Recent Advances in Bioelectrochemical Systems for Nitrogen and Phosphorus Recovery Using Membranes
Abstract
1. Introduction
2. Methodology
3. Principles of Nutrient Recovery in BESs Using Membranes
3.1. Ammonia Recovery by Stripping and Absorption
3.2. Nutrient Recovery by Precipitation
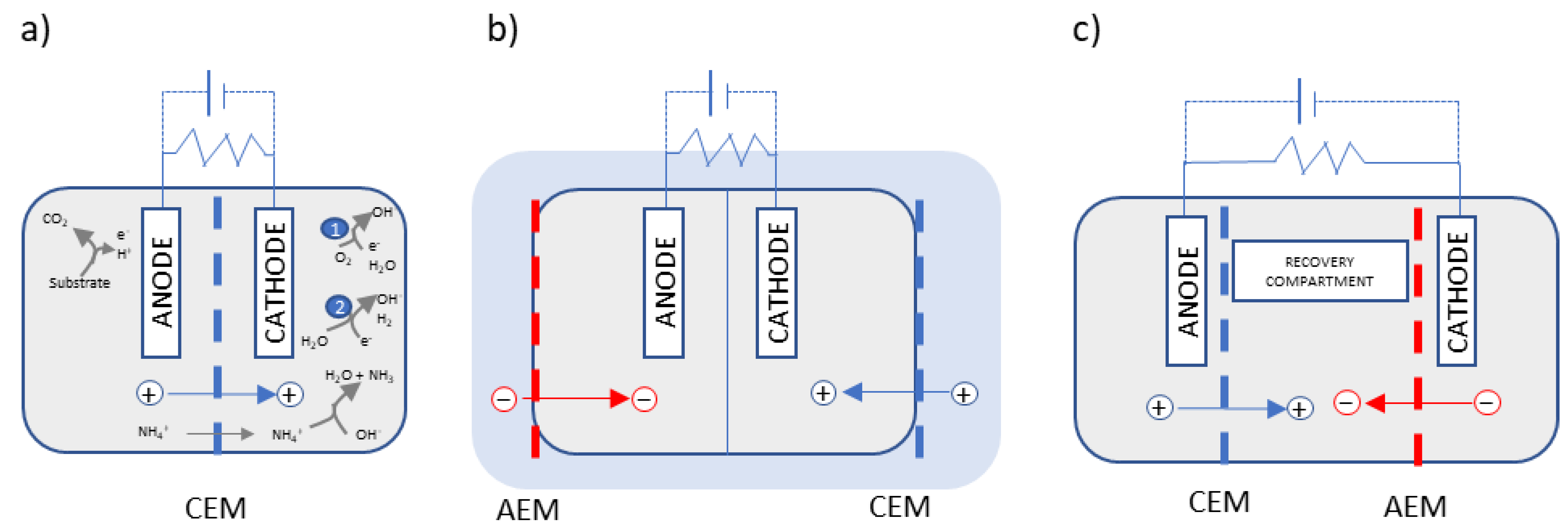
4. Nitrogen Recovery Using Membranes in BESs
4.1. Two-Chambered BESs Equipped with CEM for Ammonia Recovery
4.2. Dual-Chambered BESs Combined with Hydrophobic Membrane Modules
4.3. BESs with Three or More Chambers for Ammonia Recovery
| Configuration (Number of Chambers) | Membrane | Anode Volume (mL) | Cathode Volume (mL) | Membrane Surface (cm2) | Anolyte | Catholyte | Maximum Current Density (A m−2) | N Recovery Rate (gNH4+-N m−2 d−1) | Energy Demand (kWh kgN−1) | Combined with | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MFC (3) | PEM | 100 | 100 | n.r. | Synthetic | Fe(NO3)3 solution | n.r. | n.r. | n.r. | NO3- recovery | [46] |
| MEC (3) | CEM AEM | 35 | 35 | 35 | Synthetic | 4.8 | 38 | n.r. | [48] | ||
| MEC (3) | CEM AEM | 35 | 35 | 35 | Reject water | 4.0 | 23 | 6.1–8.2 | [48] | ||
| MEC (3) | CEM | 600 | 600 | 20 | Digested pig slurry | NaCl solution | 1.4 | 3.4 | n.r. | HM | [45] |
| MEC (3) | PEM AEM | 860 | 860 | 289 | Synthetic | Synthetic | 3.01 c | 1.3 c | n.r. | CH4 production | [47] |
| MEC (3) | PEM AEM | 860 | 860 | 289 | Synthetic | Synthetic | 5.02 c | 2.2 c | n.r. | CH4 production | [49] |
| MEC (3) | CEM AEM | 860 | 860 | 289 | Synthetic | Synthetic | 6.82 c | 19.7 c | 19.66 | CH4 production | [50] |
| MEC (3) | CEM AEM | 860 | 860 | 289 | Synthetic | Synthetic | 6.92 c | 18.2 c | 30.62 | CH4 production | [50] |
| MEC (3) | CEM AEM | 860 | 860 | 289 | Synthetic | Synthetic | 10.4 c | 24.8 c | 27.46 | CH4 production | [50] |
| Tubular MDC (3) | CEM AEM | 280 | 1600 | n.r. | Synthetic | Deionised water | n.r. | n.r. | n.r. | [24] | |
| MEC (5) | BPM, AEM, CEM, HM | 120 | 60 | 16 | Synthetic | Synthetic | 2.5 c | 22 | 2.91 | Acid-base production | [51] |
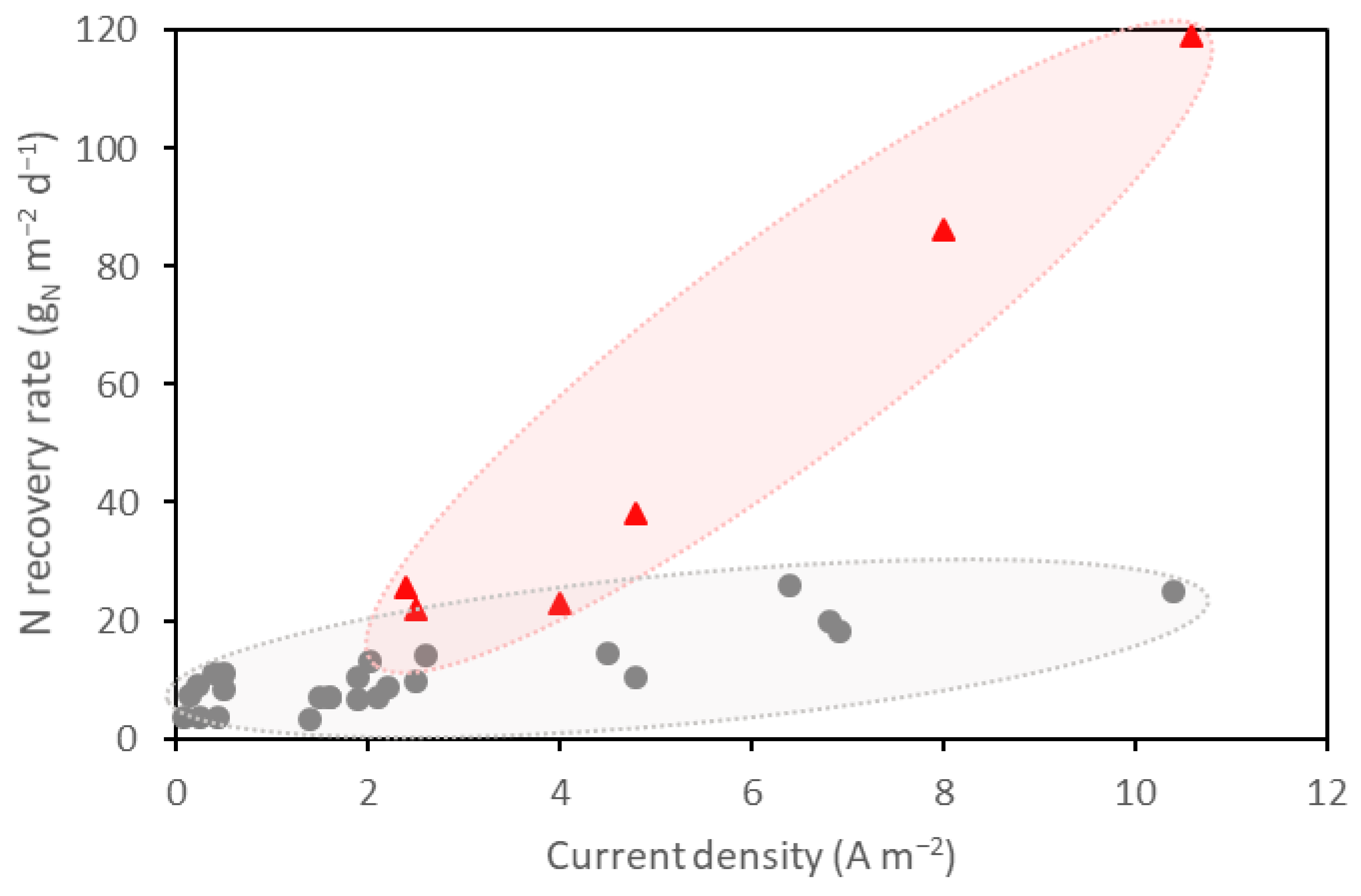
4.4. Overview of the Relationship between Nitrogen Removal Rates and Current Density
5. Concurrent Nitrogen and Phosphorus Recovery Using Membranes in BES
5.1. Dual-Chambered Cells for Nitrogen and Phosphorus Recovery
5.2. Three-Chambered Cells for Nitrogen and Phosphorus Recovery
5.3. Membrane Stack Configuration Cells for Nitrogen and Phosphorus Recovery
6. Electricity Consumption of Nutrient Recovery Using Membrane-BES
7. Challenges and Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Anaerobic digester |
| AEM | Anion-exchange membrane |
| AMNR | Advanced microbial nutrient recovery cell |
| BEC | Bioelectroconcentration cell |
| BES | Bioelectrochemical system |
| BEMAA | Bioelectrochemical membrane-absorbed ammonia system |
| BPM | Bipolar membrane |
| CEM | Cation-exchange membrane |
| COD | Chemical oxygen demand |
| EM | Electron mediator |
| FO | Forward osmosis |
| HM | Hydrophobic membranes |
| IEM | Ion-exchange membranes |
| MEC | Microbial electrolysis cell |
| MFC | Microbial fuel cell |
| MNRC | Microbial nutrient recovery cell |
| MRESC | Microbial reverse-electrodialysis electrolysis cell |
| OsBCRS | Forward osmosis integrated bioelectroconcentration and recovery system |
| PBS | Phosphate buffer solution |
| PEM | Proton-exchange membrane |
| PNRC | Photomicrobial nutrient recovery cell |
| PP | Polypropylene |
| PTFE | Polytetrafluoroethylene |
| PVDF | Polyvinylidene fluoride |
| RRMFC | Resource recovery microbial fuel cell |
| SMNRC | Stacked microbial nutrient recovery cell |
| WW | Wastewater |
| WWTPs | Wastewater treatment plant |
References
- Barbosa, S.G.; Rodrigues, T.; Peixoto, L.; Kuntke, P.; Alves, M.M.; Pereira, M.A.; Ter Heijne, A. Anaerobic biological fermentation of urine as a strategy to enhance the performance of a microbial electrolysis cell (MEC). Renew. Energy 2019, 139, 936–943. [Google Scholar] [CrossRef]
- Cerrillo, M.; Viñas, M.; Bonmatí, A. Overcoming organic and nitrogen overload in thermophilic anaerobic digestion of pig slurry by coupling a microbial electrolysis cell. Bioresour. Technol. 2016, 216, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Cerrillo, M.; Viñas, M.; Bonmatí, A. Unravelling the active microbial community in a thermophilic anaerobic digester-microbial electrolysis cell coupled system under different conditions. Water Res. 2017, 110, 192–201. [Google Scholar] [CrossRef]
- Hassanein, A.; Witarsa, F.; Guo, X.; Yong, L.; Lansing, S.; Qiu, L. Next generation digestion: Complementing anaerobic digestion (AD) with a novel microbial electrolysis cell (MEC) design. Int. J. Hydrogen Energy 2017, 42, 28681–28689. [Google Scholar] [CrossRef]
- Kumar, S.S.; Kumar, V.; Malyan, S.K.; Sharma, J.; Mathimani, T.; Maskarenj, M.S.; Ghosh, P.C.; Pugazhendhi, A. Microbial fuel cells (MFCs) for bioelectrochemical treatment of different wastewater streams. Fuel 2019, 254, 115526. [Google Scholar] [CrossRef]
- Vanotti, M.B.; Szogi, A.A.; Millner, P.D.; Loughrin, J.H. Development of a second-generation environmentally superior technology for treatment of swine manure in the USA. Bioresour. Technol. 2009, 100, 5406–5416. [Google Scholar] [CrossRef]
- Wang, Y.; Meyer, T.J. A Route to Renewable Energy Triggered by the Haber-Bosch Process. Chem 2019, 5, 496–497. [Google Scholar] [CrossRef]
- Li, B.; Boiarkina, I.; Young, B.; Yu, W.; Singhal, N. Prediction of Future Phosphate Rock: A Demand Based Model. J. Environ. Inform. 2018, 31, 41–53. [Google Scholar] [CrossRef]
- Al-Sahari, M.; Al-Gheethi, A.; Radin Mohamed, R.M.S.; Noman, E.; Naushad, M.; Rizuan, M.B.; Vo, D.-V.N.; Ismail, N. Green approach and strategies for wastewater treatment using bioelectrochemical systems: A critical review of fundamental concepts, applications, mechanism, and future trends. Chemosphere 2021, 285, 131373. [Google Scholar] [CrossRef]
- Bajracharya, S.; Sharma, M.; Mohanakrishna, G.; Dominguez Benneton, X.; Strik, D.P.B.T.B.; Sarma, P.M.; Pant, D. An overview on emerging bioelectrochemical systems (BESs): Technology for sustainable electricity, waste remediation, resource recovery, chemical production and beyond. Renew. Energy 2016, 98, 153–170. [Google Scholar] [CrossRef]
- Kelly, P.T.; He, Z. Nutrients removal and recovery in bioelectrochemical systems: A review. Bioresour. Technol. 2014, 153, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wan, Y.; Wang, X. Nutrient conversion and recovery from wastewater using electroactive bacteria. Sci. Total Environ. 2020, 706, 135690. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Arredondo, M.; Kuntke, P.; Jeremiasse, A.W.; Sleutels, T.H.J.A.; Buisman, C.J.N.; ter Heijne, A. Bioelectrochemical systems for nitrogen removal and recovery from wastewater. Environ. Sci. Water Res. Technol. 2015, 1, 22–33. [Google Scholar] [CrossRef]
- Kuntke, P.; Sleutels, T.H.J.A.; Rodríguez Arredondo, M.; Georg, S.; Barbosa, S.G.; ter Heijne, A.; Hamelers, H.V.M.; Buisman, C.J.N. (Bio)electrochemical ammonia recovery: Progress and perspectives. Appl. Microbiol. Biotechnol. 2018, 102, 3865–3878. [Google Scholar] [CrossRef]
- Yang, K.; Qin, M. The Application of Cation Exchange Membranes in Electrochemical Systems for Ammonia Recovery from Wastewater. Membranes 2021, 11, 494. [Google Scholar] [CrossRef] [PubMed]
- Zeppilli, M.; Paiano, P.; Torres, C.; Pant, D. A critical evaluation of the pH split and associated effects in bioelectrochemical processes. Chem. Eng. J. 2021, 422, 130155. [Google Scholar] [CrossRef]
- Bakonyi, P.; Koók, L.; Kumar, G.; Tóth, G.; Rózsenberszki, T.; Nguyen, D.D.; Chang, S.W.; Zhen, G.; Bélafi-Bakó, K.; Nemestóthy, N. Architectural engineering of bioelectrochemical systems from the perspective of polymeric membrane separators: A comprehensive update on recent progress and future prospects. J. Memb. Sci. 2018, 564, 508–522. [Google Scholar] [CrossRef]
- Daud, S.M.; Kim, B.H.; Ghasemi, M.; Daud, W.R.W. Separators used in microbial electrochemical technologies: Current status and future prospects. Bioresour. Technol. 2015, 195, 170–179. [Google Scholar] [CrossRef]
- San-Martín, M.I.; Carmona, F.J.; Alonso, R.M.; Prádanos, P.; Morán, A.; Escapa, A. Assessing the ageing process of cation exchange membranes in bioelectrochemical systems. Int. J. Hydrogen Energy 2019, 44, 25287–25296. [Google Scholar] [CrossRef]
- Happe, M.; Sugnaux, M.; Cachelin, C.P.; Stauffer, M.; Zufferey, G.; Kahoun, T.; Salamin, P.-A.; Egli, T.; Comninellis, C.; Grogg, A.-F.; et al. Scale-up of phosphate remobilization from sewage sludge in a microbial fuel cell. Bioresour. Technol. 2016, 200, 435–443. [Google Scholar] [CrossRef]
- Blatter, M.; Vermeille, M.; Furrer, C.; Pouget, G.; Fischer, F. Mechanisms and Model Process Parameters in Bioelectrochemical Wet Phosphate Recovery from Iron Phosphate Sewage Sludge. ACS Sustain. Chem. Eng. 2019, 7, 5856–5866. [Google Scholar] [CrossRef]
- Zhang, Y.; Angelidaki, I. Counteracting ammonia inhibition during anaerobic digestion by recovery using submersible microbial desalination cell. Biotechnol. Bioeng. 2015, 112, 1478–1482. [Google Scholar] [CrossRef] [PubMed]
- Cerrillo, M.; Oliveras, J.; Viñas, M.; Bonmatí, A. Comparative assessment of raw and digested pig slurry treatment in bioelectrochemical systems. Bioelectrochemistry 2016, 110, 69–78. [Google Scholar] [CrossRef]
- Jing, H.-P.; Li, Y.; Wang, X.; Zhao, J.; Xia, S. Simultaneous recovery of phosphate, ammonium and humic acid from wastewater using a biochar supported Mg(OH) 2 /bentonite composite. Environ. Sci. Water Res. Technol. 2019, 5, 931–943. [Google Scholar] [CrossRef]
- Sotres, A.; Cerrillo, M.; Viñas, M.; Bonmatí, A. Nitrogen recovery from pig slurry in a two-chambered bioelectrochemical system. Bioresour. Technol. 2015, 194, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Cerrillo, M.; Viñas, M.; Bonmatí, A. Removal of volatile fatty acids and ammonia recovery from unstable anaerobic digesters with a microbial electrolysis cell. Bioresour. Technol. 2016, 219, 348–356. [Google Scholar] [CrossRef]
- Cerrillo, M.; Viñas, M.; Bonmatí, A. Microbial fuel cells for polishing effluents of anaerobic digesters under inhibition, due to organic and nitrogen overloads. J. Chem. Technol. Biotechnol. 2017, 92, 2912–2920. [Google Scholar] [CrossRef]
- Barua, S.; Zakaria, B.S.; Chung, T.; Hai, F.I.; Haile, T.; Al-Mamun, A.; Dhar, B.R. Microbial electrolysis followed by chemical precipitation for effective nutrients recovery from digested sludge centrate in WWTPs. Chem. Eng. J. 2019, 361, 256–265. [Google Scholar] [CrossRef]
- Liu, F.; Worland, A.; Tang, Y.; Moustafa, H.; Hassouna, M.S.E.-D.; He, Z. Microbial electrochemical ammonia recovery from anaerobic digester centrate and subsequent application to fertilize Arabidopsis thaliana. Water Res. 2022, 220, 118667. [Google Scholar] [CrossRef]
- Cerrillo, M.; Viñas, M.; Bonmatí, A. Anaerobic digestion and electromethanogenic microbial electrolysis cell integrated system: Increased stability and recovery of ammonia and methane. Renew. Energy 2018, 120, 178–189. [Google Scholar] [CrossRef]
- Zeppilli, M.; Pavesi, D.; Gottardo, M.; Micolucci, F.; Villano, M.; Majone, M. Using effluents from two-phase anaerobic digestion to feed a methane-producing microbial electrolysis. Chem. Eng. J. 2017, 328, 428–433. [Google Scholar] [CrossRef]
- Losantos, D.; Aliaguilla, M.; Molognoni, D.; González, M.; Bosch-Jimenez, P.; Sanchis, S.; Guisasola, A.; Borràs, E. Development and optimization of a bioelectrochemical system for ammonium recovery from wastewater as fertilizer. Clean. Eng. Technol. 2021, 4, 100142. [Google Scholar] [CrossRef]
- Qin, M.; White, C.; Zou, S.; He, Z. Passive separation of recovered ammonia from catholyte for reduced energy consumption in microbial electrolysis cells. Chem. Eng. J. 2018, 334, 2303–2307. [Google Scholar] [CrossRef]
- Zhang, D.-X.; Zhai, S.-Y.; Zeng, R.; Liu, C.-Y.; Zhang, B.; Yu, Z.; Yang, L.-H.; Li, X.-Q.; Hou, Y.-N.; Wang, A.-J.; et al. A tartrate-EDTA-Fe complex mediates electron transfer and enhances ammonia recovery in a bioelectrochemical-stripping system. Environ. Sci. Ecotechnology 2022, 11, 100186. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Qin, M.; Moreau, Y.; He, Z. Nutrient-energy-water recovery from synthetic sidestream centrate using a microbial electrolysis cell - forward osmosis hybrid system. J. Clean. Prod. 2017, 154, 16–25. [Google Scholar] [CrossRef]
- Qin, M.; Molitor, H.; Brazil, B.; Novak, J.T.; He, Z. Recovery of nitrogen and water from landfill leachate by a microbial electrolysis cell–forward osmosis system. Bioresour. Technol. 2016, 200, 485–492. [Google Scholar] [CrossRef] [PubMed]
- San-Martín, M.I.; Mateos, R.; Escapa, A.; Morán, A. Understanding nitrogen recovery from wastewater with a high nitrogen concentration using microbial electrolysis cells. J. Environ. Sci. Heal. Part A 2019, 54, 472–477. [Google Scholar] [CrossRef]
- Cristiani, L.; Zeppilli, M.; Porcu, C.; Majone, M. Mauro Majone Ammonium Recovery and Biogas Upgrading in a Tubular Micro-Pilot Microbial Electrolysis Cell (MEC). Molecules 2020, 25, 2723. [Google Scholar] [CrossRef]
- Blatter, M.; Furrer, C.; Cachelin, C.P.; Fischer, F. Phosphorus, chemical base and other renewables from wastewater with three 168-L microbial electrolysis cells and other unit operations. Chem. Eng. J. 2020, 390, 124502. [Google Scholar] [CrossRef]
- Kuntke, P.; Zamora, P.; Saakes, M.; Buisman, C.J.N.; Hamelers, H.V.M. Gas-permeable hydrophobic tubular membranes for ammonia recovery in bio-electrochemical systems. Environ. Sci. Water Res. Technol. 2016, 2, 261–265. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, M.; Luo, S.; He, Z.; Qiao, R. Understanding ammonium transport in bioelectrochemical systems towards its recovery. Sci. Rep. 2016, 6, 22547. [Google Scholar] [CrossRef]
- Rodríguez Arredondo, M.; Kuntke, P.; ter Heijne, A.; Buisman, C.J.N. The concept of load ratio applied to bioelectrochemical systems for ammonia recovery. J. Chem. Technol. Biotechnol. 2019, 94, 2055–2061. [Google Scholar] [CrossRef]
- Zamora, P.; Georgieva, T.; Ter Heijne, A.; Sleutels, T.H.J.A.; Jeremiasse, A.W.; Saakes, M.; Buisman, C.J.N.; Kuntke, P. Ammonia recovery from urine in a scaled-up Microbial Electrolysis Cell. J. Power Sources 2017, 356, 491–499. [Google Scholar] [CrossRef]
- Cerrillo, M.; Burgos, L.; Bonmatí, A. Biogas upgrading and ammonia recovery from livestock manure digestates in a combined electromethanogenic biocathode—Hydrophobic membrane system. Energies 2021, 14, 503. [Google Scholar] [CrossRef]
- Cerrillo, M.; Burgos, L.; Serrano-Finetti, E.; Riau, V.; Noguerol, J.; Bonmatí, A. Hydrophobic membranes for ammonia recovery from digestates in microbial electrolysis cells: Assessment of different configurations. J. Environ. Chem. Eng. 2021, 9, 105289. [Google Scholar] [CrossRef]
- Xiao, Y.; Zheng, Y.; Wu, S.; Yang, Z.-H.; Zhao, F. Nitrogen recovery from wastewater using microbial fuel cells. Front. Environ. Sci. Eng. 2016, 10, 185–191. [Google Scholar] [CrossRef]
- Zeppilli, M.; Mattia, A.; Villano, M.; Majone, M. Three-chamber Bioelectrochemical System for Biogas Upgrading and Nutrient Recovery. Fuel Cells 2017, 17, 593–600. [Google Scholar] [CrossRef]
- Koskue, V.; Rinta-Kanto, J.M.; Freguia, S.; Ledezma, P.; Kokko, M. Optimising nitrogen recovery from reject water in a 3-chamber bioelectroconcentration cell. Sep. Purif. Technol. 2021, 264, 118428. [Google Scholar] [CrossRef]
- Zeppilli, M.; Simoni, M.; Paiano, P.; Majone, M. Two-side cathode microbial electrolysis cell for nutrients recovery and biogas upgrading. Chem. Eng. J. 2019, 370, 466–476. [Google Scholar] [CrossRef]
- Zeppilli, M.; Cristiani, L.; Dell’Armi, E.; Villano, M. Potentiostatic vs galvanostatic operation of a Microbial Electrolysis Cell for ammonium recovery and biogas upgrading. Biochem. Eng. J. 2021, 167, 107886. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Z.; Zhang, J.; Deng, R.; Peng, H.; Guo, Y.; Xiang, P.; Xia, S. Ammonia recovery from wastewater using a bioelectrochemical membrane-absorbed ammonia system with authigenic acid and base. J. Clean. Prod. 2021, 296, 126554. [Google Scholar] [CrossRef]
- Rodríguez Arredondo, M.; Kuntke, P.; ter Heijne, A.; Hamelers, H.V.M.; Buisman, C.J.N. Load ratio determines the ammonia recovery and energy input of an electrochemical system. Water Res. 2017, 111, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, A.; Limonti, C.; Curcio, G.M.; Molinari, R. Advances in struvite precipitation technologies for nutrients removal and recovery from aqueous waste and wastewater. Sustainability 2020, 12, 7538. [Google Scholar] [CrossRef]
- Yuan, P.; Kim, Y. Increasing phosphorus recovery from dewatering centrate in microbial electrolysis cells. Biotechnol. Biofuels 2017, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- Almatouq, A.; Babatunde, A.O. Concurrent phosphorus recovery and energy generation in mediator-less dual chamber microbial fuel cells: Mechanisms and influencing factors. Int. J. Environ. Res. Public Health 2016, 13, 375. [Google Scholar] [CrossRef] [PubMed]
- Almatouq, A.; Babatunde, A.O. Identifying optimized conditions for concurrent electricity production and phosphorus recovery in a mediator-less dual chamber microbial fuel cell. Appl. Energy 2018, 230, 122–134. [Google Scholar] [CrossRef]
- Ye, Y.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Liu, Y.; Ni, B.; Zhang, X. Microbial fuel cell for nutrient recovery and electricity generation from municipal wastewater under different ammonium concentrations. Bioresour. Technol. 2019, 292, 121992. [Google Scholar] [CrossRef]
- Ye, Y.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Liu, Y.; Nghiem, L.D.; Zhang, X.; Wang, J. Effect of organic loading rate on the recovery of nutrients and energy in a dual-chamber microbial fuel cell. Bioresour. Technol. 2019, 281, 367–373. [Google Scholar] [CrossRef]
- Ye, Y.; Ngo, H.H.; Guo, W.; Liu, Y.; Chang, S.W.; Nguyen, D.D.; Ren, J.; Liu, Y.; Zhang, X. Feasibility study on a double chamber microbial fuel cell for nutrient recovery from municipal wastewater. Chem. Eng. J. 2019, 358, 236–242. [Google Scholar] [CrossRef]
- Sharma, P.; Talekar, G.V.; Mutnuri, S. Demonstration of energy and nutrient recovery from urine by field-scale microbial fuel cell system. Process Biochem. 2021, 101, 89–98. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Guan, X.; She, L.; Xiang, P.; Xia, S.; Zhang, Z. Bioelectrochemical acidolysis of magnesia to induce struvite crystallization for recovering phosphorus from aqueous solution. J. Environ. Sci. 2019, 85, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Sun, F.; Chua, F.J.D.; Lu, D.; Stuckey, D.C.; Zhou, Y. In-situ power generation and nutrients recovery from waste activated sludge – Long-term performance and system optimization. Chem. Eng. J. 2019, 361, 1207–1214. [Google Scholar] [CrossRef]
- Sun, D.; Gao, Y.; Hou, D.; Zuo, K.; Chen, X.; Liang, P.; Zhang, X.; Ren, Z.J.; Huang, X. Energy-neutral sustainable nutrient recovery incorporated with the wastewater purification process in an enlarged microbial nutrient recovery cell. J. Power Sources 2018, 384, 160–164. [Google Scholar] [CrossRef]
- Lu, S.; Li, H.; Tan, G.; Wen, F.; Flynn, M.T.; Zhu, X. Resource recovery microbial fuel cells for urine-containing wastewater treatment without external energy consumption. Chem. Eng. J. 2019, 373, 1072–1080. [Google Scholar] [CrossRef]
- Ledezma, P.; Jermakka, J.; Keller, J.; Freguia, S. Recovering nitrogen as a solid without chemical dosing: Bio-electroconcentration for recovery of nutrients from urine. Environ. Sci. Technol. Lett. 2017, 4, 119–124. [Google Scholar] [CrossRef]
- Cerrillo, M.; Burgos, L.; Noguerol, J.; Riau, V.; Bonmatí, A. Ammonium and Phosphate Recovery in a Three Chambered Microbial Electrolysis Cell: Towards Obtaining Struvite from Livestock Manure. Processes 2021, 9, 1916. [Google Scholar] [CrossRef]
- Monetti, J.; Ledezma, P.; Virdis, B.; Freguia, S. Nutrient recovery by bio-electroconcentration is limited by wastewater conductivity. ACS Omega 2019, 4, 2152–2159. [Google Scholar] [CrossRef]
- El-Qelish, M.; Mahmoud, M. Overcoming organic matter limitation enables high nutrient recovery from sewage sludge reject water in a self-powered microbial nutrient recovery cell. Sci. Total Environ. 2022, 802, 149851. [Google Scholar] [CrossRef]
- Jiang, Q.; Liu, J.; Song, X.; Qiu, Y.; Xue, J.; Shao, Y.; Feng, Y. Energy efficient bioelectro-concentration and recovery system of nutrients from human urine by integrating forward osmosis. Resour. Conserv. Recycl. 2022, 181, 106253. [Google Scholar] [CrossRef]
- Jiang, Q.; Song, X.; Liu, J.; Shao, Y.; Feng, Y. Enhanced nutrients enrichment and removal from eutrophic water using a self-sustaining in situ photomicrobial nutrients recovery cell (PNRC). Water Res. 2019, 167, 115097. [Google Scholar] [CrossRef]
- Shahid, K.; Ramasamy, D.L.; Kaur, P.; Sillanpää, M.; Pihlajamäki, A. Effect of modified anode on bioenergy harvesting and nutrients removal in a microbial nutrient recovery cell. Bioresour. Technol. 2021, 332, 125077. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, D.; Zhang, X.; Liang, P.; Huang, X. Novel self-driven microbial nutrient recovery cell with simultaneous wastewater purification. Sci. Rep. 2015, 5, 15744. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhou, H.; Zuo, K.; Zhou, Y.; Wang, Q.; Sun, D.; Gao, Y.; Liang, P.; Zhang, X.; Ren, Z.J.; et al. Self-sustaining advanced wastewater purification and simultaneous in situ nutrient recovery in a novel bioelectrochemical system. Chem. Eng. J. 2017, 330, 692–697. [Google Scholar] [CrossRef]
- Chen, X.; Gao, Y.; Hou, D.; Ma, H.; Lu, L.; Sun, D.; Zhang, X.; Liang, P.; Huang, X.; Ren, Z.J. The Microbial Electrochemical Current Accelerates Urea Hydrolysis for Recovery of Nutrients from Source-Separated Urine. Environ. Sci. Technol. Lett. 2017, 4, 305–310. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, D.; Wang, H.; Lu, L.; Ma, H.; Wang, L.; Ren, Z.J.; Liang, P.; Zhang, X.; Chen, X.; et al. Urine-powered synergy of nutrient recovery and urine purification in a microbial electrochemical system. Environ. Sci. Water Res. Technol. 2018, 4, 1427–1438. [Google Scholar] [CrossRef]
- Song, Y.-H.; Hidayat, S.; Effendi, A.J.; Park, J.-Y. Simultaneous hydrogen production and struvite recovery within a microbial reverse-electrodialysis electrolysis cell. J. Ind. Eng. Chem. 2021, 94, 302–308. [Google Scholar] [CrossRef]
- Hou, D.; Lu, L.; Sun, D.; Ge, Z.; Huang, X.; Cath, T.Y.; Ren, Z.J. Microbial electrochemical nutrient recovery in anaerobic osmotic membrane bioreactors. Water Res. 2017, 114, 181–188. [Google Scholar] [CrossRef]
- Lackner, S.; Gilbert, E.M.; Vlaeminck, S.E.; Joss, A.; Horn, H.; van Loosdrecht, M.C.M. Full-scale partial nitritation/anammox experiences – An application survey. Water Res. 2014, 55, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Matassa, S.; Batstone, D.J.; Hülsen, T.; Schnoor, J.; Verstraete, W. Can Direct Conversion of Used Nitrogen to New Feed and Protein Help Feed the World? Environ. Sci. Technol. 2015, 49, 5247–5254. [Google Scholar] [CrossRef]
- Qin, M.; Liu, Y.; Luo, S.; Qiao, R.; He, Z. Integrated experimental and modeling evaluation of energy consumption for ammonia recovery in bioelectrochemical systems. Chem. Eng. J. 2017, 327, 924–931. [Google Scholar] [CrossRef]
- Kuntke, P.; Rodríguez Arredondo, M.; Widyakristi, L.; ter Heijne, A.; Sleutels, T.H.J.A.; Hamelers, H.V.M.; Buisman, C.J.N. Hydrogen gas recycling for energy efficient ammonia recovery in electrochemical systems. Environ. Sci. Technol. 2017, 51, 3110–3116. [Google Scholar] [CrossRef] [PubMed]
- AlSayed, A.; Soliman, M.; Eldyasti, A. Microbial fuel cells for municipal wastewater treatment: From technology fundamentals to full-scale development. Renew. Sustain. Energy Rev. 2020, 134, 110367. [Google Scholar] [CrossRef]
- Do, M.H.; Ngo, H.H.; Guo, W.S.; Liu, Y.; Chang, S.W.; Nguyen, D.D.; Nghiem, L.D.; Ni, B.J. Challenges in the application of microbial fuel cells to wastewater treatment and energy production: A mini review. Sci. Total Environ. 2018, 639, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, N.; Islam, N.; Ahmed, S. Progress in microbial fuel cells for sustainable management of industrial effluents. Process Biochem. 2021, 106, 20–41. [Google Scholar] [CrossRef]
- Jadhav, D.A.; Park, S.-G.; Pandit, S.; Yang, E.; Ali Abdelkareem, M.; Jang, J.-K.; Chae, K.-J. Scalability of microbial electrochemical technologies: Applications and challenges. Bioresour. Technol. 2022, 345, 126498. [Google Scholar] [CrossRef]
- Wang, A.-J.; Wang, H.-C.; Cheng, H.-Y.; Liang, B.; Liu, W.-Z.; Han, J.-L.; Zhang, B.; Wang, S.-S. Electrochemistry-stimulated environmental bioremediation: Development of applicable modular electrode and system scale-up. Environ. Sci. Ecotechnol. 2020, 3, 100050. [Google Scholar] [CrossRef]
- Goglio, A.; Tucci, M.; Rizzi, B.; Colombo, A.; Cristiani, P.; Schievano, A. Microbial recycling cells (MRCs): A new platform of microbial electrochemical technologies based on biocompatible materials, aimed at cycling carbon and nutrients in agro-food systems. Sci. Total Environ. 2019, 649, 1349–1361. [Google Scholar] [CrossRef]
- Ren, L.; Ahn, Y.; Hou, H.; Zhang, F.; Logan, B.E. Electrochemical study of multi-electrode microbial fuel cells under fed-batch and continuous flow conditions. J. Power Sources 2014, 257, 454–460. [Google Scholar] [CrossRef]
- Dhar, B.R.; Ryu, H.; Santo Domingo, J.W.; Lee, H.-S. Ohmic resistance affects microbial community and electrochemical kinetics in a multi-anode microbial electrochemical cell. J. Power Sources 2016, 331, 315–321. [Google Scholar] [CrossRef]
- Casadellà, A.; Schaetzle, O.; Loos, K. Ammonium across a Selective Polymer Inclusion Membrane: Characterization, Transport, and Selectivity. Macromol. Rapid Commun. 2016, 37, 858–864. [Google Scholar] [CrossRef]
- Khoshnevisan, B.; He, L.; Xu, M.; Valverde-Pérez, B.; Sillman, J.; Mitraka, G.-C.; Kougias, P.G.; Zhang, Y.; Yan, S.; Ji, L.; et al. From renewable energy to sustainable protein sources: Advancement, challenges, and future roadmaps. Renew. Sustain. Energy Rev. 2022, 157, 112041. [Google Scholar] [CrossRef]
- Acién Fernández, F.G.; Gómez-Serrano, C.; Fernández-Sevilla, J.M. Recovery of Nutrients From Wastewaters Using Microalgae. Front. Sustain. Food Syst. 2018, 2. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, H.; Abu-Reesh, I.M.; He, Z.; Yuan, C. Life Cycle Environmental Impact Comparison of Bioelectrochemical Systems for Wastewater Treatment. Procedia CIRP 2019, 80, 382–388. [Google Scholar] [CrossRef]
- Chin, M.Y.; Phuang, Z.X.; Woon, K.S.; Hanafiah, M.M.; Zhang, Z.; Liu, X. Life cycle assessment of bioelectrochemical and integrated microbial fuel cell systems for sustainable wastewater treatment and resource recovery. J. Environ. Manag. 2022, 320, 115778. [Google Scholar] [CrossRef]
| Configuration/Operation Mode | Membrane | Anode Volume (mL) | Cathode Volume (mL) | Membrane Surface (cm2) | Anolyte | Catholyte | Maximum Current Density (A m−2) | N Recovery Rate (gNH4+-N m−2 d−1) | Energy Demand (kWh kgN−1) | Combined with | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MFC | CEM | 500 | 500 | 168 | Pig slurry | PBS | 0.15 | 7.2 | n.r. | Stripping | [25] |
| MFC | CEM | 500 | 500 | 168 | Pig slurry | NaCl solution | 0.07 | 3.7 | n.r. | Stripping | [25] |
| MFC | CEM | 140 | 80 | 100 | Synthetic | NaCl solution | 1.6 | 6.8 | 1.6 | Stripping | [32] |
| MFC | CEM | 500 | 500 | 168 | Digested pig slurry | NaCl solution | 0.22 | 8.86 | n.r. | [23] | |
| MFC | CEM | 500 | 500 | 168 | Digested pig slurry | NaCl solution | 0.4 | 11.19 | n.r. | [27] | |
| MFC submersed | CEM AEM | 18 | 18 | 9 | Synthetic | NaCl solution | 8 | 86.2 | 0.86 b | [22] | |
| MEC | CEM | 500 | 500 | 168 | Digested pig slurry | NaCl solution | 0.43 | 3.73 | n.r. | [26] | |
| MEC | CEM | 400 | 140 | 38 | Reject water | Tap water | 6.4 | 26 | 5.8 | [28] | |
| MEC | CEM | 320 | 400 | Diluted urine | n.r. | 1.7 | n.r. | 2.48 | [40] | ||
| MEC | CEM | 200 | 180 | 42 | Synthetic | Deionised water | 1.89 | 10.2 | n.r. | [41] | |
| MEC | CEM | 336 | 336 | 96 | AD concentrate and food WW | Tap water | 2.6 c | 14 c | 2.7 | [29] | |
| MEC | PEM | 250 | 250 | n.r. | Pig slurry | PBS | 0.5 | 10.9 | n.r. | [37] | |
| MEC | PEM | 500 | 500 | n.r. | Pig slurry | PBS | 0.5 | 8.3 | n.r. | [37] | |
| MEC | CEM | 500 | 500 | 168 | Pig slurry | PBS | 4.8 c | 10.3 | n.r. | Stripping | [25] |
| MEC | CEM | 500 | 500 | 168 | Pig slurry | NaCl solution | 2.4 c | 25.5 | n.r. | Stripping | [25] |
| MEC | CEM | 500 | 500 | 168 | Digested pig slurry | NaCl solution | 2.01 | 12.97 | n.r. | Stripping | [2] |
| MEC | CEM | 140 | 80 | 100 | Synthetic | NaCl solution | 2.5 | 9.6 | 1.6 | Stripping | [32] |
| MEC | CEM | 140 | 80 | 100 | Blackwater | NaCl solution | 1.9 | 6.5 | 1.6 | Stripping | [32] |
| MEC | CEM | 100 | 180 | 42.3 | Synthetic | Deionised water | 1.6 | 7.1 | 5.7 a | Stripping | [33] |
| MEC | CEM | 120 | 180 | 96 | Synthetic | NaCl solution | 1.5 | 6.9 | n.r. | Stripping | [34] |
| MEC | CEM | 200 | 300 | 100 | Synthetic | NaCl solution | 10.6 | 119 | 1.9 | HM | [42] |
| MEC | CEM | 2500 | 2500 | 5000 | Urine | NaOH solution | 1.8 | n.r. | 1.36 | HM | [43] |
| MEC | CEM | 90 | 90 | 72 | Synthetic | NaCl solution | 0.8 c | n.r. | 1.17 | Water and P recovery, FO | [35] |
| MEC | CEM | 500 | 500 | 168 | Digested pig slurry | Synthetic | 2.2 c | 8.64 | n.r. | HM and CH4 production | [44] |
| MEC | CEM | 500 | 500 | 168 | Digested pig slurry | Synthetic | 4.5 | 14.46 | n.r. | CH4 production | [30] |
| MEC | PEM | 860 | 860 | 289 | Digestate | Synthetic | 2.1 c | 7 c | n.r. | CH4 production | [31] |
| Tubular MEC | CEM | 3140 | 5060 | 2355 | Synthetic | Synthetic | 0.25 c | 3.7 c | 2.3 | CH4 production | [38] |
| Tubular MEC | CEM | 500 | 500 | n.r. | Landfill leachate | Deionised water | 0.72 | n.r. | 8.5 | Stripping FO | [36] |
| Tubular MEC | CEM | 1159 | 1300 | 836 c | Synthetic | Deionised water | 0.15 c | n.r. | 1.3 | Passive NH3 separation | [33] |
| Configuration | Membrane | Anode Volume (mL) | Cathode Volume (mL) | Membrane Surface (cm2) | Substrate | Maximum Current Density (A m−2) | Recovered Product | Reference |
|---|---|---|---|---|---|---|---|---|
| MFC | PEM | 300 | 300 | n.r. | Synthetic | n.r. | Struvite | [56] |
| MFC | CEM | 305 | 305 | n.r. | Synthetic | n.r. | Struvite | [57] |
| MFC | CEM | 45 | 30 | n.r. | Synthetic | 1.17 | Struvite | [61] |
| MFC | CEM | 28 | 28 | n.r. | Fermented liquor | n.r. | Ammonia solution and P-rich biomass | [62] |
| MEC | AEM and CEM | 600 | 600 | 20 | Digested pig slurry | 0.26 | Nutrient solution | [66] |
| RRMFC | AEM and CEM | 28 | 28 | 7.1 | Synthetic | 1.9 c | Nutrient solution | [64] |
| MNRC | AEM and CEM | 110 | 110 | 55 | Domestic wastewater | 0.6 c | Nutrient solution | [63] |
| MNRC | AEM and CEM | 22 | 22 | n.r. | Wastewater | 6 | Nutrient solution | [71] |
| MNRC | AEM and CEM | 220 | 220 | 50 | Sludge reject water with livestock wastewater | 0.6 c | Nutrient solution | [68] |
| BEC | AEM and CEM | 200 | 200 | 100 | Synthetic | 50 | Nutrient solution | [65] |
| BEC | AEM and CEM | 200 | 200 | 100 | Domestic wastewater | 2 | Nutrient solution | [67] |
| PNRC | AEM and CEM | 20 | 20 | 7.1 c | Synthetic | 2 | Microalgal biomass | [70] |
| MEC-FO | CEM and FO | 90 | 90 | 72 | Synthetic | Struvite | [35] | |
| OsBCRS | AEM, CEM and FO | 100 | 100 | 25 | Synthetic | 1.1 c | Struvite | [69] |
| MRESC | AEM-CEM stack | 32 | 35 | 12 | Synthetic | 7.9 | Struvite | [76] |
| SMNRC | AEM and CEM stack | 21 c | 7 c | 7.1c | Urine | n.r. | Nutrient solution | [74] |
| MNRC | 3 pairs AEM-CEM | 315 | 105 | 105 | Urine | 2 c | Struvite | [75] |
| AMNR | 3 pairs AEM-CEM | 4000 | 4000 | n.r. | Domestic wastewater | n.r. | Nutrient solution | [73] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerrillo, M.; Riau, V.; Bonmatí, A. Recent Advances in Bioelectrochemical Systems for Nitrogen and Phosphorus Recovery Using Membranes. Membranes 2023, 13, 186. https://doi.org/10.3390/membranes13020186
Cerrillo M, Riau V, Bonmatí A. Recent Advances in Bioelectrochemical Systems for Nitrogen and Phosphorus Recovery Using Membranes. Membranes. 2023; 13(2):186. https://doi.org/10.3390/membranes13020186
Chicago/Turabian StyleCerrillo, Míriam, Victor Riau, and August Bonmatí. 2023. "Recent Advances in Bioelectrochemical Systems for Nitrogen and Phosphorus Recovery Using Membranes" Membranes 13, no. 2: 186. https://doi.org/10.3390/membranes13020186
APA StyleCerrillo, M., Riau, V., & Bonmatí, A. (2023). Recent Advances in Bioelectrochemical Systems for Nitrogen and Phosphorus Recovery Using Membranes. Membranes, 13(2), 186. https://doi.org/10.3390/membranes13020186








