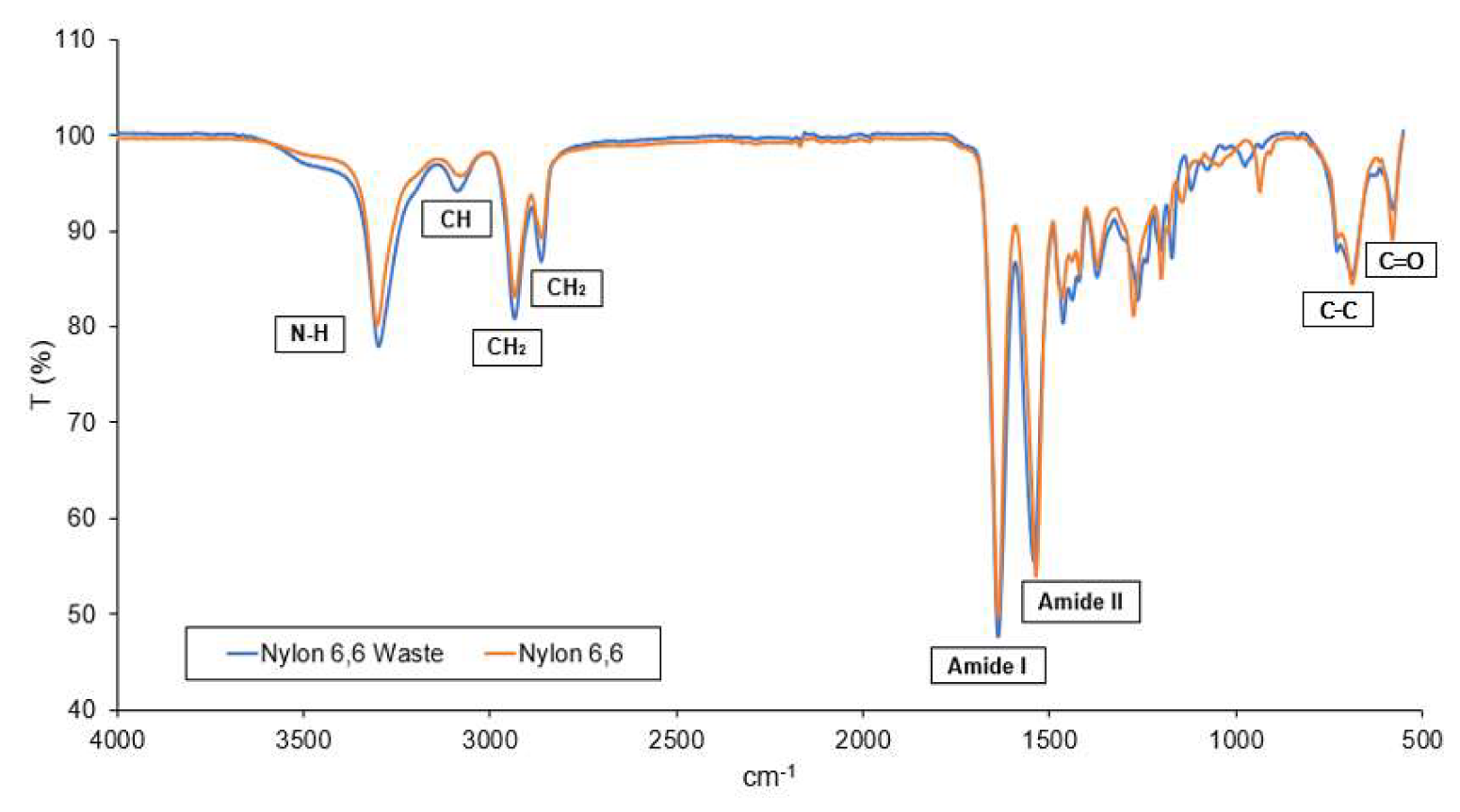
2.3. Membrane Characterization
3. Modelling and Technoeconomic Analysis Methodology
3.1. Prediction Model
- Xin = mole fraction of X inlet streams;
- Xout = mole fraction of X outlet streams.
- A = Amount of water needed to dilute the produced water feed;
- B = Amount of produced water feed;
- C = Amount of produced water solution to be filtered;
- D = Amount of permeate to be produced;
- E = Amount of retentate to be recycled to feed stream.
3.2. Techno-Economic Analysis
3.2.1. List of Assumptions
3.2.2. Capital Expenditure (CAPEX)
- I = cost index ratio for updating the cost to the recent year;
- fm = factor for pump construction material;
- fp = factor for suction pressure range;
- L = factor for labour costs;
- Q = pump flow capacity (m3/h);
- P = pump outlet pressure (kPa).
- CE = Equipment cost ($);
- CB = Base cost ($);
- Q = Design capacity (m3);
- QB = Base size;
- M = Cost exponent;
- fm = Correction factor for material;
- fp = Pressure correction factor;
- ft = Temperature correction factor.
3.2.3. Operating Expenditure (OPEX)
- QT = Targeted clean water production rate (m3/h);
- QC = Current permeate flow (m3/h).
- Scale-up factor membrane area, SCM
- QT = Targeted clean water production rate (m3/h);
- QC = Current permeate flow (m3/h);
- AC = Current membrane area (m2).
- ESP = Specific pump energy (kW/m3);
- QT = Targeted clean water production (m3/h);
- t = Plant operating hours (h/year).
- P = Power supply high voltage (kW);
- tm = Time consumption to produce membrane (h);
- Am = Size of membrane produced (m2);
- SCM = Membrane scale-up factor (m2);
- a = amortization rate.
- Ss = Amount of solvent used (g);
- Ps = Price of solvent (RM/g);
- Pm = Price of polymer used (RM).
4. Results and Discussion
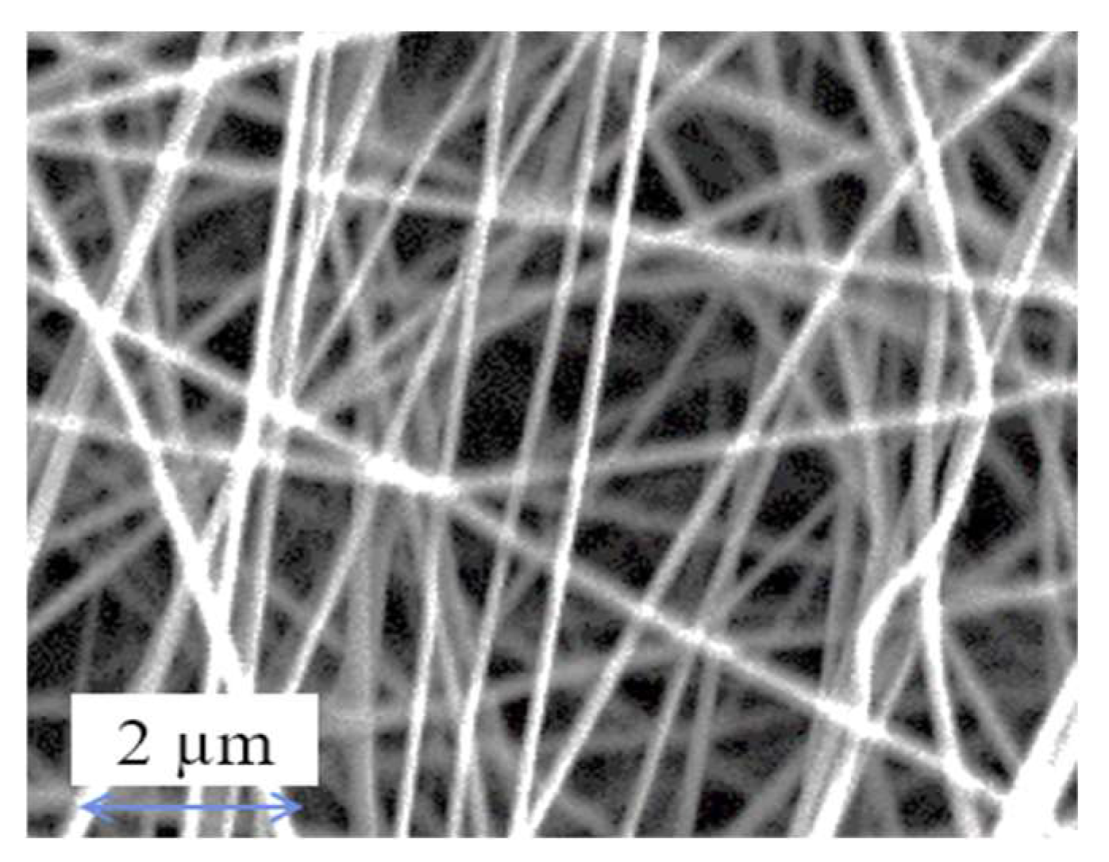
4.1. Membrane Characterization
4.2. Prediction Model
4.2.1. Permeate Concentration
4.2.2. Average Flux
4.3. Membrane Performance
4.3.1. Permeate Concentration
4.3.2. Average Flux
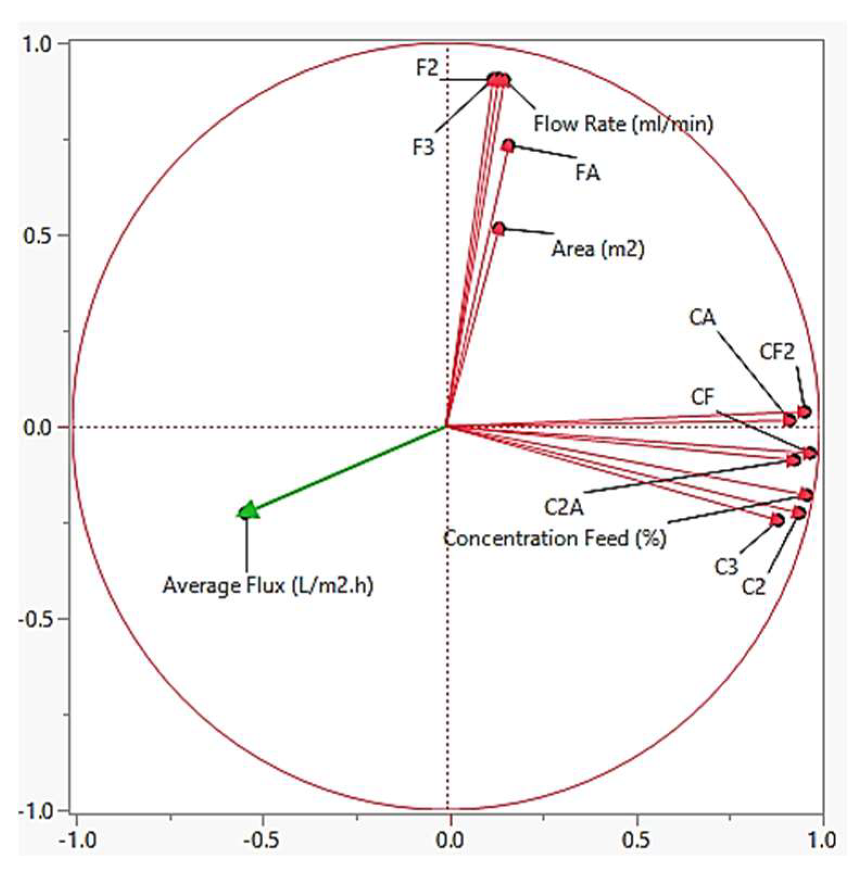
4.4. Principle Component Analysis
4.4.1. Permeate Concentration
4.4.2. Average Flux
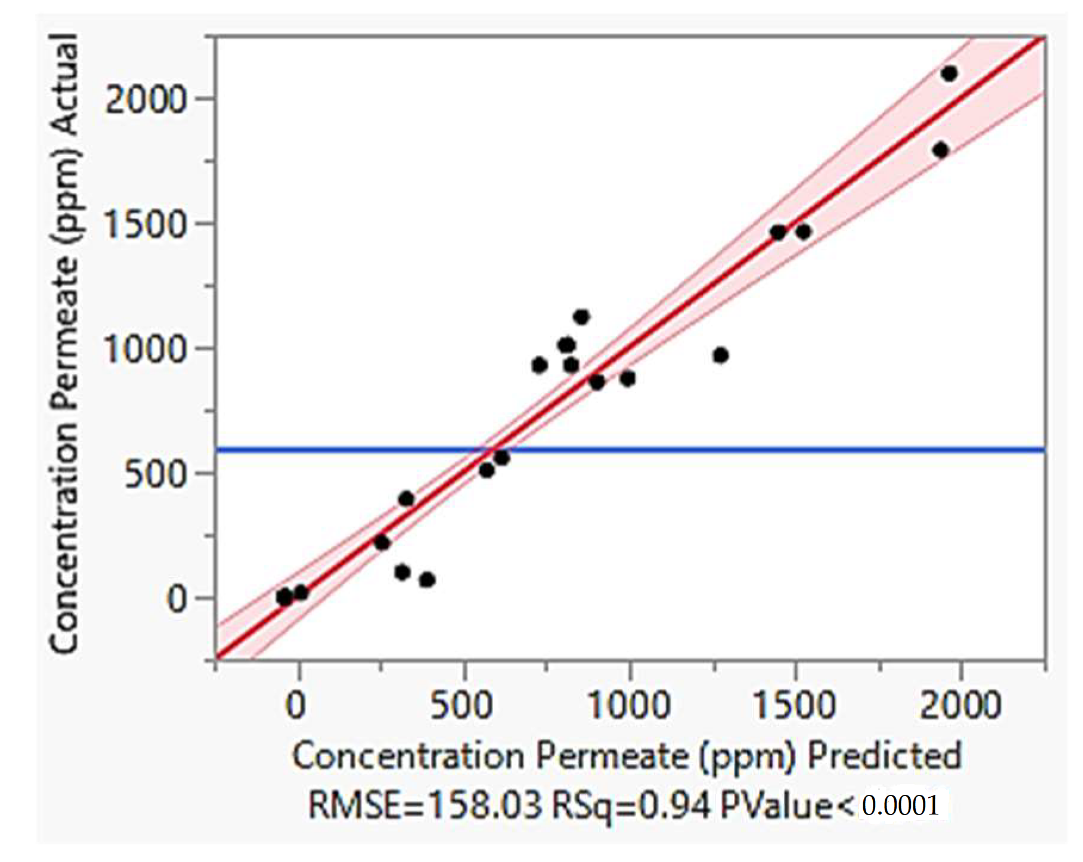
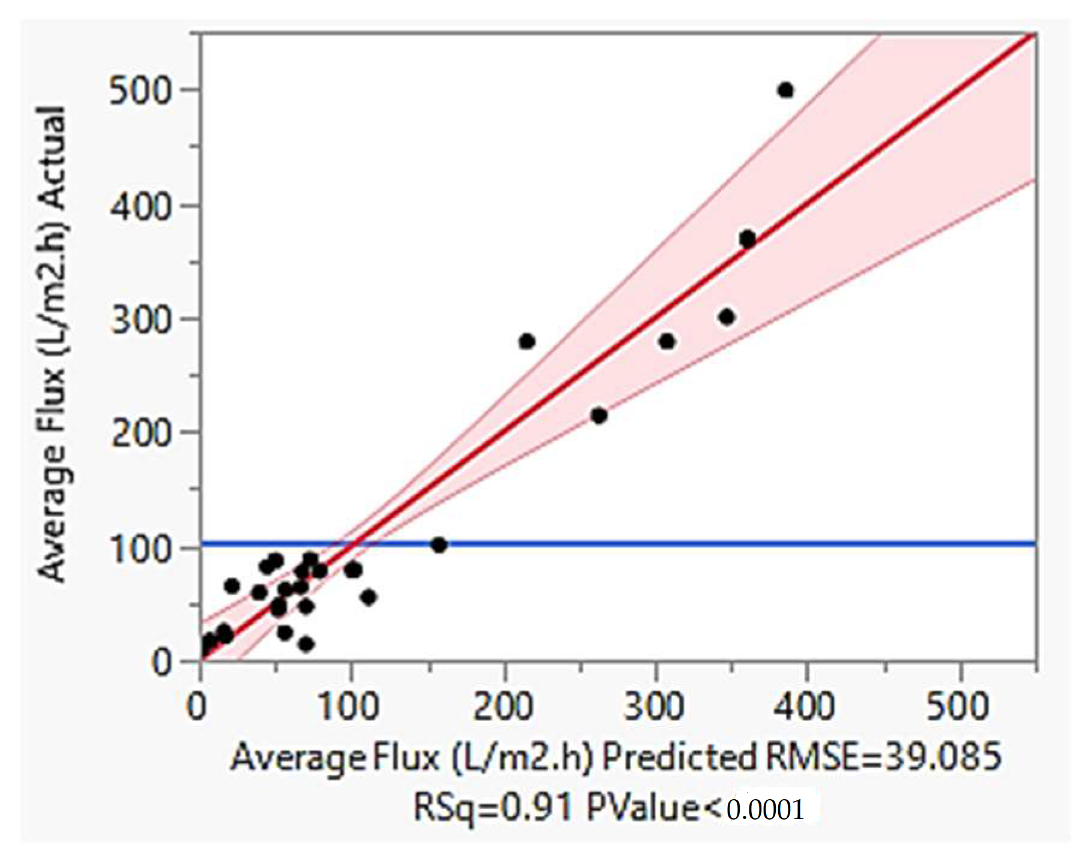
4.5. Model Validation
4.6. Economic Analysis
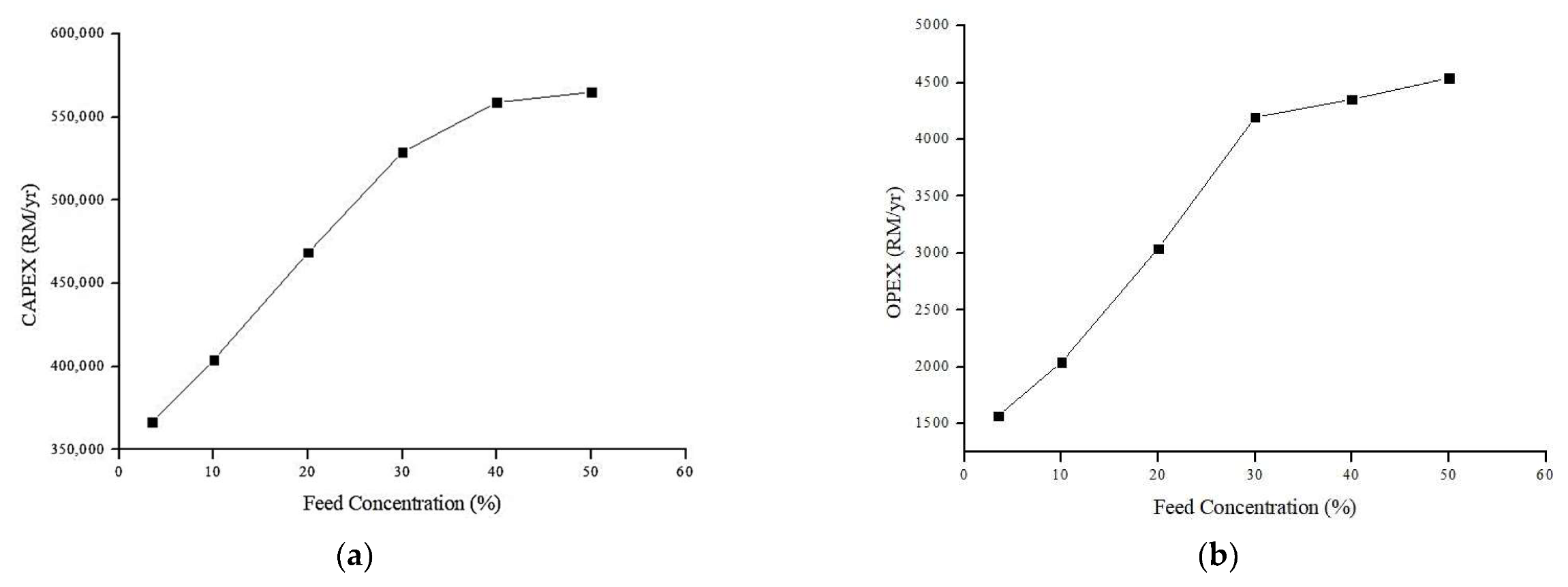
4.6.1. Effect of Feed Concentration on Annual CAPEX and OPEX (Rm/y)
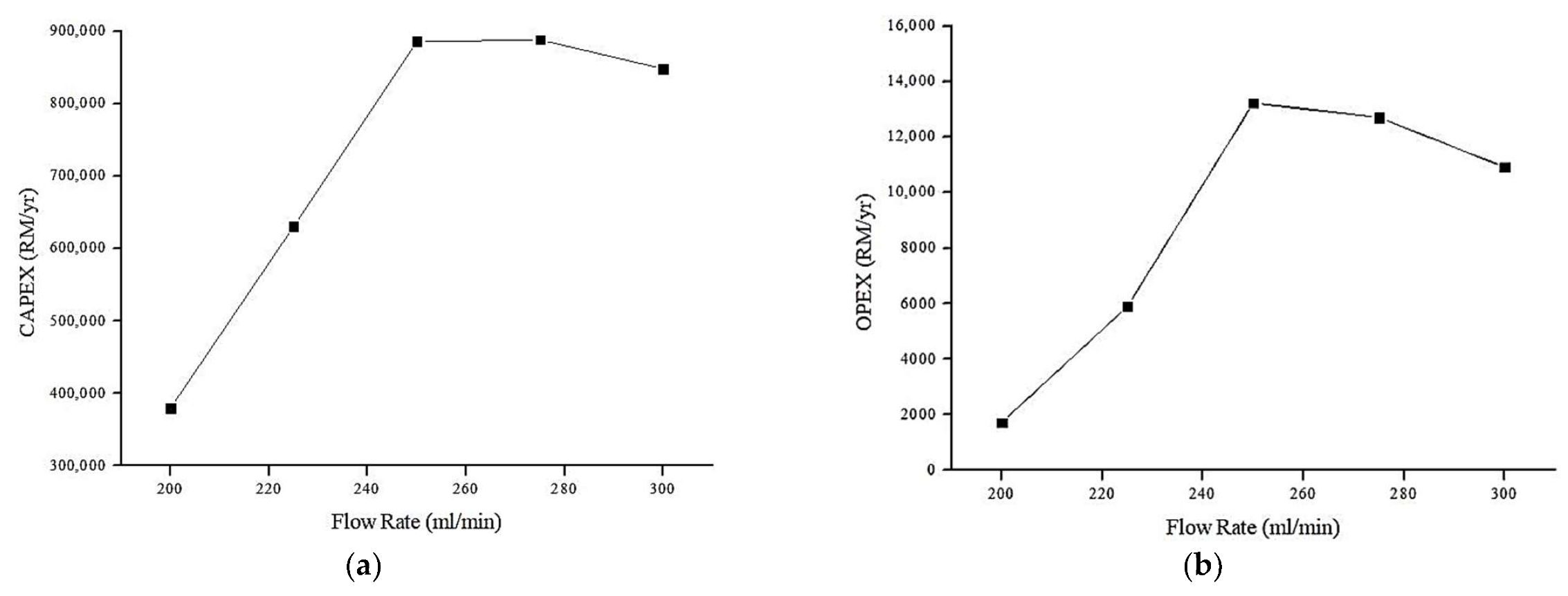
4.6.2. Effect of Flow Rate on Annual CAPEX and OPEX (RM/y)
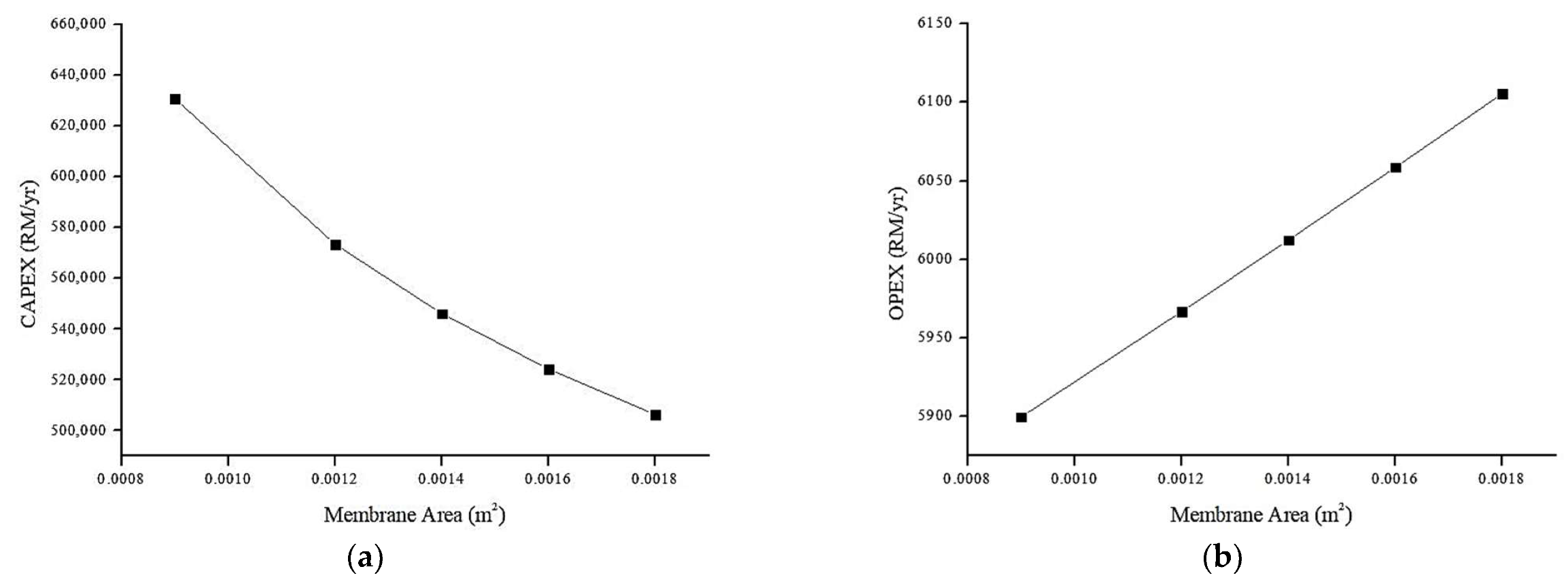
4.6.3. Effect of Membrane Area on Annual CAPEX and OPEX (RM/y)
4.6.4. Optimum Design and Scalability
4.6.5. Comparison with Other Literature
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| ANOVA | Analysis of Variance |
| CAPEX | Capital Cost/Capital Expenditure |
| FESEM | Field Emission Scanning Electron Microscope |
| FO | Forward Osmosis |
| FTIR | Fourier Transform Infrared Spectroscopy |
| MD | Membrane distillation |
| MF | Microfiltration |
| MVC | Mechanical Vapor Compression |
| NF | Nanofiltration |
| NFM | Nanofiber Membrane |
| OPEX | Operating Cost/Operating Expenditure |
| PCA | Principle Component Analysis |
| PPC | Physical Plant Cost |
| PW | Produced Water |
| RMSE | Root Mean Square Error |
| RO | Reverse Osmosis |
| TEA | Technoeconomic Analysis |
| UV-Vis | Ultraviolet-Visible |
| WCA | Water Contact Angle |
Appendix A
| Run | Feed Concentration (%) | Flow Rate (mL/min) | Area (m2) | Permeate Concentration (ppm) | Rejection (%) | Average Flux (L/m2 h) |
|---|---|---|---|---|---|---|
| 1 | 0 | 200.00 | 0.0009 | 0.00 | 0.00 | 214.44 |
| 2 | 25 | 225.00 | 0.0009 | 100.20 | 79.78 | 79.78 |
| 3 | 25 | 275.00 | 0.0009 | 67.98 | 86.28 | 101.11 |
| 4 | 50 | 200.00 | 0.0009 | 928.27 | 57.75 | 78.00 |
| 5 | 50 | 250.00 | 0.0009 | 1008.48 | 54.09 | 87.22 |
| 6 | 50 | 300.00 | 0.0009 | 1122.17 | 48.92 | 88.33 |
| 7 | 75 | 225.00 | 0.0009 | 1461.21 | 60.47 | 61.67 |
| 8 | 75 | 275.00 | 0.0009 | 967.22 | 73.83 | 64.00 |
| 9 | 100 | 250.00 | 0.0009 | 2097.84 | 66.20 | 44.67 |
| 10 | 0 | 225.00 | 0.0018 | 0.00 | 0.00 | 82.22 |
| 11 | 25 | 225.00 | 0.0018 | 217.70 | 56.08 | 25.28 |
| 12 | 25 | 275.00 | 0.0018 | 286.94 | 42.10 | 21.11 |
| 13 | 50 | 250.00 | 0.0018 | 508.13 | 76.87 | 59.44 |
| 14 | 50 | 300.00 | 0.0018 | 558.27 | 74.59 | 17.22 |
| 15 | 75 | 225.00 | 0.0018 | 861.49 | 76.69 | 79.07 |
| 16 | 75 | 275.00 | 0.0018 | 928.27 | 74.89 | 24.50 |
| 17 | 100 | 250.00 | 0.0018 | 876.53 | 85.88 | 65.00 |
References
- Fakhru’l-Razi, A.; Pendashteh, A.; Abdullah, L.C.; Biak, D.R.A.; Madaeni, S.S.; Abidin, Z.Z. Review of Technologies for Oil and Gas Produced Water Treatment. J. Hazard. Mater. 2009, 170, 530–551. [Google Scholar] [CrossRef]
- Hizam, S.M.; Bilad, M.R.; Nordin, N.A.H.; Sambudi, N.S.; Wirzal, M.D.H.; Yusof, N.; Klaysom, C.; Jaafar, J. Inclined Forward Osmosis Module System for Fouling Control in Sustainable Produced Water Treatment Using Seawater as Draw Solution. J. Water Process Eng. 2021, 40, 101752. [Google Scholar] [CrossRef]
- Lee, R.; Seright, R.; Hightower, M.; Sattler, A.; Cather, M.; Mcpherson, B.; Wrotenbery, L.; Martin, D.; Whitworth, M. Strategies for Produced Water Handling in New Mexico; Ground Water Protection Council: Denver, CO, USA, 16 October 2002; p. 13. [Google Scholar]
- Khatib, Z.; Verbeek, P. Water to Value—Produced Water Management for Sustainable Field Development of Mature and Green Fields. J. Pet. Technol. 2003, 55, 26–28. [Google Scholar] [CrossRef]
- Obeadalla, L.E.; Abdelmagd, S.E. Assessment Study of the Produced Water from Adar-Yale Oilfield in Melut Basin, for Injection. Sch. Res. Libr. 2013, 5, 30–35. [Google Scholar]
- Wang, Z.-H.; Liu, X.-Y.; Zhang, H.-Q.; Wang, Y.; Xu, Y.-F.; Peng, B.-L.; Liu, Y. Modeling of Kinetic Characteristics of Alkaline-Surfactant-Polymer-Strengthened Foams Decay under Ultrasonic Standing Wave. Pet. Sci. 2022, 19, 1825–1839. [Google Scholar] [CrossRef]
- Mousa, K.M.; Hadi, H.J. Coagulation/Flocculation Process for Produced Water Treatment. Int. J. Curr. Eng. Technol. 2016, 6, 551–555. [Google Scholar]
- Santana, C.R.; Pereira, D.F.; Sousa, S.C.S.N.; Cavalcanti, E.B.; Silva, G.F. Evaluation of The Process of Coagulation/Flocculation of Produced Water Using Moringa Oleifara Lam. as Natural Coagulants. Braz. J. Pet. Gas 2010, 4, 111–117. [Google Scholar]
- Su, Y.; Zhao, Q.; Liu, J.; Zhao, J.; Li, Y.; Jiang, Z. Improved Oil/Water Emulsion Separation Performance of PVC/CPVC Blend Ultrafiltration Membranes by Fluorination Treatment. Desalination Water Treat. 2015, 55, 304–314. [Google Scholar] [CrossRef]
- Al-Maamari, R.S.; Sueyoshi, M.; Tasaki, M.; Okamura, K.; Al-Lawati, Y.; Nabulsi, R.; Al-Battashi, M. Flotation, Filtration, and Adsorption: Pilot Trials for Oilfield Produced-Water Treatment. Oil Gas Facil. 2014, 3, 56–66. [Google Scholar] [CrossRef]
- Fathy, M.; El-Sayed, M.; Ramzi, M.; Abdelraheem, O.H. Adsorption Separation of Condensate Oil from Produced Water Using ACTF Prepared of Oil Palm Leaves by Batch and Fixed Bed Techniques. Egypt. J. Pet. 2018, 27, 319–326. [Google Scholar] [CrossRef]
- Spellman, F.R. Handbook of Water and Wastewater Treatment Plant Operations, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2013; ISBN 978-1-4665-5337-8. [Google Scholar]
- Igunnu, E.T.; Chen, G.Z. Produced Water Treatment Technologies. Int. J. Low-Carbon Technol. 2012, 9, 157–177. [Google Scholar] [CrossRef]
- Meldrum, N. Hydrocyclones: A Solution to Produced-Water Treatment. SPE Prod. Eng. 1988, 3, 669–676. [Google Scholar] [CrossRef]
- Souza, J.S.; Paiva, M.K.N.; Farias, F.P.M.; Neto, S.R.F.; Lima, A.G.B. Hydrocyclone Applications in Produced Water: A Steady-State Numerical Analysis. Braz. J. Pet. Gas 2012, 6, bjpg2012–bjpg0011. [Google Scholar] [CrossRef]
- Yu, L.; Han, M.; He, F. A Review of Treating Oily Wastewater. Arab. J. Chem. 2017, 10, S1913–S1922. [Google Scholar] [CrossRef]
- Abd Halim, N.S.; Wirzal, M.D.H.; Hizam, S.M.; Bilad, M.R.; Nordin, N.A.H.M.; Sambudi, N.S.; Putra, Z.A.; Yusoff, A.R.M. Recent Development on Electrospun Nanofiber Membrane for Produced Water Treatment: A Review. J. Environ. Chem. Eng. 2021, 9, 104613. [Google Scholar] [CrossRef]
- Gebreslase, G. Review on Membranes for the Filtration of Aqueous Based Solution: Oil in Water Emulsion. J. Membr. Sci. Technol. 2018, 8, 188. [Google Scholar] [CrossRef]
- Azizo, A.S.; Wirzal, M.D.H.; Bilad, M.R.; Yusoff, A.R.M. Assessment of Nylon 6, 6 Nanofibre Membrane for Microalgae Harvesting. AIP Conf. Proc. 2017, 1891, 020032. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Wirzal, M.D.H.; Nordin, N.A.H.; Halim, N.S.A. Development of Polyvinylidene Fluoride (PVDF)-ZIF-8 Membrane for Wastewater Treatment. IOP Conf. Ser.: Earth Environ. Sci. 2018, 140, 012021. [Google Scholar] [CrossRef]
- Zhao, C.; Nie, S.; Tang, M.; Sun, S. Polymeric PH-Sensitive Membranes—A Review. Prog. Polym. Sci. 2011, 36, 1499–1520. [Google Scholar] [CrossRef]
- Duraisamy, R.T.; Beni, A.H.; Henni, A. State of the Art Treatment of Produced Water. In Water Treatment; InTechOpen: London, UK, 2013; Volume 16, p. 199. [Google Scholar]
- Jepsen, K.; Hansen, L.; Mai, C.; Yang, Z. Challenges of Membrane Filtration for Produced Water Treatment in Offshore Oil & Gas Production. In Proceedings of the OCEANS 2016 MTS/IEEE Monterey, Monterey, CA, USA, 19–23 September 2016; IEEE: Monterey, CA, USA, 1 December 2016; pp. 1–18. [Google Scholar]
- Obaid, M.; Barakat, N.A.M.; Fadali, O.A.; Motlak, M.; Almajid, A.A.; Khalil, K.A. Effective and Reusable Oil/Water Separation Membranes Based on Modified Polysulfone Electrospun Nanofiber Mats. Chem. Eng. J. 2015, 259, 449–456. [Google Scholar] [CrossRef]
- Salahi, A.; Noshadi, I.; Badrnezhad, R.; Kanjilal, B.; Mohammadi, T. Nano-Porous Membrane Process for Oily Wastewater Treatment: Optimization Using Response Surface Methodology. J. Environ. Chem. Eng. 2013, 1, 218–225. [Google Scholar] [CrossRef]
- Munirasu, S.; Haija, M.A.; Banat, F. Use of Membrane Technology for Oil Field and Refinery Produced Water Treatment—A Review. Process Saf. Environ. Prot. 2016, 100, 183–202. [Google Scholar] [CrossRef]
- Ozgun, H.; Ersahin, M.E.; Erdem, S.; Atay, B.; Kose, B.; Kaya, R.; Altinbas, M.; Sayili, S.; Hoshan, P.; Atay, D.; et al. Effects of the Pre-Treatment Alternatives on the Treatment of Oil-Gas Field Produced Water by Nanofiltration and Reverse Osmosis Membranes. J. Chem. Technol. Biotechnol. 2013, 88, 1576–1583. [Google Scholar] [CrossRef]
- Lee, S.; Boo, C.; Elimelech, M.; Hong, S. Comparison of Fouling Behavior in Forward Osmosis (FO) and Reverse Osmosis (RO). J. Membr. Sci. 2010, 365, 34–39. [Google Scholar] [CrossRef]
- Foong, C.Y.; Wirzal, M.D.H.; Bustam, M.A. A Review on Nanofibers Membrane with Amino-Based Ionic Liquid for Heavy Metal Removal. J. Mol. Liq. 2019, 297, 111793. [Google Scholar] [CrossRef]
- Mohd, A.T.; Abd Halim, N.S.; Wirzal, M.D.H.; Bilad, M.R.; Nordin, M.N.A.H.; Adi Putra, Z.; Mohd Yusoff, A.R. EFFECT OF BMIM-CHLORIDE ON PHYSIO CHEMICAL PROPERTIES OF NANOFIBER MEMBRANE FOR DOMESTIC WASTEWATER TREATMENT (Kesan BMIM-Klorida Ke Atas Sifat-Sifat Fisio-Kimia Membran Nanogentian Untuk Rawatan Sisa Air Domestik). Malays. J. Anal. Sci. 2020, 24, 78–86. [Google Scholar]
- Saad, M.S.; Wirzal, M.D.H.; Abd Halim, N.S.; Khar, M.R. Removal Color from Palm Oil Mill Effluent (POME): Electrocoagulation Method vs Microfiltration Using Nanofiber Membrane. Int. J. Electrochem. Sci. 2020, 15, 11283–11293. [Google Scholar] [CrossRef]
- Saad, M.S.; Balasubramaniam, L.; Wirzal, M.D.H.; Abd Halim, N.S.; Bilad, M.R.; Md Nordin, N.A.H.; Adi Putra, Z.; Ramli, F.N. Integrated Membrane–Electrocoagulation System for Removal of Celestine Blue Dyes in Wastewater. Membranes 2020, 10, 184. [Google Scholar] [CrossRef]
- Asri, M.A.N.M.; Halim, N.S.A.; Wirzal, M.D.H.; Yusoff, A.R.M.; Bilad, M.R. Thermal Annealing Surface Modification: Effect on Surface and Performance of Electrospun Nylon 6,6 Nanofiber Membrane for Wastewater Treatment. Jurnal. Penelitian. Pengkajian. Ilmu. Pendidikan. E-St. 2021, 5, 56–66. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R.; Ma, H.; Hsiao, B.S.; Chu, B. High-Flux Microfiltration Filters Based on Electrospun Polyvinylalcohol Nanofibrous Membranes. Polymer 2013, 54, 548–556. [Google Scholar] [CrossRef]
- Wang, Z.; Crandall, C.; Sahadevan, R.; Menkhaus, T.J.; Fong, H. Microfiltration Performance of Electrospun Nanofiber Membranes with Varied Fiber Diameters and Different Membrane Porosities and Thicknesses. Polymer 2017, 114, 64–72. [Google Scholar] [CrossRef]
- Han, W.; Bhat, G.S.; Wang, X. Investigation of Nanofiber Breakup in the Melt-Blowing Process. Ind. Eng. Chem. Res. 2016, 55, 3150–3156. [Google Scholar] [CrossRef]
- Raghavan, B.; Soto, H.; Lozano, K. Fabrication of Melt Spun Polypropylene Nanofibers by Forcespinning. J. Eng. Fibers Fabr. 2013, 8, 155892501300800106. [Google Scholar] [CrossRef]
- Katsogiannis, K.A.G.; Vladisavljević, G.T.; Georgiadou, S. Porous Electrospun Polycaprolactone (PCL) Fibres by Phase Separation. Eur. Polym. J. 2015, 69, 284–295. [Google Scholar] [CrossRef]
- Qin, W.; Li, J.; Tu, J.; Yang, H.; Chen, Q.; Liu, H. Fabrication of Porous Chitosan Membranes Composed of Nanofibers by Low Temperature Thermally Induced Phase Separation, and Their Adsorption Behavior for Cu2+. Carbohydr. Polym. 2017, 178, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Rolandi, M.; Rolandi, R. Self-Assembled Chitin Nanofibers and Applications. Adv. Colloid Interface Sci. 2014, 207, 216–222. [Google Scholar] [CrossRef]
- Srinivasan, A.; Bandyopadhyay, S. Advances in Polymer Materials and Technology; CRC Press: Boca Raton, FL, USA, 2016; ISBN 978-1-315-35388-3. [Google Scholar]
- Li, H.; Zhu, C.; Xue, J.; Ke, Q.; Xia, Y. Enhancing the Mechanical Properties of Electrospun Nanofiber Mats through Controllable Welding at the Cross Points. Macromol. Rapid Commun. 2017, 38, 723. [Google Scholar] [CrossRef]
- Rianjanu, A.; Kusumaatmaja, A.; Suyono, E.A.; Triyana, K. Solvent Vapor Treatment Improves Mechanical Strength of Electrospun Polyvinyl Alcohol Nanofibers. Heliyon 2018, 4, e00592. [Google Scholar] [CrossRef]
- Mat Nawi, N.I.; Abd Halim, N.S.; Lee, L.C.; Wirzal, M.D.H.; Bilad, M.R.; Nordin, N.A.H.; Putra, Z.A. Improved Nylon 6,6 Nanofiber Membrane in A Tilted Panel Filtration System for Fouling Control in Microalgae Harvesting. Polymers 2020, 12, 252. [Google Scholar] [CrossRef]
- Abd Halim, N.S.; Wirzal, M.D.H.; Bilad, M.R.; Md Nordin, N.A.H.; Adi Putra, Z.; Sambudi, N.S.; Mohd Yusoff, A.R. Improving Performance of Electrospun Nylon 6,6 Nanofiber Membrane for Produced Water Filtration via Solvent Vapor Treatment. Polymers 2019, 11, 2117. [Google Scholar] [CrossRef]
- Osipi, S.R.; Secchi, A.R.; Borges, C.P. Cost Assessment and Retro-Techno-Economic Analysis of Desalination Technologies in Onshore Produced Water Treatment. Desalination 2018, 430, 107–119. [Google Scholar] [CrossRef]
- Tavakkoli, S.; Lokare, O.R.; Vidic, R.D.; Khanna, V. A Techno-Economic Assessment of Membrane Distillation for Treatment of Marcellus Shale Produced Water. Desalination 2017, 416, 24–34. [Google Scholar] [CrossRef]
- Jasni, M.J.F.; Arulkumar, M.; Sathishkumar, P.; Mohd Yusoff, A.R.; Buang, N.A.; Gu, F.L. Electrospun Nylon 6,6 Membrane as a Reusable Nano-Adsorbent for Bisphenol A Removal: Adsorption Performance and Mechanism. J. Colloid Interface Sci. 2017, 508, 591–602. [Google Scholar] [CrossRef]
- Abd Halim, N.S. Effect of Modification on Electrospun Nylon 6,6 Nanofiber Membrane for Pretreatment of Produced Water. Master’s Thesis, Universiti Teknologi PETRONAS, Bandar Seri Iskandar, Malaysia, 2019. [Google Scholar]
- Koromilas, N.D.; Anastasopoulos, C.; Oikonomou, E.K.; Kallitsis, J.K. Preparation of Porous Polymeric Membranes Based on a Pyridine Containing Aromatic Polyether Sulfone. Polymers 2019, 11, 59. [Google Scholar] [CrossRef]
- Smith, R. Chemical Process Design and Integration; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2005. [Google Scholar]
- Perry, R.; Green, D. Perry’s Chemical Engineers’ Handbook, 7th ed.; McGraw-Hill Professional: New York, NY, USA, 1997; ISBN 978-0-07-142294-9. [Google Scholar]
- Perry, J.H. Chemical Engineers’ Handbook: Prepared by a Staff of Specialists; Mcgraw-Hill Book Company: New York, NY, USA, 1950; p. 1042. [Google Scholar]
- Nguyen, T.-A.; Yoshikawa, S. Modeling and Economic Optimization of the Membrane Module for Ultrafiltration of Protein Solution Using a Genetic Algorithm. Processes 2020, 8, 4. [Google Scholar] [CrossRef]
- Patel, G.C.; Yadav, B.K. Chapter 4—Polymeric Nanofibers for Controlled Drug Delivery Applications. In Organic Materials as Smart Nanocarriers for Drug Delivery; Grumezescu, A.M., Ed.; William Andrew Publishing: Norwich, NY, USA, 2018; pp. 147–175. ISBN 978-0-12-813663-8. [Google Scholar]
- Wang, H.; Zheng, G.; Sun, D. Electrospun Nanofibrous Membrane for Air Filtration. In Proceedings of the 2007 7th IEEE conference on nanotechnology (IEEE NANO), Hong Kong, 2–5 August 2007; pp. 1244–1247. [Google Scholar] [CrossRef]
- Parlayıcı, Ş.; Avcı, A.; Pehlivan, E. Electrospinning of Polymeric Nanofiber (Nylon 6,6/Graphene Oxide) for Removal of Cr (VI): Synthesis and Adsorption Studies. J. Anal. Sci. Technol. 2019, 10, 13. [Google Scholar] [CrossRef]
- Bilad, M.R.; Azizo, A.S.; Wirzal, M.D.H.; Jia Jia, L.; Putra, Z.A.; Nordin, N.A.H.M.; Mavukkandy, M.O.; Jasni, M.J.F.; Yusoff, A.R.M. Tackling Membrane Fouling in Microalgae Filtration Using Nylon 6,6 Nanofiber Membrane. J. Environ. Manag. 2018, 223, 23–28. [Google Scholar] [CrossRef]
- Mahbobi, M.; Tiemann, T.K. Chapter 8. Regression Basics. In Introductory Business Statistics with Interactive Spreadsheet; BCcampus Open Publishing: Vancouver, BC, Canada, 2015. [Google Scholar]
- Eliseus, A.; Adi Putra, Z.; Bilad, M.R.; Nordin, M.N.A.H.; Wirzal, M.D.H.; Jaafar, J.; Khan, A.L.; Aqsha, A. Energy Minimization of a Tilted Panel Filtration System for Microalgae Filtration: Performance Modeling and Optimization. Algal Res. Biomass Biofuels Bioprod. 2018, 34, 104–115. [Google Scholar] [CrossRef]
- Harlacher, T.; Wessling, M. Chapter Thirteen—Gas–Gas Separation by Membranes. In Progress in Filtration and Separation; Tarleton, S., Ed.; Academic Press: Oxford, UK, 2015; pp. 557–584. ISBN 978-0-12-384746-1. [Google Scholar]
- Manickam, M.; Kwon, T.; Kim, J.; Moon, I. Factors Affecting Flux and Water Separation Performance in Air Gap Membrane Distillation. J. Ind. Eng. Chem. 2007, 13, 965–970. [Google Scholar]
- Abdelrasoul, A.; Doan, H.; Lohi, A. Fouling in Membrane Filtration and Remediation Methods. In Mass Transfer—Advances in Sustainable Energy and Environment Oriented Numerical Modeling; IntechOpen: London, UK, 2013; ISBN 978-953-51-1170-2. [Google Scholar]
- Okamoto, Y.; Lienhard, J.H. How RO Membrane Permeability and Other Performance Factors Affect Process Cost and Energy Use: A Review. Desalination 2019, 470, 114064. [Google Scholar] [CrossRef]
- Iorhemen, O.T.; Hamza, R.A.; Tay, J.H. Membrane Bioreactor (MBR) Technology for Wastewater Treatment and Reclamation: Membrane Fouling. Membranes 2016, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Abd Halim, N.S.; Wirzal, M.D.H.; Bilad, M.R.; Md Nordin, N.A.H.; Adi Putra, Z.; Mohd Yusoff, A.R.; Narkkun, T.; Faungnawakij, K. Electrospun Nylon 6,6/ZIF-8 Nanofiber Membrane for Produced Water Filtration. Water 2019, 11, 2111. [Google Scholar] [CrossRef]
- Embas, D.U. Environmental Quality (Industrial Effluent) Regulations 2009; Environmental Quality Act 1974; Percetakan Nasional Berhad: Kuala Lumpur, Malaysia, 2009. [Google Scholar]
- Al-Husaini, I.S.; Yusoff, A.R.M.; Lau, W.-J.; Ismail, A.F.; Al-Abri, M.Z.; Wirzal, M.D.H. Iron Oxide Nanoparticles Incorporated Polyethersulfone Electrospun Nanofibrous Membranes for Effective Oil Removal. Chem. Eng. Res. Des. 2019, 148, 142–154. [Google Scholar] [CrossRef]





| Parameters | Assumptions |
|---|---|
| Clean Water Production Rate (m3/day) | 100 |
| Plant operating hours per year (hours/year) | 7200 |
| Plant Lifetime (years) | 20 |
| Interest rate (%) | 8 |
| Pump efficiency | 0.8 |
| Electricity Tariff (RM/kWh) | 0.441 |
| Power Supply High Voltage (Watt) | 30 |
| Time Consumption for Membrane Fabrication (hour) | 30 |
| Membrane Area Produced from Spinning (cm2) | 200 |
| Price of Formic Acid (RM/kg) | 21.15 |
| Price of Acetic Acid (RM/kg) | 0.000423 |
| Parameters | Assumptions | Factor |
|---|---|---|
| f1 | 100 | 0.4 |
| f2 | 7200 | 0.7 |
| f3 | 20 | 0.2 |
| f4 | 8 | 0.1 |
| Physical Plant Factor | 1.4 | |
| f5 | 0.441 | 0.3 |
| f6 | 30 | 0.05 |
| f7 | Contingency Cost | 0.1 |
| Fixed Capital Factor | 0.45 |
| Sample Name | Nylon 6,6 Waste |
|---|---|
| Thickness (mm) | 0.29 ± 0.05 |
| Porosity (%) | 81.34 |
| Pore Size (µm) | 0.20 |
| Fibre Diameter (nm) | 104.65 ± 65.49 |
| Source | Logworth | p-Value |
|---|---|---|
| Feed Concentration (%) × Feed Concentration (%) × Area (m2) (C2A) | 8.540 | 0.00000 |
| Feed Concentration (%) × Feed Concentration (%) (C2) | 7.603 | 0.00000 |
| Feed Concentration (%) × Flow Rate (mL/min) × Flow Rate (mL/min) (CF2) | 6.847 | 0.00000 |
| Feed Concentration (%) × Feed Concentration (%) × Flow Rate (mL/min) (C2F) | 4.668 | 0.00000 |
| Source | Logworth | p-Value |
|---|---|---|
| Feed Concentration (%) × Area (m2) (CA) | 6.850 | 0.00000 |
| Feed Concentration (%) × Feed Concentration (C2) | 4.979 | 0.00001 |
| Feed Concentration (%) × Feed Concentration (%) × Area (m2) (C2A) | 3.885 | 0.00013 |
| Feed Concentration (%) × Feed Concentration (%) × Feed Concentration (%) (C3) | 1.972 | 0.01067 |
| Feed Concentration (%) × Flow Rate (mL/min) (CF) | 1.498 | 0.03179 |
| Flow Rate (mL/min) × Area (m2) (FA) | 1.466 | 0.03423 |
| Feed Concentration (%) × Flow Rate (mL/min) × Flow Rate (mL/min) (CF2) | 1.405 | 0.03938 |
| Flow Rate (mL/min) × Flow Rate (mL/min) × Flow Rate (mL/min) (F3) | 0.866 | 0.13603 |
| Flow Rate (mL/min) × Flow Rate (mL/min) (F2) | 0.766 | 0.17120 |
| Feed Concentration (%) (C) | 0.669 | 0.21440 |
| Flow Rate (mL/min) (F) | 0.640 | 0.22928 |
| Area (m2) (A) | 0.044 | 0.90366 |
| Source | Feed (%) | Flowrate (mL/min) | Area (m2) | Predicted | Experimental | Percentage Error (%) |
|---|---|---|---|---|---|---|
| 1 | 3.5 | 248 | 0.00175 | 9.94 | 10.27 | 3.30 |
| 2 | 69 | 258.7 | 0.0009 | 1196.14 | 1161.79 | 2.87 |
| 3 | 100 | 200 | 0.0009 | 2517.17 | 2389.26 | 5.00 |
| Source | Feed (%) | Flowrate (mL/min) | Area (m2) | Predicted | Experimental | Percentage Error (%) |
|---|---|---|---|---|---|---|
| 1 | 3.5 | 248 | 0.00175 | 69.77 | 66.67 | 4.45 |
| 2 | 69 | 258.7 | 0.0009 | 48.98 | 50.56 | 3.22 |
| 3 | 100 | 200 | 0.0009 | 208 | 197.64 | 4.98 |
| Conc. Feed (%) | Flow Rate (mL/min) | Area (m2) | Average Flux (L/m2h) | Power Consumption (kWh/m3) | Con Permeate (ppm) | CAPEX (RM) | OPEX (RM/y) |
|---|---|---|---|---|---|---|---|
| 4.9 | 200 | 0.0009 | 216.5 | 0.09 | 10 | 3.743 M | 1660 |
| No | Type of Membrane | Flowrate (mL/min) | Membrane Area (m2) | Feed Conc (ppm) | Permeate Conc (ppm) | Average Flux (L/m2 h) | Specific Energy Consumption (kW/m3) | OPEX in Terms of Utilities (RM/y) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | MF | 200 | 0.0009 | 304.13 | 10 | 217 | 0.09 | 1126 | This study |
| 2 | MF | 237.6 | 0.0009 | 88.43 | 4.93 | 80 | 0.45 | 5929 | [45] |
| 3 | UF | 1000 | 0.0025 | 12,000 | 660 | 347 | 1.61 | 21,346 | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd Halim, N.S.; Mohd Hizam, S.; Wan Suhaimi, W.M.S.; Ahmad Farid, A.S.; Abd Rahman, P.N.K.; Wirzal, M.D.H.; Sambudi, N.S.; Md Nordin, N.A.H. Nylon 6,6 Waste Nanofiber Membrane for Produced Water Filtration: Experimental, Performance Modelling, Optimization and Techno-Economic Analysis. Membranes 2023, 13, 224. https://doi.org/10.3390/membranes13020224
Abd Halim NS, Mohd Hizam S, Wan Suhaimi WMS, Ahmad Farid AS, Abd Rahman PNK, Wirzal MDH, Sambudi NS, Md Nordin NAH. Nylon 6,6 Waste Nanofiber Membrane for Produced Water Filtration: Experimental, Performance Modelling, Optimization and Techno-Economic Analysis. Membranes. 2023; 13(2):224. https://doi.org/10.3390/membranes13020224
Chicago/Turabian StyleAbd Halim, Nur Syakinah, Shafiq Mohd Hizam, Wan Mohamad Syameer Wan Suhaimi, Ahmad Syahmi Ahmad Farid, Puteri Nur Khaliesah Abd Rahman, Mohd Dzul Hakim Wirzal, Nonni Soraya Sambudi, and Nik Abdul Hadi Md Nordin. 2023. "Nylon 6,6 Waste Nanofiber Membrane for Produced Water Filtration: Experimental, Performance Modelling, Optimization and Techno-Economic Analysis" Membranes 13, no. 2: 224. https://doi.org/10.3390/membranes13020224
APA StyleAbd Halim, N. S., Mohd Hizam, S., Wan Suhaimi, W. M. S., Ahmad Farid, A. S., Abd Rahman, P. N. K., Wirzal, M. D. H., Sambudi, N. S., & Md Nordin, N. A. H. (2023). Nylon 6,6 Waste Nanofiber Membrane for Produced Water Filtration: Experimental, Performance Modelling, Optimization and Techno-Economic Analysis. Membranes, 13(2), 224. https://doi.org/10.3390/membranes13020224







