Treatment of Spent Pickling Solutions by Diffusion Dialysis Using Anion-Exchange Membrane Neosepta-AFN
Abstract
:1. Introduction
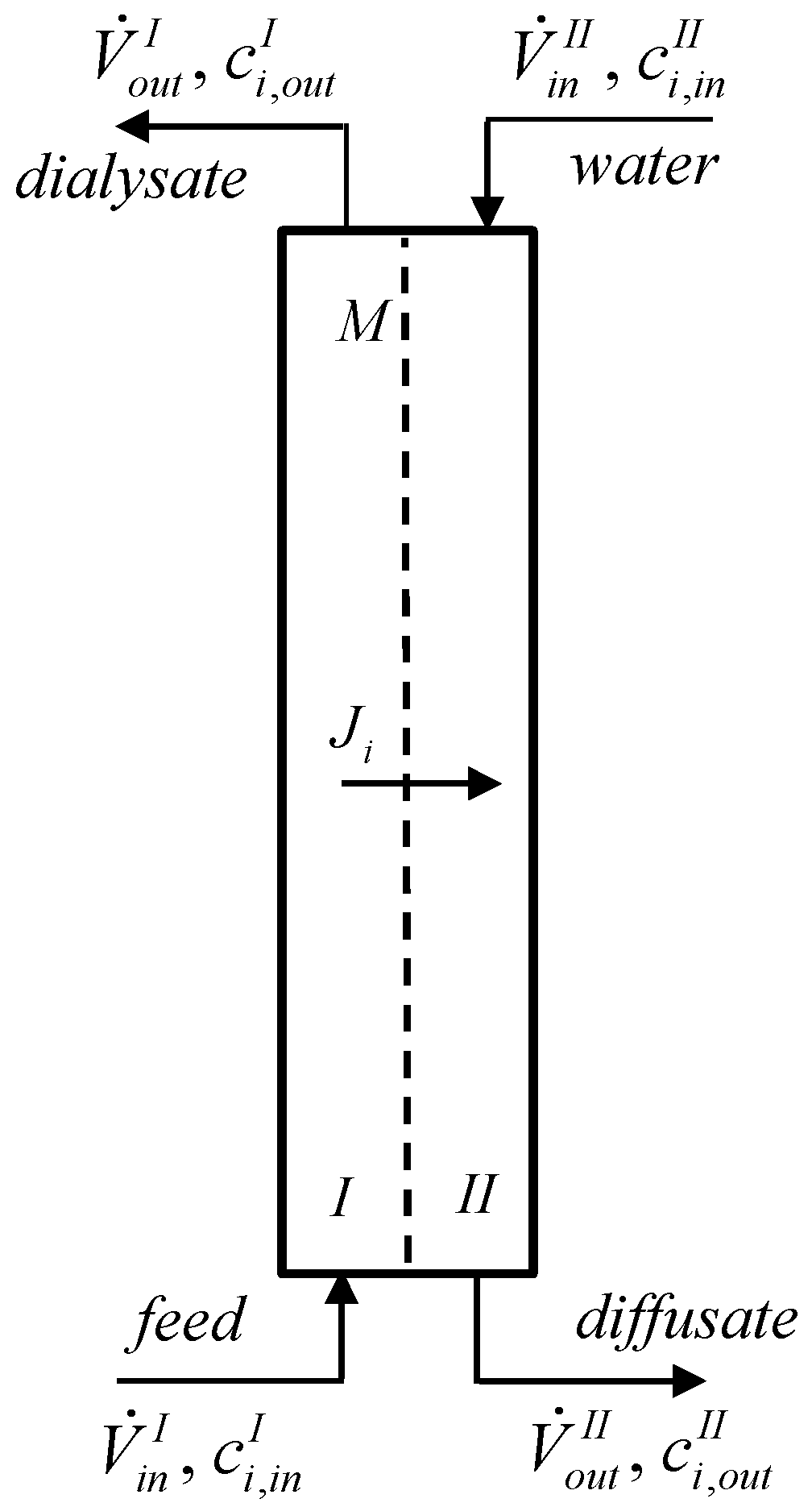
2. Theory
3. Materials and Methods
4. Results and Discussion
4.1. Comparison of the Model Solution and the Spent Pickling Solution
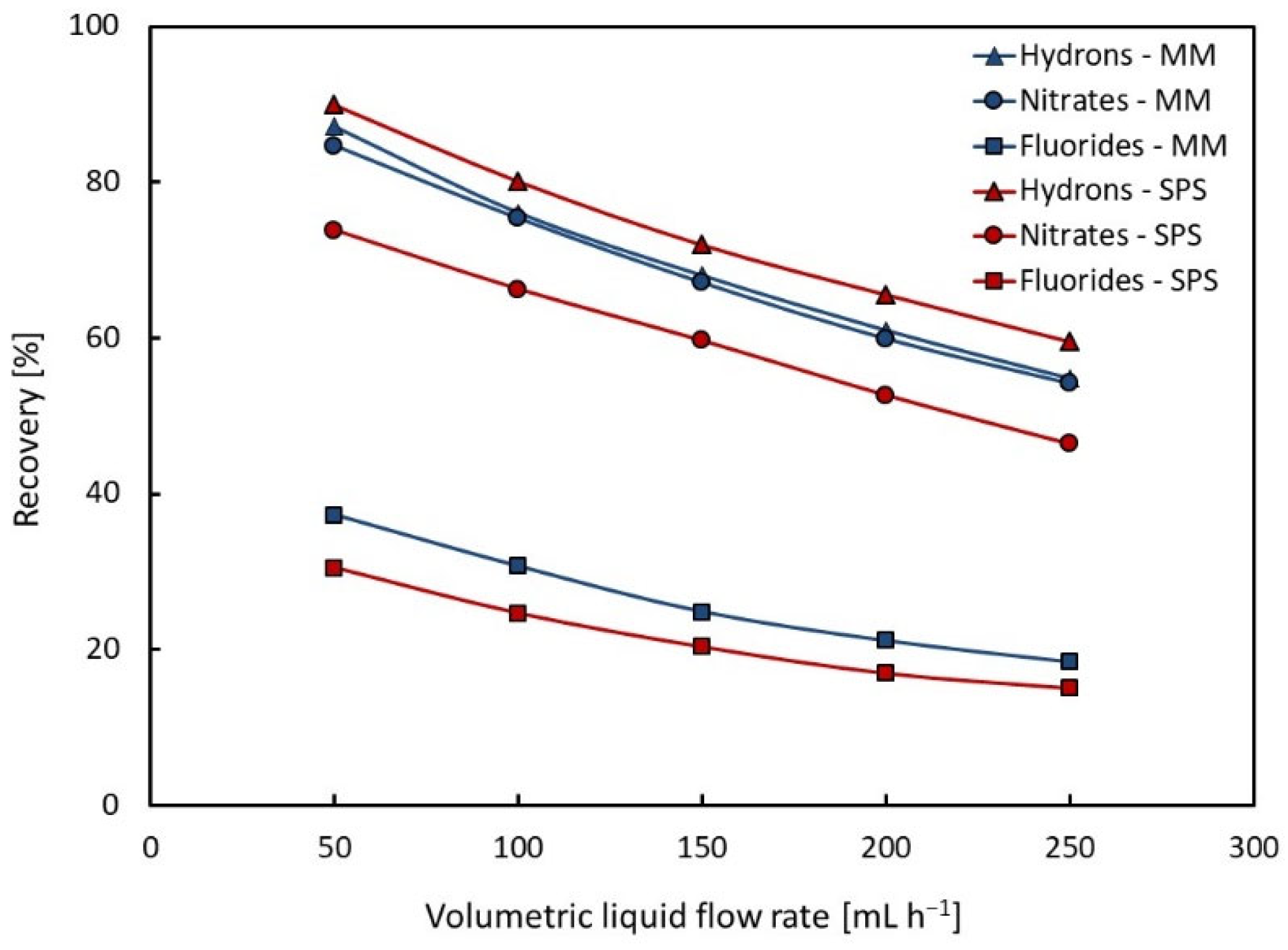
4.1.1. Recovery Yield
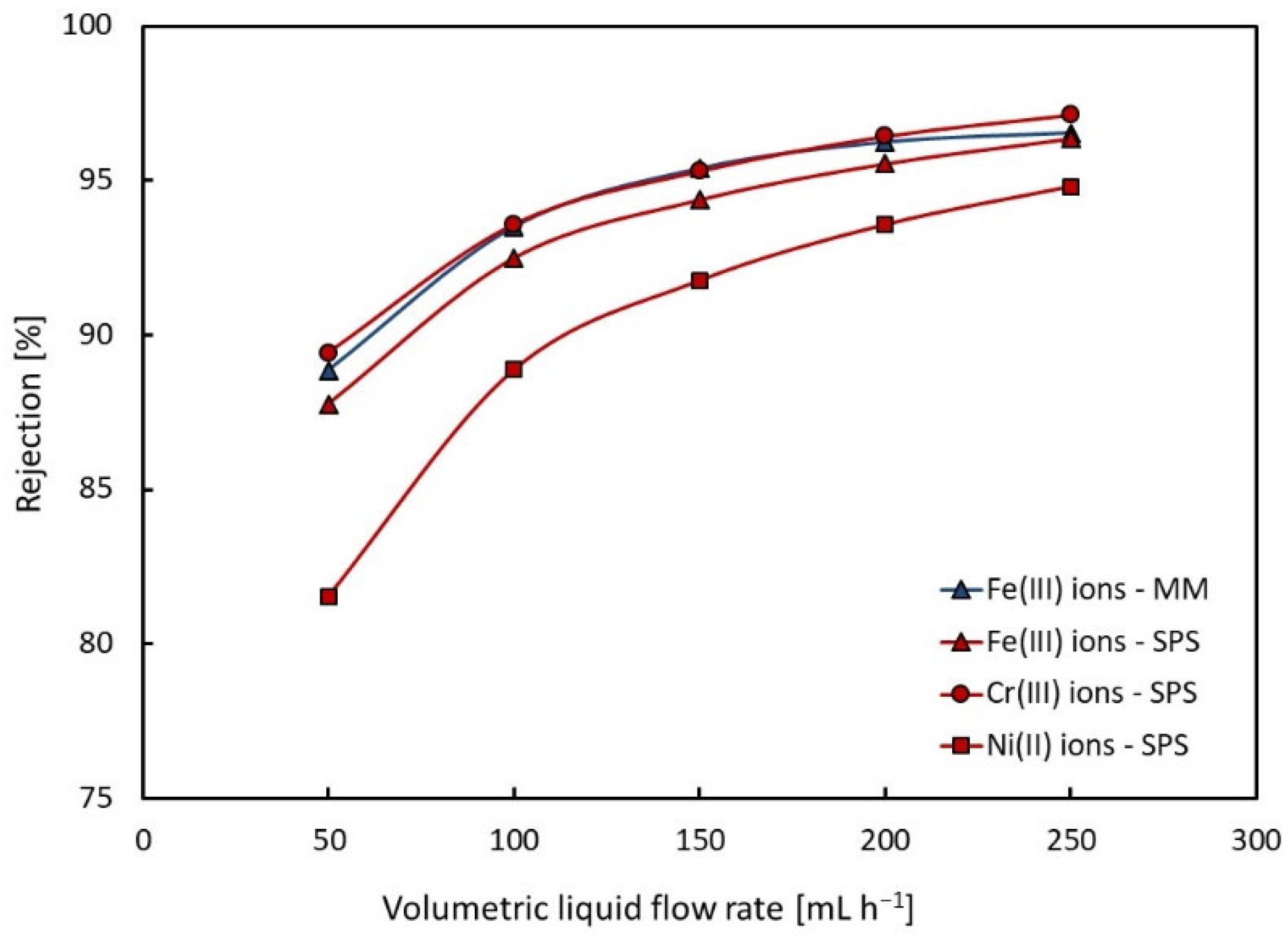
4.1.2. Rejection Coefficient
4.1.3. Permeability of Membrane and Separation Factor
HF + FeF(NO3)2 ⇔ HNO3 + FeF2NO3
HF + FeF2NO3 ⇔ HNO3 + FeF3
4.2. Recovery and Rejection for Different Acid and Salt Concentrations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| A | membrane area, m2 |
| C | constant in Equation (7) |
| c | molar concentration, mol L−1 |
| J | molar flux, kmol m−2 s−1 |
| kL | liquid mass transfer coefficient, m s−1 |
| P | permeability coefficient, m s−1 |
| R | rejection coefficient, % |
| Re | Reynolds number |
| Sc | Schmidt number |
| Sh | Sherwood number |
| volumetric liquid flow rate, m3 s−1 (mL h−1) | |
| ν | recovery yield, % |
| z | length coordinate, m |
| Subscripts and Superscripts | |
| I | referred to compartment I |
| II | referred to compartment II |
| i | referred to i ion |
| f | referred to solution/membrane interface |
| in | inlet |
| out | outlet |
References
- Regel-Rosocka, M. A review on methods of regeneration of spent pickling solutions from steel processing. J. Hazard. Mater. 2010, 177, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Devi, A.; Singhal, A.; Gupta, R.; Panzade, P. A study on treatment methods of spent pickling liquor generated by pickling process of steel. Clean Technol. Environ. Policy 2014, 16, 1515–1527. [Google Scholar] [CrossRef]
- Lan, S.J.; Wen, X.M.; Zhu, Z.H.; Shao, F.; Zhu, C.L. Recycling of spent nitric acid solution from electrodialysis by diffusion dialysis. Desalination 2011, 278, 227–230. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.M.; Huang, J.; Zhu, X.B.; Wang, Y. Separation and recovery of sulfuric acid from acidic vanadium leaching solution by diffusion dialysis. Sep. Purif. Technol. 2012, 96, 44–49. [Google Scholar] [CrossRef]
- Kim, J.Y.; Shin, C.H.; Choi, H.; Bae, W. Recovery of phosphoric acid from mixed waste acids of semiconductor industry by diffusion dialysis and vacuum distillation. Sep. Purif. Technol. 2012, 90, 64–68. [Google Scholar] [CrossRef]
- Ahn, J.W.; Ryu, S.H.; Kim, T.Y. Recovery of tin and nitric acid from spent solder stripping solutions. Korean J. Met. Mater. 2015, 53, 426–431. [Google Scholar] [CrossRef]
- Xiao, H.F.; Chen, Q.; Cheng, H.; Li, X.M.; Qin, W.M.; Chen, B.S.; Xiao, D.; Zhang, W.M. Selective removal of halides from spent zinc sulfate electrolyte by diffusion dialysis. J. Membr. Sci. 2017, 537, 111–118. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Y.M.; Huang, J.; Liu, T.; Wang, J.P. Recovery of sulfuric acid from a stone coal acid leaching solution by diffusion dialysis. Hydrometallurgy 2017, 173, 9–14. [Google Scholar] [CrossRef]
- Amrane, C.; Lalmi, A.; Bouhidel, K.E. Coupling diffusion dialysis with precipitation cementation to separate and recover nitric acid, Cu plus plus, Zn plus plus and Pb plus plus from the wastewater of a brass pickling bath. Int. J. Glob. Warm. 2017, 11, 337–357. [Google Scholar] [CrossRef]
- Bendova, H.; Weidlich, T. Application of diffusion dialysis in hydrometallurgical separation of nickel from spent Raney Ni catalyst. Sep. Sci. Technol. 2018, 53, 1218–1222. [Google Scholar] [CrossRef]
- Gueccia, R.; Aguirre, A.R.; Randazzo, S.; Cipollina, A.; Micale, G. Diffusion Dialysis for Separation of Hydrochloric Acid, Iron and Zinc Ions from Highly Concentrated Pickling Solutions. Membranes 2020, 10, 129. [Google Scholar] [CrossRef] [PubMed]
- Culcasi, A.; Gueccia, R.; Randazzo, S.; Cipollina, A.; Micale, G. Design of a novel membrane-integrated waste acid recovery process from pickling solution. J. Clean Prod. 2019, 236, 117623. [Google Scholar] [CrossRef]
- Gueccia, R.; Winter, D.; Randazzo, S.; Cipollina, A.; Koschikowski, J.; Micale, G.D.M. An integrated approach for the HCl and metals recovery from waste pickling solutions: Pilot plant and design operations. Chem. Eng. Res. Des. 2021, 168, 383–396. [Google Scholar] [CrossRef]
- Gueccia, R.; Bogle, D.; Randazzo, S.; Tamburini, A.; Cipollina, A.; Winter, D.; Koschikowski, J.; Micale, G. Economic Benefits of Waste Pickling Solution Valorization. Membranes 2022, 12, 114. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, M.Q.; Li, W.J.; Wu, C.M.; Han, X.Z.; Zhong, S.; Chen, Y.S. Application and modeling of pressure-concentration double-driven diffusion dialysis. J. Membr. Sci. 2020, 595, 117478. [Google Scholar] [CrossRef]
- Du, M.G.; Chen, Q.; Gao, W.T.; Li, X.M.; Zhang, W.M. Selective removal of chloride from the adipate formation bath in foil industry by diffusion dialysis. Sep. Purif. Technol. 2020, 230, 115871. [Google Scholar] [CrossRef]
- Hammache, Z.; Bensaadi, S.; Berbar, Y.; Audebrand, N.; Szymczyk, A.; Amara, M. Recovery of rare earth elements from electronic waste by diffusion dialysis. Sep. Purif. Technol. 2021, 254, 117641. [Google Scholar] [CrossRef]
- Loza, S.; Loza, N.; Korzhov, A.; Romanyuk, N.; Kovalchuk, N.; Melnikov, S. Hybrid Membrane Technology for Acid Recovery from Wastewater in Coated Steel Wire Production: A Pilot Scale Study. Membranes 2022, 12, 1196. [Google Scholar] [CrossRef]
- Merkel, A.; Copak, L.; Dvorak, L.; Golubenko, D.; Seda, L. Recovery of Spent Sulphuric Acid by Diffusion Dialysis Using a Spiral Wound Module. Int. J. Mol. Sci. 2021, 22, 11819. [Google Scholar] [CrossRef]
- Merkel, A.; Copak, L.; Golubenko, D.; Dvorak, L.; Vavro, M.; Yaroslavtsev, A.; Seda, L. Recovery of Hydrochloric Acid from Industrial Wastewater by Diffusion Dialysis Using a Spiral-Wound Module. Int. J. Mol. Sci. 2022, 23, 6212. [Google Scholar] [CrossRef]
- Bendová, H.; Palatý, Z.; Žáková, A. Continuous dialysis of inorganic acids: Permeability of Neosepta-AFN membrane. Desalination 2009, 240, 333–340. [Google Scholar] [CrossRef]
- Palatý, Z.; Bendová, H. Continuous dialysis of sulphuric acid and sodium sulphate mixture. J. Membr. Sci. 2016, 497, 36–46. [Google Scholar] [CrossRef]
- Coulson, J.M.; Richardson, J.F. Coulson & Richardson Chemical Engineering, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 1. [Google Scholar]
- Bendová, H.; Šnejdrla, P.; Palatý, Z. Continuous dialysis of selected salts of sulphuric acid. Membr. Water Treat. 2010, 1, 171–179. [Google Scholar] [CrossRef]


| Tested Model Mixture (3 M HF + 0.7 M Fe(NO3)3) | Tested Spent Pickling Solution | Typical Composition of Spent Pickling Solution [2] | |
|---|---|---|---|
| NO3− F− H+ Fe3+ Cr3+ Ni2+ | 2.1 | 3.32 | 1.9–2.6 |
| 3.0 | 2.52 | 3.2–4.2 | |
| 3.0 0.7 − − | 3.04 0.68 0.21 0.089 | 2.6–4.0 0.6–0.8 0.1–0.2 0.05–0.1 |
| Dialysate | Diffusate | |||
|---|---|---|---|---|
| Model Mixture | Spent Pickling Solution | Model Mixture | Spent Pickling Solution | |
| NO3− F− H+ Fe3+ Cr3+ Ni2+ | 0.70 | 1.51 | 1.37 | 1.95 |
| 2.26 | 2.04 | 0.72 | 0.50 | |
| 0.98 0.68 − − | 0.86 0.66 0.020 0.083 | 1.98 0.031 − − | 2.15 0.038 0.0096 0.0072 | |
| Model Mixture | Spent pickling Solution | ||
|---|---|---|---|
| NO3− | 54–85% | 46–74% | |
| Recovery | F− H+ | 18–37% 55–87% | 15–31% 60–90% |
| Rejection | Fe3+ Cr3+ Ni2+ | 89–97% − − | 88–96% 89–97% 82–95% |
| Model Mixture | Spent Pickling Solution | ||
|---|---|---|---|
| NO3− | 2.54 | 1.73 | |
| Permeability | F− Fe3+ | 0.54 0.097 | 0.40 0.11 |
| Separation factor | NO3−/Fe3+ F−/Fe3+ | 26.2 5.6 | 15.7 3.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bendová, H.; Dušek, L. Treatment of Spent Pickling Solutions by Diffusion Dialysis Using Anion-Exchange Membrane Neosepta-AFN. Membranes 2023, 13, 9. https://doi.org/10.3390/membranes13010009
Bendová H, Dušek L. Treatment of Spent Pickling Solutions by Diffusion Dialysis Using Anion-Exchange Membrane Neosepta-AFN. Membranes. 2023; 13(1):9. https://doi.org/10.3390/membranes13010009
Chicago/Turabian StyleBendová, Helena, and Libor Dušek. 2023. "Treatment of Spent Pickling Solutions by Diffusion Dialysis Using Anion-Exchange Membrane Neosepta-AFN" Membranes 13, no. 1: 9. https://doi.org/10.3390/membranes13010009
APA StyleBendová, H., & Dušek, L. (2023). Treatment of Spent Pickling Solutions by Diffusion Dialysis Using Anion-Exchange Membrane Neosepta-AFN. Membranes, 13(1), 9. https://doi.org/10.3390/membranes13010009







