Sorption of Polar Sorbents into GO Powders and Membranes
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of HGO and BGO Powders and Fabrication of the Membranes
2.2. Instruments
2.3. Sorption Measurements
2.4. Orientational Order Parameters of Spin Probes in the Membranes
3. Results and Discussion
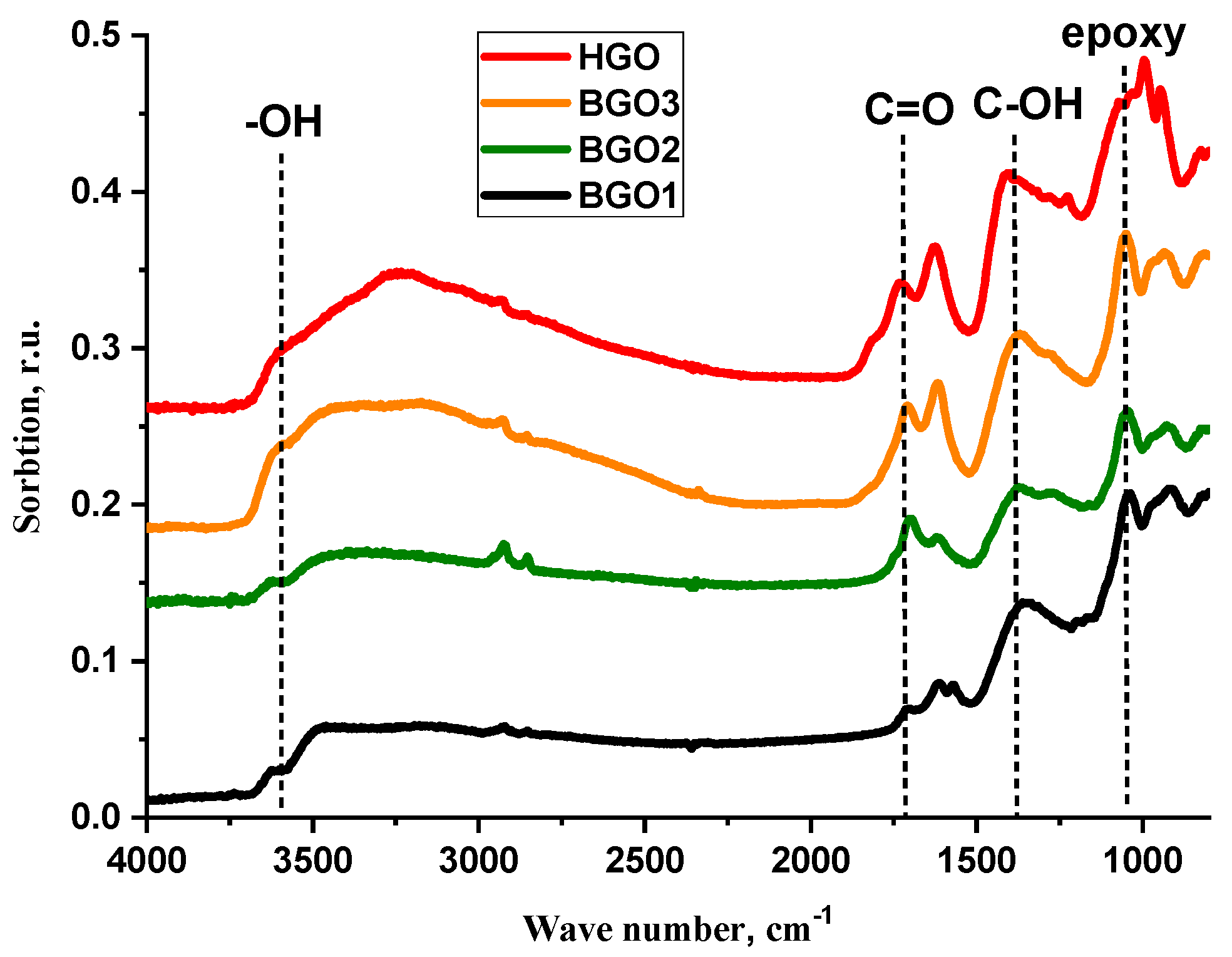
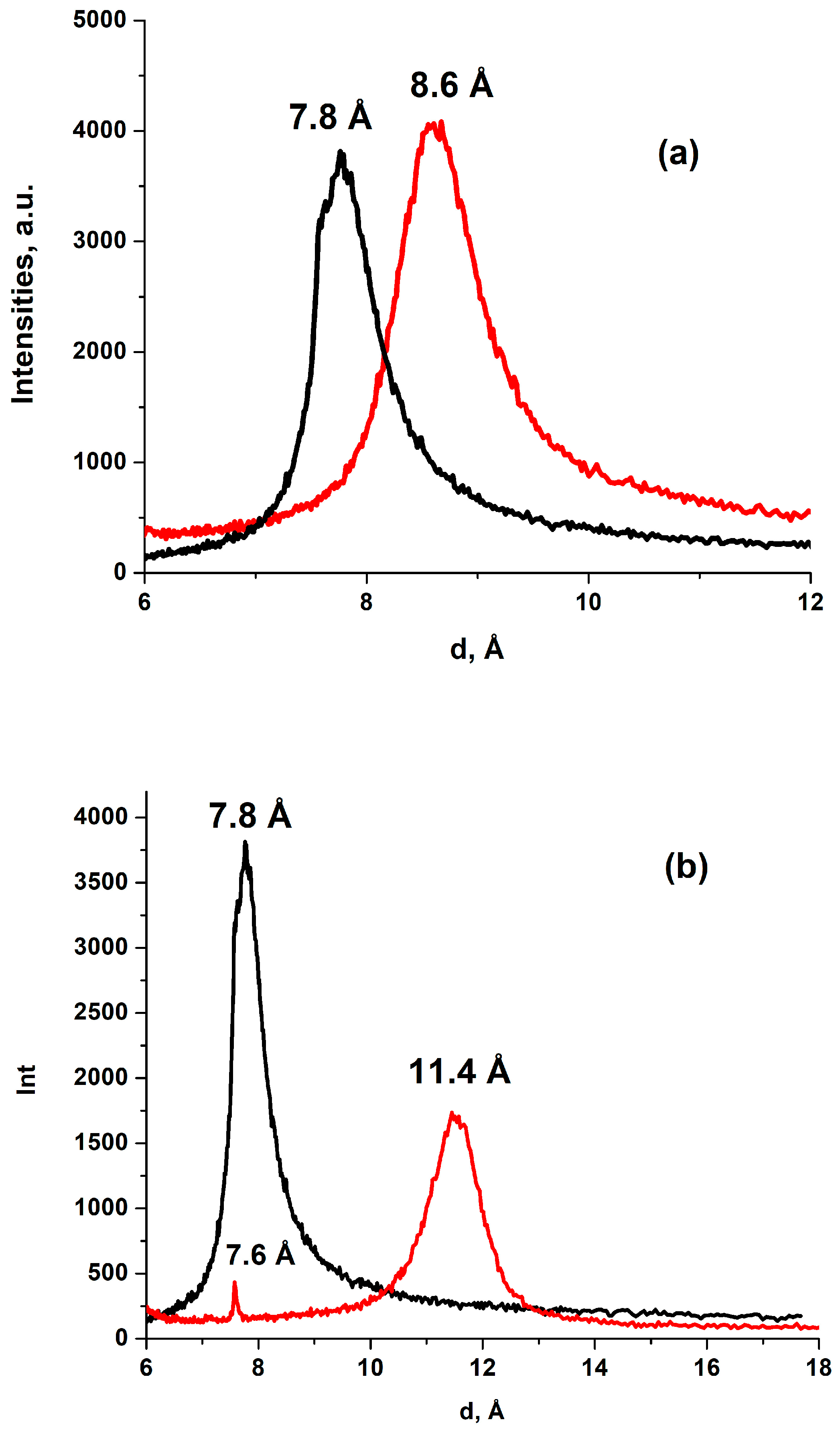
3.1. Properties of the Dry HGO and BGO Powders
3.2. Properties of the Dry Membranes, mHGO and mBGO
3.3. Sorption Properties
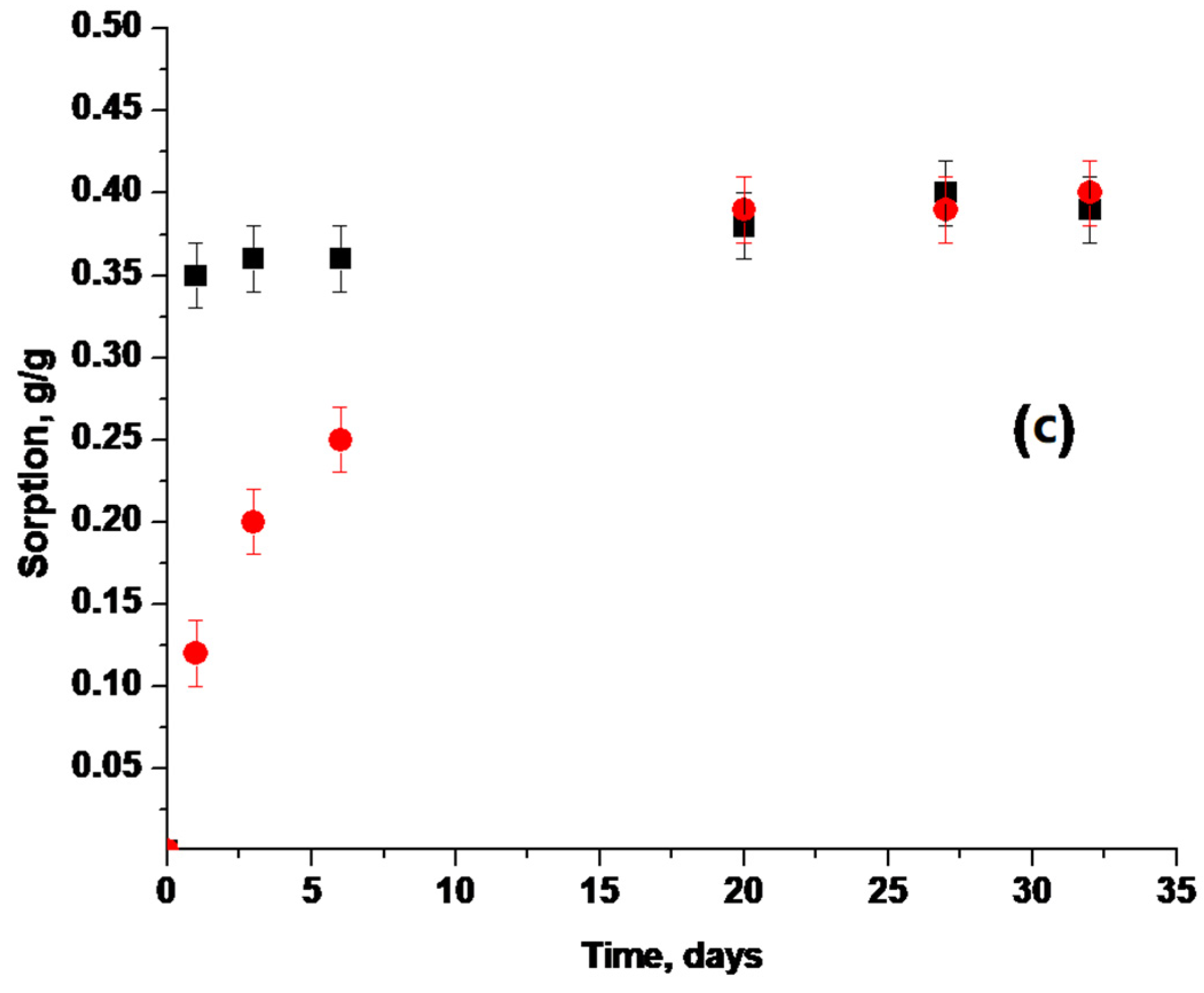
3.4. Sorption Properties of Water
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, P.; Wang, K.; Zhu, H. Recent Developments in Graphene-Based Membranes: Structure, Mass-Transport Mechanism and Potential Applications. Adv. Mater. 2016, 28, 2287–2310. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Jin, W.; Xu, N. Graphene-Based Membranes. Chem. Soc. Rev. 2015, 44, 5016–5030. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Ping, D.; Dong, X. Recent Developments of Graphene Oxide-Based Membranes: A Review. Membranes 2017, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Song, Z.; Zhang, X.; Huang, Y.; Li, S.; Mao, Y.; Ploehn, H.J.; Bao, Y.; Yu, M. Ultrathin, Molecular-Sieving Graphene Oxide Membranes for Selective Hydrogen Separation. Science 2013, 342, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Yoon, H.W.; Yoo, B.M.; Park, J.S.; Gleason, K.L.; Freeman, B.D.; Park, H.B. High-Performance CO2-Philic Graphene Oxide Membranes under Wet-Conditions. Chem. Commun. 2014, 50, 13563–13566. [Google Scholar] [CrossRef]
- Fatemi, S.M.; Arabieh, M.; Sepehrian, H. Nanoporous Graphene Oxide Membrane and Its Application in Molecular Sieving. Carbon Lett. 2015, 16, 183–191. [Google Scholar] [CrossRef]
- Burress, J.W.; Gadipelli, S.; Ford, J.; Simmons, J.M.; Zhou, W.; Yildirim, T. Graphene Oxide Framework Materials: Theoretical Predictions and Experimental Results. Angew. Chemie Int. Ed. 2010, 49, 8902–8904. [Google Scholar] [CrossRef]
- Kumar, R.; Suresh, V.M.; Maji, T.K.; Rao, C.N.R. Porous Graphene Frameworks Pillared by Organic Linkers with Tunable Surface Area and Gas Storage Properties. Chem. Commun. 2014, 50, 2015. [Google Scholar] [CrossRef]
- Nair, R.R.; Wu, H.A.; Jayaram, P.N.; Grigorieva, I.V.; Geim, A.K. Unimpeded Permeation of Water Through Helium-Leak–Tight Graphene-Based Membranes. Science 2012, 335, 442–444. [Google Scholar] [CrossRef]
- Iakunkov, A.; Talyzin, A.V. Swelling Properties of Graphite Oxides and Graphene Oxide Multilayered Materials. Nanoscale 2020, 12, 21060–21093. [Google Scholar] [CrossRef]
- Gupta, O.; Roy, S.; Rao, L.; Mitra, S. Graphene Oxide-Carbon Nanotube (GO-CNT) Hybrid Mixed Matrix Membrane for Pervaporative Dehydration of Ethanol. Membranes 2022, 12, 1227. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.; Vasu, K.S.; Williams, C.D.; Gopinadhan, K.; Su, Y.; Cherian, C.T.; Dix, J.; Prestat, E.; Haigh, S.J.; Grigorieva, I.V.; et al. Tunable Sieving of Ions Using Graphene Oxide Membranes. Nat. Nanotechnol. 2017, 12, 546–550. [Google Scholar] [CrossRef]
- Barroso-Bujans, F.; Cerveny, S.; Verdejo, R.; del Val, J.J.; Alberdi, J.M.; Alegría, A.; Colmenero, J. Permanent Adsorption of Organic Solvents in Graphite Oxide and Its Effect on the Thermal Exfoliation. Carbon N. Y. 2010, 48, 1079–1087. [Google Scholar] [CrossRef]
- Barroso-Bujans, F.; Cerveny, S.; Alegría, A.; Colmenero, J. Sorption and Desorption Behavior of Water and Organic Solvents from Graphite Oxide. Carbon N. Y. 2010, 48, 3277–3286. [Google Scholar] [CrossRef]
- Korobov, M.V.; Talyzin, A.V.; Rebrikova, A.T.; Shilayeva, E.A.; Avramenko, N.V.; Gagarin, A.N.; Ferapontov, N.B. Sorption of Polar Organic Solvents and Water by Graphite Oxide: Thermodynamic Approach. Carbon N. Y. 2016, 102, 297–303. [Google Scholar] [CrossRef]
- Rebrikova, A.T.; Klechikov, A.; Iakunkov, A.; Sun, J.; Talyzin, A.V.; Avramenko, N.V.; Korobov, M. Swollen Structures of Brodie Graphite Oxide as Solid Solvates. J. Phys. Chem. C 2020, 124, 23410–23418. [Google Scholar] [CrossRef]
- Talyzin, A.V.; Luzan, S.M.; Szabó, T.; Chernyshev, D.; Dmitriev, V. Temperature Dependent Structural Breathing of Hydrated Graphite Oxide in H2O. Carbon N. Y. 2011, 49, 1894–1899. [Google Scholar] [CrossRef]
- Talyzin, A.V.; Solozhenko, V.L.; Kurakevych, O.O.; Szabó, T.; Dékány, I.; Kurnosov, A.; Dmitriev, V. Colossal Pressure-Induced Lattice Expansion of Graphite Oxide in the Presence of Water. Angew. Chemie 2008, 120, 8392–8395. [Google Scholar] [CrossRef]
- You, S.; Luzan, S.M.; Szabó, T.; Talyzin, A.V. Effect of Synthesis Method on Solvation and Exfoliation of Graphite Oxide. Carbon N. Y. 2013, 52, 171–180. [Google Scholar] [CrossRef]
- You, S.; Sundqvist, B.; Talyzin, A.V. Enormous Lattice Expansion of Hummers Graphite Oxide in Alcohols at Low Temperatures. ACS Nano 2013, 7, 1395–1399. [Google Scholar] [CrossRef]
- Klechikov, A.; Sun, J.; Vorobiev, A.; Talyzin, A.V. Swelling of Thin Graphene Oxide Films Studied by in Situ Neutron Reflectivity. J. Phys. Chem. C 2018, 122, 13106–13116. [Google Scholar] [CrossRef]
- Klechikov, A.; Yu, J.; Thomas, D.; Sharifi, T.; Talyzin, A.V. Structure of Graphene Oxide Membranes in Solvents and Solutions. Nanoscale 2015, 7, 15374–15384. [Google Scholar] [CrossRef] [PubMed]
- Talyzin, A.V.; Hausmaninger, T.; You, S.; Szabó, T. The Structure of Graphene Oxide Membranes in Liquid Water, Ethanol and Water–Ethanol Mixtures. Nanoscale 2014, 6, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Talyzin, A.V.; Mercier, G.; Klechikov, A.; Hedenström, M.; Johnels, D.; Wei, D.; Cotton, D.; Opitz, A.; Moons, E. Brodie vs Hummers Graphite Oxides for Preparation of Multi-Layered Materials. Carbon N. Y. 2017, 115, 430–440. [Google Scholar] [CrossRef]
- Chumakova, N.A.; Rebrikova, A.T.; Talyzin, A.V.; Paramonov, N.A.; Vorobiev, A.K.; Korobov, M.V. Properties of Graphite Oxide Powders and Membranes as Revealed by Electron Paramagnetic Resonance Spectroscopy. J. Phys. Chem. C 2018, 122, 22750–22759. [Google Scholar] [CrossRef]
- Szabó, T.; Berkesi, O.; Forgó, P.; Josepovits, K.; Sanakis, Y.; Petridis, D.; Dékány, I. Evolution of Surface Functional Groups in a Series of Progressively Oxidized Graphite Oxides. Chem. Mater. 2006, 18, 2740–2749. [Google Scholar] [CrossRef]
- Cerveny, S.; Barroso-Bujans, F.; Alegría, Á.; Colmenero, J. Dynamics of Water Intercalated in Graphite Oxide. J. Phys. Chem. C 2010, 114, 2604–2612. [Google Scholar] [CrossRef]
- Chumakova, N.A.; Vorobiev, A.K. Orientation Distribution of Molecules: Characterization and Experimental Determination by Means of Magnetic Resonance. Appl. Magn. Reson. 2020, 51, 1145–1175. [Google Scholar] [CrossRef]
- Marcus, Y.; Smith, A.L.; Korobov, M.V.; Mirakyan, A.L.; Avramenko, N.V.; Stukalin, E.B. Solubility of C 60 Fullerene. J. Phys. Chem. B 2001, 105, 2499–2506. [Google Scholar] [CrossRef]
- Iakunkov, A.; Skrypnychuk, V.; Nordenström, A.; Shilayeva, E.A.; Korobov, M.; Prodana, M.; Enachescu, M.; Larsson, S.H.; Talyzin, A.V. Activated Graphene as a Material for Supercapacitor Electrodes: Effects of Surface Area, Pore Size Distribution and Hydrophilicity. Phys. Chem. Chem. Phys. 2019, 21, 17901–17912. [Google Scholar] [CrossRef]
- McClellan, A.; Harnsberger, H. Cross-Sectional Areas of Molecules Adsorbed on Solid Surfaces. J. Colloid Interface Sci. 1967, 23, 577–599. [Google Scholar] [CrossRef]
- Paredes, J.I.; Villar-Rodil, S.; Martínez-Alonso, A.; Tascón, J.M.D. Graphene Oxide Dispersions in Organic Solvents. Langmuir 2008, 24, 10560–10564. [Google Scholar] [CrossRef] [PubMed]
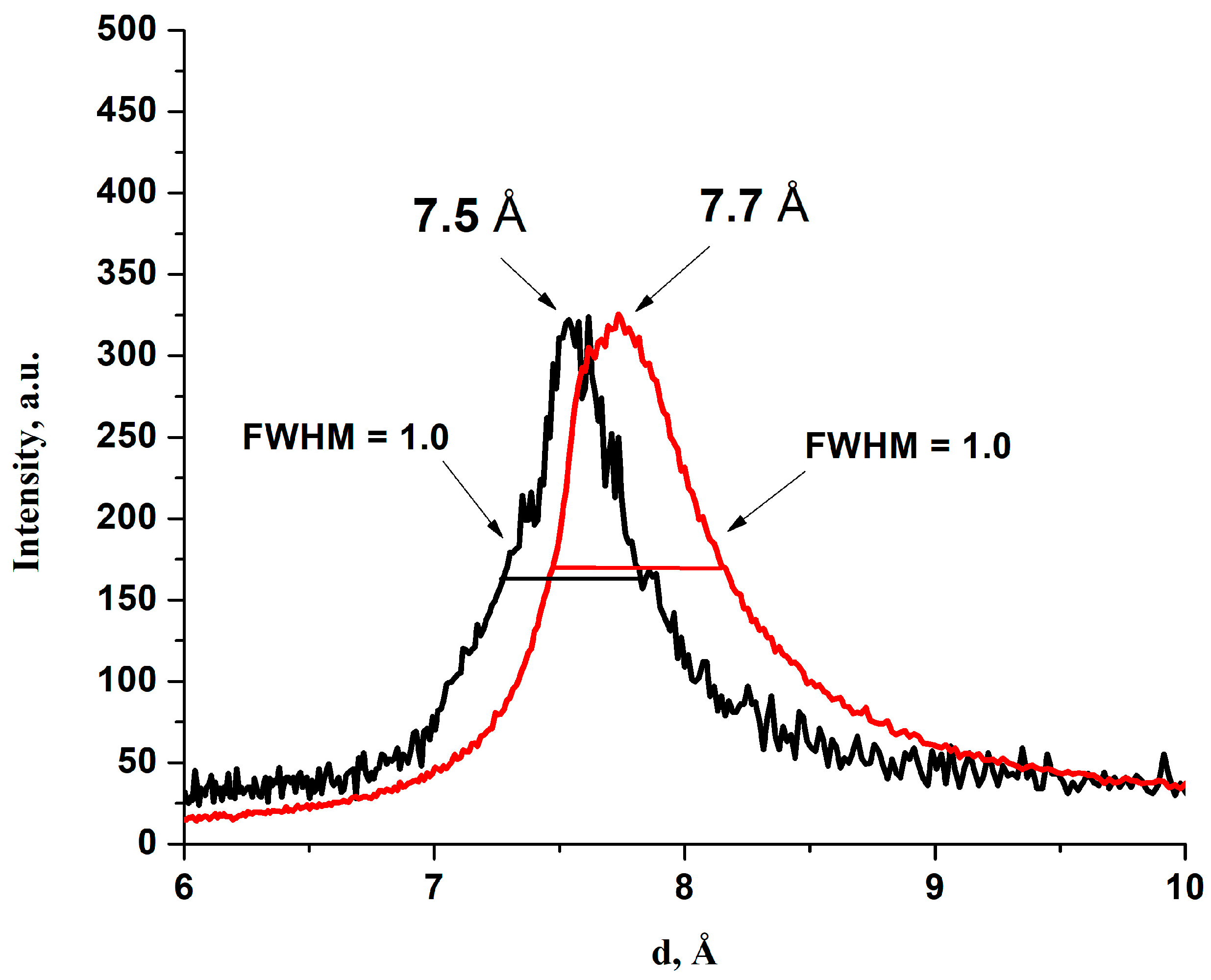

| GO Material | Method of the Membrane Preparation | d (001). Å a (±0.3) | C/O. b (±0.2) | P20 (±0.01) |
|---|---|---|---|---|
| HGO (9:1) | 7.5 | 2.5 | ||
| mHGO (9:1) | Evaporation | 7.7 | 2.5 | |
| mHGO (9:1) | Vacuum filtration | 7.8 | 2.5 | 0.33 (TEMPOL) |
| HGO (9:3) | 7.8 | 2.2 | ||
| mHGO (9:3) | Vacuum filtration | 7.8 | 2.7 | 0.35 (TEMPOL) 0.41 (A3) |
| HGO (9:1→9:3) | 7.3 | 2.3 | ||
| mHGO (9:1→9:3) | Vacuum filtration | 7.7 | 2.3 | 0.34 (TEMPOL) |
| HGO c [17] | 7.18 | 2.47 | ||
| mHGO [21] | Vacuum filtration | 7.7 | 2.49 | |
| mHGO [9.21] | Vacuum filtration | 7.57 | 2.81 | |
| BGO (1) | 6.0 | 3.3 | ||
| BGO (2) | 6.6 | 2.8 | ||
| mBGO (2) | Vacuum filtration (pH = 12) | 6.6 | 2.8 | 0.21 (A3) |
| mBGO (2) | Vacuum filtration (pH = 7) | 6.4 | 2.8 | ≈0 (A3) |
| BGO (3) | 7.0 | 2.5 | ||
| BGO [17] | 6.35 | 2.85 | ||
| mBGO(1) [21.22] | Vacuum filtration (pH = 12) | 3.84 d |
| Sorbent | a ϵ | b ET (30) | HGO | mHGO | BGO | mBGO |
|---|---|---|---|---|---|---|
| CH3CN c | 38.0 | 45.6 | 0.35 | 0.17 | 0.53 | d 0.14; e 0 |
| C6H6 | 2.2 | 34.3 | 0 | 0 | 0 | 0 |
| C5H5N | 1.1 | 40.5 | 0.25 | 0.11 | 0.43 | 0 |
| 1-octanol | 9.9 | 48.1 | 0.71 | 0.42 | 1.05 | 0 |
| H2O c | 78.5 | 63.1 | 0.40 | 0.38 | 0.31 | 0.33 |
| Samples | Sorption of CH3CN | Sorption of H2O | ||
|---|---|---|---|---|
| T = 298 K | T = 219 K | T = 298 K | T = 273 K | |
| HGO (9:1) | 0.38 ± 0.02 | 0.47 ± 0.08 | 0.38 ± 0.02 | 0.63 ± 0.06 |
| mHGO (9:1) Vacuum filtration | 0.19 ± 0.02 | 0.48 ± 0.03 | 0.74 ± 0.02 | |
| mHGO (9:1) Evaporation | 0.18 ± 0.02 | 0.46 ± 0.07 | 0.38 ± 0.02 | 0.61 ± 0.06 |
| HGO (9:3) | 0.34 ± 0.02 | 0.44 ± 0.02 | ||
| mHGO (9:3) Evaporation | 0.19 ± 0.02 | 0.41 ± 0.03 | ||
| HGO (9:3;1) | 0.33 ± 0.02 | 0.42± 0.02 | ||
| mHGO (9:3;1) Evaporation | 0.11 ± 0.02 | |||
| HGO [15] | 0.34 ± 0.02 | 0.48 ± 0.03 | ||
| HGO [30] | 0.47± 0.03 | |||
| mHGO [21] | 0.26 ± 0.02 | |||
| BGO1 | 0.21 ± 0.02 | 0.46 ± 0.07 | 0.29 ± 0.02 | |
| BGO2 | 0.20 ± 0.02 | 0.54 ± 0.09 | 0.30 ± 0.02 | 0.36 ± 0.06 |
| BGO [15] | 0.25 ± 0.02 | 0.53 ± 0.04 | 0.33 ± 0.02 | 0.33 ± 0.02 |
| mBGO2 Vacuum filtration (pH = 12) | <0.02 | ≈0 | 0.34 ± 0.02 | 0.35 ± 0.06 |
| mBGO2 Vacuum filtration (pH = 7) | 0.14 ± 0.02 | 0.53 ± 0.03 | 0.32 ± 0.02 | |
| BGO3 | 0.25 ± 0.02 | 0.53 ± 0.05 | 0.31 ± 0.02 | |
| Sample | Sorption of CH3CN mg/mg GO | Inter- Plane Distance, d001, A |
|---|---|---|
| HGO, powder | 0.35± 0.03 | 12.0 a |
| HGO, membrane | 0.16± 0.03 | 8.8 a |
| HGO, powder [15] | 0.34± 0.01 | |
| HGO, powder [22] | 14.0 b | |
| HGO, membrane [22] | 9.0 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaplin, A.V.; Rebrikova, A.T.; Eremina, E.A.; Chumakova, N.A.; Avramenko, N.V.; Korobov, M.V. Sorption of Polar Sorbents into GO Powders and Membranes. Membranes 2023, 13, 53. https://doi.org/10.3390/membranes13010053
Kaplin AV, Rebrikova AT, Eremina EA, Chumakova NA, Avramenko NV, Korobov MV. Sorption of Polar Sorbents into GO Powders and Membranes. Membranes. 2023; 13(1):53. https://doi.org/10.3390/membranes13010053
Chicago/Turabian StyleKaplin, A. V., A. T. Rebrikova, E. A. Eremina, N. A. Chumakova, N. V. Avramenko, and M. V. Korobov. 2023. "Sorption of Polar Sorbents into GO Powders and Membranes" Membranes 13, no. 1: 53. https://doi.org/10.3390/membranes13010053
APA StyleKaplin, A. V., Rebrikova, A. T., Eremina, E. A., Chumakova, N. A., Avramenko, N. V., & Korobov, M. V. (2023). Sorption of Polar Sorbents into GO Powders and Membranes. Membranes, 13(1), 53. https://doi.org/10.3390/membranes13010053







