Bipolar Membrane Electrodialysis for Cleaner Production of Diprotic Malic Acid: Separation Mechanism and Performance Evaluation
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. BMED Setup
2.3. Experimental Procedure
2.4. Data Analysis
3. Results and Discussion
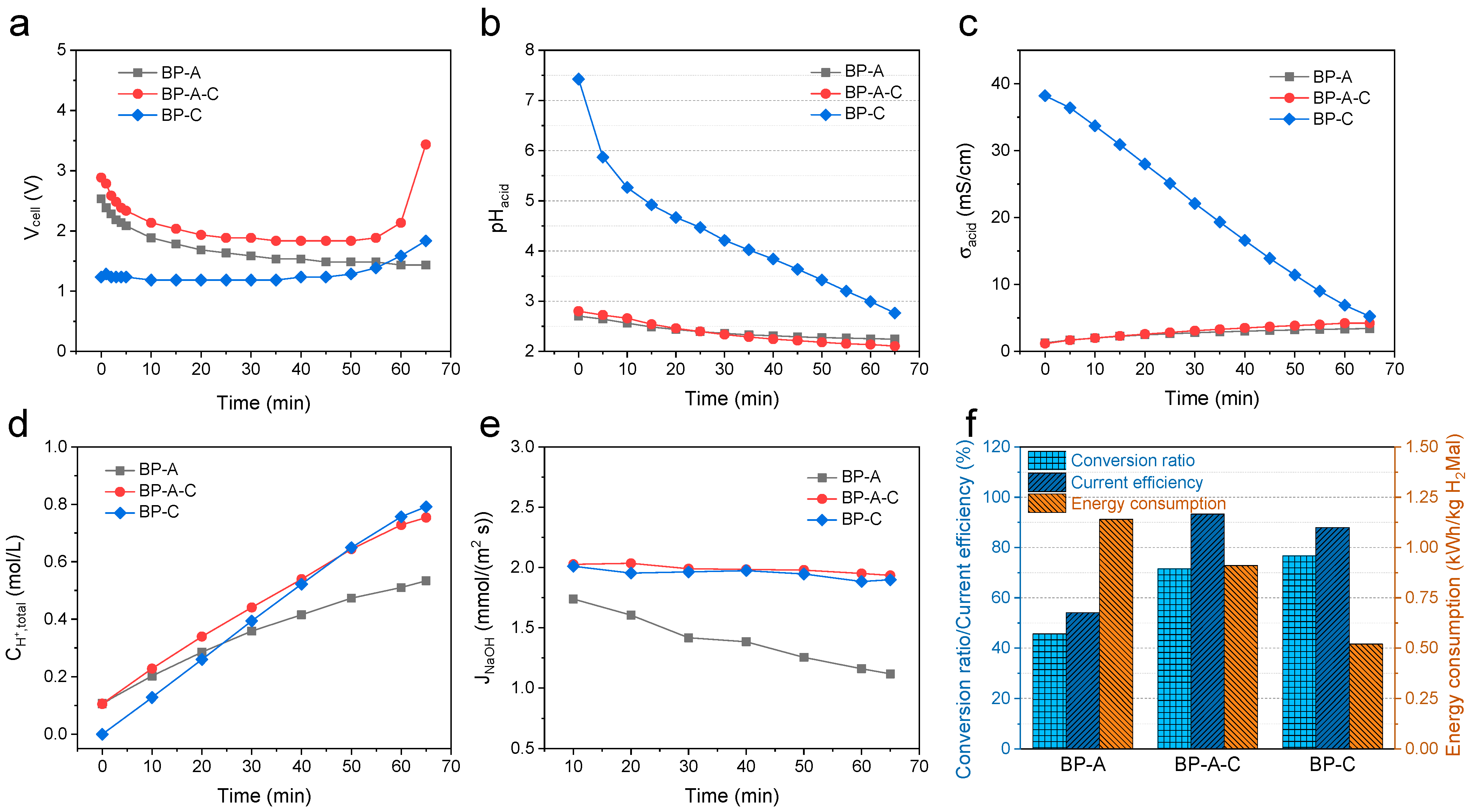
3.1. Effect of the Cell Configuration
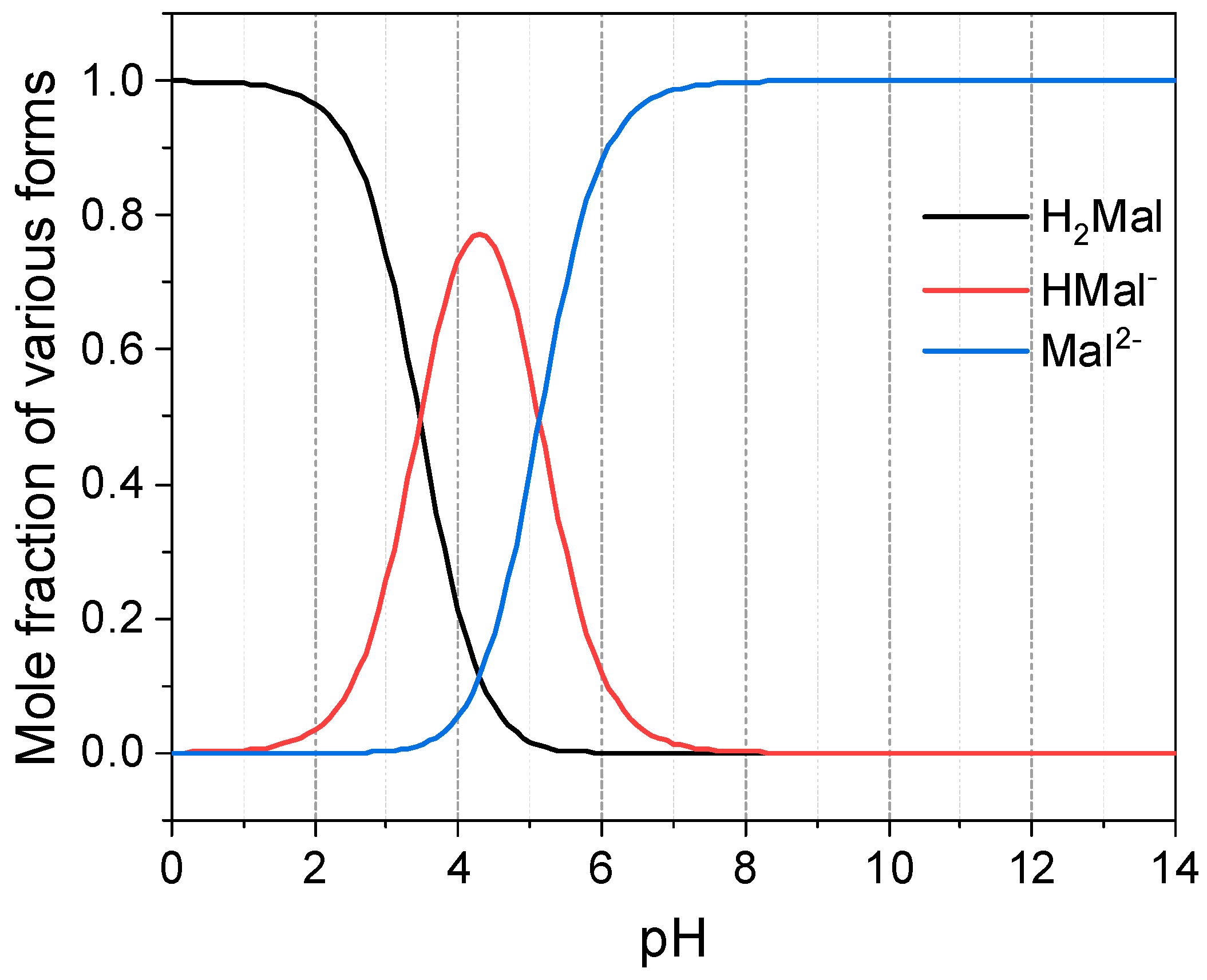
3.1.1. Separation Mechanism for Different Cell Configurations
3.1.2. Conversion Ratio, Current Efficiency and Energy Consumption
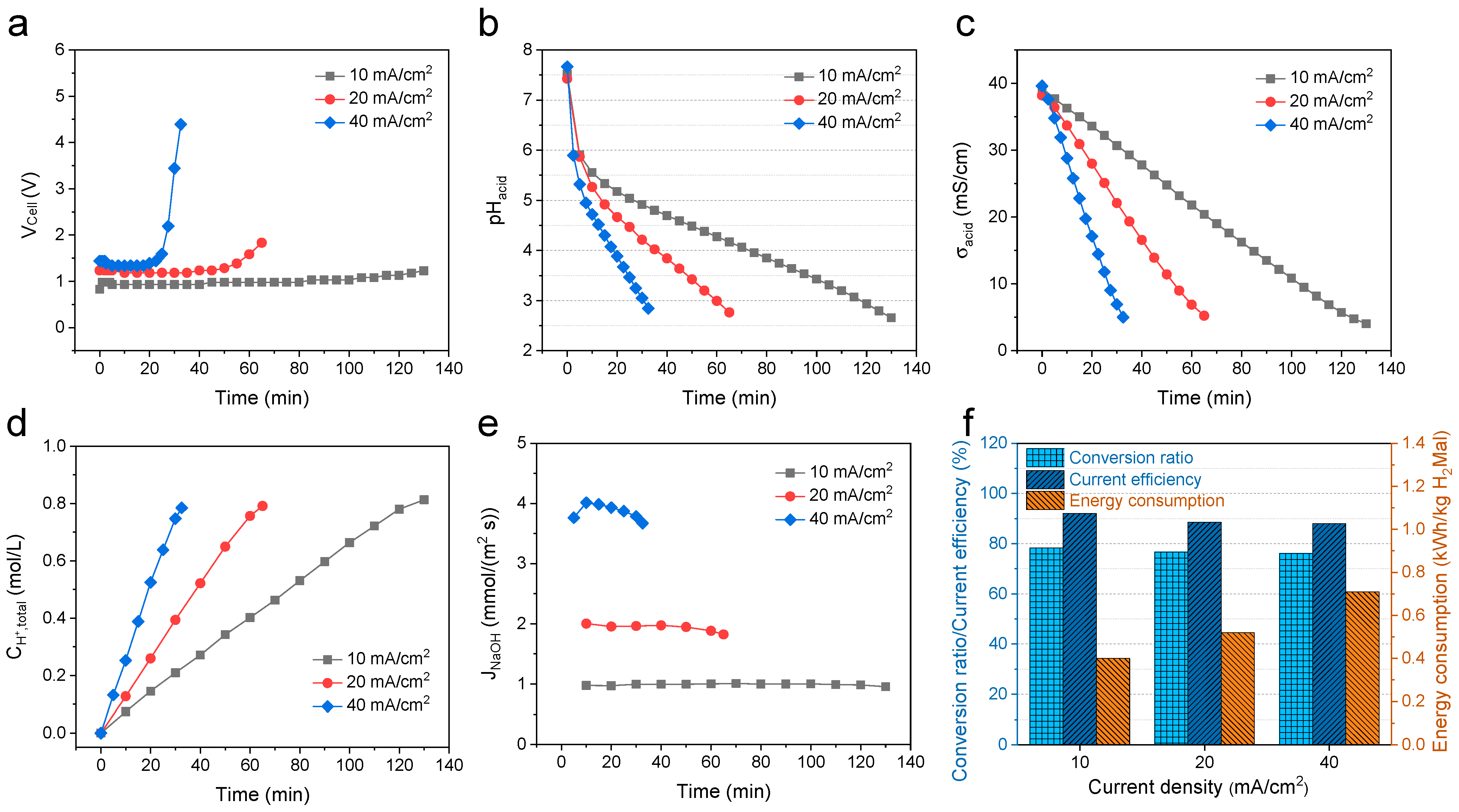
3.2. Effect of the Current Density
3.3. Effect of the Feed Concentration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chi, Z.; Wang, Z.-P.; Wang, G.-Y.; Khan, I.; Chi, Z.-M. Microbial biosynthesis and secretion of l-malic acid and its applications. Crit. Rev. Biotechnol. 2016, 36, 99–107. [Google Scholar] [CrossRef]
- Kövilein, A.; Kubisch, C.; Cai, L.; Ochsenreither, K. Malic acid production from renewables: A review. J. Chem. Technol. Biotechnol. 2020, 95, 513–526. [Google Scholar] [CrossRef]
- Lameloise, M.-L.; Matinier, H.; Fargues, C. Concentration and purification of malate ion from a beverage industry waste water using electrodialysis with homopolar membranes. J. Membr. Sci. 2009, 343, 73–81. [Google Scholar] [CrossRef]
- Lameloise, M.-L.; Lewandowski, R. Recovering l-malic acid from a beverage industry waste water: Experimental study of the conversion stage using bipolar membrane electrodialysis. J. Membr. Sci. 2012, 403–404, 196–202. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, N.; Huang, C.; Xu, T. Production of monoprotic, diprotic, and triprotic organic acids by using electrodialysis with bipolar membranes: Effect of cell configurations. J. Membr. Sci. 2011, 385–386, 226–233. [Google Scholar] [CrossRef]
- Korngold, E.; Belayev, N.; Aronov, L.; Oren, Y. Conversion of dipotassium malate to malic acid by electromembrane processes. Desalination 2006, 195, 153–159. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, C.; Xu, T. Which is more competitive for production of organic acids, ion-exchange or electrodialysis with bipolar membranes? J. Membr. Sci. 2011, 374, 150–156. [Google Scholar] [CrossRef]
- Kumar, A.; Phillips, K.R.; Cai, J.; Schröder, U.; Lienhard, J.H. Integrated Valorization of Desalination Brine through NaOH Recovery: Opportunities and Challenges. Angew. Chem. Int. Ed. 2019, 58, 6502–6511. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Yan, H.; Jiang, C.; Ge, L.; Xu, T. Bipolar membrane electrodialysis for cleaner production of N-methylated glycine derivative amino acids. AIChE J. 2020, 66, e17023. [Google Scholar] [CrossRef]
- Yan, J.; Yan, H.; Wang, H.; Li, Q.; Zhang, H.; Jiang, C.; Ye, B.; Wang, Y.; Xu, T. Bipolar membrane electrodialysis for clean production of L-10-camphorsulfonic acid: From laboratory to industrialization. AIChE J. 2022, 68, e17490. [Google Scholar] [CrossRef]
- Giesbrecht, P.K.; Freund, M.S. Recent Advances in Bipolar Membrane Design and Applications. Chem. Mater. 2020, 32, 8060–8090. [Google Scholar] [CrossRef]
- Pärnamäe, R.; Mareev, S.; Nikonenko, V.; Melnikov, S.; Sheldeshov, N.; Zabolotskii, V.; Hamelers, H.V.M.; Tedesco, M. Bipolar membranes: A review on principles, latest developments, and applications. J. Membr. Sci. 2021, 617, 118538. [Google Scholar] [CrossRef]
- Shehzad, M.A.; Yasmin, A.; Ge, X.; Ge, Z.; Zhang, K.; Liang, X.; Zhang, J.; Li, G.; Xiao, X.; Jiang, B.; et al. Shielded goethite catalyst that enables fast water dissociation in bipolar membranes. Nat. Commun. 2021, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yan, J.; Fu, R.; Yan, H.; Jiang, C.; Wang, Y.; Xu, T. Bipolar Membrane Electrodialysis for Cleaner Production of Gluconic Acid: Valorization of the Regenerated Base for the Upstream Enzyme Catalysis. Ind. Eng. Chem. Res. 2022, 61, 7634–7644. [Google Scholar] [CrossRef]
- Lei, C.; Li, Z.; Gao, Q.; Fu, R.; Wang, W.; Li, Q.; Liu, Z. Comparative study on the production of gluconic acid by electrodialysis and bipolar membrane electrodialysis: Effects of cell configurations. J. Membr. Sci. 2020, 608, 118192. [Google Scholar] [CrossRef]
- Huang, C.; Xu, T.; Zhang, Y.; Xue, Y.; Chen, G. Application of electrodialysis to the production of organic acids: State-of-the-art and recent developments. J. Membr. Sci. 2007, 288, 1–12. [Google Scholar] [CrossRef]
- Sun, X.; Lu, H.; Wang, J. Recovery of citric acid from fermented liquid by bipolar membrane electrodialysis. J. Clean. Prod. 2017, 143, 250–256. [Google Scholar] [CrossRef]
- Fu, L.; Gao, X.; Yang, Y.; Aiyong, F.; Hao, H.; Gao, C. Preparation of succinic acid using bipolar membrane electrodialysis. Sep. Purif. Technol. 2014, 127, 212–218. [Google Scholar] [CrossRef]
- Li, C.; Wang, G.; Feng, H.; He, T.; Wang, Y.; Xu, T. Cleaner production of Niacin using bipolar membranes electrodialysis (BMED). Sep. Purif. Technol. 2015, 156, 391–395. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, M.; Gao, C. Ion conductive spacers for the energy-saving production of the tartaric acid in bipolar membrane electrodialysis. J. Membr. Sci. 2012, 387–388, 48–53. [Google Scholar] [CrossRef]
- Quoc, A.L.; Mondor, M.; Lamarche, F.; Ippersiel, D.; Bazinet, L.; Makhlouf, J. Effect of a combination of electrodialysis with bipolar membranes and mild heat treatment on the browning and opalescence stability of cloudy apple juice. Food Res. Int. 2006, 39, 755–760. [Google Scholar] [CrossRef]
- Quoc, A.L.; Mondor, M.; Lamarche, F.; Makhlouf, J. Optimization of electrodialysis with bipolar membranes applied to cloudy apple juice: Minimization of malic acid and sugar losses. Innov. Food Sci. Emerg. Technol. 2011, 12, 45–49. [Google Scholar] [CrossRef]
- Liu, G.; Luo, H.; Wang, H.; Wang, B.; Zhang, R.; Chen, S. Malic acid production using a biological electrodialysis with bipolar membrane. J. Membr. Sci. 2014, 471, 179–184. [Google Scholar] [CrossRef]
- Vera, E.; Sandeaux, J.; Persin, F.; Pourcelly, G.; Dornier, M.; Ruales, J. Deacidification of clarified tropical fruit juices by electrodialysis. Part I. Influence of operating conditions on the process performances. J. Food Eng. 2007, 78, 1427–1438. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Jiang, C.; Xu, T. Recovery of gamma-aminobutyric acid (GABA) from reaction mixtures containing salt by electrodialysis. Sep. Purif. Technol. 2016, 170, 353–359. [Google Scholar] [CrossRef]
- Yan, H.; Wang, Y.; Wu, L.; Shehzad, M.A.; Jiang, C.; Fu, R.; Liu, Z.; Xu, T. Multistage-batch electrodialysis to concentrate high-salinity solutions: Process optimisation, water transport, and energy consumption. J. Membr. Sci. 2019, 570–571, 245–257. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Abraham, M.H.; Zissimos, A.M. Fast Calculation of van der Waals Volume as a Sum of Atomic and Bond Contributions and Its Application to Drug Compounds. J. Org. Chem. 2003, 68, 7368–7373. [Google Scholar] [CrossRef]
- Nightingale, E.R. Phenomenological Theory of Ion Solvation. Effective Radii of Hydrated Ions. J. Phys. Chem. 1959, 63, 1381–1387. [Google Scholar] [CrossRef]
- Sheng, F.; Wu, B.; Li, X.; Xu, T.; Shehzad, M.A.; Wang, X.; Ge, L.; Wang, H.; Xu, T. Efficient Ion Sieving in Covalent Organic Framework Membranes with Sub-2-Nanometer Channels. Adv. Mater. 2021, 33, 2104404. [Google Scholar] [CrossRef]
- Huang, C.; Xu, T.; Yang, X. Regenerating Fuel-Gas Desulfurizing Agents by Using Bipolar Membrane Electrodialysis (BMED): Effect of Molecular Structure of Alkanolamines on the Regeneration Performance. Environ. Sci. Technol. 2007, 41, 984–989. [Google Scholar] [CrossRef]
- Zhu, Y.; Yan, H.; Lu, F.; Su, Y.; Li, W.; An, J.; Wang, Y.; Xu, T. Electrodialytic concentration of landfill leachate effluent: Lab- and pilot-scale test, and assessment. Sep. Purif. Technol. 2021, 276, 119311. [Google Scholar] [CrossRef]
- Fu, R.; Wang, H.; Yan, J.; Li, R.; Jiang, C.; Wang, Y.; Xu, T. Asymmetric bipolar membrane electrodialysis for acid and base production. AIChE J. 2022, e17957. [Google Scholar] [CrossRef]
- Yan, H.; Wu, L.; Wang, Y.; Irfan, M.; Jiang, C.; Xu, T. Ammonia capture from wastewater with a high ammonia nitrogen concentration by water splitting and hollow fiber extraction. Chem. Eng. Sci. 2020, 227, 115934. [Google Scholar] [CrossRef]
- Liu, H.; She, Q. Influence of membrane structure-dependent water transport on conductivity-permselectivity trade-off and salt/water selectivity in electrodialysis: Implications for osmotic electrodialysis using porous ion exchange membranes. J. Membr. Sci. 2022, 650, 120398. [Google Scholar] [CrossRef]
- Liu, G.; Wu, D.; Chen, G.; Halim, R.; Liu, J.; Deng, H. Comparative study on tartaric acid production by two-chamber and three-chamber electro-electrodialysis. Sep. Purif. Technol. 2021, 263, 118403. [Google Scholar] [CrossRef]



| Membrane Name | Thickness (μm) | IEC (meq/g) | Water Uptake (%) | Burst Strength (MPa) | Area Resistance (Ω·cm2) | Transport Number (%) |
|---|---|---|---|---|---|---|
| a CIS | 70 | 0.90–1.10 | 20–30 | ≥0.22 | ≤4.0 | ≥95 |
| a AIS | 70 | 0.90–1.10 | 20–30 | ≥0.17 | ≤4.0 | ≥98 |
| Membrane | Thickness (μm) | Water splitting voltage (V) | Water splitting efficiency | Burst strength (MPa) | ||
| b BP-1E | 220 | 1.2 | ≥0.98 | ≥0.40 |
| Molecular Formula | Mal2− (C4H4O52−) | HMal− (C4H5O5−) | Na+ | H+ | OH− |
|---|---|---|---|---|---|
| Molecular weight (g/mol) | 132.09 | 133.09 | 22.99 | 1.00 | 17.00 |
| Van der Waals volume (Å3) | a 113.79 | a 115.11 | - | - | - |
| Bare radius (Å) | b 3.01 | b 3.02 | c 0.95 | c 0.28 | c 1.76 |
| Hydrated radius (Å) | - | - | c 3.58 | c 2.82 | c 3.00 |
| Diffusion coefficient (m2/s) | d 9.46 × 10−10 | d 9.40 × 10−10 | e 1.33 × 10−9 | f 9.31 × 10−9 | f 5.27 × 10−9 |
| Parameters | Remarks | |
|---|---|---|
| Membrane area of CIS (cm2) | 594 | 11 × 27 × 2 |
| Membrane area of BPM (cm2) | 594 | 11 × 27 × 2 |
| a Membrane price of CIS (USD/m2) | 122 | |
| b Membrane price of BPM (USD/m2) | 1350 | |
| Membrane cost (USD) | 87.44 | |
| Membrane lifetime and amortization of the peripheral equipment (year) | 3 | |
| Stack cost (USD) | 131.16 | ×1.5 membrane cost |
| Peripheral equipment cost (USD) | 196.73 | ×1.5 stack cost |
| Total investment cost (USD) | 327.89 | Stack cost + peripheral equipment cost |
| Amortization (USD/year) | 109.30 | 3 years |
| Interest (USD/year) | 26.23 | Interest rate, 8% |
| Maintenance (USD/year) | 32.79 | 10% of total investment cost |
| Total fixed cost (USD/year) | 168.32 | |
| Current Density (mA/cm2) | 10 | 20 | 40 |
|---|---|---|---|
| Energy consumption (kWh/kg) | 0.40 | 0.52 | 0.71 |
| Treatment capacity (kg/year) | 81.3 | 166.6 | 323.4 |
| Total fixed cost (USD/year) | 168.32 | 168.32 | 168.32 |
| Total fixed cost (USD/kg) | 2.07 | 1.01 | 0.52 |
| a Total process cost (USD/kg) | 2.10 | 1.05 | 0.58 |
| Feed Concentration (mol/L) | 0.25 | 0.50 | 1.00 |
|---|---|---|---|
| Energy consumption (kWh/kg) | 1.25 | 0.71 | 0.66 |
| Treatment capacity (kg/year) | 294.3 | 323.4 | 312.0 |
| Total fixed cost (USD/year) | 168.32 | 168.32 | 168.32 |
| Total fixed cost (USD/kg) | 0.57 | 0.52 | 0.54 |
| a Total process cost (USD/kg) | 0.67 | 0.58 | 0.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Zhou, R.; Dong, Z.; Yan, J.; Ma, X.; Liu, W.; Sun, L.; Li, C.; Yan, H.; Wang, Y.; et al. Bipolar Membrane Electrodialysis for Cleaner Production of Diprotic Malic Acid: Separation Mechanism and Performance Evaluation. Membranes 2023, 13, 197. https://doi.org/10.3390/membranes13020197
He J, Zhou R, Dong Z, Yan J, Ma X, Liu W, Sun L, Li C, Yan H, Wang Y, et al. Bipolar Membrane Electrodialysis for Cleaner Production of Diprotic Malic Acid: Separation Mechanism and Performance Evaluation. Membranes. 2023; 13(2):197. https://doi.org/10.3390/membranes13020197
Chicago/Turabian StyleHe, Jinfeng, Rong Zhou, Zhiguo Dong, Junying Yan, Xixi Ma, Wenlong Liu, Li Sun, Chuanrun Li, Haiyang Yan, Yaoming Wang, and et al. 2023. "Bipolar Membrane Electrodialysis for Cleaner Production of Diprotic Malic Acid: Separation Mechanism and Performance Evaluation" Membranes 13, no. 2: 197. https://doi.org/10.3390/membranes13020197
APA StyleHe, J., Zhou, R., Dong, Z., Yan, J., Ma, X., Liu, W., Sun, L., Li, C., Yan, H., Wang, Y., & Xu, T. (2023). Bipolar Membrane Electrodialysis for Cleaner Production of Diprotic Malic Acid: Separation Mechanism and Performance Evaluation. Membranes, 13(2), 197. https://doi.org/10.3390/membranes13020197









