Comparative Study of Membrane Fouling with Aeration Shear Stress in Filtration of Different Substances
Abstract
:1. Introduction
2. Methods
2.1. Experimental Study
2.1.1. Large-Scale FSMBR System
- Humic acid solution: mixing 500 g humic acid with 10,000 kg tap water in a water tank for more than 2 h using a gas with a maximum aeration intensity of 24 L/(m2·min).
- Humic acid + Ca2+ solution: mixing 500 g humic acid and 7350 g calcium chloride dihydrate with 10,000 kg tap water in a water tank for more than 2 h using a gas with a maximum aeration intensity of 24 L/(m2·min).
- Humic acid + Ca2+ + yeast solution: mixing 500 g humic acid, 7350 g calcium chloride dihydrate and 100 kg yeast with 10,000 kg tap water in a water tank for more than 2 h using a gas with a maximum aeration intensity of 24 L/(m2·min).
2.1.2. Data Collection and Analysis
- A is the membrane area, m2
- T is the filtering time, s
- J is the membrane flux, m3/ (m2·s)
- P is the constant filtration pressure difference, kPa
- μ is the viscosity of the transmission fluid, Pa·s
- Rm is the intrinsic resistance of membrane system, m−1
- Rf is the membrane fouling resistance, m−1
- V is the cumulative effluent volume, m3
- Qi is the instantaneous effluent flow, m3/h
- Δt is the recording interval, h.
2.2. Numerical Simulation Method
2.2.1. Physical Model and Meshing
2.2.2. Numerical Methods
- : function of the mean strain, rotation and turbulence fields;
- : turbulence kinetic energy due to the mean velocity gradients;
- : turbulence kinetic energy due to buoyancy;
- : fluctuating dilatation in compressible turbulence to the overall dissipation rate;
- , , : constant;
- : turbulent Prandtl numbers for k;
- : turbulent Prandtl numbers for ε;
- and : user-defined source terms.
3. Results and Discussion
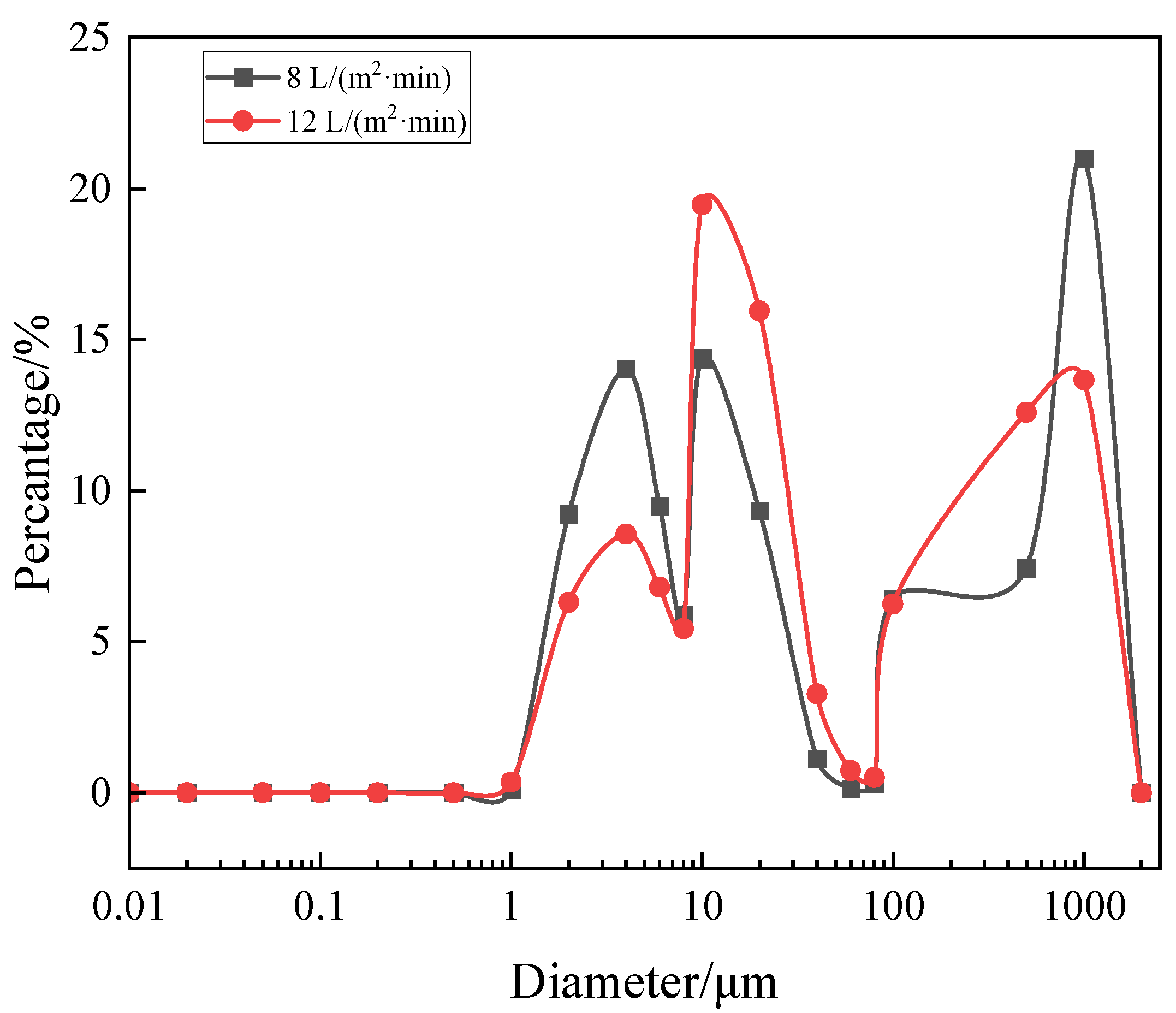
3.1. Size Distribution
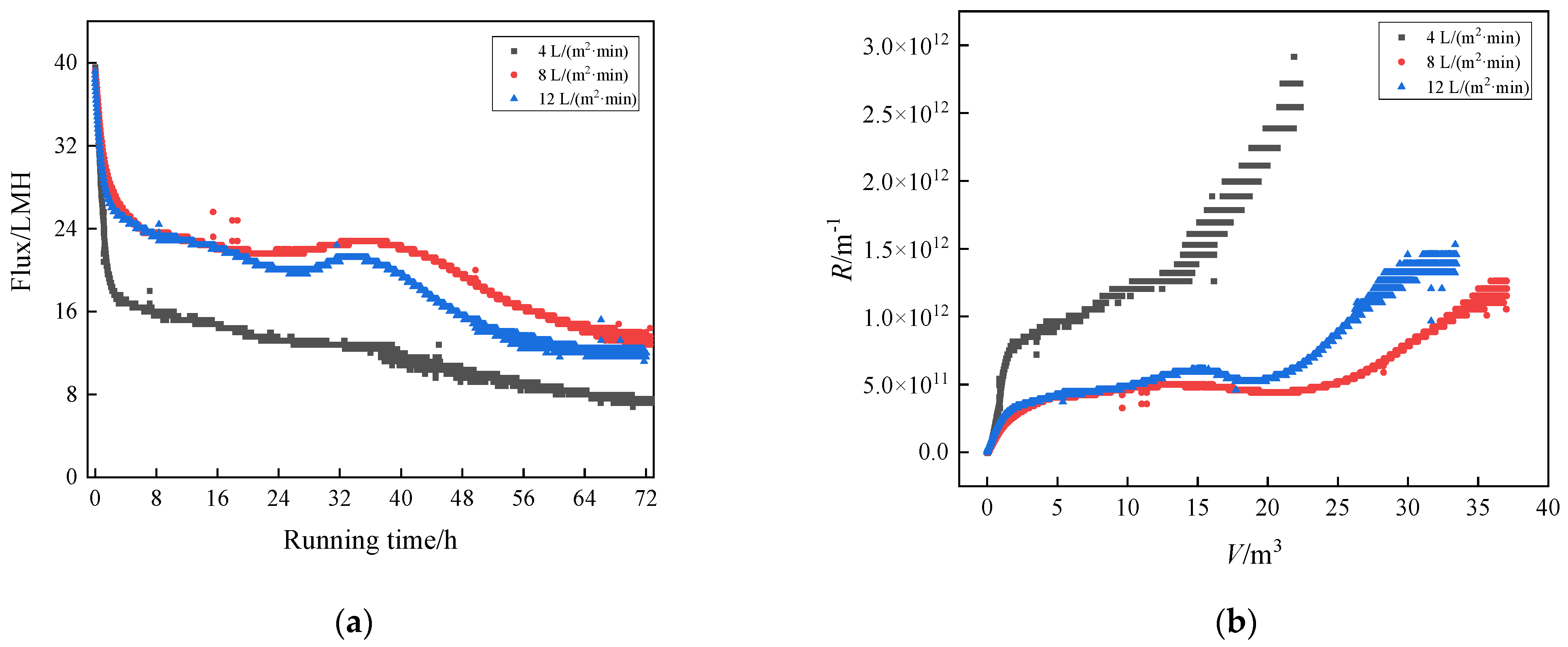
3.2. Flux Decline and Fouling Resistance
3.2.1. Humic Acid
3.2.2. Humic Acid + Ca2+
3.2.3. Humic Acid + Ca2+ + Yeast
3.3. Bubble and Shear Stress Distribution
3.4. Fouling Rate and Shear Stress
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.; Wang, B.; Chen, Z.; Ma, B.; Chen, J.P. Ultrafiltration membrane fouling by microplastics with raw water: Behaviors and alleviation methods. Chem. Eng. J. 2021, 410, 128174. [Google Scholar] [CrossRef]
- Tong, K.; Lin, A.; Ji, G.; Wang, D.; Wang, X. The effects of adsorbing organic pollutants from super heavy oil wastewater by lignite activated coke. J. Hazard. Mater. 2016, 308, 113–119. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, J.; Tang, C.Y.; Kimura, K.; Wang, Q.; Han, X. Membrane cleaning in membrane bioreactors: A review. J. Memb. Sci. 2014, 468, 276–307. [Google Scholar] [CrossRef]
- Braak, E.; Alliet, M.; Schetrite, S.; Albasi, C. Aeration and hydrodynamics in submerged membrane bioreactors. J. Memb. Sci. 2011, 379, 1–18. [Google Scholar] [CrossRef]
- Hu, G.; Liu, X.; Wang, Z.; Du, X.; Wang, X. Comparison of fouling behaviors between activated sludge suspension in MBR and EPS model solutions: A new combined model. J. Memb. Sci. 2021, 621, 119020. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Cui, Z.; Yao, Y. Modeling of filtration characteristics during submerged hollow fiber membrane microfiltration of yeast suspension under aeration condition. J. Memb. Sci. 2016, 510, 455–465. [Google Scholar] [CrossRef]
- Meng, F.; Yang, F.; Shi, B.; Zhang, H. A comprehensive study on membrane fouling in submerged membrane bioreactors operated under different aeration intensities. Sep. Purif. Technol. 2008, 59, 91–100. [Google Scholar] [CrossRef]
- Gao, W.; Liang, H.; Ma, J.; Han, M.; Chen, Z.L.; Han, Z.S.; Li, G.B. Membrane fouling control in ultrafiltration technology for drinking water production: A review. Desalination 2011, 272, 1–8. [Google Scholar] [CrossRef]
- Yuan, W.; Zydney, A.L. Humic acid fouling during microfiltration. J. Memb. Sci. 1999, 157, 1–12. [Google Scholar] [CrossRef]
- Jermann, D.; Pronk, W.; Kägi, R.; Halbeisen, M.; Boller, M. Influence of interactions between NOM and particles on UF fouling mechanisms. Water Res. 2008, 42, 3870–3878. [Google Scholar] [CrossRef]
- Yuan, W.; Zydney, A.L. Effects of solution environment on humic acid fouling during microfiltration. Desalination 1999, 122, 63–76. [Google Scholar] [CrossRef]
- Taheri, A.H.; Sim, L.N.; Haur, C.T.; Akhondi, E.; Fane, A.G. The fouling potential of colloidal silica and humic acid and their mixtures. J. Memb. Sci. 2013, 433, 112–120. [Google Scholar] [CrossRef]
- Xu, H.; Xiao, K.; Wang, X.; Liang, S.; Wei, C.; Wen, X.; Huang, X. Outlining the Roles of Membrane-Foulant and Foulant-Foulant Interactions in Organic Fouling during Microfiltration and Ultrafiltration: A Mini-Review. Front. Chem. 2020, 8, 417. [Google Scholar] [CrossRef] [PubMed]
- Teychene, B.; Collet, G.; Gallard, H. Modeling of combined particles and natural organic matter fouling of ultrafiltration membrane. J. Memb. Sci. 2016, 505, 185–193. [Google Scholar] [CrossRef]
- Sioutopoulos, D.C.; Goudoulas, T.B.; Kastrinakis, E.G.; Nychas, S.G.; Karabelas, A.J. Rheological and permeability characteristics of alginate fouling layers developing on reverse osmosis membranes during desalination. J. Memb. Sci. 2013, 434, 74–84. [Google Scholar] [CrossRef]
- Patsios, S.I.; Goudoulas, T.B.; Kastrinakis, E.G.; Nychas, S.G.; Karabelas, A.J. A novel method for rheological characterization of biofouling layers developing in Membrane Bioreactors (MBR). J. Memb. Sci. 2015, 482, 13–24. [Google Scholar] [CrossRef]
- Lasisi, K.H.; Yao, W.; Ajibade, T.F.; Tian, H.; Fang, F.; Zhang, K. Impacts of sulfuric acid on the stability and separation performance of polymeric pvdf-based membranes at mild and high concentrations: An experimental study. Membranes 2020, 10, 375. [Google Scholar] [CrossRef] [PubMed]
- Arabi, S.; Nakhla, G. Impact of calcium on the membrane fouling in membrane bioreactors. J. Memb. Sci. 2008, 314, 134–142. [Google Scholar] [CrossRef]
- Hirt, C.W.; Nichols, B.D. Volume of fluid (VOF) method for the dynamics of free boundaries. J. Comput. Phys. 1981, 39, 201–225. [Google Scholar] [CrossRef]
- Brackbill, J.U.; Kothe, D.B.; Zemach, C. A continuum method for modeling surface tension. J. Comput. Phys. 1992, 100, 335–354. [Google Scholar] [CrossRef]
- Benedetti, M.F.; Milne, C.J.; Kinniburgh, D.G.; Van Riemsdijk, W.H.; Koopal, L.K. Metal Ion Binding to Hemic Substances: Application of the Non-Ideal Competitive Adsorption Model. Environ. Sci. Technol. 1995, 29, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lv, G.; Liao, L.; He, M.; Li, Z.; Wang, M. Removal of Low Concentrations of Ammonium and Humic Acid from Simulated Groundwater by Vermiculite/Palygorskite Mixture. Water Environ. Res. 2012, 84, 682–688. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; He, X.; Wang, P.; Liu, Q.; Qiu, W.; Ma, J. Opposite impacts of K+ and Ca2+ on membrane fouling by humic acid and cleaning process: Evaluation and mechanism investigation. Water Res. 2020, 183, 116006. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Hou, J.; Song, H. Comparison of humic acid rejection and flux decline during filtration with negatively charged and uncharged ultrafiltration membranes. Water Res. 2011, 45, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Kromkamp, J.; Faber, F.; Schroen, K.; Boom, R. Effects of particle size segregation on crossflow microfiltration performance: Control mechanism for concentration polarisation and particle fractionation. J. Memb. Sci. 2006, 268, 189–197. [Google Scholar] [CrossRef]
- Altmann, J.; Ripperger, S. Particle deposition and layer formation at the crossflow microfiltration. J. Memb. Sci. 1997, 124, 119–128. [Google Scholar] [CrossRef]
- Zhang, K.; Cui, Z.; Field, R.W. Effect of bubble size and frequency on mass transfer in flat sheet MBR. J. Memb. Sci. 2009, 332, 30–37. [Google Scholar] [CrossRef]

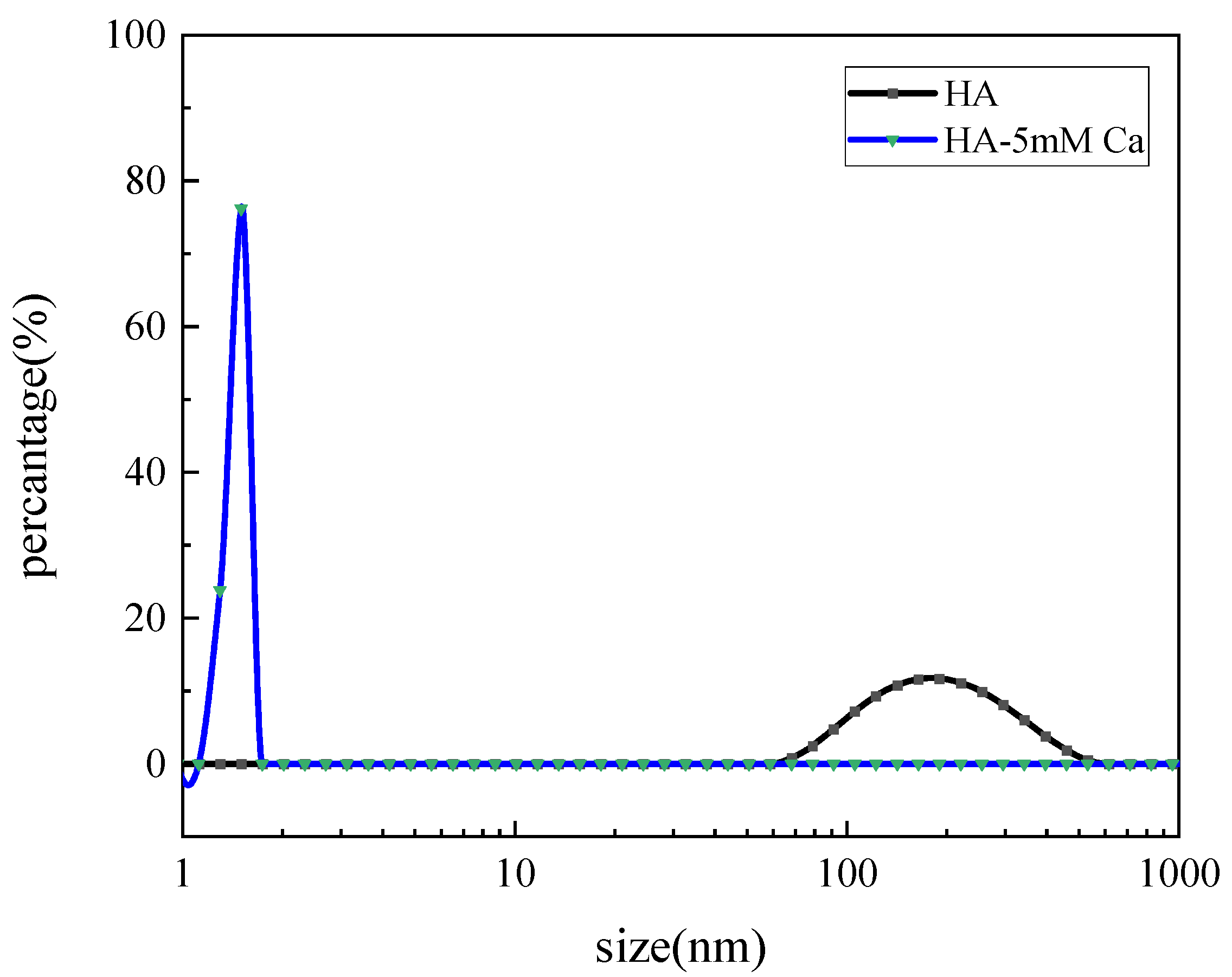
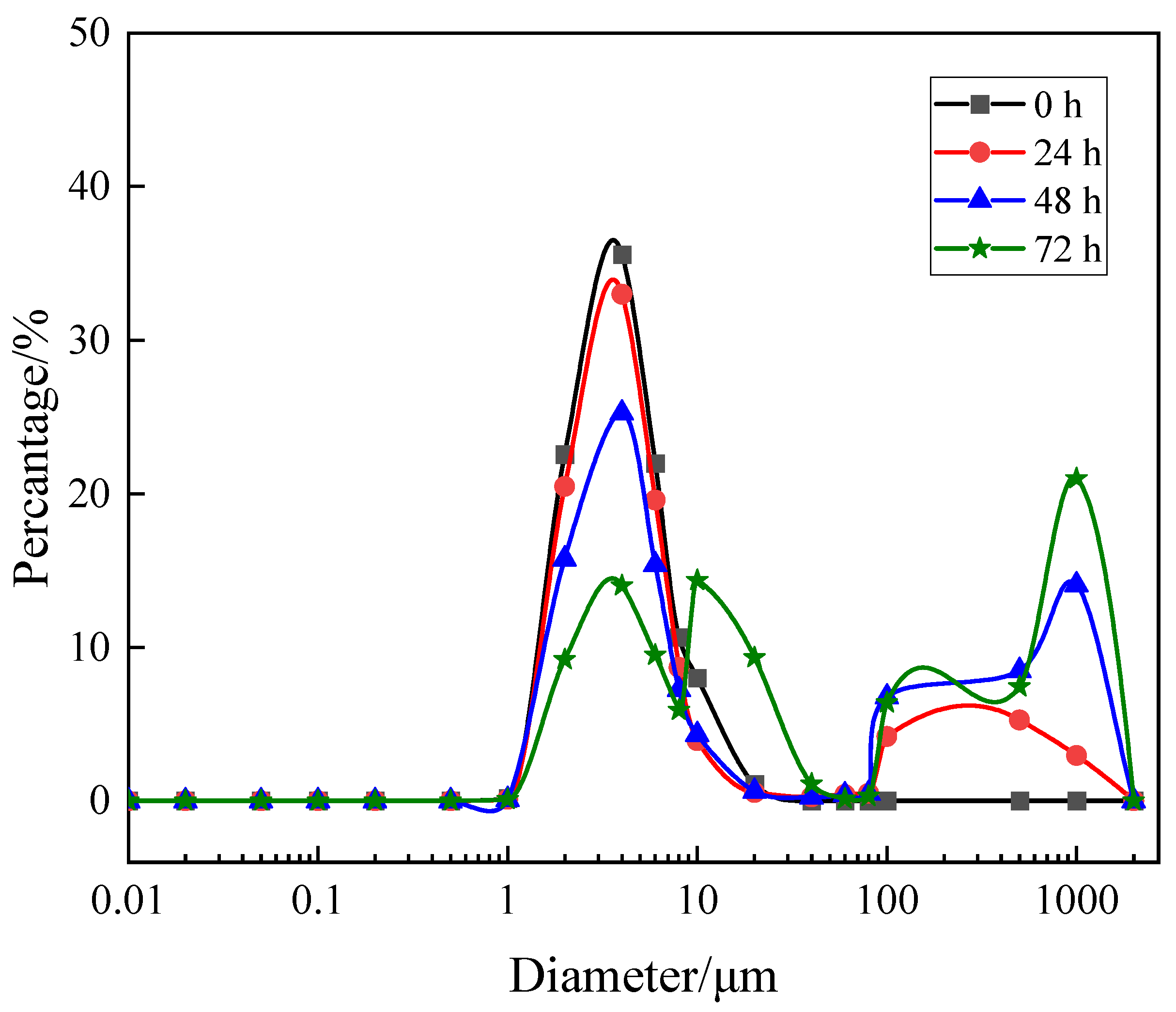
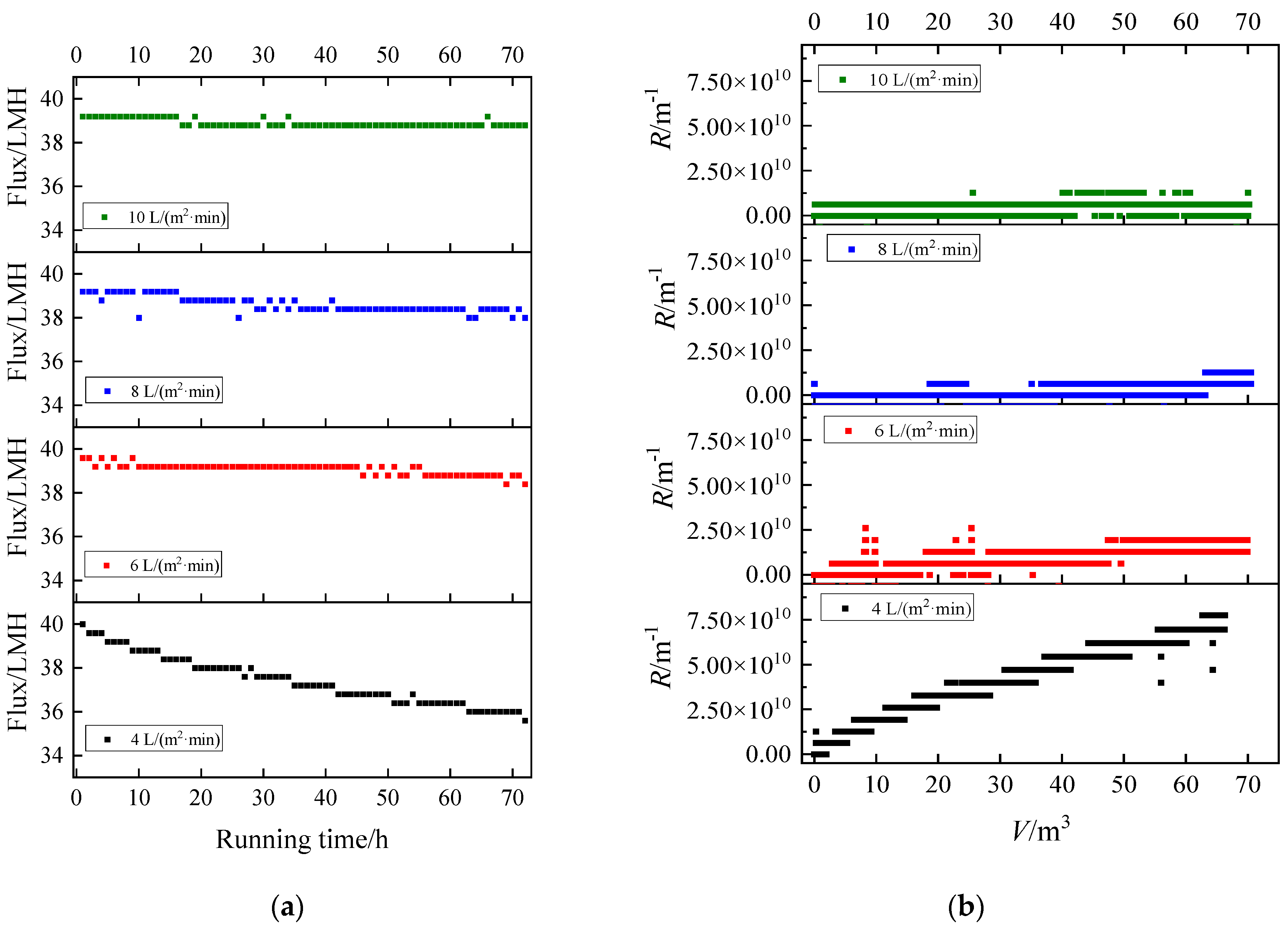
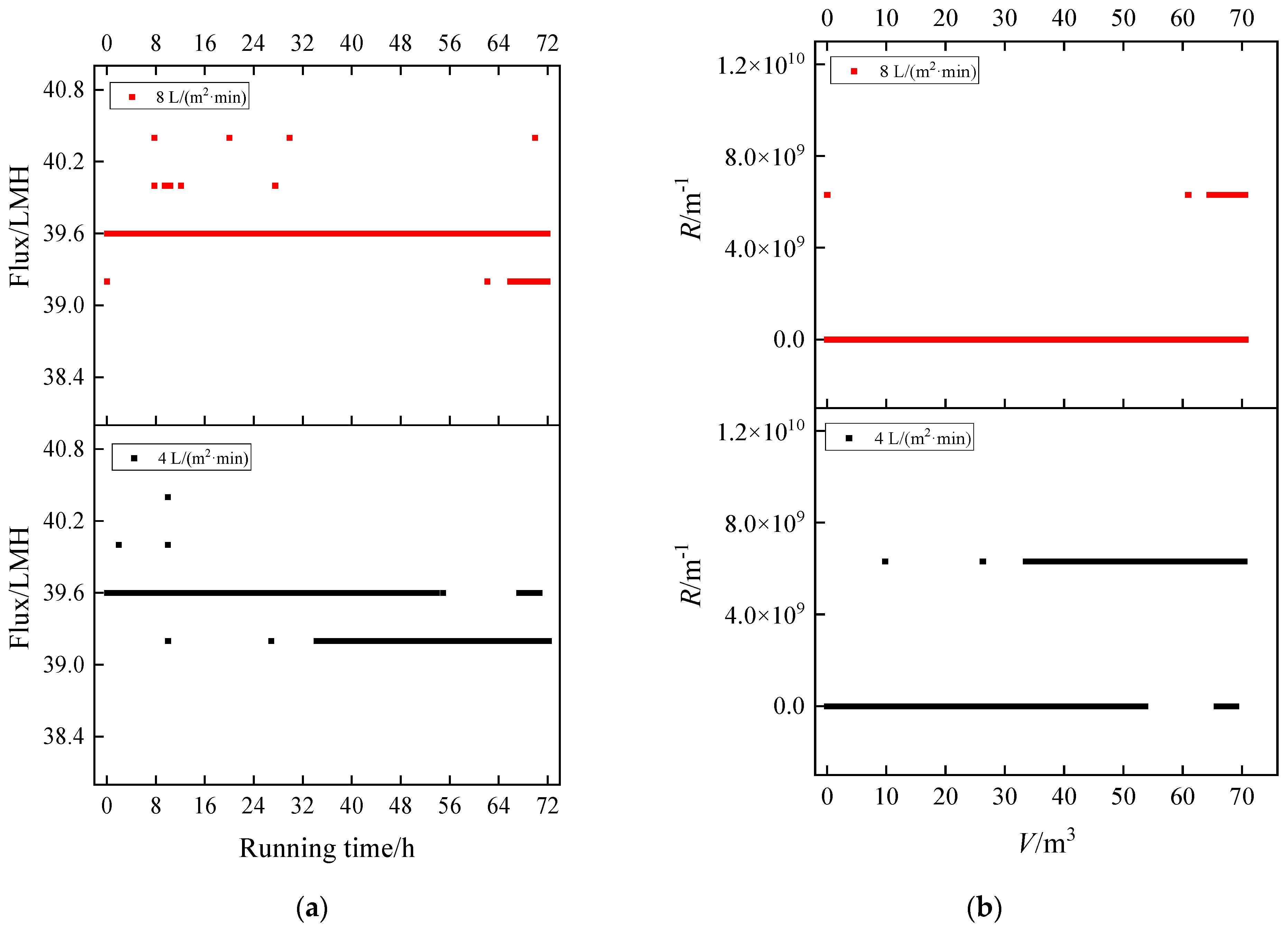


| No. | Component | Aeration Intensity/(L/m2·min) |
|---|---|---|
| 1 | HA | 4 |
| 2 | 6 | |
| 3 | 8 | |
| 4 | 10 | |
| 5 | HA + Ca2+ | 4 |
| 6 | 8 | |
| 7 | HA + Ca2+ + Yeast | 4 |
| 8 | 8 | |
| 9 | 12 |
| Aeration Intensity/(L/m2·min) | D [4, 3]/μm | D10/μm | D50/μm | D90/μm |
|---|---|---|---|---|
| 8 | 414 | 4.09 | 17.1 | 1500 |
| 12 | 308 | 4.75 | 22.3 | 1150 |
| Aeration Intensity/(L/m2·min) | 4 | 6 | 8 | 10 | 12 |
|---|---|---|---|---|---|
| Average shear stress/Pa | 0.644 | 0.982 | 1.410 | 1.642 | 1.556 |
| Maximum shear stress/Pa | 4.486 | 6.573 | 7.794 | 13.512 | 8.849 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, W.; Wang, B.; Zhang, K. Comparative Study of Membrane Fouling with Aeration Shear Stress in Filtration of Different Substances. Membranes 2023, 13, 867. https://doi.org/10.3390/membranes13110867
Yao W, Wang B, Zhang K. Comparative Study of Membrane Fouling with Aeration Shear Stress in Filtration of Different Substances. Membranes. 2023; 13(11):867. https://doi.org/10.3390/membranes13110867
Chicago/Turabian StyleYao, Weihao, Bing Wang, and Kaisong Zhang. 2023. "Comparative Study of Membrane Fouling with Aeration Shear Stress in Filtration of Different Substances" Membranes 13, no. 11: 867. https://doi.org/10.3390/membranes13110867
APA StyleYao, W., Wang, B., & Zhang, K. (2023). Comparative Study of Membrane Fouling with Aeration Shear Stress in Filtration of Different Substances. Membranes, 13(11), 867. https://doi.org/10.3390/membranes13110867





