Recent Advances in Transition Metal Tellurides (TMTs) and Phosphides (TMPs) for Hydrogen Evolution Electrocatalysis
Abstract
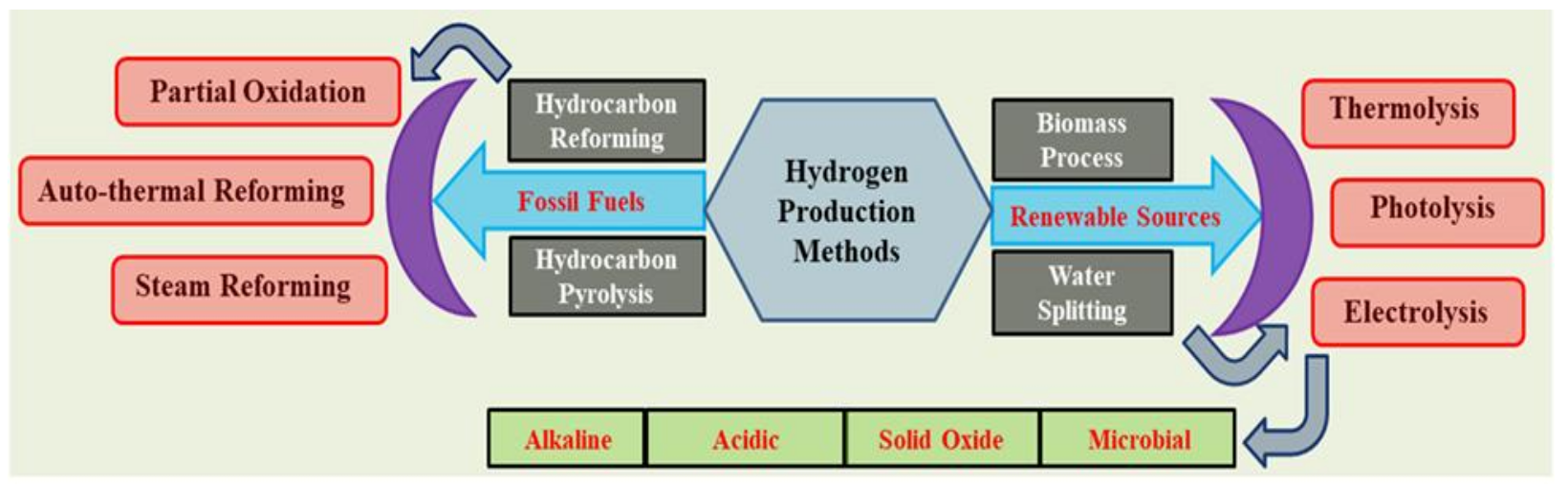
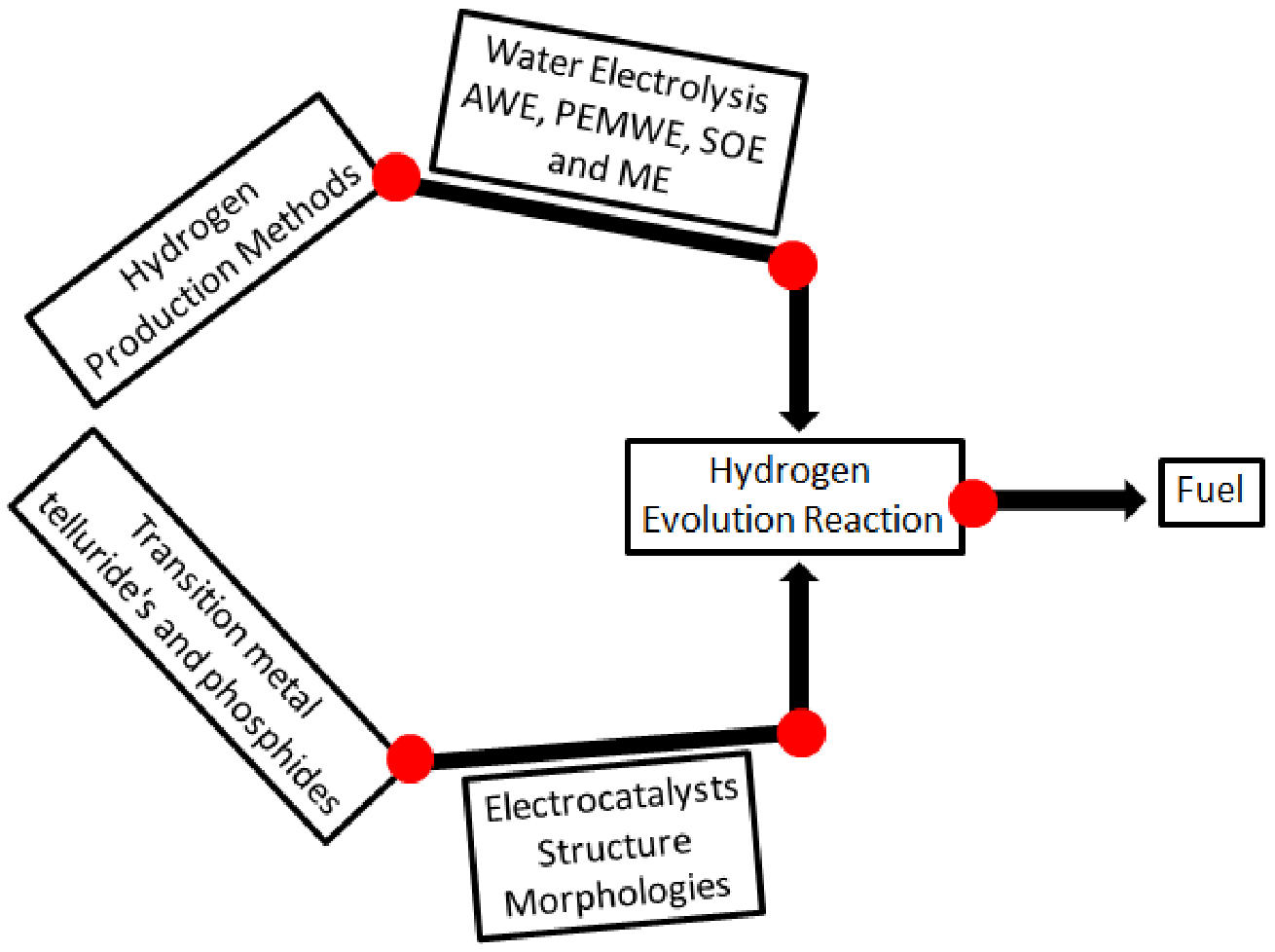
1. Introduction
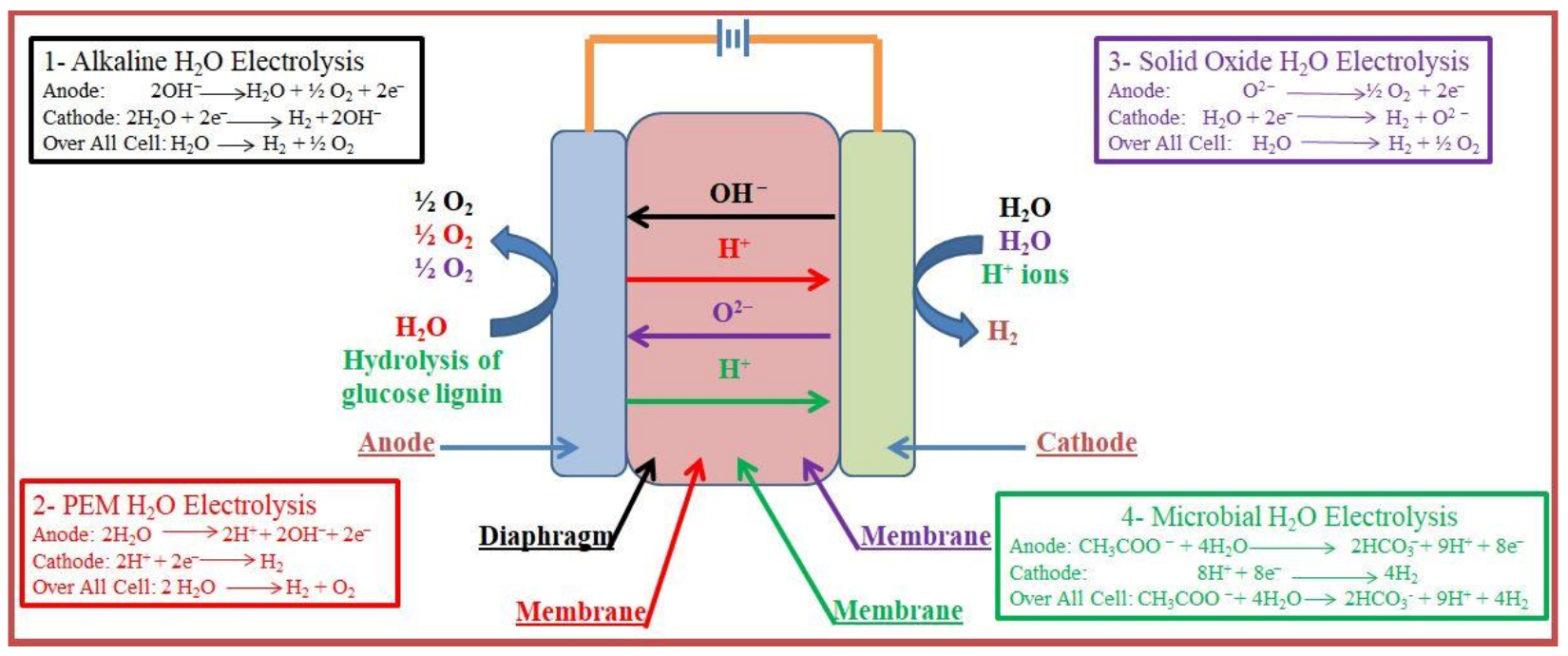
2. Water Electrolysis
2.1. Alkaline Water Electrolysis (AWE)
2.2. Proton-Exchange Membrane Water Electrolysis (PEMWE)
2.3. Solid Oxide Electrolysis (SOE)
2.4. Microbial Electrolysis (ME)
3. Transition Metal Tellurides (TMTs)-Based Electrocatalysts for HER
3.1. Nanostructuring
3.2. In Situ Development
3.3. Nanostructure Engineering
3.4. Dopants
3.5. Heterostructure
3.6. Nanocomposites
4. Transition Metal Phosphide (TMP)-Based Electrocatalysts for (HER)
4.1. Dopant
4.2. Two-Dimensional Nanosheet Structure
4.3. Heterostructure
4.4. Core–Shell Structure
5. Concluding Remarks
- Transition metal-based catalysts require some improvements for industrial-scale water electrolysis in terms of current density (greater than 500 mA cm−2) and long-term stability.
- TMT-based electrocatalysts have high electrical conductivity, due to their metallic character, but conductivity is not the only characteristic needed for enhancing the HER performance of the electrocatalysts. Therefore, improvements of the electronic structure of electrocatalysts are still required to change the composition; and of the structural engineering to achieve binding energy regulation of the reaction intermediates and lower the reaction energy barrier.
- The development of novel and advanced synthesis methods is required for large-scale production with suitable structural properties.
- More research is needed to explain the self-construction mechanism in the transition metal tellurides and phosphides. Furthermore, to understand the self-construction mechanisms and intrinsic properties, tests under different conditions should be performed for the self-constructions of TMT and TMP. By controlling the construction mechanism of the electrocatalysts, HER performance can be boosted.
- Development of simple but more accurate characterization techniques for the structural elucidation of the material may be beneficial for designing efficient electrocatalysts for HER performance.
- Transition metal telluride and phosphide electrocatalysts have greater electrical conductivity than other electrocatalysts due to the metallic character of transition metals.
- TMT and TMP electrocatalysts are better than conventional platinum-group electrocatalysts, due to their low cost.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahmood, A.; Hu, J.-Y.; Xiao, B.; Tang, A.; Wang, X.; Zhou, E. Recent progress in porphyrin-based materials for organic solar cells. J. Mater. Chem. A 2018, 6, 16769–16797. [Google Scholar] [CrossRef]
- Mahmood, A. Recent research progress on quasi-solid-state electrolytes for dye-sensitized solar cells. J. Energy Chem. 2015, 24, 686–692. [Google Scholar] [CrossRef]
- Mahmood, A.; Irfan, A. Effect of fluorination on exciton binding energy and electronic coupling in small molecule acceptors for organic solar cells. Comput. Theor. Chem. 2020, 1179, 112797. [Google Scholar] [CrossRef]
- Mahmood, A. Understanding the effect of terminal electron-deficient group on the performance of small molecule acceptor in organic solar cells. J. Ultra Chem. 2019, 15, 54–65. [Google Scholar] [CrossRef]
- Kazim, A.; Veziroglu, T.N. Utilization of solar–hydrogen energy in the UAE to maintain its share in the world energy market for the 21st century. Renew. Energy 2001, 24, 259–274. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H. Water electrolysis based on renewable energy for hydrogen production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I. Comparative assessment of hydrogen production methods from renewable and non-renewable sources. Int. J. Hydrogen Energy 2014, 39, 1–12. [Google Scholar] [CrossRef]
- Rand, D.A. A journey on the electrochemical road to sustainability. J. Solid State Electrochem. 2011, 15, 1579–1622. [Google Scholar] [CrossRef]
- Züttel, A. Hydrogen storage methods. Naturwissenschaften 2004, 91, 157–172. [Google Scholar] [CrossRef]
- Rakib, M.A.; Grace, J.R.; Lim, C.J.; Elnashaie, S.S.; Ghiasi, B. Steam reforming of propane in a fluidized bed membrane reactor for hydrogen production. Int. J. Hydrogen Energy 2010, 35, 6276–6290. [Google Scholar] [CrossRef]
- Lim, H. Hydrogen selectivity and permeance effect on the water gas shift reaction (WGSR) in a membrane reactor. Korean J. Chem. Eng. 2015, 32, 1522–1527. [Google Scholar] [CrossRef]
- Lee, B.; Chae, H.; Choi, N.H.; Moon, C.; Moon, S.; Lim, H. Economic evaluation with sensitivity and profitability analysis for hydrogen production from water electrolysis in Korea. Int. J. Hydrogen Energy 2017, 42, 6462–6471. [Google Scholar] [CrossRef]
- Trane, R.; Dahl, S.; Skjøth-Rasmussen, M.S.; Jensen, A.D. Catalytic steam reforming of bio-oil. Int. J. Hydrogen Energy 2012, 37, 6447–6472. [Google Scholar] [CrossRef]
- Iranshahi, D.; Pourazadi, E.; Paymooni, K.; Rahimpour, M.R.; Jahanmiri, A.; Moghtaderi, B. A dynamic membrane reactor concept for naphtha reforming, considering radial-flow patterns for both sweeping gas and reacting materials. Chem. Eng. J. 2011, 178, 264–275. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Jafari, M.; Iranshahi, D. Progress in catalytic naphtha reforming process: A review. Appl. Energy 2013, 109, 79–93. [Google Scholar] [CrossRef]
- Boyano, A.; Blanco-Marigorta, A.; Morosuk, T.; Tsatsaronis, G. Exergoenvironmental analysis of a steam methane reforming process for hydrogen production. Energy 2011, 36, 2202–2214. [Google Scholar] [CrossRef]
- Xu, J.; Chen, L.; Tan, K.F.; Borgna, A.; Saeys, M. Effect of boron on the stability of Ni catalysts during steam methane reforming. J. Catal. 2009, 261, 158–165. [Google Scholar] [CrossRef]
- Ligthart, D.; Van Santen, R.; Hensen, E. Influence of particle size on the activity and stability in steam methane reforming of supported Rh nanoparticles. J. Catal. 2011, 280, 206–220. [Google Scholar] [CrossRef]
- Veziroğlu, T.N.; Barbir, F. Hydrogen Energy Technologies; United Nations Industrial Development Organization: Vienna, Austria, 1998. [Google Scholar]
- Muhammad, A.M. Hydrogen and syngas production by superadiabatic combustion–A review. Appl. Energy 2016, 173, 210–224. [Google Scholar]
- Kalinci, Y.; Hepbasli, A.; Dincer, I. Biomass-based hydrogen production: A review and analysis. Int. J. Hydrogen Energy 2009, 34, 8799–8817. [Google Scholar] [CrossRef]
- Huang, J.; Dincer, I. Parametric analysis and assessment of a coal gasification plant for hydrogen production. Int. J. Hydrogen Energy 2014, 39, 3294–3303. [Google Scholar] [CrossRef]
- Seyitoglu, S.S.; Dincer, I.; Kilicarslan, A. Energy and exergy analyses of hydrogen production by coal gasification. Int. J. Hydrogen Energy 2017, 42, 2592–2600. [Google Scholar] [CrossRef]
- Burmistrz, P.; Chmielniak, T.; Czepirski, L.; Gazda-Grzywacz, M. Carbon footprint of the hydrogen production process utilizing subbituminous coal and lignite gasification. J. Clean. Prod. 2016, 139, 858–865. [Google Scholar] [CrossRef]
- Levin David, B.; Pitt, L.; Murray, L. Biohydrogen production: Prospects and limitations to practical application. Int. J. Hydrogen Energy 2004, 29, 173–185. [Google Scholar] [CrossRef]
- Das, D.; Veziroǧlu, T.N. Hydrogen production by biological processes: A survey of literature. Int. J. Hydrogen Energy 2001, 26, 13–28. [Google Scholar] [CrossRef]
- Elsharnouby, O.; Hafez, H.; Nakhla, G.; El Naggar, M.H. A critical literature review on biohydrogen production by pure cultures. Int. J. Hydrogen Energy 2013, 38, 4945–4966. [Google Scholar] [CrossRef]
- Sivagurunathan, P.; Kumar, G.; Kim, S.-H.; Kobayashi, T.; Xu, K.-Q.; Guo, W.; Hao Ngo, H. Enhancement Strategies for Hydrogen Production from Wastewater: A Review. Curr. Org. Chem. 2016, 20, 2744–2752. [Google Scholar] [CrossRef]
- Barbir, F. PEM electrolysis for production of hydrogen from renewable energy sources. Solar Energy 2005, 78, 661–669. [Google Scholar] [CrossRef]
- Atlam, O.; Kolhe, M. Equivalent electrical model for a proton exchange membrane (PEM) electrolyser. Energy Convers. Manag. 2011, 52, 2952–2957. [Google Scholar] [CrossRef]
- Siracusano, S.; Baglio, V.; Briguglio, N.; Brunaccini, G.; Di Blasi, A.; Stassi, A.; Ornelas, R.; Trifoni, E.; Antonucci, V.; Aricò, A.S. An electrochemical study of a PEM stack for water electrolysis. Int. J. Hydrogen Energy 2012, 37, 1939–1946. [Google Scholar] [CrossRef]
- Lee, B.; Heo, J.; Kim, S.; Sung, C.; Moon, C.; Moon, S.; Lim, H. Economic feasibility studies of high pressure PEM water electrolysis for distributed H2 refueling stations. Energy Convers. Manag. 2018, 162, 139–144. [Google Scholar] [CrossRef]
- Borgschulte, A. The Hydrogen Grand Challenge. Front. Energy Res. 2016, 4. [Google Scholar] [CrossRef]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Damyanova, S.; Pawelec, B.; Arishtirova, K.; Fierro, J.L.G. Ni-based catalysts for reforming of methane with CO2. Int. J. Hydrogen Energy 2012, 37, 15966–15975. [Google Scholar] [CrossRef]
- Rashid, M.; Al Mesfer, M.K.; Naseem, H.; Danish, M. Hydrogen production by water electrolysis: A review of alkaline water electrolysis, PEM water electrolysis and high temperature water electrolysis. Int. J. Eng. Adv. Technol. 2015, 4. [Google Scholar]
- Sapountzi, F.M.; Gracia, J.M.; Weststrate, C.J.; Fredriksson, H.O.A.; Niemantsverdriet, J.W. Electrocatalysts for the generation of hydrogen, oxygen and synthesis gas. Prog. Energy Combust. Sci. 2017, 58, 1–35. [Google Scholar] [CrossRef]
- Das Gouri, S.; Bhatnagar, A.; Yli-Pirilä, P.; Tripathi, K.M.; Kim, T. Sustainable nitrogen-doped functionalized graphene nanosheets for visible-light-induced photocatalytic water splitting. Chem. Commun. 2020, 56, 6953–6956. [Google Scholar]
- Li, S.-H.; Ming-Yu, Q.; Tang, Z.-R.; Xu, Y.-J. Nanostructured metal phosphides: From controllable synthesis to sustainable catalysis. Chem. Soc. Rev. 2021, 50, 7539–7586. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Xia, X.; Shi, F.; Zhan, J.; Tu, J.; Fan, H.J. Transition Metal Carbides and Nitrides in Energy Storage and Conversion. Adv. Sci. 2016, 3, 1500286. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, S.; Pan, K.; Dong, Z.; Zhang, B.; Wu, H.-H.; Zhang, Q.; Lin, J.; Pang, H. Self-supporting transition metal chalcogenides on metal substrates for catalytic water splitting. Chem. Eng. J. 2021, 421, 129645. [Google Scholar] [CrossRef]
- Gao, Q.; Huang, C.-Q.; Ju, Y.-M.; Gao, M.-R.; Liu, J.-W.; An, D.; Cui, C.-H.; Zheng, Y.-R.; Li, W.-X.; Yu, S.-H. Phase-Selective Syntheses of Cobalt Telluride Nanofleeces for Efficient Oxygen Evolution Catalysts. Angew. Chem. Int. Ed. 2017, 56, 7769–7773. [Google Scholar] [CrossRef] [PubMed]
- Dehane, A.; Merouani, S.; Hamdaoui, O.; Alghyamah, A. A complete analysis of the effects of transfer phenomenons and reaction heats on sono-hydrogen production from reacting bubbles: Impact of ambient bubble size. Int. J. Hydrogen Energy 2021, 46, 18767–18779. [Google Scholar] [CrossRef]
- Masud, J.; Ioannou, P.-C.; Levesanos, N.; Kyritsis, P.; Nath, M. A Molecular Ni-complex Containing Tetrahedral Nickel Selenide Core as Highly Efficient Electrocatalyst for Water Oxidation. Chemsuschem 2016, 9, 3128–3132. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, F.A.; Thygesen, K.S. Computational 2D Materials Database: Electronic Structure of Transition-Metal Dichalcogenides and Oxides. J. Phys. Chem. C 2015, 119, 13169–13183. [Google Scholar] [CrossRef]
- Ji, D.; Peng, L.; Shen, J.; Deng, M.; Mao, Z.; Tan, L.; Wang, M.; Xiang, R.; Wang, J.; Shah, S.S.A. Inert V2O3 oxide promotes the electrocatalytic activity of Ni metal for alkaline hydrogen evolution. Chem. Commun. 2019, 55, 3290–3293. [Google Scholar] [CrossRef] [PubMed]
- Laghari, A.J.; Aftab, U.; Tahira, A.; Shah, A.A.; Gradone, A.; Solangi, M.Y.; Samo, A.H.; Kumar, M.; Abro, M.I.; Akhtar, M.W.; et al. MgO as promoter for electrocatalytic activities of Co3O4–MgO composite via abundant oxygen vacancies and Co2+ ions towards oxygen evolution reaction. Int. J. Hydrogen Energy 2022. [Google Scholar] [CrossRef]
- Najam, T.; Ibraheem, S.; Nazir, M.A.; Shaheen, A.; Waseem, A.; Javed, M.S.; Shah, S.S.A.; Cai, X. Partially oxidized cobalt species in nitrogen-doped carbon nanotubes: Enhanced catalytic performance to water-splitting. Int. J. Hydrogen Energy 2021, 46, 8864–8870. [Google Scholar] [CrossRef]
- Shah, S.S.A.; Jery, A.E.; Najam, T.; Nazir, M.A.; Wei, L.; Hussain, E.; Hussain, S.; Rebah, F.B.; Javed, M.S. Surface engineering of MOF-derived FeCo/NC core-shell nanostructures to enhance alkaline water-splitting. Int. J. Hydrogen Energy 2022, 47, 5036–5043. [Google Scholar] [CrossRef]
- Ortiz-Rodríguez, J.C.; Singstock, N.R.; Perryman, J.T.; Hyler, F.P.; Jones, S.J.; Holder, A.M.; Musgrave, C.B.; Velázquez, J.M. Stabilizing Hydrogen Adsorption through Theory-Guided Chalcogen Substitution in Chevrel-Phase Mo6X8 (X=S, Se, Te) Electrocatalysts. ACS Appl. Mater. Interfaces 2020, 12, 35995–36003. [Google Scholar] [CrossRef]
- Lee, J.; Kang, S.; Yim, K.; Kim, K.Y.; Jang, H.W.; Kang, Y.; Han, S. Hydrogen Evolution Reaction at Anion Vacancy of Two-Dimensional Transition-Metal Dichalcogenides: Ab Initio Computational Screening. J. Phys. Chem. Lett. 2018, 9, 2049–2055. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ai, H.; Chen, M.; Zhou, P.; Li, B.; Liu, D.; Du, X.; Lo, K.H.; Ng, K.-W.; Wang, S.-P. Multi-Phase Heterostructure of CoNiP/CoxP for Enhanced Hydrogen Evolution Under Alkaline and Seawater Conditions by Promoting H2O Dissociation. Small 2021, 17, 2007557. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Wang, G.; Yang, Z.; Li, B.; Wang, Q.; Kuliiev, R.; Orlovskaya, N.; Gu, M.; Du, Y.; Wang, G.; et al. Dual-Doping and Synergism toward High-Performance Seawater Electrolysis. Adv. Mater. 2021, 33, 2101425. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Yu, L.; McElhenny, B.; Xing, X.; Luo, D.; Zhang, F.; Bao, J.; Chen, S.; Ren, Z. Rational design of core-shell-structured CoPx@FeOOH for efficient seawater electrolysis. Appl. Catal. B Environ. 2021, 294, 120256. [Google Scholar] [CrossRef]
- Wu, L.; Yu, L.; Zhang, F.; McElhenny, B.; Luo, D.; Karim, A.; Chen, S.; Ren, Z. Heterogeneous Bimetallic Phosphide Ni2P-Fe2P as an Efficient Bifunctional Catalyst for Water/Seawater Splitting. Adv. Funct. Mater. 2021, 31, 2006484. [Google Scholar] [CrossRef]
- Yang, M.; Wang, Y.; Gu, Y.; Xue, Z.; Shi, J.; An, W.; Rui, Y. Electro-deposited copper nanoclusters on leaf-shaped cobalt phosphide for boosting hydrogen evolution reaction. J. Alloys Compd. 2022, 902, 163771. [Google Scholar] [CrossRef]
- Ding, Z.; Yu, H.; Liu, X.; He, N.; Chen, X.; Li, H.; Wang, M.; Yamauchi, Y.; Xu, X.; Amin, M.A.; et al. Prussian blue analogue derived cobalt–nickel phosphide/carbon nanotube composite as electrocatalyst for efficient and stable hydrogen evolution reaction in wide-pH environment. J. Colloid Interface Sci. 2022, 616, 210–220. [Google Scholar] [CrossRef]
- Xiao, Z.; Yang, M.; Wang, J.; Xu, Z.; Zhang, S.; Tang, A.; Gao, R.; Yang, H. FeNiP/MoOx integrated electrode grown on monocrystalline NiMoO4 nanorods with multi-interface for accelerating alkaline hydrogen evolution reaction. Appl. Catal. B Environ. 2022, 303, 120913. [Google Scholar] [CrossRef]
- Zhang, H.; Li, W.; Feng, X.; Zhu, L.; Fang, Q.; Li, S.; Wang, L.; Li, Z.; Kou, Z. A chainmail effect of ultrathin N-doped carbon shell on Ni2P nanorod arrays for efficient hydrogen evolution reaction catalysis. J. Colloid Interface Sci. 2022, 607, 281–289. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, Z.; Zhao, Y.; Yu, W.; Jiang, X.; He, M.; Li, Z.; Ma, T.; Wu, Z.; Wang, L. Facile synthesis of MoP-Ru2P on porous N, P co-doped carbon for efficiently electrocatalytic hydrogen evolution reaction in full pH range. Appl. Catal. B Environ. 2022, 303, 120879. [Google Scholar] [CrossRef]
- Samal, R.; Debbarma, C.; Rout, C.S. Transition metal tellurides/2D Ti3C2Tx MXene: Investigation towards active alkaline hydrogen evolution reaction. Catal. Today 2022, in press. [Google Scholar] [CrossRef]
- Dang, C.; Yun, S.; Zhang, Y.; Dang, J.; Wang, Y.; Liu, Z.; Deng, Y.; Yang, G.; Yang, J. A tailored interface engineering strategy designed to enhance the electrocatalytic activity of NiFe2O4/NiTe heterogeneous structure for advanced energy conversion applications. Mater. Today Nano 2022, 20, 100242. [Google Scholar] [CrossRef]
- Li, T.; Wu, J.; Qiao, L.; Zhu, Q.; Fu, Z.; Lin, J.; Chen, J.; Peng, L.; Wang, B.; Chen, Z. Bimetallic Ni-Hf tellurides as an advanced electrocatalyst for overall water splitting with layered g-C3N4 modification. Mater. Today Energy 2022, 26, 101002. [Google Scholar] [CrossRef]
- Gao, B.; Du, X.; Zhao, Y.; Seok Cheon, W.; Ding, S.; Xiao, C.; Song, Z.; Won Jang, H. Electron strain-driven phase transformation in transition-metal-co doped MoTe2 for electrocatalytic hydrogen evolution. Chem. Eng. J. 2022, 433, 133768. [Google Scholar] [CrossRef]
- Xing, M.; Liu, H.; Dong, X.; Liang, Z.; Huang, S.; Ding, X.; Yang, L.; Liu, Z.; Wang, S.; Cao, D. Co(OH)2 promoting the catalytic activity of CoP/Ni2P heterojunction for hydrogen evolution in both alkaline and acid media. Mater. Today Energy 2022, 30, 101142. [Google Scholar] [CrossRef]
- Cipriani, G.; Di Dio, V.; Genduso, F.; La Cascia, D.; Liga, R.; Miceli, R.; Ricco Galluzzo, G. Perspective on hydrogen energy carrier and its automotive applications. Int. J. Hydrogen Energy 2014, 39, 8482–8494. [Google Scholar] [CrossRef]
- Dunn, S. Hydrogen futures: Toward a sustainable energy system. Int. J. Hydrogen Energy 2002, 27, 235–264. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C. Technological development of hydrogen production by solid oxide electrolyzer cell (SOEC). Int. J. Hydrogen Energy 2008, 33, 2337–2354. [Google Scholar] [CrossRef]
- Laguna-Bercero, M.A. Recent advances in high temperature electrolysis using solid oxide fuel cells: A review. J. Power Sources 2012, 203, 4–16. [Google Scholar] [CrossRef]
- Zeng, K.; Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Kumar, S.S.; Ramakrishna, S.U.B.; Krishna, S.V.; Srilatha, K.; Devi, B.R.; Himabindu, V. Synthesis of titanium (IV) oxide composite membrane for hydrogen production through alkaline water electrolysis. S. Afr. J. Chem. Eng. 2018, 25, 54–61. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Ramakrishna, S.; Srinivasulu Reddy, D.; Bhagawan, D.; Himabindu, V. Synthesis of polysulfone and zirconium oxide coated asbestos composite separators for alkaline water electrolysis. Chem. Eng. Process Technol. 2017, 3, 1–1035. [Google Scholar]
- Kadier, A.; Simayi, Y.; Abdeshahian, P.; Azman, N.F.; Chandrasekhar, K.; Kalil, M.S. A comprehensive review of microbial electrolysis cells (MEC) reactor designs and configurations for sustainable hydrogen gas production. Alex. Eng. J. 2016, 55, 427–443. [Google Scholar] [CrossRef]
- Naik, S.; Chandrasekhar, K.; Jujjavarapu, S.E. Synthesis of Green Polymeric Nanocomposites Using Electrospinning. In Green Polymeric Nanocomposites; CPR Press: Hong Kong, China, 2020; p. 24. [Google Scholar]
- Trasatti, S. Water electrolysis: Who first? J. Electroanal. Chem. 1999, 476, 90–91. [Google Scholar] [CrossRef]
- Seetharaman, S.; Balaji, R.; Ramya, K.; Dhathathreyan, K.S.; Velan, M. Graphene oxide modified non-noble metal electrode for alkaline anion exchange membrane water electrolyzers. Int. J. Hydrogen Energy 2013, 38, 14934–14942. [Google Scholar] [CrossRef]
- Vermeiren, P.; Adriansens, W.; Moreels, J.P.; Leysen, R. Evaluation of the Zirfon® separator for use in alkaline water electrolysis and Ni-H2 batteries. Int. J. Hydrogen Energy 1998, 23, 321–324. [Google Scholar] [CrossRef]
- Burnat, D.; Schlupp, M.; Wichser, A.; Lothenbach, B.; Gorbar, M.; Züttel, A.; Vogt, U.F. Composite membranes for alkaline electrolysis based on polysulfone and mineral fillers. J. Power Sources 2015, 291, 163–172. [Google Scholar] [CrossRef]
- Marini, S.; Salvi, P.; Nelli, P.; Pesenti, R.; Villa, M.; Berrettoni, M.; Zangari, G.; Kiros, Y. Advanced alkaline water electrolysis. Electrochim. Acta 2012, 82, 384–391. [Google Scholar] [CrossRef]
- Ohmori, T.; Tachikawa, K.; Tsuji, K.; Anzai, K. Nickel oxide water electrolysis diaphragm fabricated by a novel method. Int. J. Hydrogen Energy 2007, 32, 5094–5097. [Google Scholar] [CrossRef]
- Chakrabarty, B.; Ghoshal, A.K.; Purkait, M.K. Preparation, characterization and performance studies of polysulfone membranes using PVP as an additive. J. Membr. Sci. 2008, 315, 36–47. [Google Scholar] [CrossRef]
- Seetharaman, S.; Ravichandran, S.; Davidson, D.J.; Vasudevan, S.; Sozhan, G. Polyvinyl Alcohol Based Membrane as Separator for Alkaline Water Electrolyzer. Sep. Sci. Technol. 2011, 46, 1563–1570. [Google Scholar] [CrossRef]
- Hwang, G.-J.; Lim, S.-G.; Bong, S.-Y.; Ryu, C.-H.; Choi, H.-S. Preparation of anion exchange membrane using polyvinyl chloride (PVC) for alkaline water electrolysis. Korean J. Chem. Eng. 2015, 32, 1896–1901. [Google Scholar] [CrossRef]
- Sandeep, K.C.; Kamath, S.; Mistry, K.; Kumar, M.A.; Bhattacharya, S.K.; Bhanja, K.; Mohan, S. Experimental studies and modeling of advanced alkaline water electrolyser with porous nickel electrodes for hydrogen production. Int. J. Hydrogen Energy 2017, 42, 12094–12103. [Google Scholar] [CrossRef]
- Grubb, W.T. Batteries with Solid Ion Exchange Electrolytes: I. Secondary Cells Employing Metal Electrodes. J. Electrochem. Soc. 1959, 106, 275. [Google Scholar] [CrossRef]
- Grubb, W.T.; Niedrach, L.W. Batteries with Solid Ion-Exchange Membrane Electrolytes: II. Low-Temperature Hydrogen-Oxygen Fuel Cells. J. Electrochem. Soc. 1960, 107, 131. [Google Scholar] [CrossRef]
- Nuttall, L.J.; Fickett, A.P.; Titterington, W.A. Hydrogen Generation by Solid Polymer Electrolyte Water Electrolysis. In Hydrogen Energy: Part A; Veziroğlu, T.N., Ed.; Springer: Boston, MA, USA, 1975; pp. 441–455. [Google Scholar]
- Baykara, S.Z. Hydrogen: A brief overview on its sources, production and environmental impact. Int. J. Hydrogen Energy 2018, 43, 10605–10614. [Google Scholar] [CrossRef]
- Khan, M.A.; Zhao, H.; Zou, W.; Chen, Z.; Cao, W.; Fang, J.; Xu, J.; Zhang, L.; Zhang, J. Recent Progresses in Electrocatalysts for Water Electrolysis. Electrochem. Energy Rev. 2018, 1, 483–530. [Google Scholar] [CrossRef]
- Ju, H.; Badwal, S.; Giddey, S. A comprehensive review of carbon and hydrocarbon assisted water electrolysis for hydrogen production. Appl. Energy 2018, 231, 502–533. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A comparative overview of hydrogen production processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Abdol Rahim, A.H.; Tijani, A.S.; Kamarudin, S.K.; Hanapi, S. An overview of polymer electrolyte membrane electrolyzer for hydrogen production: Modeling and mass transport. J. Power Sources 2016, 309, 56–65. [Google Scholar] [CrossRef]
- Grigoriev, S.A.; Millet, P.; Fateev, V.N. Evaluation of carbon-supported Pt and Pd nanoparticles for the hydrogen evolution reaction in PEM water electrolysers. J. Power Sources 2008, 177, 281–285. [Google Scholar] [CrossRef]
- Grigoriev, S.A.; Porembsky, V.I.; Fateev, V.N. Pure hydrogen production by PEM electrolysis for hydrogen energy. Int. J. Hydrogen Energy 2006, 31, 171–175. [Google Scholar] [CrossRef]
- Millet, P.; Ngameni, R.; Grigoriev, S.A.; Mbemba, N.; Brisset, F.; Ranjbari, A.; Etiévant, C. PEM water electrolyzers: From electrocatalysis to stack development. Int. J. Hydrogen Energy 2010, 35, 5043–5052. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, H.; Chen, G.; Zhang, Y. Study of IrxRu1−xO2 oxides as anodic electrocatalysts for solid polymer electrolyte water electrolysis. Electrochim. Acta 2009, 54, 6250–6256. [Google Scholar] [CrossRef]
- Xu, W.; Scott, K. The effects of ionomer content on PEM water electrolyser membrane electrode assembly performance. Int. J. Hydrogen Energy 2010, 35, 12029–12037. [Google Scholar] [CrossRef]
- Dönitz, W.; Erdle, E. High-temperature electrolysis of water vapor—Status of development and perspectives for application. Int. J. Hydrogen Energy 1985, 10, 291–295. [Google Scholar] [CrossRef]
- Brisse, A.; Schefold, J.; Zahid, M. High temperature water electrolysis in solid oxide cells. Int. J. Hydrogen Energy 2008, 33, 5375–5382. [Google Scholar] [CrossRef]
- Liang, M.; Yu, B.; Wen, M.; Chen, J.; Xu, J.; Zhai, Y. Preparation of LSM–YSZ composite powder for anode of solid oxide electrolysis cell and its activation mechanism. J. Power Sources 2009, 190, 341–345. [Google Scholar] [CrossRef]
- Moçoteguy, P.; Brisse, A. A review and comprehensive analysis of degradation mechanisms of solid oxide electrolysis cells. Int. J. Hydrogen Energy 2013, 38, 15887–15902. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J.; Christiansen, L. Concepts in syngas preparation. In Catalytic Science Series; Imperial College Press: London, UK, 2011. [Google Scholar]
- Knibbe, R.; Traulsen, M.L.; Hauch, A.; Ebbesen, S.D.; Mogensen, M. Solid Oxide Electrolysis Cells: Degradation at High Current Densities. J. Electrochem. Soc. 2010, 157, B1209. [Google Scholar] [CrossRef]
- Liu, H.; Grot, S.; Logan, B.E. Electrochemically Assisted Microbial Production of Hydrogen from Acetate. Environ. Sci. Technol. 2005, 39, 4317–4320. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, N.K.; Sani, R.K.; Salem, D. Rewiring Extremophilic Electrocatalytic Processes for Production of Biofuels and Value-Added Compounds from Lignocellulosic Biomass. In Extremophilic Microbial Processing of Lignocellulosic Feedstocks to Biofuels, Value-Added Products, and Usable Power; Sani, R.K., Krishnaraj Rathinam, N., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 229–245. [Google Scholar]
- Dorezco, D.; Jaqueline Carreno, D.L.; Maria, D.C.; Fernandez, V.; Suilma, M. Construccion y Operacion de un Sistema Bioelectroquimico Para la Disminucion de DQO; Tecnológico Nacional de México: Mexico City, Mexico, 2019. [Google Scholar]
- Chia, X.; Ambrosi, A.; Lazar, P.; Sofer, Z.; Pumera, M. Electrocatalysis of layered Group 5 metallic transition metal dichalcogenides (MX2, M = V, Nb, and Ta; X = S, Se, and Te). J. Mater. Chem. A 2016, 4, 14241–14253. [Google Scholar] [CrossRef]
- Qiao, H.; Huang, Z.; Liu, S.; Liu, Y.; Li, J.; Qi, X. Liquid-exfoliated molybdenum telluride nanosheets with superior electrocatalytic hydrogen evolution performances. Ceram. Int. 2018, 44, 21205–21209. [Google Scholar] [CrossRef]
- Zhuang, P.; Sun, Y.; Dong, P.; Smith, W.; Sun, Z.; Ge, Y.; Pei, Y.; Cao, Z.; Ajayan, P.M.; Shen, J.; et al. Revisiting the Role of Active Sites for Hydrogen Evolution Reaction through Precise Defect Adjusting. Adv. Funct. Mater. 2019, 29, 1901290. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, L. Nickel Ditelluride Nanosheet Arrays: A Highly Efficient Electrocatalyst for the Oxygen Evolution Reaction. ChemElectroChem 2018, 5, 1153–1158. [Google Scholar] [CrossRef]
- De Silva, U.; Masud, J.; Zhang, N.; Hong, Y.; Liyanage, W.P.R.; Asle Zaeem, M.; Nath, M. Nickel telluride as a bifunctional electrocatalyst for efficient water splitting in alkaline medium. J. Mater. Chem. A 2018, 6, 7608–7622. [Google Scholar] [CrossRef]
- Zhang, F.; Eom, T.-Y.; Cho, M.; Lee, H.-J.; Pang, H. Fabrication of defect-rich bifunctional hollow NiTe2 nanotubes for high performance hydrogen evolution electrocatalysts and supercapacitors. J. Energy Storage 2021, 42, 103098. [Google Scholar] [CrossRef]
- Zheng, Z.; Wu, L.; Han, Y.; Chen, J.; Zhu, S.; Yao, Y.; Wang, B.; Li, L. Gut Microbiota-Controlled Tryptophan Metabolism Improves D-Gal/LPS-Induced Acute Liver Failure in C57BL/6 Mice. Engineering 2022, 14, 134–146. [Google Scholar] [CrossRef]
- Sivanantham, A.; Hyun, S.; Son, M.; Shanmugam, S. Nanostructured core-shell cobalt chalcogenides for efficient water oxidation in alkaline electrolyte. Electrochim. Acta 2019, 312, 234–241. [Google Scholar] [CrossRef]
- Anantharaj, S.; Karthick, K.; Kundu, S. NiTe2 Nanowire Outperforms Pt/C in High-Rate Hydrogen Evolution at Extreme pH Conditions. Inorg. Chem. 2018, 57, 3082–3096. [Google Scholar] [CrossRef]
- Wang, X.; Huang, X.; Gao, W.; Tang, Y.; Jiang, P.; Lan, K.; Yang, R.; Wang, B.; Li, R. Metal–organic framework derived CoTe2 encapsulated in nitrogen-doped carbon nanotube frameworks: A high-efficiency bifunctional electrocatalyst for overall water splitting. J. Mater. Chem. A 2018, 6, 3684–3691. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Tan, L.; Fang, L.; Yang, X.; Huang, Z.; Li, J.; Zhang, H.; Wang, Y. Component-controllable cobalt telluride nanoparticles encapsulated in nitrogen-doped carbon frameworks for efficient hydrogen evolution in alkaline conditions. Appl. Catal. B Environ. 2019, 244, 568–575. [Google Scholar] [CrossRef]
- He, B.; Wang, X.-C.; Xia, L.-X.; Guo, Y.-Q.; Tang, Y.-W.; Zhao, Y.; Hao, Q.-L.; Yu, T.; Liu, H.-K.; Su, Z. Metal-Organic Framework-Derived Fe-Doped Co1.11Te2 Embedded in Nitrogen-Doped Carbon Nanotube for Water Splitting. Chemsuschem 2020, 13, 5239–5247. [Google Scholar] [CrossRef] [PubMed]
- Xue, Z.; Li, X.; Liu, Q.; Cai, M.; Liu, K.; Liu, M.; Ke, Z.; Liu, X.; Li, G. Interfacial Electronic Structure Modulation of NiTe Nanoarrays with NiS Nanodots Facilitates Electrocatalytic Oxygen Evolution. Adv. Mater. 2019, 31, 1900430. [Google Scholar] [CrossRef]
- Amorim, I.; Xu, J.; Zhang, N.; Yu, Z.; Araújo, A.; Bento, F.; Liu, L. Dual-phase CoP−CoTe2 nanowires as an efficient bifunctional electrocatalyst for bipolar membrane-assisted acid-alkaline water splitting. Chem. Eng. J. 2021, 420, 130454. [Google Scholar] [CrossRef]
- Li, Y.; Wei, B.; Yu, Z.; Bondarchuk, O.; Araujo, A.; Amorim, I.; Zhang, N.; Xu, J.; Neves, I.C.; Liu, L. Bifunctional Porous Cobalt Phosphide Foam for High-Current-Density Alkaline Water Electrolysis with 4000-h Long Stability. ACS Sustain. Chem. Eng. 2020, 8, 10193–10200. [Google Scholar] [CrossRef]
- Li, W.; Xiong, D.; Gao, X.; Song, W.-G.; Xia, F.; Liu, L. Self-supported Co-Ni-P ternary nanowire electrodes for highly efficient and stable electrocatalytic hydrogen evolution in acidic solution. Catal. Today 2017, 287, 122–129. [Google Scholar] [CrossRef]
- Xiong, D.; Zhang, Q.; Thalluri, S.M.; Xu, J.; Li, W.; Fu, X.; Liu, L. One-Step Fabrication of Monolithic Electrodes Comprising Co9S8 Particles Supported on Cobalt Foam for Efficient and Durable Oxygen Evolution Reaction. Chem.–A Eur. J. 2017, 23, 8749–8755. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Gao, X.; Xiong, D.; Wei, F.; Song, W.-G.; Xu, J.; Liu, L. Hydrothermal Synthesis of Monolithic Co3Se4 Nanowire Electrodes for Oxygen Evolution and Overall Water Splitting with High Efficiency and Extraordinary Catalytic Stability. Adv. Energy Mater. 2017, 7, 1602579. [Google Scholar] [CrossRef]
- Wu, X.; Lu, L.; Liu, H.; Feng, L.; Li, W.; Sun, L. Metalloid Te-Doped Fe-Based Catalysts Applied for Electrochemical Water Oxidation. Chem. Sel. 2021, 6, 6154–6158. [Google Scholar] [CrossRef]
- Qi, Y.; Wu, J.; Xu, J.; Gao, H.; Du, Z.; Liu, B.; Liu, L.; Xiong, D. One-step fabrication of a self-supported Co@CoTe2 electrocatalyst for efficient and durable oxygen evolution reactions. Inorg. Chem. Front. 2020, 7, 2523–2532. [Google Scholar] [CrossRef]
- Wang, R.; Liu, Y.; Tian, Z.; Shi, Y.; Xu, Q.; Zhang, G.; Chen, J.; Zheng, W. Copper Telluride Nanosheet/Cu Foil Electrode: Facile Ionic Liquid-Assisted Synthesis and Efficient Oxygen Evolution Performance. J. Phys. Chem. C 2020, 124, 22117–22126. [Google Scholar] [CrossRef]
- Li, W.; Xiong, D.; Gao, X.; Liu, L. The oxygen evolution reaction enabled by transition metal phosphide and chalcogenide pre-catalysts with dynamic changes. Chem. Commun. 2019, 55, 8744–8763. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Xu, H.; Liu, H.; Cheng, D.; Cao, D. Active Site Identification and Evaluation Criteria of In Situ Grown CoTe and NiTe Nanoarrays for Hydrogen Evolution and Oxygen Evolution Reactions. Small Methods 2019, 3, 1900113. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, Y.; Huang, L.; Wu, Y.; Ren, S. Se Doping Regulates the Activity of NiTe2 for Electrocatalytic Hydrogen Evolution Reaction. J. Phys. Chem. C 2020, 124, 26793–26800. [Google Scholar] [CrossRef]
- Wang, Q.; Cui, K.; Li, J.; Wu, Y.; Yang, Y.; Zhou, X.; Ma, G.; Yang, Z.; Lei, Z.; Ren, S. Phosphorus-doped CoTe2/C nanoparticles create new Co–P active sites to promote the hydrogen evolution reaction. Nanoscale 2020, 12, 9171–9177. [Google Scholar] [CrossRef]
- Zhong, L.; Bao, Y.; Yu, X.; Feng, L. An Fe-doped NiTe bulk crystal as a robust catalyst for the electrochemical oxygen evolution reaction. Chem. Commun. 2019, 55, 9347–9350. [Google Scholar] [CrossRef]
- Li, W.; Chen, J.; Zhang, Y.; Gong, W.; Sun, M.; Wang, Y.; Wang, X.; Rao, H.; Ye, J.; Lu, Z. Hollow Fe/Ni–CoTe@NCFs nanoarchitecture derived from MOF@MOF as high-efficiency electrocatalysts for boosting oxygen evolution reaction. Int. J. Hydrogen Energy 2021, 46, 39912–39920. [Google Scholar] [CrossRef]
- He, R.; Li, M.; Qiao, W.; Feng, L. Fe doped Mo/Te nanorods with improved stability for oxygen evolution reaction. Chem. Eng. J. 2021, 423, 130168. [Google Scholar] [CrossRef]
- Pan, U.N.; Paudel, D.R.; Kumar Das, A.; Singh, T.I.; Kim, N.H.; Lee, J.H. Ni-nanoclusters hybridized 1T–Mn–VTe2 mesoporous nanosheets for ultra-low potential water splitting. Appl. Catal. B Environ. 2022, 301, 120780. [Google Scholar] [CrossRef]
- Xu, J.; Li, J.; Xiong, D.; Zhang, B.; Liu, Y.; Wu, K.-H.; Amorim, I.; Li, W.; Liu, L. Trends in activity for the oxygen evolution reaction on transition metal (M = Fe, Co, Ni) phosphide pre-catalysts. Chem. Sci. 2018, 9, 3470–3476. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shen, Y.; Xiao, X.; Dai, L.; Yao, S.; An, C. Topology conversion of 1T MoS2 to S-doped 2H-MoTe2 nanosheets with Te vacancies for enhanced electrocatalytic hydrogen evolution. Sci. China Mater. 2021, 64, 2202–2211. [Google Scholar] [CrossRef]
- Qian, G.; Mo, Y.; Yu, C.; Zhang, H.; Yu, T.; Luo, L.; Yin, S. Free-standing bimetallic CoNiTe2 nanosheets as efficient catalysts with high stability at large current density for oxygen evolution reaction. Renew. Energy 2020, 162, 2190–2196. [Google Scholar] [CrossRef]
- Sadaqat, M.; Manzoor, S.; Nisar, L.; Hassan, A.; Tyagi, D.; Shah, J.H.; Ashiq, M.N.; Joya, K.S.; Alshahrani, T.; Najam-ul-Haq, M. Iron doped nickel ditelluride hierarchical nanoflakes arrays directly grown on nickel foam as robust electrodes for oxygen evolution reaction. Electrochim. Acta 2021, 371, 137830. [Google Scholar] [CrossRef]
- Sarwar, S.; Ali, A.; Wang, Y.; Ahasan, M.R.; Wang, R.; Adamczyk, A.J.; Zhang, X. Enhancement of hydrogen evolution reaction activity using metal–rich molybdenum sulfotelluride with graphene support: A combined experimental and computational study. Nano Energy 2021, 90, 106599. [Google Scholar] [CrossRef]
- Kosmala, T.; Coy Diaz, H.; Komsa, H.P.; Ma, Y.; Krasheninnikov, A.V.; Batzill, M.; Agnoli, S. Metallic twin boundaries boost the hydrogen evolution reaction on the basal plane of molybdenum selenotellurides. Adv. Energy Mater. 2018, 8, 1800031. [Google Scholar] [CrossRef]
- Perryman, J.T.; Ortiz-Rodríguez, J.C.; Jude, J.W.; Hyler, F.P.; Davis, R.C.; Mehta, A.; Kulkarni, A.R.; Patridge, C.J.; Velázquez, J.M. Metal-promoted Mo6S8 clusters: A platform for probing ensemble effects on the electrochemical conversion of CO2 and CO to methanol. Mater. Horiz. 2020, 7, 193–202. [Google Scholar] [CrossRef]
- Kwon, T.; Jun, M.; Joo, J.; Lee, K. Nanoscale hetero-interfaces between metals and metal compounds for electrocatalytic applications. J. Mater. Chem. A 2019, 7, 5090–5110. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, X.; Wang, L.; Cui, G.; Wang, H.; Sun, X. A hierarchical CoTe2–MnTe2 hybrid nanowire array enables high activity for oxygen evolution reactions. Chem. Commun. 2018, 54, 10993–10996. [Google Scholar] [CrossRef]
- Qi, Y.; Yang, Z.; Peng, S.; Dong, Y.; Wang, M.; Bao, X.-Q.; Li, H.; Xiong, D. CoTe2–NiTe2 heterojunction directly grown on CoNi alloy foam for efficient oxygen evolution reaction. Inorg. Chem. Front. 2022, 9, 332–342. [Google Scholar] [CrossRef]
- Majhi, K.C.; Yadav, M. Transition metal chalcogenides based nanocomposites as efficient electrocatalyst for hydrogen evolution reaction over the entire pH range. Int. J. Hydrogen Energy 2020, 45, 24219–24231. [Google Scholar] [CrossRef]
- Mingli, F.; Xue, L.; Dandan, W.; Yinling, W.; Maoguo, L. Fabrication of Te@NiTe2/NiS heterostructures for electrocatalytic hydrogen evolution reaction. Electrochim. Acta 2019, 328, 135075. [Google Scholar] [CrossRef]
- Hu, L.; Zeng, X.; Wei, X.; Wang, H.; Wu, Y.; Gu, W.; Shi, L.; Zhu, C. Interface engineering for enhancing electrocatalytic oxygen evolution of NiFe LDH/NiTe heterostructures. Appl. Catal. B Environ. 2020, 273, 119014. [Google Scholar] [CrossRef]
- Zhang, P.-Y.; Zhong-Liang, L. Experimental study of the microbial fuel cell internal resistance. J. Power Sources 2010, 195, 8013–8018. [Google Scholar] [CrossRef]
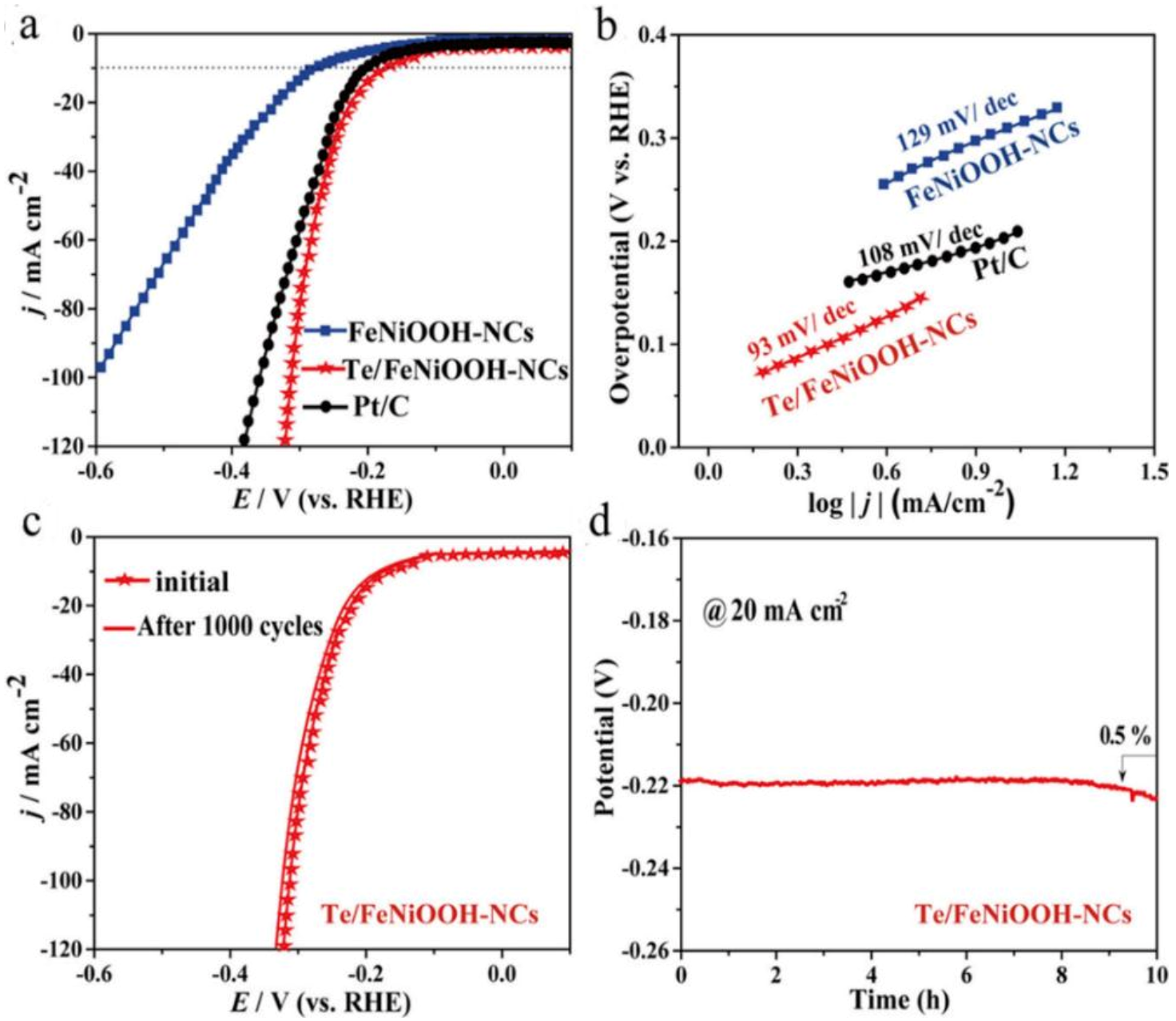
- Ibraheem, S.; Li, X.; Shah, S.S.A.; Najam, T.; Yasin, G.; Iqbal, R.; Hussain, S.; Ding, W.; Shahzad, F. Tellurium Triggered Formation of Te/Fe-NiOOH Nanocubes as an Efficient Bifunctional Electrocatalyst for Overall Water Splitting. ACS Appl. Mater. Interfaces 2021, 13, 10972–10978. [Google Scholar] [CrossRef]
- Li, Y.; Tan, X.; Tan, H.; Ren, H.; Chen, S.; Yang, W.; Smith, S.C.; Zhao, C. Phosphine vapor-assisted construction of heterostructured Ni2P/NiTe2 catalysts for efficient hydrogen evolution. Energy Environ. Sci. 2020, 13, 1799–1807. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y. Circular economy is game-changing municipal wastewater treatment technology towards energy and carbon neutrality. Chem. Eng. J. 2022, 429, 132114. [Google Scholar] [CrossRef]
- Xu, J.; Yin, Y.; Xiong, H.; Du, X.; Jiang, Y.; Guo, W.; Wang, Z.; Xie, Z.; Qu, D.; Tang, H. Improving catalytic activity of metal telluride by hybridization: An efficient Ni3Te2-CoTe composite electrocatalyst for oxygen evolution reaction. Appl. Surf. Sci. 2019, 490, 516–521. [Google Scholar] [CrossRef]
- Sun, H.; Yang, J.-M.; Li, J.-G.; Li, Z.; Ao, X.; Liu, Y.-Z.; Zhang, Y.; Li, Y.; Wang, C.; Tang, J. Synergistic coupling of NiTe nanoarrays with RuO2 and NiFe-LDH layers for high-efficiency electrochemical-/photovoltage-driven overall water splitting. Appl. Catal. B Environ. 2020, 272, 118988. [Google Scholar] [CrossRef]
- Li, Y.; Chen, B.; Zhang, H.; Gao, J.; Sun, H.; Habibi, Y.A.; Wang, C. Synergistic Coupling of NiTe Nanoarrays with FeOOH Nanosheets for Highly Efficient Oxygen Evolution Reaction. ChemElectroChem 2021, 8, 3643–3650. [Google Scholar] [CrossRef]
- Ibraheem, S.; Chen, S.; Li, J.; Wang, Q.; Wei, Z. In situ growth of vertically aligned FeCoOOH-nanosheets/nanoflowers on Fe, N co-doped 3D-porous carbon as efficient bifunctional electrocatalysts for rechargeable zinc–O2 batteries. J. Mater. Chem. A 2019, 7, 9497–9502. [Google Scholar] [CrossRef]
- Karthick, K.; Mansoor, B.; Abdul, B.; Sivakumaran, A.; Kundu, S. Enhancement of HER kinetics with RhNiFe for high-rate water electrolysis. Catal. Sci. Technol. 2020, 10, 3681–3693. [Google Scholar] [CrossRef]
- Tian, J.; Liu, Q.; Asiri, A.M.; Sun, X. Self-Supported Nanoporous Cobalt Phosphide Nanowire Arrays: An Efficient 3D Hydrogen-Evolving Cathode over the Wide Range of pH 0–14. J. Am. Chem. Soc. 2014, 136, 7587–7590. [Google Scholar] [CrossRef]
- Jiang, H.; Yan, L.; Zhang, S.; Zhao, Y.; Yang, X.; Wang, Y.; Shen, J.; Zhao, X.; Wang, L. Electrochemical Surface Restructuring of Phosphorus-Doped Carbon@MoP Electrocatalysts for Hydrogen Evolution. Nano-Micro Lett. 2021, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, Z.; Jiao, L. Multifunctional Transition Metal-Based Phosphides in Energy-Related Electrocatalysis. Adv. Energy Mater. 2020, 10, 1902104. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, B. Recent advances in transition metal phosphide nanomaterials: Synthesis and applications in hydrogen evolution reaction. Chem. Soc. Rev. 2016, 45, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Fei, B.; Cai, G.; Ha, Y.; Liu, J.; Jia, H.; Zhang, J.; Liu, M.; Wu, R. Boronization-Induced Ultrathin 2D Nanosheets with Abundant Crystalline–Amorphous Phase Boundary Supported on Nickel Foam toward Efficient Water Splitting. Adv. Energy Mater. 2020, 10, 1902714. [Google Scholar] [CrossRef]
- Cai, J.; Song, Y.; Zang, Y.; Niu, S.; Wu, Y.; Xie, Y.; Zheng, X.; Liu, Y.; Lin, Y.; Liu, X.; et al. N-induced lattice contraction generally boosts the hydrogen evolution catalysis of P-rich metal phosphides. Sci. Adv. 2020, 6, eaaw8113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kim, D.; Guo, X.; Zhu, Y.; Lee, L.Y.S. Impacts of boron doping on the atomic structure, stability, and photocatalytic activity of Cu3P nanocrystals. Appl. Catal. B Environ. 2021, 298, 120515. [Google Scholar] [CrossRef]
- Luo, Y.; Tang, L.; Khan, U.; Yu, Q.; Cheng, H.-M.; Zou, X.; Liu, B. Morphology and surface chemistry engineering toward pH-universal catalysts for hydrogen evolution at high current density. Nat. Commun. 2019, 10, 269. [Google Scholar] [CrossRef] [PubMed]


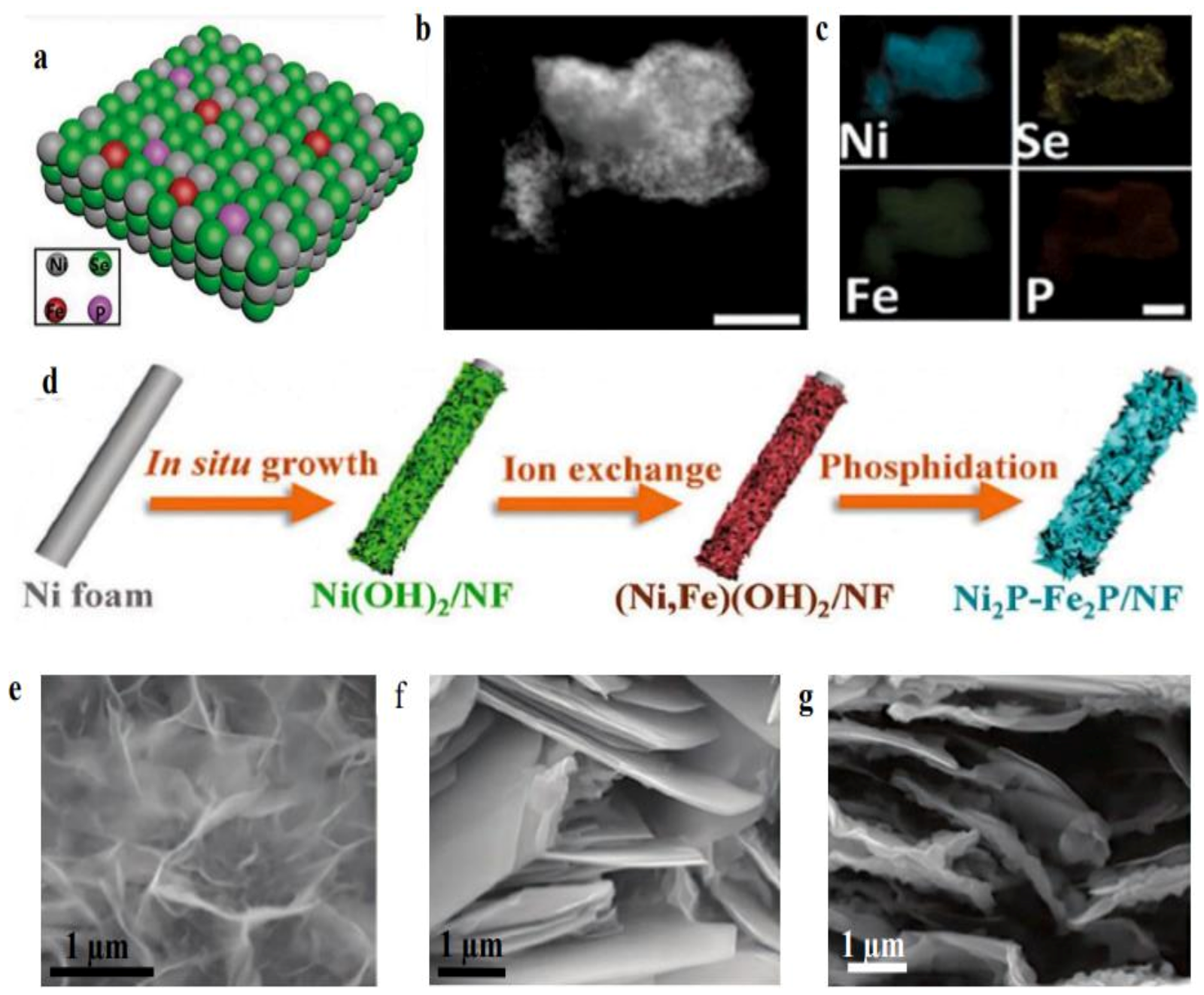
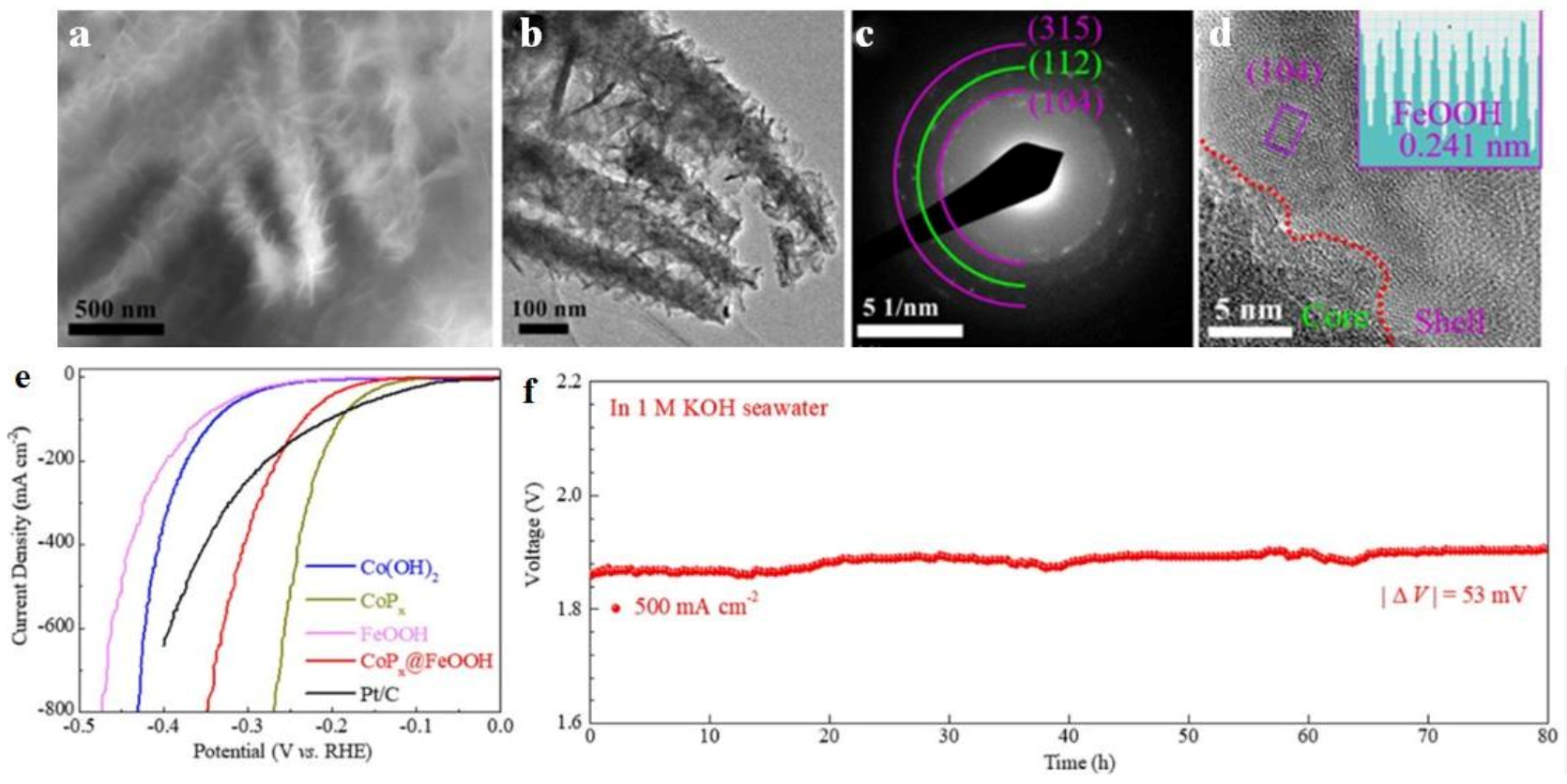
| Sr. No | Electrocatalysts | HER Medium | Current Density (mA cm−2) | Overpotential (mV) | Tafel Slope (mV dec−1) | Refs |
|---|---|---|---|---|---|---|
| 1 | Co2P@Cu nanostructure | Alkaline | 10 100 | 99.7 303.2 | 48.8 | [56] |
| 2 | Co2P/Ni2P/CNT | Acidic Alkaline | 10 10 | 151 202 | 41.64 ---- | [57] |
| 3 | FeNiP/MoOx/NiMoO4/NF | Alkaline | 10 100 | 16 97 | 21.2 | [58] |
| 4 | Ni2P@NC/NF | Alkaline | 10 | 93 | ---- | [59] |
| 5 | MoP-Ru2P/NPC | Alkaline | 10 10 | 47 82 | 36.93 64.99 | [60] |
| 6 | CoTe2/Ti3C2Tx | Alkaline | 10 | 200 | 95 | [61] |
| 7 | NiFe2O4/NiTe | Alkaline | 10 | 148.8 | 73.67 | [62] |
| 8 | NiTe-HfTe2/g-C3N4 | Alkaline | 10 | 71 | 75 | [63] |
| 9 | Co,Ni-MoTe2 | Acidic | 10 | −82 | ---- | [64] |
| 10 | CoP/Ni2P@Co(OH)2 | Acidic Alkaline | 10 10 | 68 39 | 68 55 | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, S.S.A.; Khan, N.A.; Imran, M.; Rashid, M.; Tufail, M.K.; Rehman, A.u.; Balkourani, G.; Sohail, M.; Najam, T.; Tsiakaras, P. Recent Advances in Transition Metal Tellurides (TMTs) and Phosphides (TMPs) for Hydrogen Evolution Electrocatalysis. Membranes 2023, 13, 113. https://doi.org/10.3390/membranes13010113
Shah SSA, Khan NA, Imran M, Rashid M, Tufail MK, Rehman Au, Balkourani G, Sohail M, Najam T, Tsiakaras P. Recent Advances in Transition Metal Tellurides (TMTs) and Phosphides (TMPs) for Hydrogen Evolution Electrocatalysis. Membranes. 2023; 13(1):113. https://doi.org/10.3390/membranes13010113
Chicago/Turabian StyleShah, Syed Shoaib Ahmad, Naseem Ahmad Khan, Muhammad Imran, Muhammad Rashid, Muhammad Khurram Tufail, Aziz ur Rehman, Georgia Balkourani, Manzar Sohail, Tayyaba Najam, and Panagiotis Tsiakaras. 2023. "Recent Advances in Transition Metal Tellurides (TMTs) and Phosphides (TMPs) for Hydrogen Evolution Electrocatalysis" Membranes 13, no. 1: 113. https://doi.org/10.3390/membranes13010113
APA StyleShah, S. S. A., Khan, N. A., Imran, M., Rashid, M., Tufail, M. K., Rehman, A. u., Balkourani, G., Sohail, M., Najam, T., & Tsiakaras, P. (2023). Recent Advances in Transition Metal Tellurides (TMTs) and Phosphides (TMPs) for Hydrogen Evolution Electrocatalysis. Membranes, 13(1), 113. https://doi.org/10.3390/membranes13010113









