Treatment Trends and Combined Methods in Removing Pharmaceuticals and Personal Care Products from Wastewater—A Review
Abstract
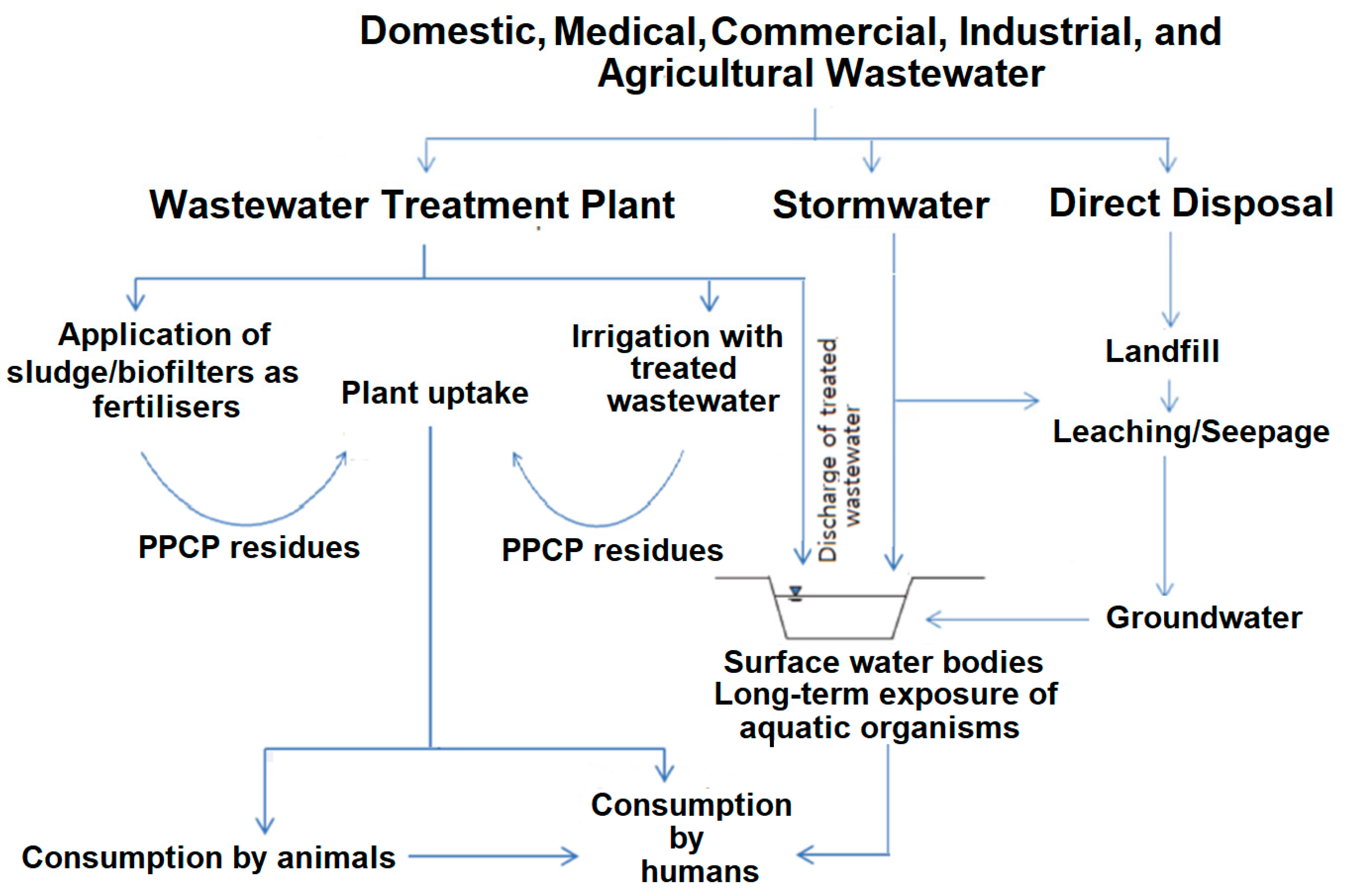
1. Introduction
2. PPCP Removal Methods
2.1. Conventional Biological Treatment Process
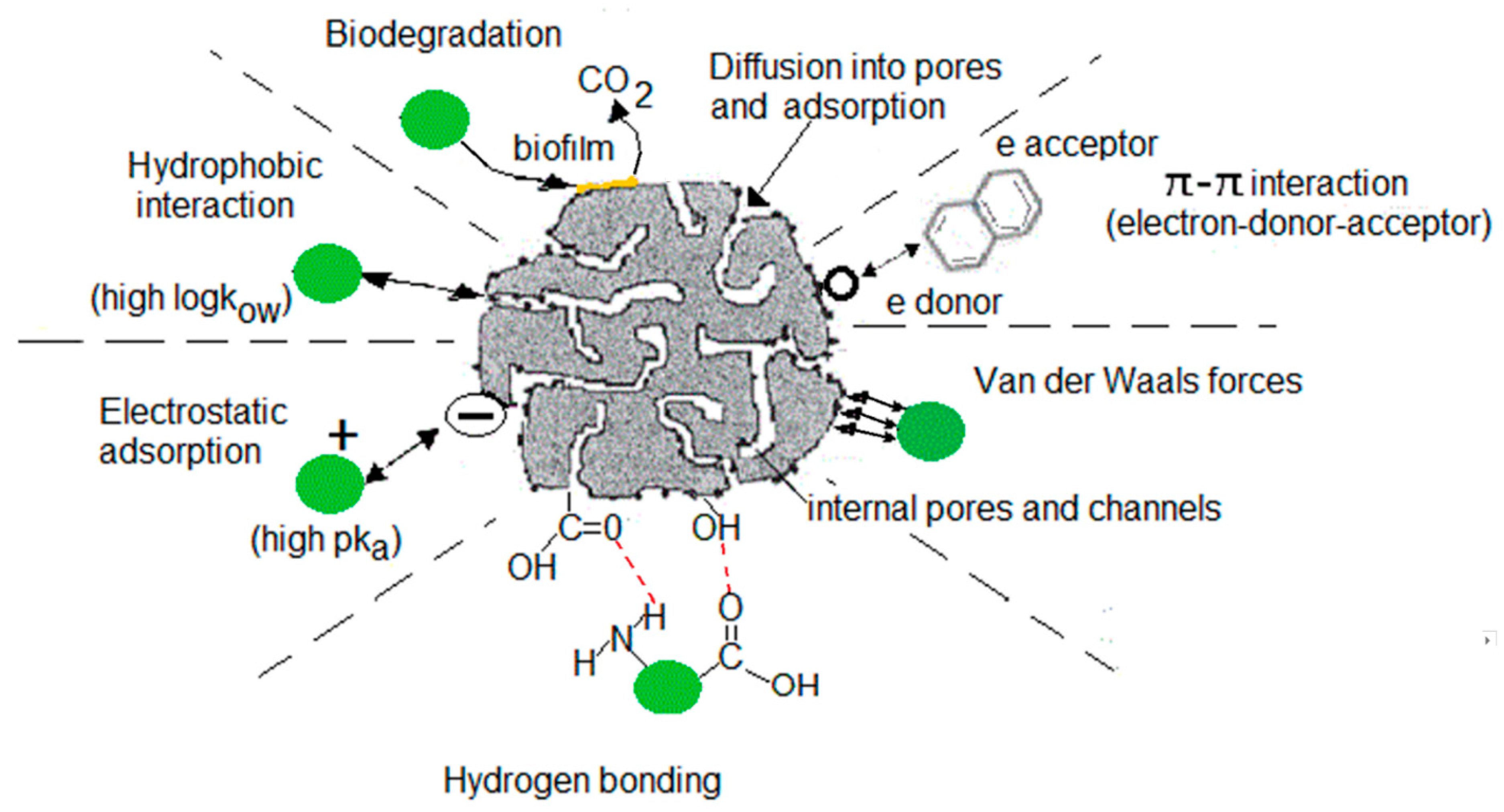
2.2. Adsorption
2.2.1. Activated Carbon (AC)
2.2.2. Graphene
2.2.3. Carbon Nanotubes (CNT)
- (1)
- The costs of graphene and graphene oxide are still prohibitive, and further research is required to lower them [58];
- (2)
- The aggregation of graphene sheets should be prevented to avoid a reduction in adsorption capacity by loading it onto low-cost materials [58];
- (3)
- Similarly, more research is required to simplify and lower the cost of CNT production;
2.3. Membrane Process
2.4. Advanced Oxidation Processes
2.4.1. Ozonation
2.4.2. Fenton Oxidation
2.4.3. UV Oxidation Treatment
2.4.4. Electrochemical Advanced Oxidation Processes (EAOPs)
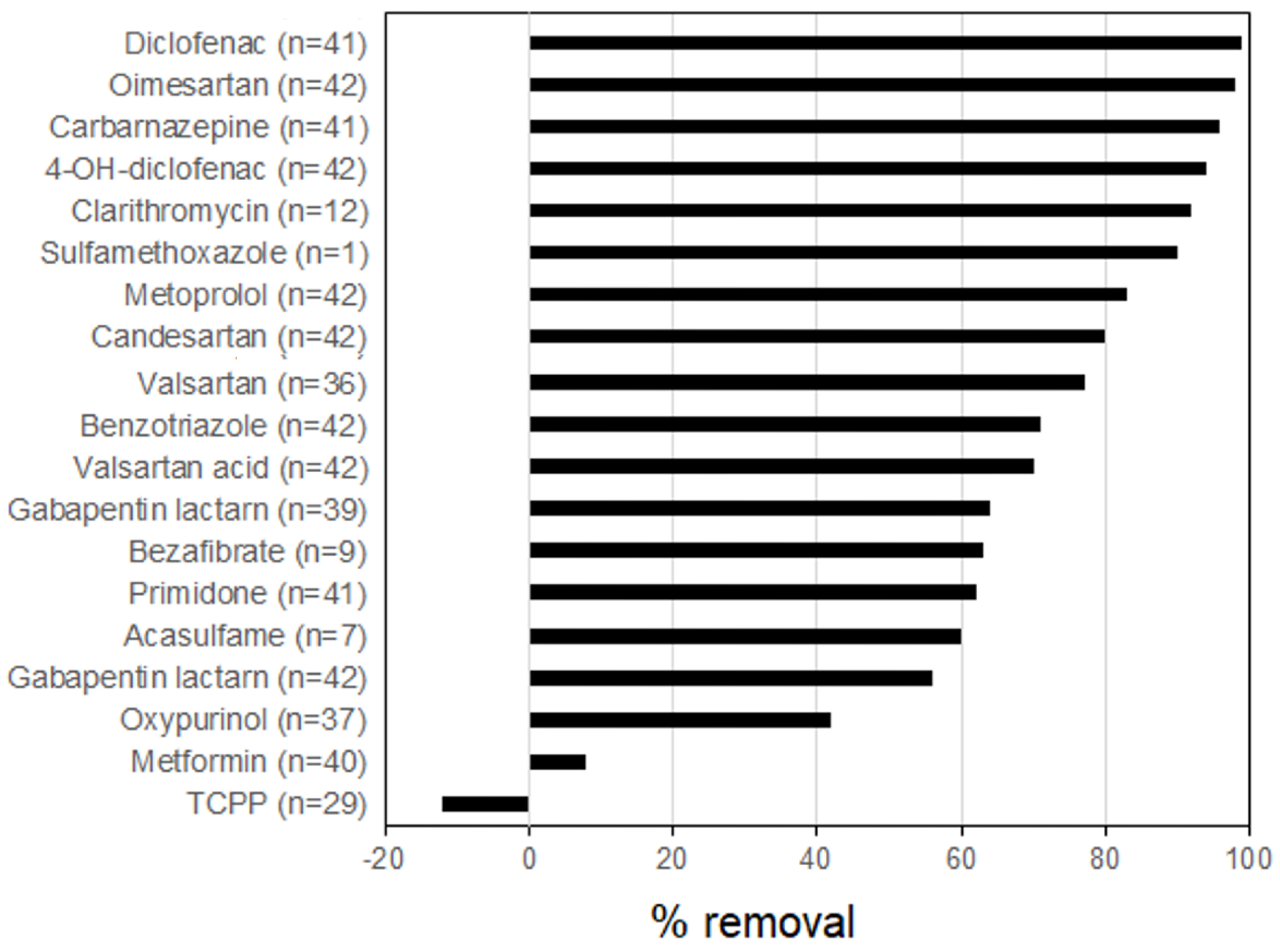
2.5. Removal of PPCPs by Combined Methods
2.5.1. Combined Chemical and Biological Methods
2.5.2. Combined Chemical and Physical Methods (Ozonation and Adsorption)
2.5.3. Combined Membrane Processes
- Membrane anti-fouling: An air diffuser placed at the bottom of the influent tank creates coarse air bubbles that are used to keep the adsorbent in suspension. The bubbles also flow past the membrane surface, inducing shear stress across it and removing the membrane foulant. The energy requirement of immersed membrane systems used in wastewater treatment plants is currently very low (less than 20% of the total energy requirement).
- Optimisation of backwash: For the successful long-term operation of the membrane process, it is necessary to optimise the frequency and duration of the backwash. Adaptive backwash initiation and duration schemes with new control systems can lead to a 40–50% reduction in backwash water and energy consumption [87,88].
- Incorporation of adsorbent in SMAHS: The adsorbent added to the SMAHS creates an additional shearing effect that reduces particle deposition on the membrane surface and reduces membrane resistance. It directly removes organics that would otherwise deposit on the membrane and cause fouling. The periodic daily substitution of adsorbent is as little as 2–5%, which is equivalent to an average adsorbent residence time of 20–50 days in the tank. This helps economise the adsorbent without it becoming exhausted.
2.5.4. Advanced Membrane Bioreactor Hybrid Systems
Osmotic Membrane Bioreactor
Membrane distillation bioreactor
Comparison of Hybrid MBRs
3. Concluding Remarks and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Adeleye, A.S.; Xue, J.; Zhao, Y.; Taylor, A.A.; Zenobio, J.E.; Sun, Y.; Han, Z.; Salawu, O.A.; Zhu, Y. Abundance, fate, and effects of pharmaceuticals and personal care products in aquatic environments. J. Hazard. Mater. 2022, 424, 127284. [Google Scholar] [CrossRef]
- Reyes, N.J.D.G.; Geronimo, F.K.F.; Yano, K.A.V.; Guerra, H.B.; Kim, L.-H. Pharmaceutical and Personal Care Products in Different Matrices: Occurrence, Pathways, and Treatment Processes. Water 2021, 13, 1159. [Google Scholar] [CrossRef]
- Zhang, M.; Shen, J.; Zhong, Y.; Ding, T.; Dissanayake, P.D.; Yang, Y.; Tsang, Y.F. Sorption of pharmaceuticals and personal care products (PPCPs) from water and wastewater by carbonaceous materials: A review. Crit. Rev. Environ. Sci. 2022, 52, 727–766. [Google Scholar] [CrossRef]
- Water, J.P.I. Water Joint Programming Initiative Knowledge Hub on Contaminants of Emerging Concern, Continuous increase of CECs in the anthroposphere as a stressor for water resources. Stakeholder Brief, Water Joint Programming Initiative Knowledge Hub on Contaminants of Emerging Concern; Water JPI: 2020; Agence Nationale de la Recherche (ANR): Paris, France, 2020; 21p. [Google Scholar]
- Tousova, Z.; Oswald, P.; Slobodnik, J.; Blaha, L.; Muz, M.; Hu, M.; Brack, W.; Krauss, M.; Di Paolo, C.; Tarcai, Z.; et al. European demonstration program on the effect-based and chemical identification and monitoring of organic pollutants in European surface waters. Sci. Total Environ. 2017, 601–602, 1849–1868. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Removal of pharmaceuticals and personal care products (PPCPs) from wastewater: A review. J. Environ. Manag. 2016, 182, 620–640. [Google Scholar] [CrossRef]
- Yang, Y.C.; Ok, Y.S.; Kim, K.H.; Kwon, E.E.; Tsang, Y.F. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review. Sci. Total. Environ. 2017, 596, 303–320. [Google Scholar] [CrossRef]
- Bartelt-Hunt, S.L.; Snow, D.D.; Damon, T.; Shockley, J.; Hoagland, K. The occurrence of illicit and therapeutic pharmaceuticals in wastewater effluent and surface waters in Nebraska. Environ. Pollut. 2009, 157, 786–791. [Google Scholar] [CrossRef]
- Kim, Y.; Choi, K.; Jung, J.; Park, S.; Kim, S.-C.; Carlson, K. Temporal and spatial trends in the occurrence of human and veterinary antibiotics in aqueous and river sediment matrices. Environ. Sci. Technol. 2017, 41, 50–57. [Google Scholar] [CrossRef]
- Kim, S.D.; Cho, J.; Kim, I.S.; Vanderford, B.J.; Snyder, S.A. Occurrence and removal of pharmaceuticals and endocrine disruptors in South Korean surface, drinking, and waste waters. Water Res. 2017, 41, 1013–1021. [Google Scholar] [CrossRef]
- Petrie, B.; Barden, R.; Kasprzyk-Hordern, B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring. Water Res. 2015, 72, 3–27. [Google Scholar] [CrossRef]
- Stumpf, M.; Ternes, T.A.; Wilken, R.-D.; Rodrigues, S.V.; Baumann, W. Polar drug residues in sewage and natural waters in the state of Rio de Janeiro, Brazil. Sci. Total Environ. 1999, 225, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Mompelat, S.; Le Bot, B.; Thomas, O. Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water. Environ. Int. 2009, 35, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Cizmas, L.; Sharma, V.K.; Gray, C.M.; McDonald, T.J. Pharmaceuticals and personal care products in waters: Occurrence, toxicity, and risk. Environ. Chem. Lett. 2015, 13, 381–394. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Gin, K.Y.H.; Lin, A.Y.C.; Reinhard, M. Impacts of emerging organic contaminants on freshwater resources: Review of recent occurrences, sources, fate, and effects. Sci. Total Environ. 2010, 408, 6062–6069. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Fick, J.; Lindberg, R.H.; Tysklind, M.; Larsson, D.G.J. Predicted critical environmental concentrations for 500 pharmaceuticals. Regul. Toxicol. Pharmacol. 2010, 58, 516–523. [Google Scholar] [CrossRef]
- Celiz, M.D.; Tso, J.; Aga, D.S. Pharmaceutical metabolites in the environment: Analytical challenges and ecological risks. Environ. Toxicol. Chem. 2009, 28, 2473–2484. [Google Scholar] [CrossRef]
- Cleuvers, M. Aquatic ecotoxicity of pharmaceuticals including the assessment of combination effects. Toxicol. Lett. 2003, 142, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-L.; Wong, M.-H. Pharmaceuticals and personal care products (PPCPs): A review on environmental contamination in China. Environ. Int. 2013, 59, 208–224. [Google Scholar] [CrossRef] [PubMed]
- Balakrishna, K.; Rath, A.; Praveenkumarreddy, Y.; Guruge, K.S.; Subedi, B. A review of the occurrence of pharmaceuticals and personal care products in Indian water bodies. Ecotoxicol. Environ. Saf. 2017, 137, 113–120. [Google Scholar] [CrossRef]
- Arpin-Pont, L.; Bueno, M.; Gomez, E.; Fenet, H. Occurrence of PPCPs in the marine environment: A review. Environ. Sci. Pollut. Res. 2016, 23, 4978–4991. [Google Scholar] [CrossRef]
- Deo, R.P. Pharmaceuticals in the surface water of the USA: A review. Curr. Environ. Health Rep. 2014, 1, 113–122. [Google Scholar] [CrossRef]
- Sui, Q.; Cao, X.; Lu, S.; Zhao, W.; Qiu, Z.; Yu, G. Occurrence, sources and fate of pharmaceuticals and personal care products in the groundwater: A review. Emerg. Contam. 2015, 1, 14–24. [Google Scholar] [CrossRef]
- Vieno, N.; Sillanpää, M. Fate of diclofenac in municipal wastewater treatment plant—A review. Environ. Int. 2014, 69, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Antibiotics in the aquatic environment—A review–Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Huang, G.; Zhang, B. Review of aquatic toxicity of pharmaceuticals and personal care products to algae. J. Hazard. Mater. 2021, 410, 124619. [Google Scholar] [CrossRef]
- Corcoran, J.; Winter, M.J.; Tyler, C.R. Pharmaceuticals in the aquatic environment: A critical review of the evidence for health effects in fish. Crit. Rev. Toxicol. 2010, 40, 287–304. [Google Scholar] [CrossRef]
- Neha, R.; Adithya, S.; Jayaraman, R.S.; Gopinath, K.P.; Pandimadevi, M.; Praburaman, L.; Arun, J. Nano-adsorbents an effective candidate for removal of toxic pharmaceutical compounds from aqueous environment: A critical review on emerging trends. Chemosphere 2021, 272, 129852. [Google Scholar] [CrossRef]
- Sophia, C.; Eder, A.; Lima, C. Removal of emerging contaminants from the environment by adsorption. Ecotoxicol. Environ. Saf. 2018, 150, 1–17. [Google Scholar] [CrossRef]
- Zhang, M.; Igalavithana, A.D.; Xu, L.; Sarkar, B.; Hou, D.; Zhang, M.; Bhatnagar, A. Engineered/designer hierarchical porous carbon materials for organic pollutant removal from water and wastewater: A critical review. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2295–2328. [Google Scholar] [CrossRef]
- Derco, J.; Gotvajn, A.Ž.; Cižmárová, O.; Dudáš, J.; Sumegová, L.; Šimovičová, K. Removal of Micropollutants by Ozone-Based Processes. Processes 2021, 9, 1013. [Google Scholar] [CrossRef]
- Rekhate, C.V.; Srivastava, J.K. Recent advances in ozone-based advanced oxidation processes for treatment of wastewater: A review. Chem. Eng. J. Adv. 2020, 3, 100031. [Google Scholar] [CrossRef]
- Sauter, D.; Dąbrowska, A.; Bloch, R.; Stapf, M.; Miehe, U.; Sperlich, A.; Gnirss, R.; Wintgens, T. Deep-bed filters as post-treatment for ozonation in tertiary municipal wastewater treatment: Impact of design and operation on treatment goals. Environ. Sci. Water Res. Tech. 2021, 7, 197–211. [Google Scholar] [CrossRef]
- Couto, C.F.; Lange, L.C.; Amaral, M.C.S. A critical review on membrane separation processes applied to remove pharmaceutically active compounds from water and wastewater. J. Water Process Eng. 2018, 26, 156–175. [Google Scholar] [CrossRef]
- Jamil, S.; Loganathan, P.; Khan, S.J.; McDonald, J.A.; Kandasamy, J.; Vigneswaran, S. Enhanced nanofiltration rejection of inorganic and organic compounds from a wastewater-reclamation plant’s micro-filtered water using adsorption pre-treatment. Sep. Purif. Tech. 2021, 260, 118207. [Google Scholar] [CrossRef]
- Lama, G.; Meijide, J.; Sanromán, A.; Pazos, M. Heterogeneous Advanced Oxidation Processes: Current Approaches for Wastewater Treatment. Catalysts 2022, 12, 344. [Google Scholar] [CrossRef]
- Roberts, J.; Kumar, A.; Du, J.; Hepplewhite, C.; Ellis, D.J.; Christy, A.G.; Beavis, S.G. Pharmaceuticals and personal care products (PPCPs) in Australia’s largest inland sewage treatment plant, and its contribution to a major Australian river during high and low flow. Sci. Total Environ. 2016, 541, 1625–1637. [Google Scholar] [CrossRef]
- Li, Q.; Wang, P.; Chen, L.; Gao, H.; Wu, L. Acute toxicity and histopathological effects of naproxen in zebrafish (Danio rerio) early life stages. Environ. Sci. Pollut. Res. 2016, 23, 18832–18841. [Google Scholar] [CrossRef] [PubMed]
- Blair, B.; Nikolaus, A.; Hedman, C.; Klaper, R.; Grundl, T. Evaluating the degradation, sorption, and negative mass balances of pharmaceuticals and personal care products during wastewater treatment. Chemosphere 2015, 134, 395–401. [Google Scholar] [CrossRef]
- Wu, M.; Xiang, J.; Que, C.; Chen, F.; Xu, G. Occurrence and fate of psychiatric pharmaceuticals in the urban water system of Shanghai, China. Chemosphere 2015, 138, 486–493. [Google Scholar] [CrossRef]
- Carballa, M.; Omil, F.; Lema, J.M.; Llompart, M.A.; García-Jares, C.; Rodríguez, I.; Gomez, M.; Ternes, T. Behavior of pharmaceuticals, cosmetics and hormones in a sewage treatment plant. Water Res. 2004, 38, 2918–2926. [Google Scholar] [CrossRef]
- Lindqvist, N.; Tuhkanen, T.; Kronberg, L. Occurrence of acidic pharmaceuticals in raw and treated sewages and in receiving waters. Water Res. 2005, 39, 2219–2228. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters. Water Res. 2009, 43, 363–380. [Google Scholar] [CrossRef]
- Kosma, C.I.; Lambropoulou, D.A.; Albanis, T.A. Investigation of PPCPs in wastewater treatment plants in Greece: Occurrence, removal and environmental risk assessment. Sci. Total Environ. 2014, 466, 421–438. [Google Scholar] [CrossRef]
- Quednow, K.; Püttmann, W. Organophosphates and synthetic musk fragrances in freshwater streams in Hessen/Germany. CLEAN Soil Air Water 2008, 36, 70–77. [Google Scholar] [CrossRef]
- Ma, C.; Yu, S.; Shi, W.; Heijman, S.G.; Rietveld, L.C. Effect of different temperatures on performance and membrane fouling in high concentration PACeMBR system treating micro-polluted surface water. Bioresour. Technol. 2013, 141, 19–24. [Google Scholar] [CrossRef]
- Jamil, S.; Loganathan, P.; Listowski, A.; Kandasamy, J.; Khourshed, C.; Vigneswaran, S. Simultaneous removal of natural organic matter and micro-organic pollutants from reverse osmosis concentrate using granular activated carbon. Water Res. 2019, 155, 106–114. [Google Scholar] [CrossRef]
- Kennedy, A.M.; Reinert, A.M.; Knappe, D.R.U.; Ferrer, I.; Summers, R.S. Full- and pilot-scale GAC adsorption of organic micropollutants. Water Res. 2015, 68, 238–248. [Google Scholar] [CrossRef]
- Ye, N.; Cimetiere, N.; Heim, V.; Fauchon, N.; Feliers, C.; Wolbert, D. Upscaling fixed bed adsorption behaviors towards emerging micropollutants in treated natural waters with aging activated carbon: Model development and validation. Water Res. 2019, 148, 30–40. [Google Scholar] [CrossRef]
- Boehler, M.; Zwickenpflug, B.; Hollender, J.; Ternes, T.; Joss, A.; Siegrist, H. Removal of micropollutants in municipal wastewater treatment plants by powder-activated carbon. Water Sci. Technol. 2012, 66, 2115–2121. [Google Scholar] [CrossRef]
- Liu, Z.H.; Kanjo, Y.; Mizutani, S. Removal mechanisms for endocrine disrupting compounds (EDCs) in wastewater treatmentdphysical means, biodegradation, and chemical advanced oxidation: A review. Sci. Total Environ. 2009, 407, 731–748. [Google Scholar] [CrossRef] [PubMed]
- Loganathan, P.; Kandasamy, J.; Jamil, S.; Ratnaweera, H.; Vigneswaran, S. Ozonation/adsorption hybrid treatment system for improved removal of natural organic matter and organic micropollutants from water—A mini review and future perspectives. Chemosphere 2022, 296, 133961. [Google Scholar] [CrossRef] [PubMed]
- Meinel, F.; Ruhl, A.S.; Sperlich, A.; Zietzschmann, F.; Jekel, M. Pilot-scale investigation of micropollutant removal with granular and powdered activated carbon. Water Air Soil Pollut. 2015, 226, 2260. [Google Scholar] [CrossRef]
- Rodriguez, E.; Campinas, M.; Acero, J.L.; Rosa, M.J. Investigating PPCP removal from wastewater by powdered activated carbon/ultrafiltration. Water Air Soil Pollut. 2016, 227, 177. [Google Scholar]
- Stoller, M.D.; Park, S.; Zhu, Y.; An, J.; Ruoff, R.S. Graphene-Based Ultracapacitors. Nano Lett. 2008, 8, 3498–3502. [Google Scholar] [CrossRef]
- Hao, J.; Zhang, Q.; Chen, P.; Zheng, X.; Wu, Y.; Ma, D.; Wei, D.; Liu, H.; Liu, G.; Lv, W. Removal of pharmaceuticals and personal care products (PPCPs) from water and wastewater using novel sulfonic acid (–SO 3 H) functionalized covalent organic frameworks. Environ. Sci. Nano 2019, 6, 3374–3387. [Google Scholar] [CrossRef]
- Prathibha, C.; Biswas, A.; Chunduri, L.A.A.; Reddy, S.K.; Loganathan, P.; Kalaruban, M.; Venkatarmaniah, K. Zr(IV) functionalized graphene oxide anchored sand as potential and economic adsorbent for fluoride removal from water. Diam. Relat. Mater. 2020, 109, 108081. [Google Scholar] [CrossRef]
- Liu, F.-f.; Zhao, J.; Wang, S.; Du, P.; Xing, B. Effects of solution chemistry on adsorption of selected pharmaceuticals and personal care products (PPCPs) by graphenes and carbon nanotubes. Environ. Sci. Technol. 2014, 48, 13197–13206. [Google Scholar] [CrossRef]
- Liu, Y.K.; Hu, J.; Wang, J.L. Fe2þ enhancing sulfamethazine degradation in aqueous solution by gamma irradiation. Radiat. Phys. Chem. 2014, 96, 81–87. [Google Scholar] [CrossRef]
- Ji, L.; Chen, W.; Zheng, S.; Xu, Z.; Zhu, D. Adsorption of sulfonamide antibiotics to multiwalled carbon nanotubes. Langmuir 2009, 25, 11608–11613. [Google Scholar] [CrossRef]
- Cho, H.H.; Huang, H.; Schwab, K. Effects of solution chemistry on the adsorption of ibuprofen and triclosan onto carbon nanotubes. Langmuir 2011, 27, 12960–12967. [Google Scholar] [CrossRef]
- Taheran, M.; Brar, S.K.; Verma, M.; Surampalli, R.Y.; Zhang, T.C.; Valero, J.R. Membrane processes for removal of pharmaceutically active compounds (PhACs) from water and wastewaters. Sci. Total Environ. 2016, 547, 60–77. [Google Scholar] [CrossRef]
- Parida, V.K.; Saidulub, D.; Majumder, A.; Srivastava, A.; Gupta, P.; Gupta, A.K. Emerging contaminants in wastewater: A critical review on occurrence, existing legislations, risk assessment, and sustainable treatment alternatives. J. Environ. Chem. Eng. 2021, 9, 105966. [Google Scholar] [CrossRef]
- Urtiaga, A.M.; Pérez, G.; Ibáñez, R.; Ortiz, I. Removal of pharmaceuticals from a WWTP secondary effluent by ultrafiltration/reverse osmosis followed by electrochemical oxidation of the RO concentrate. Desalination 2013, 331, 26–34. [Google Scholar] [CrossRef]
- Simon, A.; McDonald, J.A.; Khan, S.J.; Price, W.E.; Nghiem, L.D. Effect of caustic cleaning on pore size of nanofiltration membranes and their rejection of trace organic chemicals. J. Membr. Sci. 2013, 447, 153–162. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, L.J. Advanced oxidation processes for wastewater treatment: Formation of hydroxyl radical and application. Crit. Rev. Environ. Sci. Technol. 2012, 42, 251–325. [Google Scholar] [CrossRef]
- Esplugas, S.; Bila, D.M.; Krause, L.G.T.; Dezotti, M. Ozonation and advanced oxidation technologies to remove endocrine disrupting chemicals (EDCs) and pharmaceuticals and personal care products (PPCPs) in water effluents. J. Hazard. Mater. 2007, 149, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; von Gunten, U. Oxidative transformation of micropollutants during municipal wastewater treatment: Comparison of kinetic aspects of selective (chlorine, chlorine dioxide, ferrateVI, and ozone) and non-selective oxidants (hydroxyl radical). Water Res. 2010, 44, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Van Gijn, K.; Sohier, J.; Maasdam, R.; de Wilt, H.A.; Rijnaarts, H.H.M.; Langenhoff, A.A.M. Optimizing micropollutant removal by ozonation; Interference of effluent organic matter fractions. Ozone Sci. Eng. 2021, 43, 579–591. [Google Scholar] [CrossRef]
- Feng, L.; van Hullebusch, E.D.; Rodrigo, M.A.; Esposito, G.; Oturan, M.A. Removal of residual anti-inflammatory and analgesic pharmaceuticals from aqueous systems by electrochemical advanced oxidation processes. A review. Chem. Eng. J. 2013, 228, 944–964. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.J.; Wang, J.L. Magnetic Nanoscaled Fe3O4/CeO2 composite as an efficient Fenton-like heterogeneous catalyst for degradation of 4-chlorophenol. Environ. Sci. Technol. 2012, 46, 10145–10153. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Naoyuki, Y.; Hiroaki, T. Performance of UV and UV/H2O2 processes for the removal of pharmaceuticals detected in secondary effluent of a sewage treatment plant in Japan. J. Hazard. Mater. 2009, 166, 1134–1140. [Google Scholar] [CrossRef]
- Vogna, D.; Marotta, R.; Napolitano, A.; Andreozzi, R.; d’Ischia, M. Advanced oxidation of the pharmaceutical drug diclofenac with UV/H2O2 and ozone. Water Res. 2004, 38, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Hu, C.; Hu, X.; Wei, D.; Chen, Y.; Qu, J. Photodegradation and toxicity changes of antibiotics in UV and UV/H2O2 process. J. Hazard. Mater. 2011, 185, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Ganiyu, S.O.; Martínez-Huitle, C.A.; Oturan, M.A. Electrochemical advanced oxidation processes for wastewater treatment: Advances in formation and detection of reactive species and mechanisms. Curr. Opin. Electrochem. 2021, 27, 100678. [Google Scholar] [CrossRef]
- Moreiraa, F.C.; Boaventura, R.A.; Brillas, E.; Vítor, J.P. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Appl. Catal. B Environ. 2017, 207, 217–261. [Google Scholar] [CrossRef]
- Lozano, I.; Pérez-Guzmán, C.J.; Mora, I.; Mahlknecht, J.; Aguilar, C.L.; Cervantes-Avilés, P. Pharmaceuticals and personal care products in water streams: Occurrence, detection, and removal by electrochemical advanced oxidation processes. Sci. Total Environ. 2022, 827, 154348. [Google Scholar] [CrossRef]
- Li, S.; Wu, Y.; Zheng, H.; Li, H.; Zheng, Y.; Nan, J.; Ma, J.; Nagarajan, D.; Chang, J.S. Antibiotics degradation by advanced oxidation process (AOPs): Recent advances in ecotoxicity and antibiotic-resistance genes induction of degradation products. Chemosphere 2022, 311 Pt 2, 136977. [Google Scholar] [CrossRef]
- He, S.J.; Wang, J.L.; Ye, L.F.; Zhang, Y.X.; Yu, J. Removal of diclofenac from surface water by electron beam irradiation combined with a biological aerated filter. Radiat. Phys. Chem. 2014, 105, 104–108. [Google Scholar] [CrossRef]
- de Wilt, A.; van Gijn, K.; Verhoek, T.; Vergnes, A.; Hoek, M.; Rijnaarts, H.; Alette Langenhoff, A. Enhanced pharmaceutical removal from water in a three step bio-ozone-bio process. Water Res. 2018, 138, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Zietzschmann, F.; Mitchell, R.L.; Jekel, M. Impacts of ozonation on the competition between organic micropollutants and effluent organic matter in powdered activated carbon adsorption. Water Res. 2015, 84, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Bourgin, M.; Beck, B.; Boehler, M.; Borowska, E.; Fleiner, J.; Salhi, E.; Teichler, R.; von Gunten, U.; Siegrist, H.; McArdell, C.S. Evaluation of a full-scale wastewater treatment plant upgraded with ozonation and biological post-treatments: Abatement of micropollutants, formation of transformation products and oxidation by-products. Water Res. 2018, 129, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Ullberg, M.; Lavonen, E.; Köhler, S.J.; Golovkoa, O.; Wiberg, K. Pilot-scale removal of organic micropollutants and natural organic matter from drinking water using ozonation followed by granular activated carbon. Environ. Sci. Water Res. Tech. 2021, 7, 535–548. [Google Scholar] [CrossRef]
- Loganathan, P.; Kandasamy, J.; Ratnaweera, H.; Vigneswaran, S. Submerged membrane/adsorption hybrid process in water reclamation and concentrate management—A mini review. Environ. Sci. Pollut. Res. 2022. [Google Scholar] [CrossRef]
- Smith, P.J.; Vigneswaran, S.; Ngo, H.; Nguyen, H.T.; Aim, R.B. A New Approach to backwash initiation in Membrance Systems. J. Membr. Sci. 2006, 278, 381–389. [Google Scholar] [CrossRef]
- Smith, P.J.; Vigneswaran, S.; Ngo, H.; Ben-Aim, R.; Nguyen, H.T. Design of a generic control system for optimising back flush durations in a submerged membrane hybrid reactor. J. Membr. Sci. 2005, 255, 99–106. [Google Scholar] [CrossRef]
- Ma, L.; Gutierrez, L.; Van Vooren, T.; Vanoppen, M.; Kazemabad, M.; Verliefde, A.; Cornelissen, E. Fate of organic micropollutants in reverse electrodialysis: Influence of membrane fouling and channel clogging. Desalination 2021, 512, 115114. [Google Scholar] [CrossRef]
- Jamil, S.; Loganathan, P.; Kazner, C.; Vigneswaran, S. Forward osmosis treatment for volume minimisation of reverse osmosis concentrate from a water reclamation plant and removal of organic micropollutants. Desalination 2015, 372, 32–38. [Google Scholar] [CrossRef]
- Hai, F.I.; Yamamoto, K.; Lee, C.-H. Membrane Biological Reactors: Theory, Modeling, Design, Management and Applications to Wastewater Reuse; IWA Publishing: London, UK, 2018. [Google Scholar]
- Neoh, C.H.; Noor, Z.Z.; Mutamim, N.S.A.; Lim, C.K. Green technology in wastewater treatment technologies: Integration of membrane bioreactor with various wastewater treatment systems. Chem. Eng. J. 2016, 283, 582–594. [Google Scholar] [CrossRef]
- Pathak, N.; Shon, H.; Vigneswaran, S. Advanced Membrane Bioreactor Hybrid Systems, Sustainable Technologies for Water and Wastewater Treatment; CRC Press: Boca Raton, FL, USA, 2021; pp. 317–342. [Google Scholar]
- Luo, W.; Arhatari, B.; Gray, S.R.; Xie, M. Seeing is believing: Insights from synchrotron infrared mapping for membrane fouling in osmotic membrane bioreactors. Water Res. 2018, 137, 355–361. [Google Scholar] [CrossRef]
- Wijekoon, K.C.; Hai, F.I.; Kang, J.; Price, W.E.; Guo, W.; Ngo, H.H.; Cath, T.Y.; Nghiem, L.D. A novel membrane distillation–thermophilic bioreactor system: Biological stability and trace organic compound removal. Bioresour. Technol. 2014, 159, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Krzeminski, P.; Leverette, L.; Malamis, S.; Katsou, E. Membrane bioreactors—A review on recent developments in energy reduction, fouling control, novel configurations, LCA and market prospects. J. Membr. Sci. 2017, 527, 207–227. [Google Scholar] [CrossRef]
- Holloway, R.W.; Miller-Robbie, L.; Patel, M.; Stokes, J.R.; Munakata-Marr, J.; Dadakis, J.; Cath, T.Y. Life-cycle assessment of two potable water reuse technologies: MF/RO/UV–AOP treatment and hybrid osmotic membrane bioreactors. J. Membr. Sci. 2016, 507, 165–178. [Google Scholar] [CrossRef]
- Sun, Q.; Lv, M.; Hu, A.; Yang, X.; Yu, C.-P. Seasonal variation in the occurrence and removal of pharmaceuticals and personal care products in a wastewater treatment plant in Xiamen, China. J. Hazard. Mater. 2014, 277, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Snyder, S.A.; Adham, S.; Redding, A.M.; Cannon, F.S.; DeCarolis, J.; Oppenheimer, J.; Wert, E.C.; Yoon, Y. Role of membranes and activated carbon in the removal of endocrine disruptors and pharmaceuticals. Desalination 2007, 202, 156–181. [Google Scholar] [CrossRef]
- Altmann, J.; Ruhl, A.S.; Zietzschmann, F.; Jekel, M. Direct comparison of ozonation and adsorption onto powdered activated carbon for micropollutant removal in advanced wastewater treatment. Water Res. 2014, 55, 185–193. [Google Scholar] [CrossRef]
- Rostamian, R.; Behnejad, H. A comparative adsorption study of sulfamethoxazole onto graphene and graphene oxide nanosheets through equilibrium, kinetic and thermodynamic modeling. Process Saf. Environ. Prot. 2016, 102, 20–29. [Google Scholar] [CrossRef]
- Rizzo, L.; Fiorentino, A.; Grassi, M.; Attanasio, D.; Guida, M. Advanced treatment of urban wastewater by sand filtration and graphene adsorption for wastewater reuse: Effect on a mixture of pharmaceuticals and toxicity. J. Environ. Chem. Eng. 2015, 3, 122–128. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Koltsakidou, A.; Nanaki, S.G.; Bikiaris, D.N.; Lambropoulou, D.A. Removal of beta-blockers from aqueous media by adsorption onto graphene oxide. Sci. Total Environ. 2015, 537, 411–420. [Google Scholar] [CrossRef]
- Michael, I.; Hapeshi, E.; Michael, C.; Fatta-Kassinos, D. Solar Fenton and solar TiO2 catalytic treatment of ofloxacin in secondary treated effluents: Evaluation of operational and kinetic parameters. Water Res. 2010, 44, 5450–5462. [Google Scholar] [CrossRef]
- Trovo, A.G.; Melo, S.A.S.; Nogueira, R.F.P. Photodegradation of the pharmaceuticals amoxicillin, bezafibrate and paracetamol by the photo-Fenton processed application to sewage treatment plant effluent. J. Photochem. Photobiol. A Chem. 2008, 198, 215–220. [Google Scholar] [CrossRef]
- Mendez-Arriaga, F.; Esplugas, S.; Gimenez, J. Degradation of the emerging contaminant ibuprofen in water by photo-Fenton. Water Res. 2010, 44, 589–595. [Google Scholar] [CrossRef]
- Bae, S.; Kim, D.; Lee, W. Degradation of diclofenac by pyrite catalyzed Fenton oxidation. Appl. Catal. B Environ. 2013, 134, 93–102. [Google Scholar] [CrossRef]
- Trovo, A.G.; Nogueira, R.F. Diclofenac abatement using modified solar photo-Fenton process with ammonium iron (III) citrate. J. Braz. Chem. Soc. 2011, 22, 1033–1039. [Google Scholar] [CrossRef]
- Perez-Estrada, L.A.; Malato, S.; Gernjak, W.; Agüera, A.; Thurman, E.M.; Ferrer, I.; Fern_andez-Alba, A.R. Photo-Fenton degradation of diclofenac: Identification of main intermediates and degradation pathway. Environ. Sci. Technol. 2005, 39, 8300–8306. [Google Scholar] [CrossRef] [PubMed]
- Velichkova, F.; Julcour-Lebigue, C.; Koumanova, B.; Delmas, H. Heterogeneous Fenton oxidation of paracetamol using iron oxide (nano) particles. J. Environ.Chem. Eng. 2013, 1, 1214–1222. [Google Scholar] [CrossRef]
- Lan, R.-J.; Li, J.-T.; Sun, H.-W.; Su, W.-B. Degradation of naproxen by combination of Fenton reagent and ultrasound irradiation: Optimization using response surface methodology. Water Sci. Technol. 2012, 66, 2695–2701. [Google Scholar] [CrossRef]
- Goi, A.; Veressinina, Y.; Trapido, M. Degradation of salicylic acid by Fenton and modified Fenton treatment. Chem. Eng. J. 2008, 143, 1–9. [Google Scholar] [CrossRef]
- Veloutsou, S.; Bizani, E.; Fytianos, K. Photo-Fenton decomposition of blockers atenolol and metoprolol; study and optimization of system parameters and identification of intermediates. Chemosphere 2014, 107, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Ghauch, A.; Tuqan, A.; Assi, H.A. Antibiotic removal from water: Elimination of amoxicillin and ampicillin by microscale and nanoscale iron particles. Environ. Pollut. 2009, 157, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, D.; Brar, S.; Tyagi, R.; Picard, P.; Surampalli, R. A comparative study of ultrasonication, Fenton’s oxidation and ferro-sonication treatment for degradation of carbamazepine from wastewater and toxicity test by Yeast Estrogen Screen (YES) assay. Sci. Total Environ. 2013, 447, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.P.; Zeng, X.; Li, C.; Lemley, A.T. Enhanced heterogeneous and homogeneous Fenton-like degradation of carbamazepine by nano-Fe3O4/H2O2 with nitrilotriacetic acid. Chem. Eng. J. 2014, 244, 44–49. [Google Scholar] [CrossRef]
- Ferrag-Siagh, F.; Fourcade, F.; Soutrel, I.; Aït-Amar, H.; Djelal, H.; Amrane, A. Electro-Fenton pretreatment for the improvement of tylosin biodegradability. Environ. Sci. Pollut. Res. 2014, 21, 8534–8542. [Google Scholar] [CrossRef] [PubMed]
- Annabi, C.; Fourcade, F.; Soutrel, I.; Geneste, F.; Floner, D.; Bellakhal, N.; Amrane, A. Degradation of enoxacin antibiotic by the electro-Fenton process: Optimization, biodegradability improvement and degradation mechanism. J. Environ. Manag. 2016, 165, 96–105. [Google Scholar] [CrossRef]
- Sires, I.; Arias, C.; Cabot, P.L.; Centellas, F.; Garrido, J.A.; Rodríguez, R.M.; Brillas, E. Degradation of clofibric acid in acidic aqueous medium by electro-Fenton and photoelectro-Fenton. Chemosphere 2007, 66, 1660–1669. [Google Scholar] [CrossRef] [PubMed]
- Klamerth, N.; Rizzo, L.; Malato, S.; Maldonado, M.I.; Agüera, A.; Fernandez-Alba, A. Degradation of fifteen emerging contaminants at mgL_1 initial concentrations by mild solar photo-Fenton in MWTP effluents. Water Res. 2010, 44, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Isarain-Chavez, E.; Garrido, J.A.; Rodríguez, R.M.; Centellas, F.; Arias, C.; Cabot, P.L.; Brillas, E. Mineralization of metoprolol by electro-Fenton and photoelectro-Fenton processes. J. Phys. Chem. A 2011, 115, 1234–1242. [Google Scholar] [CrossRef]
- Ahmed, M.M.; Chiron, S. Solar photo-Fenton like using persulphate for carbamazepine removal from domestic wastewater. Water Res. 2014, 48, 229–236. [Google Scholar] [CrossRef]
- De la Cruz, N.; Gimenez, J.; Esplugas, S.; Grandjean, D.; De Alencastro, L.F.; Pulgarin, C. Degradation of 32 emergent contaminants by UV and neutral photo-fenton in domestic wastewater effluent previously treated by activated sludge. Water Res. 2012, 46, 1947–1957. [Google Scholar] [CrossRef]
- Dogan, S.; Kidak, R. A Plug flow reactor model for UV-based oxidation of amoxicillin. Desalination Water Treat. 2016, 57, 13586–13599. [Google Scholar] [CrossRef]
- The Swedish Environmental Protection Agency. Advanced Wastewater Treatment for Separation and Removal of Pharmaceutical Residues and Other Hazardous Substances–Needs, Technologies and Impacts. A Government-Commissioned Report; Report 6803; The Swedish Environmental Protection Agency: Stockholm, Sweden, 2017. [Google Scholar]
- Yao, M.; Duan, L.; Wei, J.; Qian, F.; Hermanowicz, S.W. Carbamazepine removal from wastewater and the degradation mechanism in a submerged forward osmotic membrane bioreactor. Bioresour. Technol. 2020, 314, 123732. [Google Scholar] [CrossRef] [PubMed]
- Pathak, N.; Li, S.; Kim, Y.; Chekli, L.; Phuntsho, S.; Jang, A.; Ghaffour, N.; Leiknes, T.; Shon, H.K. Assessing the removal of organic micropollutants by a novel baffled osmotic membrane bioreactor-microfiltration hybrid system. Bioresour. Technol. 2018, 262, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Luo, W.; Mcdonald, J.; Khan, S.J.; Hai, F.I.; Price, W.E.; Nghiem, L.D. An anaerobic membrane bioreactor–membrane distillation hybrid system for energy recovery and water reuse: Removal performance of organic carbon, nutrients, and trace organic contaminants. Sci. Total Environ. 2018, 628, 358–365. [Google Scholar] [CrossRef] [PubMed]

| PPCP | By NF alone | By GAC + NF | By Purolite + NF |
|---|---|---|---|
| Benzotriazole | 35 | 94 | 99 |
| Carbamazepine | 96 | 96 | >98 |
| Diclofenac | >93 | >93 | >93 |
| Diuron | 77 | >94 | >94 |
| Gemfibrozil | >95 | >95 | >95 |
| Ibuprofen | >90 | >90 | >90 |
| Naproxen | >98 | >98 | >98 |
| Saccharin | 88 | >92 | >92 |
| Triclosan | >92 | >92 | >92 |
| Trimethoprim | >97 | >97 | >97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loganathan, P.; Vigneswaran, S.; Kandasamy, J.; Cuprys, A.K.; Maletskyi, Z.; Ratnaweera, H. Treatment Trends and Combined Methods in Removing Pharmaceuticals and Personal Care Products from Wastewater—A Review. Membranes 2023, 13, 158. https://doi.org/10.3390/membranes13020158
Loganathan P, Vigneswaran S, Kandasamy J, Cuprys AK, Maletskyi Z, Ratnaweera H. Treatment Trends and Combined Methods in Removing Pharmaceuticals and Personal Care Products from Wastewater—A Review. Membranes. 2023; 13(2):158. https://doi.org/10.3390/membranes13020158
Chicago/Turabian StyleLoganathan, Paripurnanda, Saravanamuthu Vigneswaran, Jaya Kandasamy, Agnieszka Katarzyna Cuprys, Zakhar Maletskyi, and Harsha Ratnaweera. 2023. "Treatment Trends and Combined Methods in Removing Pharmaceuticals and Personal Care Products from Wastewater—A Review" Membranes 13, no. 2: 158. https://doi.org/10.3390/membranes13020158
APA StyleLoganathan, P., Vigneswaran, S., Kandasamy, J., Cuprys, A. K., Maletskyi, Z., & Ratnaweera, H. (2023). Treatment Trends and Combined Methods in Removing Pharmaceuticals and Personal Care Products from Wastewater—A Review. Membranes, 13(2), 158. https://doi.org/10.3390/membranes13020158







