Poly(alkyl-biphenyl pyridinium)-Based Anion Exchange Membranes with Alkyl Side Chains Enable High Anion Permselectivity and Monovalent Ion Flux
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of Poly(alkyl-biphenyl pyridine) (PAB)
2.3. Quaternization of DPAB and Preparation of Homologous Membranes
2.4. Membrane Characterizations
2.4.1. Structure Characterization
2.4.2. Ion Exchange Capacity, Water Uptake and Swelling Ratio
2.4.3. Membrane Transport Number
2.4.4. Mechanical Properties
2.4.5. Current–Voltage (I–V) Curves and Membrane Area Resistance
2.4.6. Electrodialysis Experiment
3. Results and Discussion
3.1. Chemical Structure Characterization of DPAB and QDPAB-Cx Polymers and the Homologous Membranes
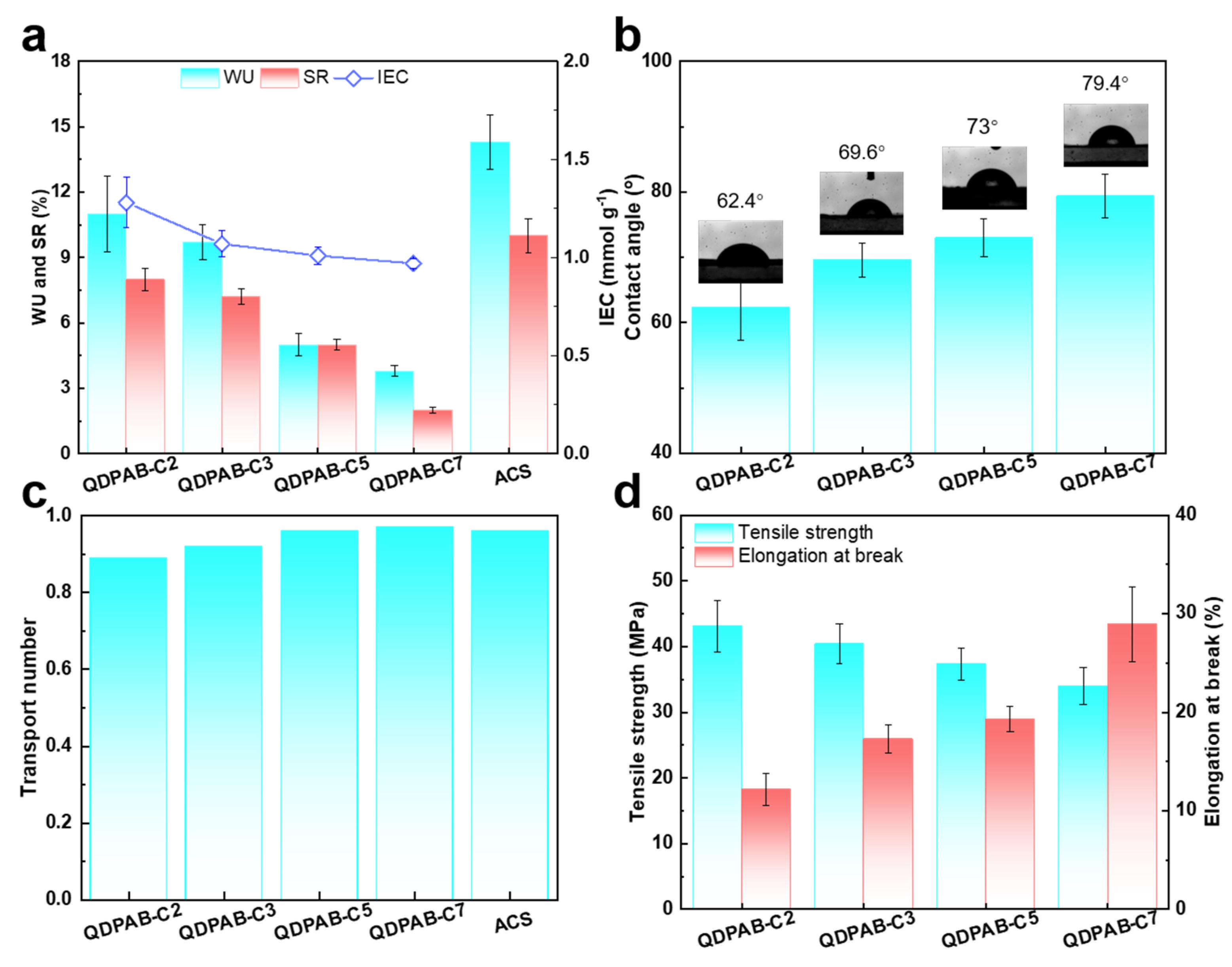
3.2. Physicochemical Properties of QDPAB-Cx Membranes
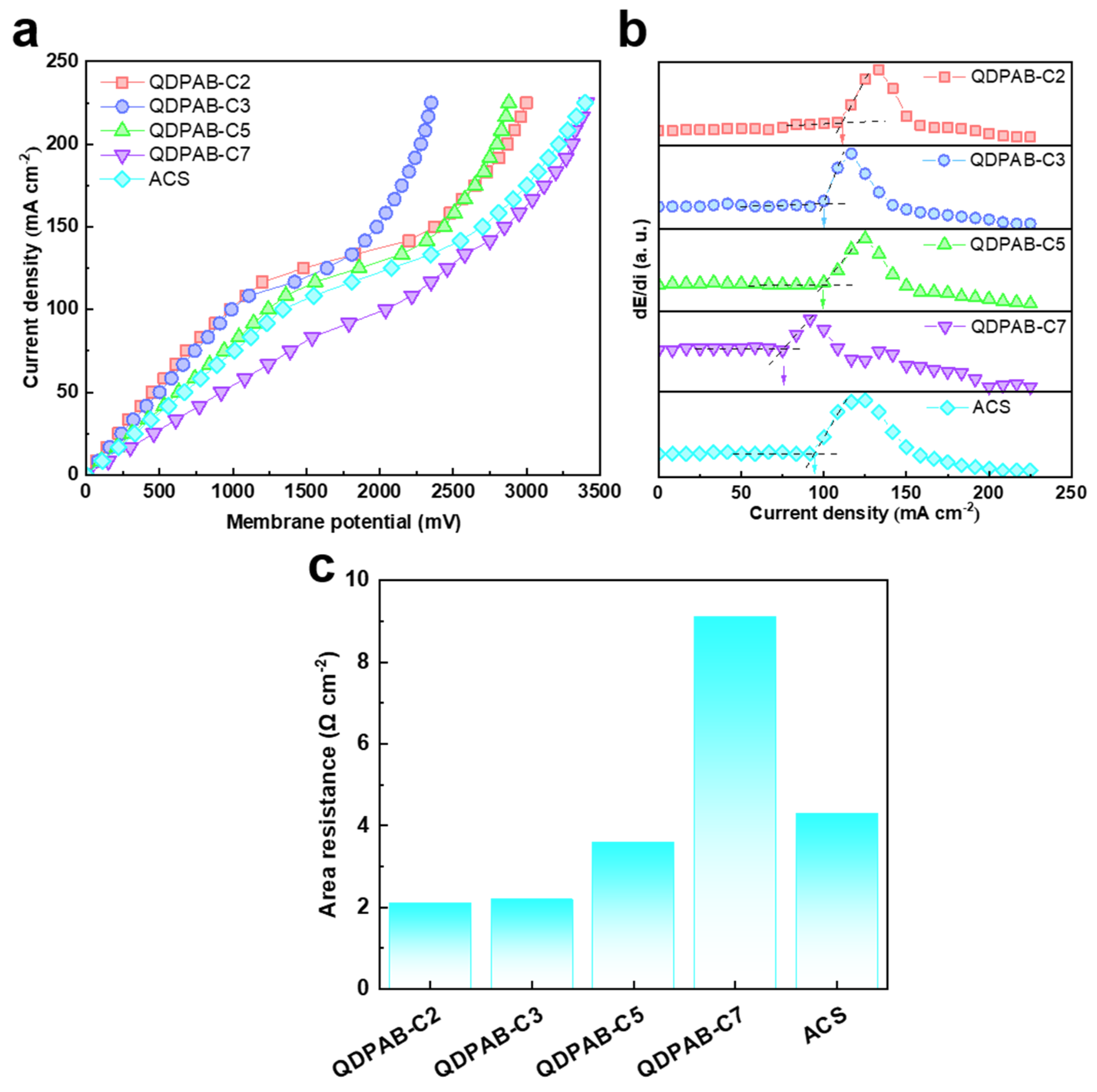
3.3. Electrochemical Properties of QDPAB-Cx Membranes
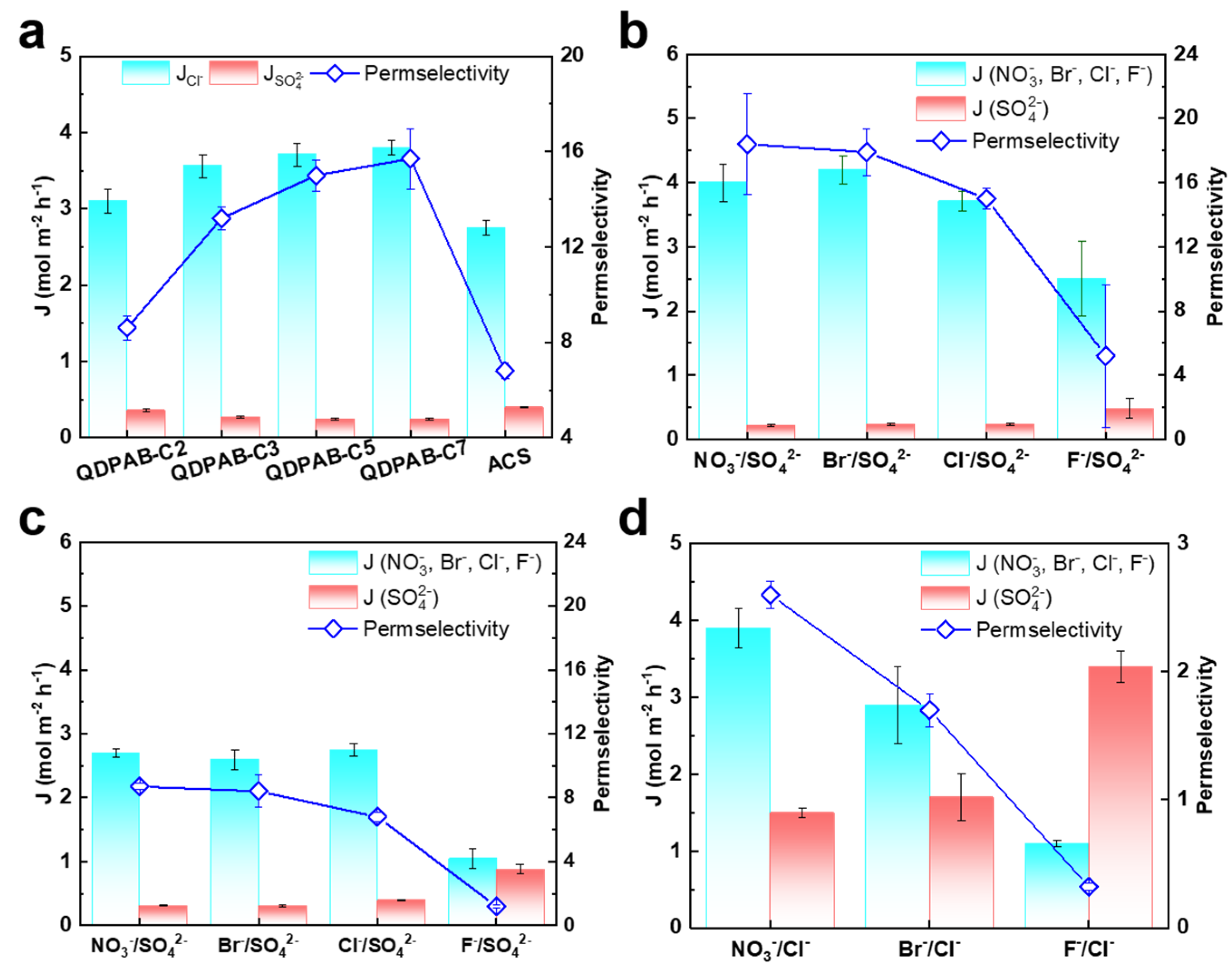
3.4. Evaluation and Comparison of Anion Permselectivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- He, Y.; Ge, L.; Ge, Z.; Zhao, Z.; Sheng, F.; Liu, X.; Ge, X.; Yang, Z.; Fu, R.; Liu, Z. Monovalent cations permselective membranes with zwitterionic side chains. J. Membr. Sci. 2018, 563, 320–325. [Google Scholar] [CrossRef]
- Tekinalp, Ö.; Zimmermann, P.; Burheim, O.S.; Deng, L. Designing monovalent selective anion exchange membranes for the simultaneous separation of chloride and fluoride from sulfate in an equimolar ternary mixture. J. Membr. Sci. 2023, 666, 121–148. [Google Scholar] [CrossRef]
- Zhao, Y.; Lu, W.; Mamrol, N.; Croes, T.; Mai, Z.; Houtmeyers, S.; Dewil, R.; Zhang, Y.; Yang, X.; Van der Bruggen, B. Self-assembled embedding of ion exchange materials into nanofiber-based hydrogel framework for fluoride capture. Chem. Eng. J. 2022, 431, 134–201. [Google Scholar] [CrossRef]
- Lan, Y.; Zhou, D.; Lai, L.; Qi, H.; Xia, L.; Depuydt, S.; Van der Bruggen, B.; Zhao, Y. A monovalent selective anion exchange membrane made by poly(2,6-dimethyl-1,4-phenyl oxide) for bromide recovery. Sep. Purif. Technol. 2023, 305, 122–377. [Google Scholar] [CrossRef]
- Wang, C.; Pan, N.; Liao, J.; Ruan, H.; Sotto, A.; Shen, J. Effect of microstructures of side-chain-type anion exchange membranes on mono-/bivalent anion permselectivity in electrodialysis. ACS Appl. Polym. Mater. 2020, 3, 342–353. [Google Scholar] [CrossRef]
- Xia, L.; Zhao, Y.; Zhang, X.; Qiu, Y.; Shao, J.; Dewil, R.; der Bruggen, B.V.; Yang, X. Ionic control of functional zeolitic imidazolate framework-based membrane for tailoring selectivity toward target ions. ACS Appl. Mater. Interfaces 2022, 14, 11038–11049. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Abdu, S.; Wessling, M. Selectivity of ion exchange membranes: A review. J. Membr. Sci. 2018, 555, 429–454. [Google Scholar] [CrossRef]
- Liu, H.; Ruan, H.; Zhao, Y.; Pan, J.; Sotto, A.; Gao, C.; van der Bruggen, B.; Shen, J. A facile avenue to modify polyelectrolyte multilayers on anion exchange membranes to enhance monovalent selectivity and durability simultaneously. J. Membr. Sci. 2017, 543, 310–318. [Google Scholar] [CrossRef]
- Jin, Y.; Zhao, Y.; Liu, H.; Sotto, A.; Gao, C.; Shen, J. A durable and antifouling monovalent selective anion exchange membrane modified by polydopamine and sulfonated reduced graphene oxide. Sep. Purif. Technol. 2018, 207, 116–123. [Google Scholar] [CrossRef]
- Zhang, H.; Ding, R.; Zhang, Y.; Shi, B.; Wang, J.; Liu, J. Stably coating loose and electronegative thin layer on anion exchange membrane for efficient and selective monovalent anion transfer. Desalination 2017, 410, 55–65. [Google Scholar] [CrossRef]
- Liao, J.; Yu, X.; Pan, N.; Li, J.; Shen, J.; Gao, C. Amphoteric ion-exchange membranes with superior mono-/bi-valent anion separation performance for electrodialysis applications. J. Membr. Sci. 2019, 577, 153–164. [Google Scholar] [CrossRef]
- Goel, P.; Bhuvanesh, E.; Mandal, P.; Shahi, V.K.; Bandyopadhyay, A.; Chattopadhyay, S. Di-quaternized graphene oxide based multi-cationic cross-linked monovalent selective anion exchange membrane for electrodialysis. Sep. Purif. Technol. 2021, 276, 119361. [Google Scholar] [CrossRef]
- Sahin, S.; Dykstra, J.E.; Zuilhof, H.; Zornitta, R.L.; de Smet, L. Modification of cation-exchange membranes with polyelectrolyte multilayers to tune ion selectivity in capacitive deionization. ACS Appl. Mater. Interfaces 2020, 12, 34746–34754. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Tao, Y.; Xu, Y.; Pan, J.; Shen, J.; Gao, C. Enhanced monovalent selectivity of cation exchange membranes via adjustable charge density on functional layers. J. Membr. Sci. 2020, 595, 117–544. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, K.; Liu, H.; Van der Bruggen, B.; Sotto Díaz, A.; Shen, J.; Gao, C. An anion exchange membrane modified by alternate electro-deposition layers with enhanced monovalent selectivity. J. Membr. Sci. 2016, 520, 262–271. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, Y.; Ding, J.; Shi, W.; Pan, J.; Gao, C.; Shen, J.; van der Bruggen, B. Surface layer modification of AEMs by infiltration and photo-cross-linking to induce monovalent selectivity. AIChE J. 2018, 64, 993–1000. [Google Scholar] [CrossRef]
- Irfan, M.; Wang, Y.; Xu, T. Novel electrodialysis membranes with hydrophobic alkyl spacers and zwitterion structure enable high monovalent/divalent cation selectivity. Chem. Eng. J. 2020, 383, 123–171. [Google Scholar] [CrossRef]
- Irfan, M.; Xu, T.; Ge, L.; Wang, Y.; Xu, T. Zwitterion structure membrane provides high monovalent/divalent cation electrodialysis selectivity: Investigating the effect of functional groups and operating parameters. J. Membr. Sci. 2019, 588, 117–211. [Google Scholar] [CrossRef]
- Zhang, H.; Hou, X.; Zeng, L.; Yang, F.; Li, L.; Yan, D.; Tian, Y.; Jiang, L. Bioinspired artificial single ion pump. J. Am. Chem. Soc. 2013, 135, 16102–16110. [Google Scholar] [CrossRef]
- Morth, J.P.; Pedersen, B.P.; Buch-Pedersen, M.J.; Andersen, J.P.; Vilsen, B.; Palmgren, M.G.; Nissen, P. A structural overview of the plasma membrane Na+, K+-ATPase and H+-ATPase ion pumps. Nat. Rev. Mol. Cell Biol. 2011, 12, 60–70. [Google Scholar] [CrossRef]
- Sata, T. Studies on anion exchange membranes having permselectivity for specific anions in electrodialysis—Effect of hydrophilicity of anion exchange membranes on permselectivity of anions. J. Membr. Sci. 2000, 167, 1–31. [Google Scholar] [CrossRef]
- Mubita, T.; Porada, S.; Aerts, P.; van der Wal, A. Heterogeneous anion exchange membranes with nitrate selectivity and low electrical resistance. J. Membr. Sci. 2020, 607, 118000. [Google Scholar] [CrossRef]
- Ge, Q.; Ran, J.; Miao, J.; Yang, Z.; Xu, T. Click chemistry finds its way in constructing an ionic highway in anion-exchange membrane. ACS Appl. Mater. Interfaces 2015, 7, 28545–28553. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhou, J.; Wang, S.; Hou, J.; Wu, L.; Xu, T. A strategy to construct alkali-stable anion exchange membranes bearing ammonium groups via flexible spacers. J. Mater. Chem. A 2015, 3, 15015–15019. [Google Scholar] [CrossRef]
- Li, M.; Li, W.; Zhang, X.; Wu, C.; Han, X.; Chen, Y. Polyvinyl alcohol-based monovalent anion selective membranes with excellent permselectivity in selectrodialysis. J. Membr. Sci. 2021, 620, 118–889. [Google Scholar] [CrossRef]
- Sang, J.; Yang, L.; Li, Z.; Wang, F.; Wang, Z.; Zhu, H. Comb-shaped SEBS-based anion exchange membranes with obvious microphase separation morphology. Electrochim. Acta 2022, 403, 139–500. [Google Scholar] [CrossRef]
- Ran, J.; Fu, C.; Ding, L.; Cao, P.; Xu, T. Dual hydrophobic grafted chains facilitating quaternary ammonium aggregations of hydroxide conducting polymers: A theoretical and experimental investigation. J. Mater. Chem. A 2018, 6, 5714–5723. [Google Scholar] [CrossRef]
- Liao, J.; Zhu, J.; Yang, S.; Pan, N.; Yu, X.; Wang, C.; Li, J.; Shen, J. Long-side-chain type imidazolium-functionalized fluoro-methyl poly(arylene ether ketone) anion exchange membranes with superior electrodialysis performance. J. Membr. Sci. 2019, 574, 181–195. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Wu, C.; Han, X.; Xu, C. Anion exchange membranes with excellent monovalent anion perm-selectivity for electrodialysis applications. Chem. Eng. Res. Des. 2020, 158, 24–32. [Google Scholar] [CrossRef]
- Irfan, M.; Ge, L.; Wang, Y.; Yang, Z.; Xu, T. Hydrophobic side chains impart anion exchange membranes with high monovalent–divalent anion selectivity in electrodialysis. ACS Sustain. Chem. Eng. 2019, 7, 4429–4442. [Google Scholar] [CrossRef]
- Wei, H.; Li, Y.; Wang, S.; Tao, G.; Wang, T.; Cheng, S.; Yang, S.; Ding, Y. Side-chain-type imidazolium-functionalized anion exchange membranes: The effects of additional hydrophobic side chains and their hydrophobicity. J. Membr. Sci. 2019, 579, 219–229. [Google Scholar] [CrossRef]
- Cetina-Mancilla, E.; Olvera, L.I.; Balmaseda, J.; Forster, M.; Ruiz-Treviño, F.A.; Cárdenas, J.; Vivaldo-Lima, E.; Zolotukhin, M.G. Well-defined, linear, wholly aromatic polymers with controlled content and position of pyridine moieties in macromolecules from one-pot, room temperature, metal-free step-polymerizations. Polym. Chem. 2020, 11, 6194–6205. [Google Scholar] [CrossRef]
- Li, C.; Wang, G.; Yu, D.; Sheng, F.; Shehzad, M.A.; He, T.; Xu, T.; Ren, X.; Cao, M.; Wu, B. Cross-linked anion exchange membranes with hydrophobic side-chains for anion separation. J. Membr. Sci. 2019, 581, 150–157. [Google Scholar] [CrossRef]
- Nightingale, E. Phenomenological theory of ion solvation. Effective radii of hydrated ions. Biochim. Biophys. Acta 1959, 63, 566–567. [Google Scholar] [CrossRef]

| Anion Species | ||
|---|---|---|
| I− | 257 | 3.31 |
| NO3− | 270 | 3.35 |
| Br− | 303 | 3.30 |
| Cl− | 317 | 3.32 |
| F− | 434 | 3.52 |
| SO42− | 1000 | 3.79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Chen, Q.; Afsar, N.U.; Ge, L.; Xu, T. Poly(alkyl-biphenyl pyridinium)-Based Anion Exchange Membranes with Alkyl Side Chains Enable High Anion Permselectivity and Monovalent Ion Flux. Membranes 2023, 13, 188. https://doi.org/10.3390/membranes13020188
Yang J, Chen Q, Afsar NU, Ge L, Xu T. Poly(alkyl-biphenyl pyridinium)-Based Anion Exchange Membranes with Alkyl Side Chains Enable High Anion Permselectivity and Monovalent Ion Flux. Membranes. 2023; 13(2):188. https://doi.org/10.3390/membranes13020188
Chicago/Turabian StyleYang, Jin, Qian Chen, Noor Ul Afsar, Liang Ge, and Tongwen Xu. 2023. "Poly(alkyl-biphenyl pyridinium)-Based Anion Exchange Membranes with Alkyl Side Chains Enable High Anion Permselectivity and Monovalent Ion Flux" Membranes 13, no. 2: 188. https://doi.org/10.3390/membranes13020188
APA StyleYang, J., Chen, Q., Afsar, N. U., Ge, L., & Xu, T. (2023). Poly(alkyl-biphenyl pyridinium)-Based Anion Exchange Membranes with Alkyl Side Chains Enable High Anion Permselectivity and Monovalent Ion Flux. Membranes, 13(2), 188. https://doi.org/10.3390/membranes13020188








