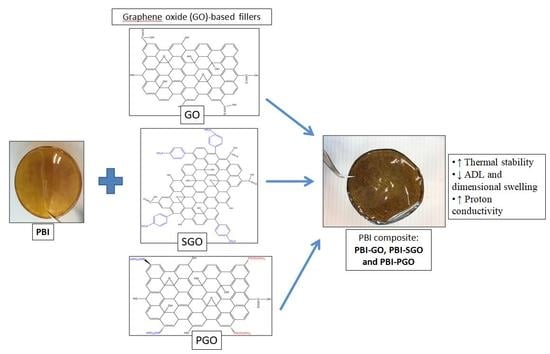
Proton Conductivity Enhancement at High Temperature on Polybenzimidazole Membrane Electrolyte with Acid-Functionalized Graphene Oxide Fillers
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
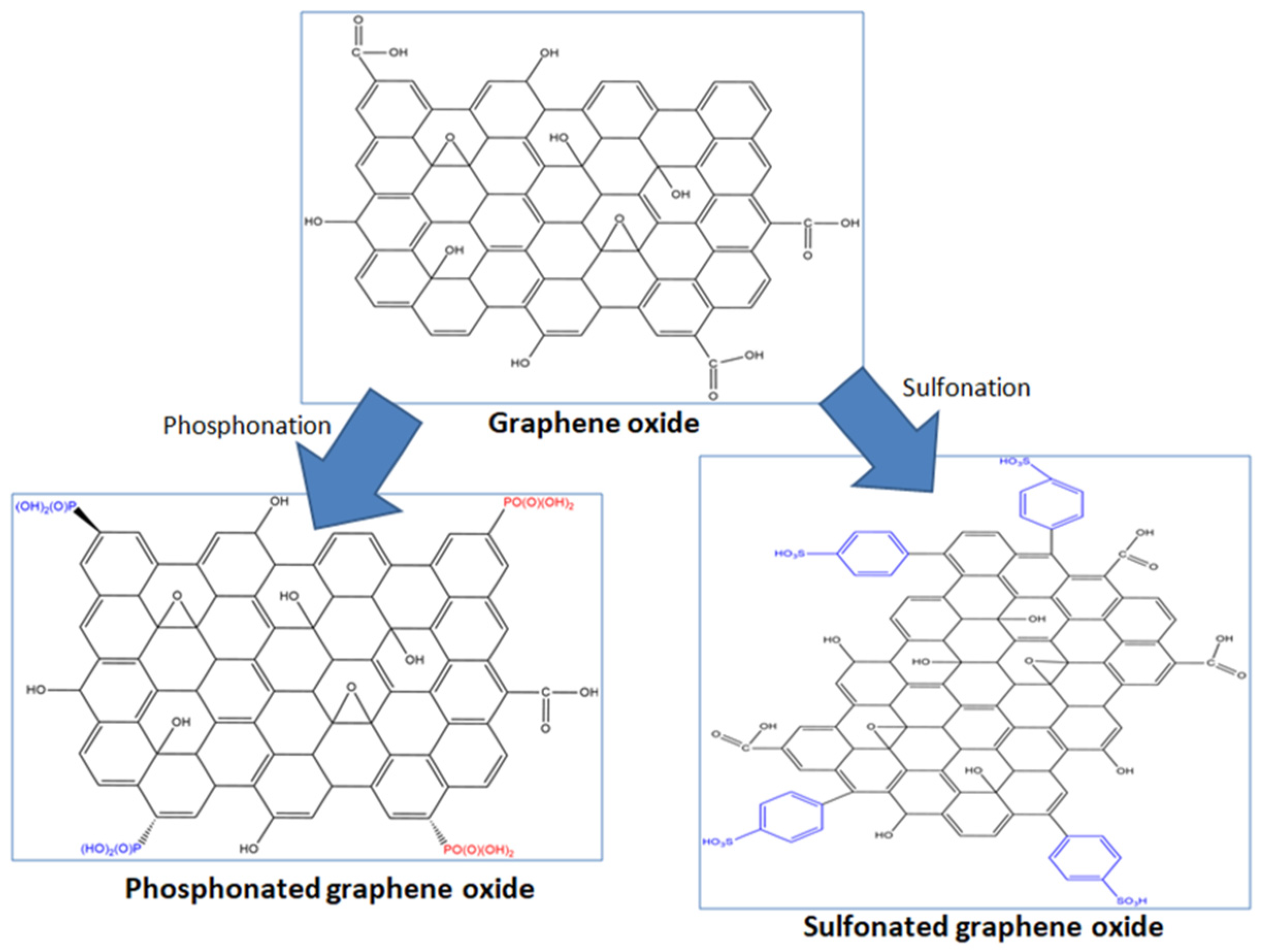
2.2. Synthesis of GO
2.3. Synthesis of SGO and PGO
2.4. PBI and Composite PBI Membrane Preparation
2.5. Physicochemical Characterization
2.6. PA Doping, ADL, Dimensional Swelling, and Acid Leaching Test
2.7. Proton Conductivity Measurements
3. Results
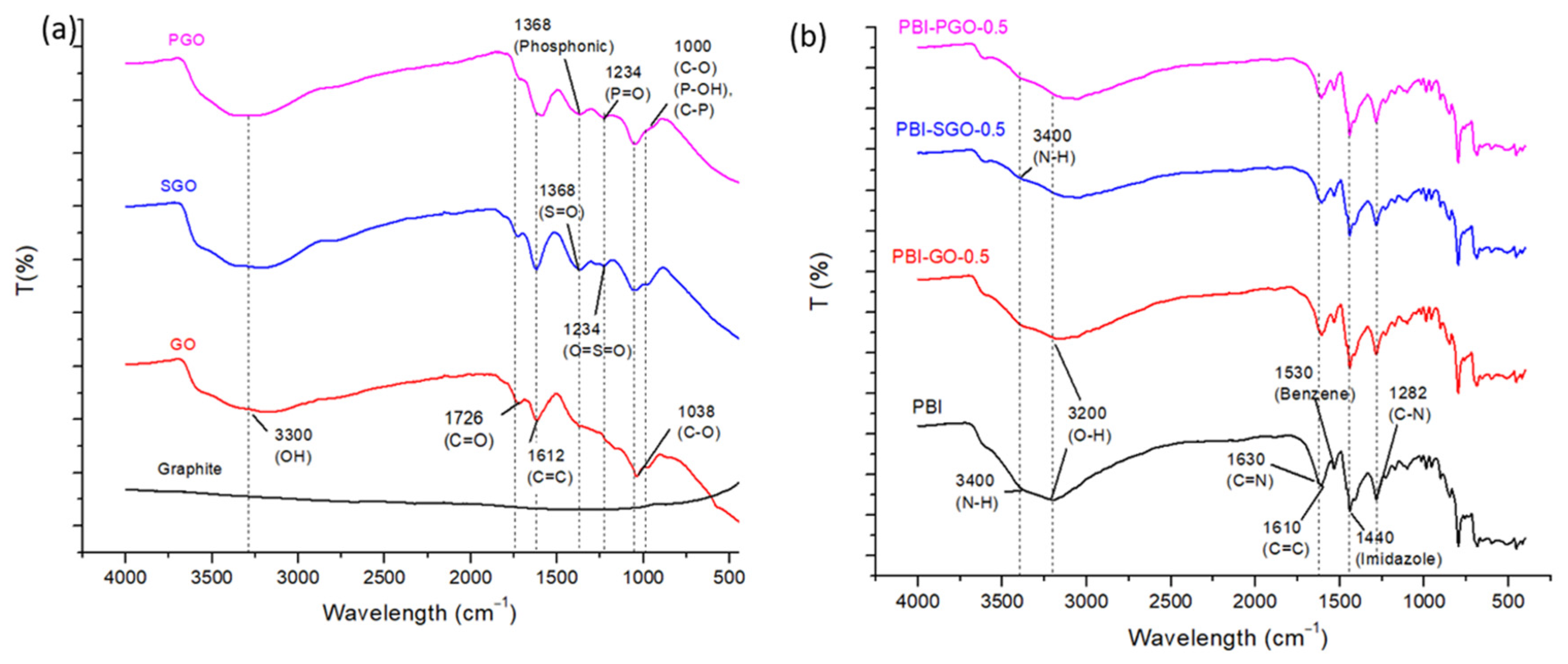
3.1. FTIR
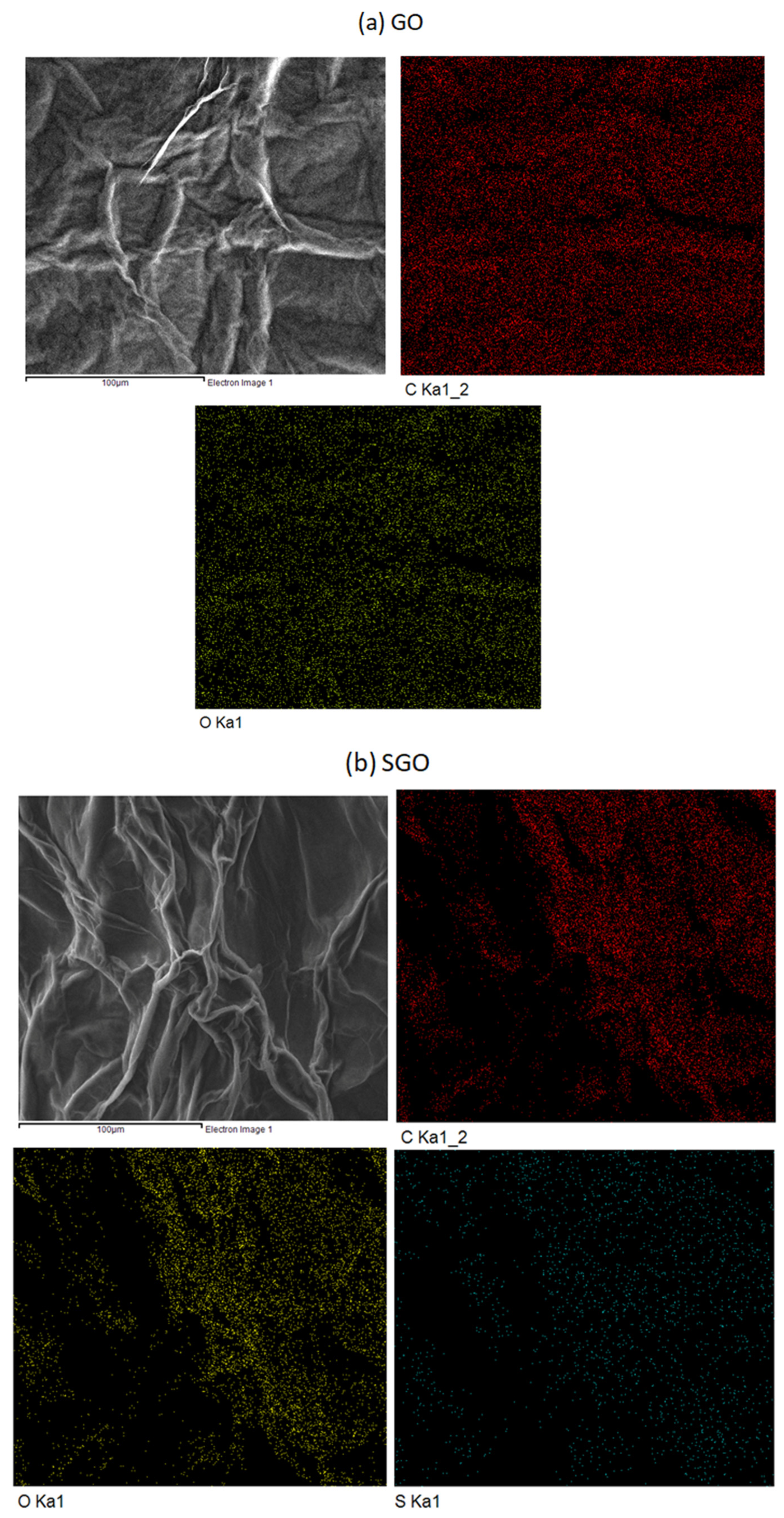
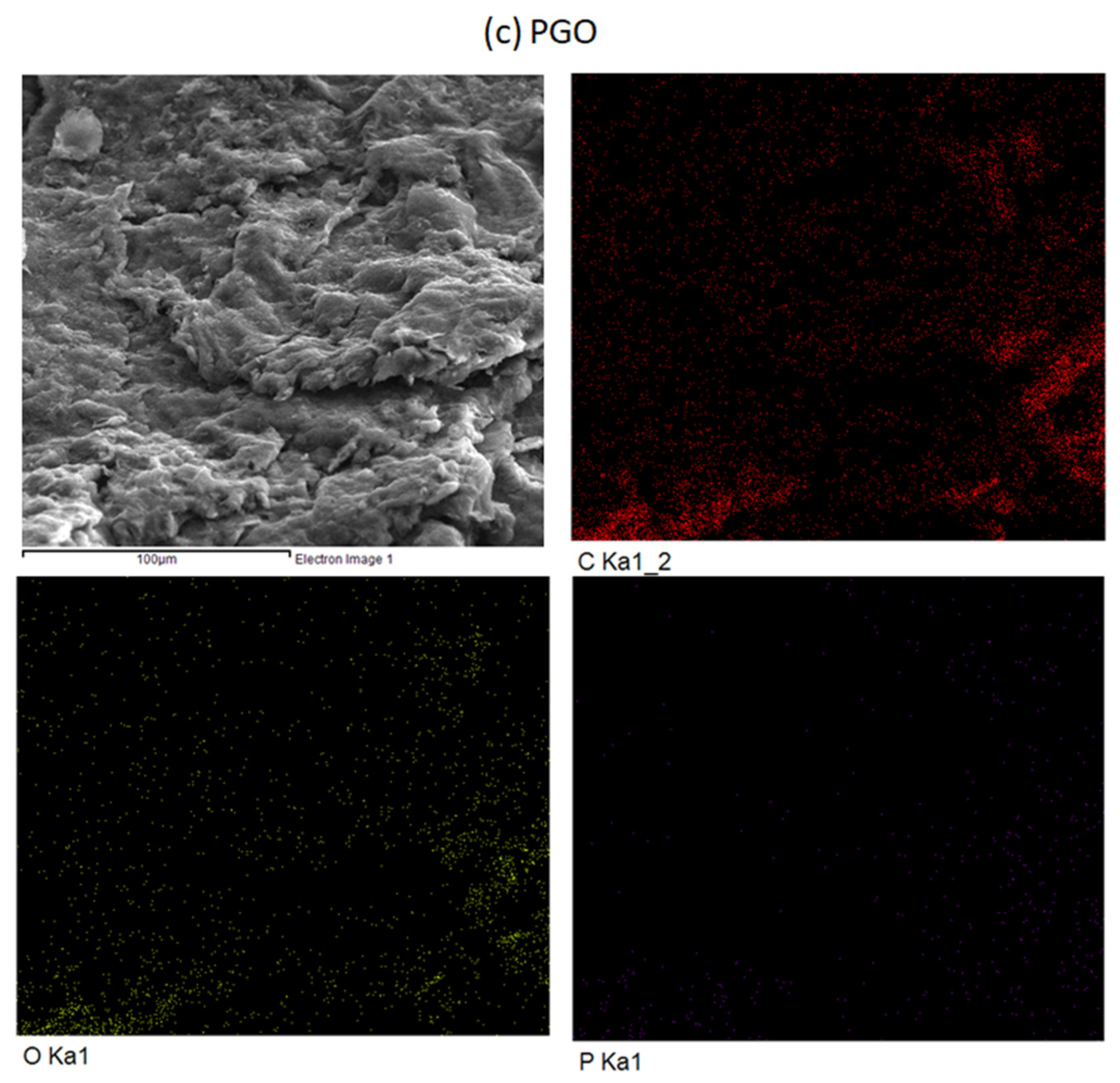
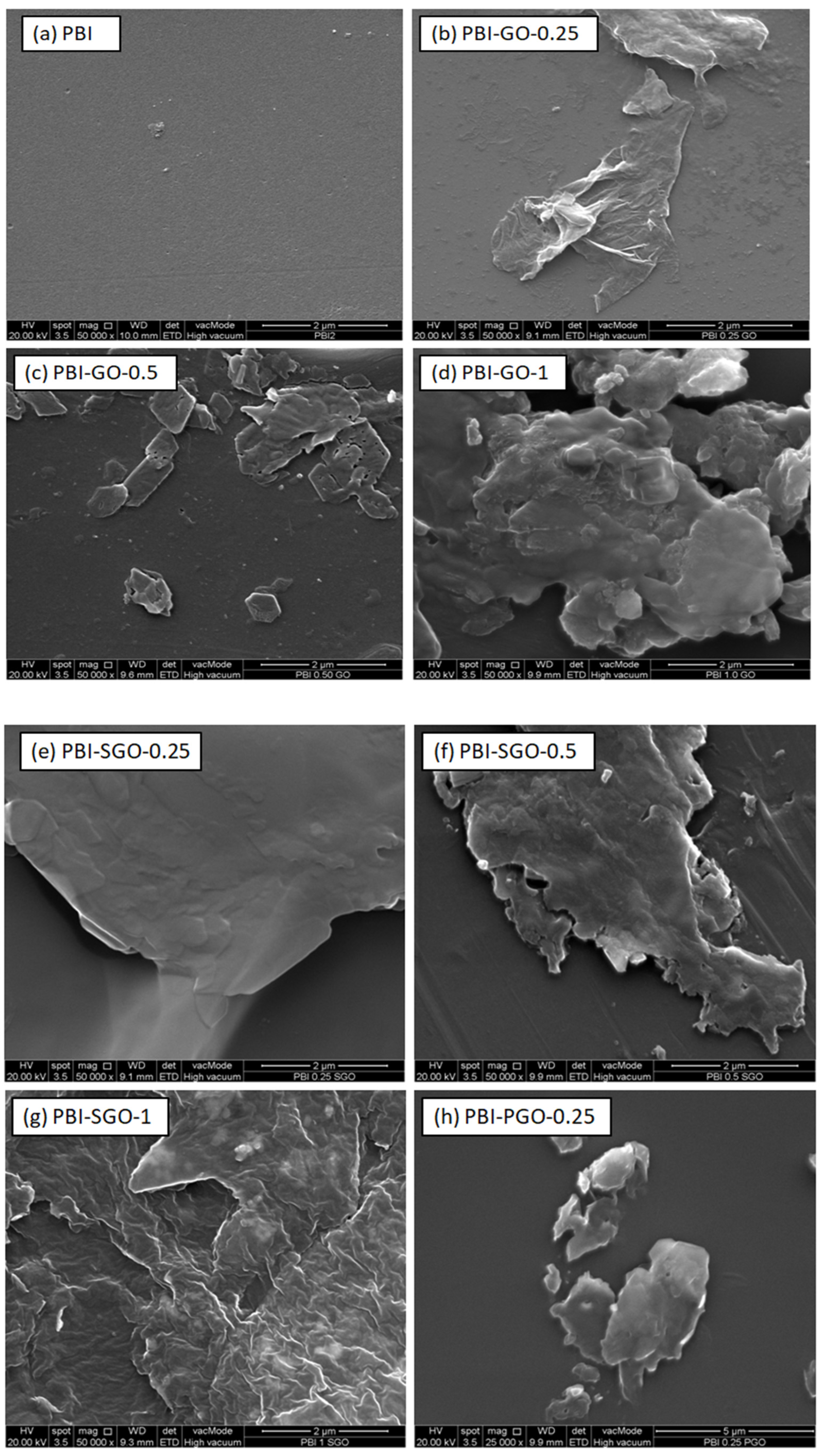
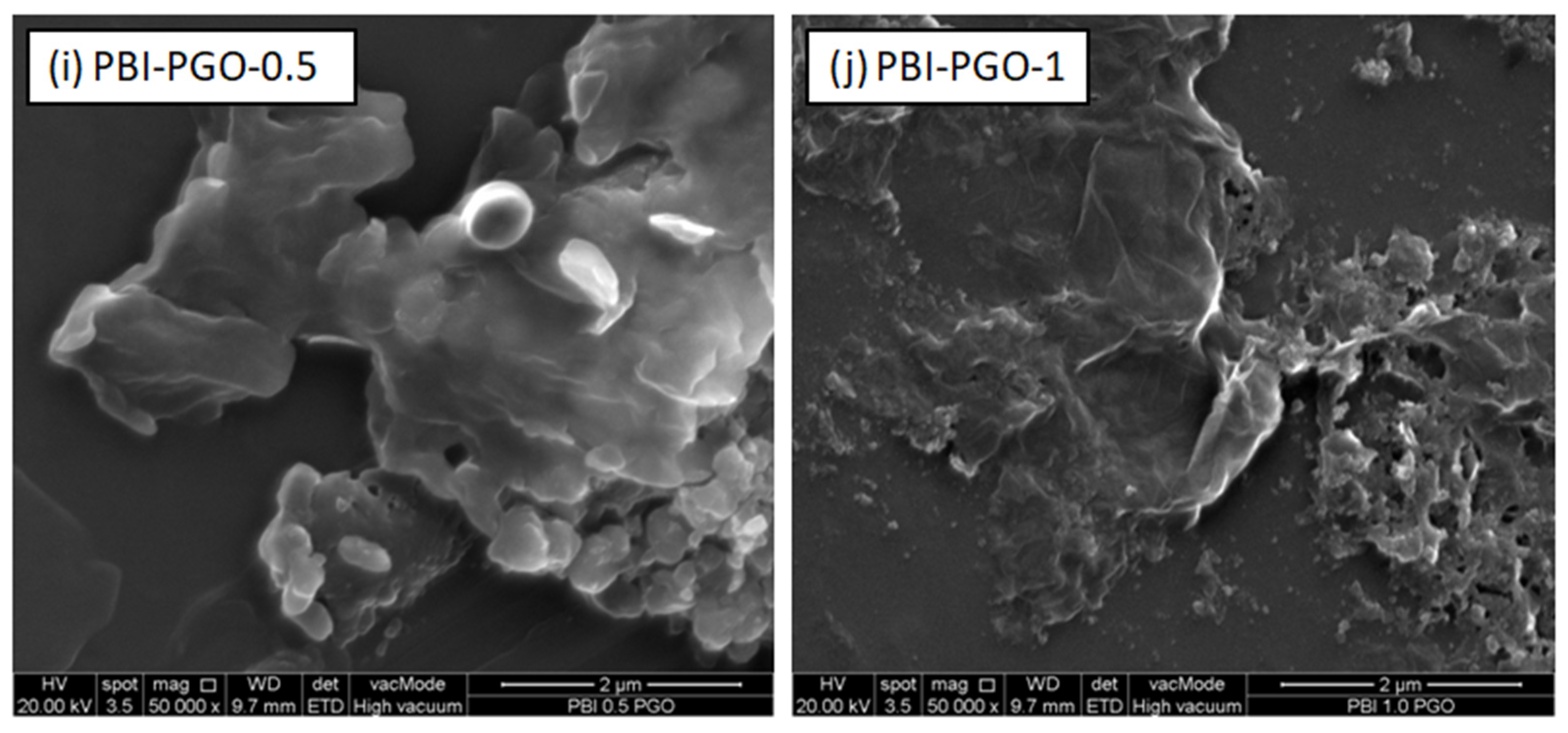
3.2. FESEM and EDX Mapping
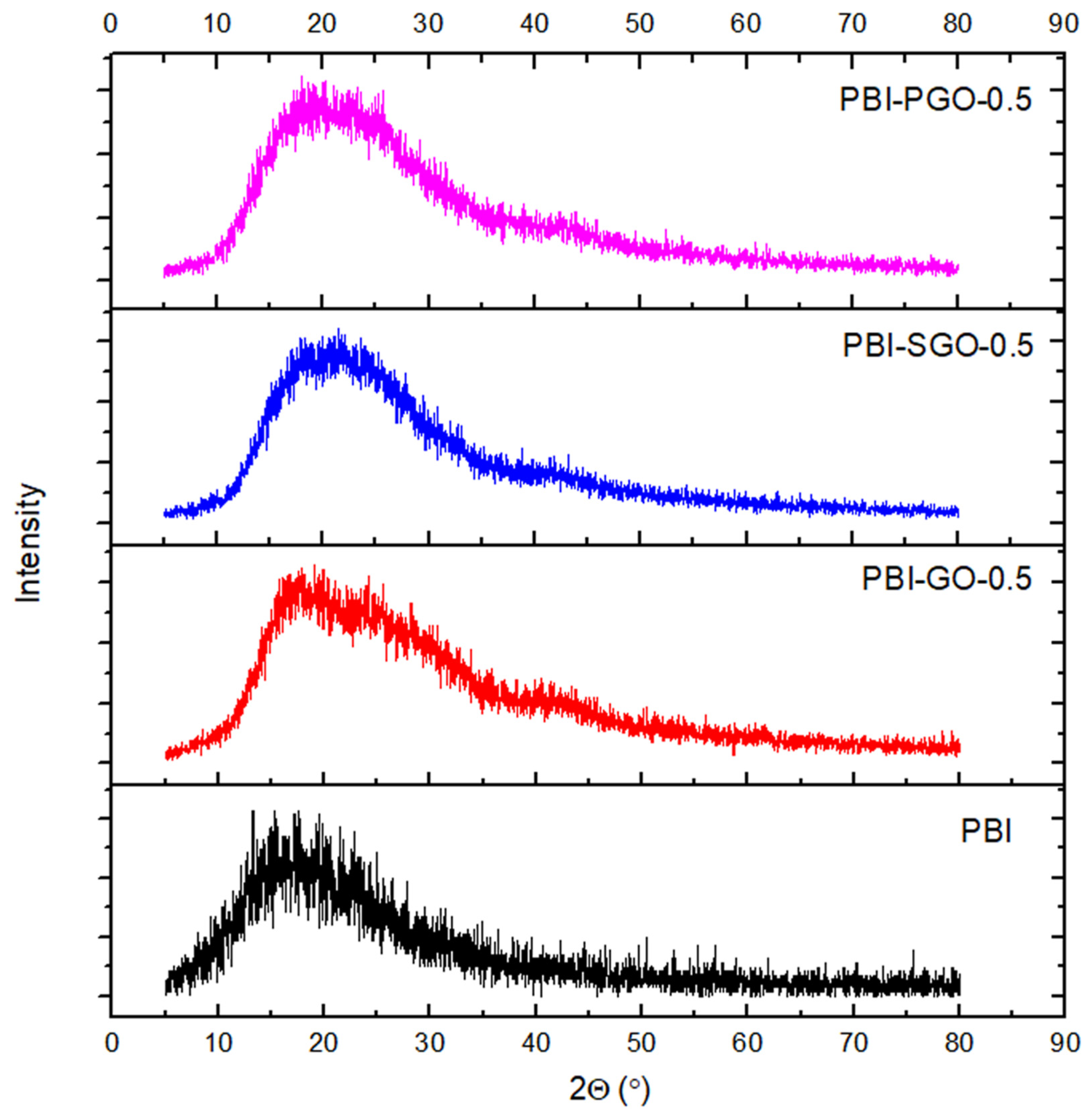
3.3. XRD
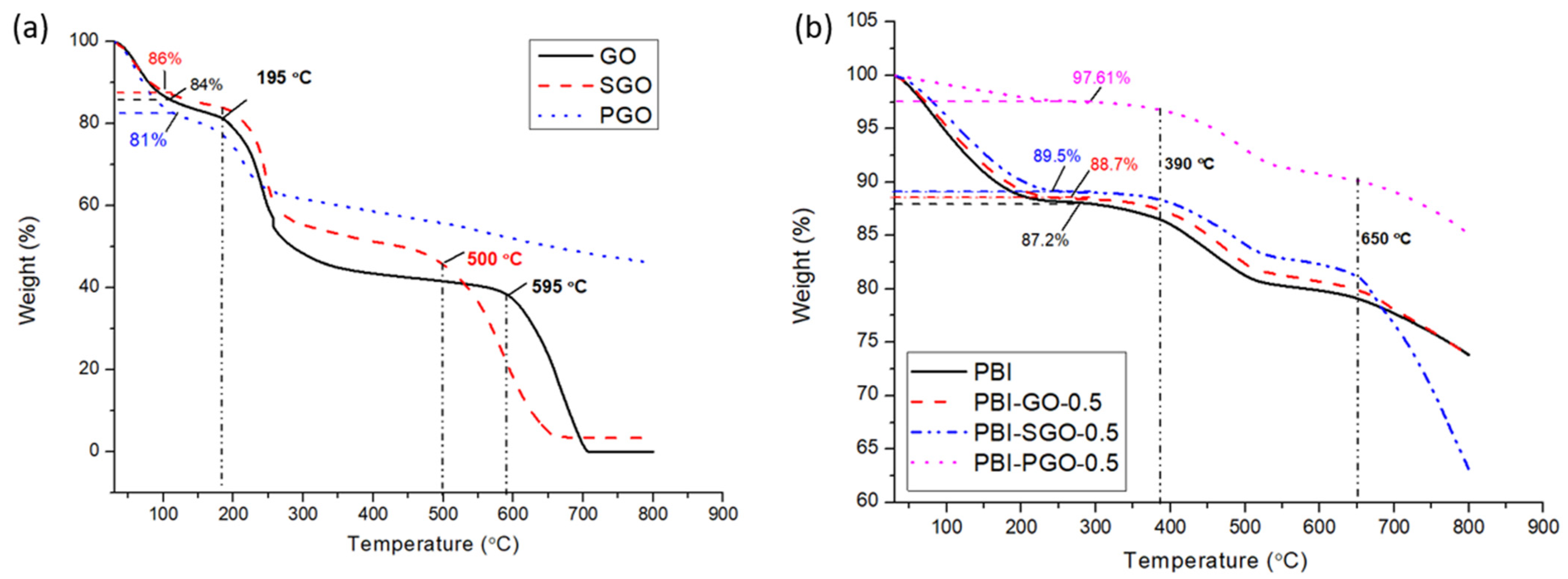
3.4. TGA
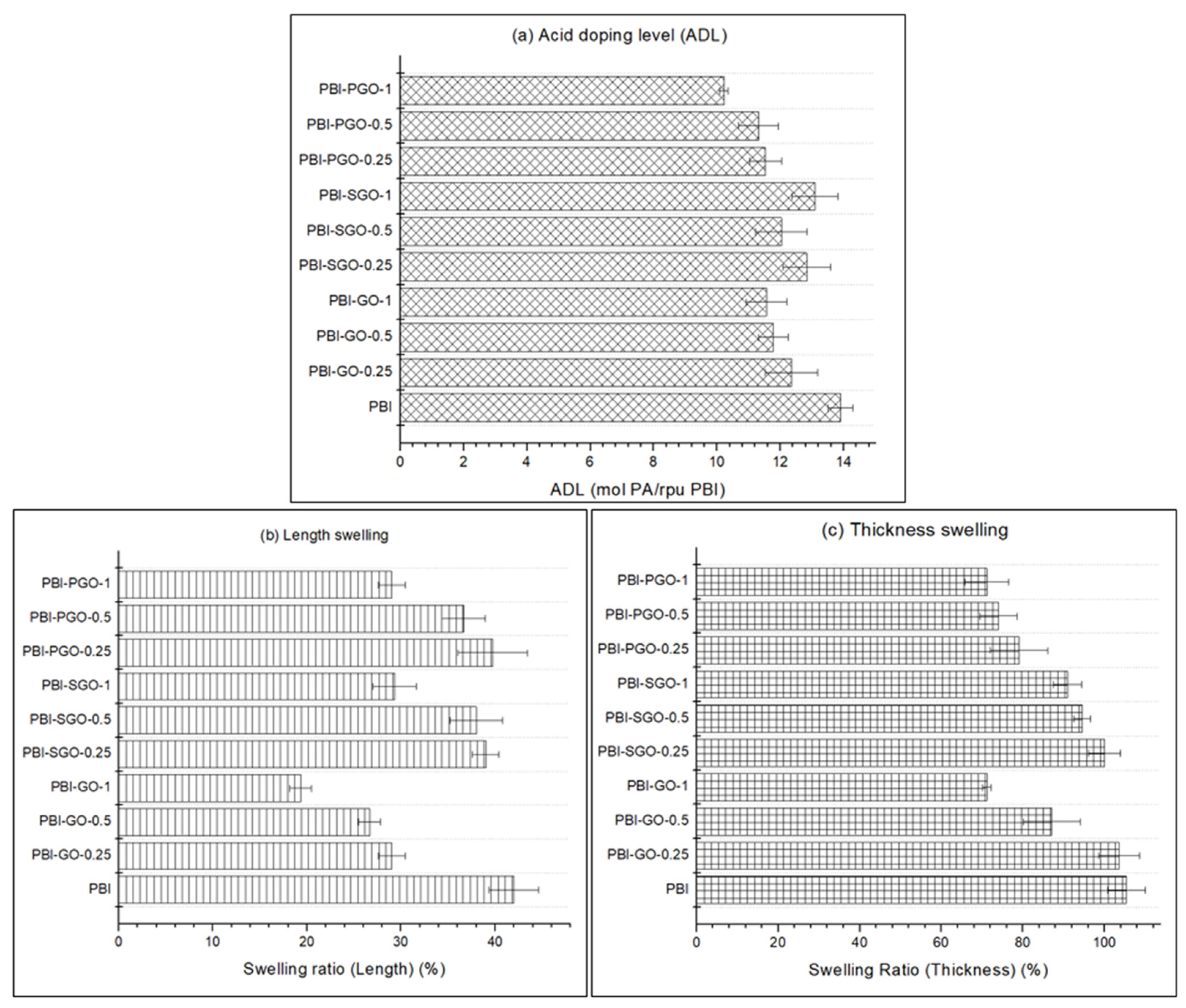
3.5. ADL and Dimensional Swelling
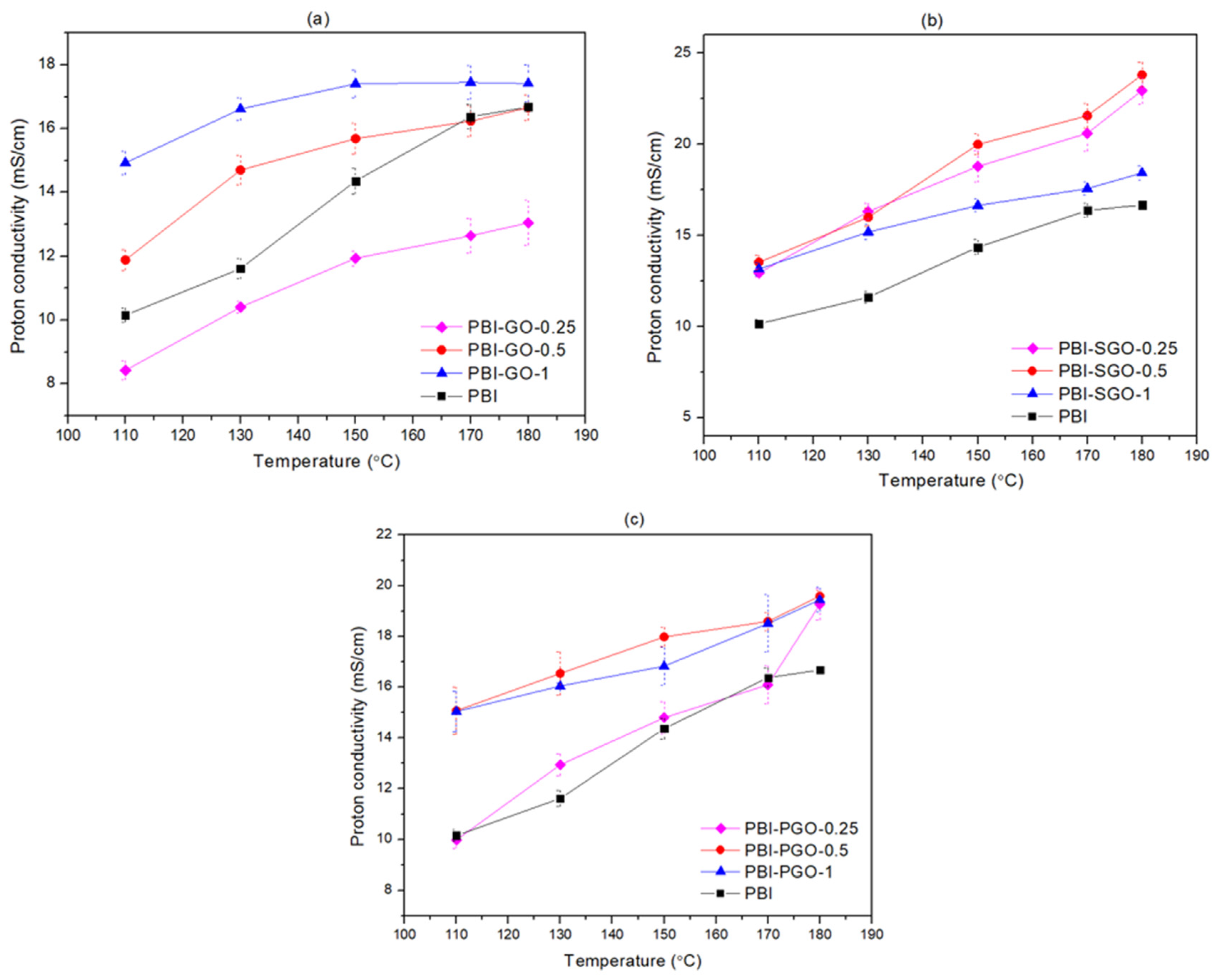
3.6. Proton Conductivity
3.7. Acid Retention
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Sharaf, O.Z.; Orhan, M.F. An overview of fuel cell technology: Fundamentals and applications. Renew. Sustain. Energy Rev. 2014, 32, 810–853. [Google Scholar] [CrossRef]
- Authayanun, S.; Im-orb, K.; Arpornwichanop, A. A review of the development of high temperature proton exchange membrane fuel cells. Chin. J. Catal. 2015, 36, 473–483. [Google Scholar] [CrossRef]
- Araya, S.S.; Zhou, F.; Liso, V.; Sahlin, S.L.; Vang, J.R.; Thomas, S.; Gao, X.; Jeppesen, C.; Kær, S.K. A comprehensive review of PBI-based high temperature PEM fuel cells. Int. J. Hydrogen Energy 2016, 41, 21310–21344. [Google Scholar] [CrossRef]
- Escorihuela, J.; Olvera-Mancilla, J.; Alexandrova, L.; Del Castillo, L.F.; Compan, V. Recent Progress in the Development of Composite Membranes Based on Polybenzimidazole for High Temperature Proton Exchange Membrane (PEM) Fuel Cell Applications. Polymers 2020, 12, 1861. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.A.; Sulong, A.B.; Loh, K.S.; Majlan, E.H.; Husaini, T.; Rosli, R.E. Acid doped polybenzimidazoles based membrane electrode assembly for high temperature proton exchange membrane fuel cell: A review. Int. J. Hydrogen Energy 2017, 42, 9156–9179. [Google Scholar] [CrossRef]
- Zeis, R. Materials and characterization techniques for high-temperature polymer electrolyte membrane fuel cells. Beilstein J. Nanotechnol. 2015, 6, 68–83. [Google Scholar] [CrossRef] [PubMed]
- Farooqui, U.R.; Ahmad, A.L.; Hamid, N.A. Graphene oxide: A promising membrane material for fuel cells. Renew. Sustain. Energy Rev. 2018, 82, 714–733. [Google Scholar] [CrossRef]
- Üregen, N.; Pehlivanoğlu, K.; Özdemir, Y.; Devrim, Y. Development of polybenzimidazole/graphene oxide composite membranes for high temperature PEM fuel cells. Int. J. Hydrogen Energy 2017, 42, 2636–2647. [Google Scholar] [CrossRef]
- Cai, Y.; Yue, Z.; Xu, S. A novel polybenzimidazole composite modified by sulfonated graphene oxide for high temperature proton exchange membrane fuel cells in anhydrous atmosphere. J. Appl. Polym. Sci. 2017, 134, 44986. [Google Scholar] [CrossRef]
- Yusoff, Y.N.; Loh, K.S.; Wong, W.Y.; Daud, W.R.W.; Lee, T.K. Sulfonated graphene oxide as an inorganic filler in promoting the properties of a polybenzimidazole membrane as a high temperature proton exchange membrane. Int. J. Hydrogen Energy 2020, 45, 27510–27526. [Google Scholar] [CrossRef]
- Abouzari-Lotf, E.; Zakeri, M.; Nasef, M.M.; Miyake, M.; Mozarmnia, P.; Bazilah, N.A.; Emelin, N.F.; Ahmad, A. Highly durable polybenzimidazole composite membranes with phosphonated graphene oxide for high temperature polymer electrolyte membrane fuel cells. J. Power Sources 2019, 412, 238–245. [Google Scholar] [CrossRef]
- Abouzari-Lotf, E.; Ghassemi, H.; Shockravi, A.; Zawodzinski, T.; Schiraldi, D. Phosphonated poly(arylene ether)s as potential high temperature proton conducting materials. Polymer 2011, 52, 4709–4717. [Google Scholar] [CrossRef]
- Zhang, B.; Cao, Y.; Jiang, S.; Li, Z.; He, G.; Wu, H. Enhanced proton conductivity of Nafion nanohybrid membrane incorporated with phosphonic acid functionalized graphene oxide at elevated temperature and low humidity. J. Membr. Sci. 2016, 518, 243–253. [Google Scholar] [CrossRef]
- Wu, X.; Scott, K. A H2SO4 Loaded Polybenzimidazole (PBI) Membrane for High Temperature PEMFC. Fuel Cells 2012, 12, 583–588. [Google Scholar] [CrossRef]
- Mauritz, K.A.; Moore, R.B. State of Understanding of Nafion. Chem. Rev. 2004, 104, 4535–4586. [Google Scholar] [CrossRef] [PubMed]
- Mathesh, M.; Liu, J.; Nam, N.D.; Lam, S.K.H.; Zheng, R.; Barrow, C.J.; Yang, W. Facile synthesis of graphene oxide hybrids bridged by copper ions for increased conductivity. J. Mater. Chem. C 2013, 1, 3084–3090. [Google Scholar] [CrossRef]
- Li, C.; Xiao, L.; Jiang, Z.; Tian, X.; Luo, L.; Liu, W.; Xu, Z.-L.; Yang, H.; Jiang, Z.-J. Sulfonic acid functionalized graphene oxide paper sandwiched in sulfonated poly(ether ether ketone): A proton exchange membrane with high performance for semi-passive direct methanol fuel cells. Int. J. Hydrogen Energy 2017, 42, 16731–16740. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Q.; Xia, L.; Huang, D.; Fu, X.; Zhang, R.; Hu, S.; Zhao, F.; Li, X.; Bao, X. Poly(2,5-benzimidazole)/sulfonated sepiolite composite membranes with low phosphoric acid doping levels for PEMFC applications in a wide temperature range. J. Membr. Sci. 2019, 574, 282–298. [Google Scholar] [CrossRef]
- Cai, Y.; Yue, Z.; Teng, X.; Xu, S. Radiation grafting graphene oxide reinforced polybenzimidazole membrane with a sandwich structure for high temperature proton exchange membrane fuel cells in anhydrous atmosphere. Eur. Polym. J. 2018, 103, 207–213. [Google Scholar] [CrossRef]
- Lee, D.C.; Yang, H.N.; Park, S.H.; Kim, W.J. Nafion/graphene oxide composite membranes for low humidifying polymer electrolyte membrane fuel cell. J. Membr. Sci. 2014, 452, 20–28. [Google Scholar] [CrossRef]
- Chaiyakun, S.; Witit-anun, N.; Nuntawong, N.; Chindaudom, P.; Oaew, S.; Kedkeaw, C.; Limsuwan, P. Preparation and characterization of graphene oxide nanosheets. Procedia Eng. 2012, 32, 759–764. [Google Scholar] [CrossRef]
- Li, J.; Zhang, B.; Wu, H.; Cao, L.; He, X.; Li, Y.; Xu, M.; Jiang, Z. Incorporating imidazolium-functionalized graphene oxide into imidazolium-functionalized poly(ether ether ketone) for enhanced hydroxide conductivity. J. Membr. Sci. 2018, 565, 233–240. [Google Scholar] [CrossRef]
- Papiya, F.; Pattanayak, P.; Kumar, V.; Das, S.; Kundu, P.P. Sulfonated graphene oxide and titanium dioxide coated with nanostructured polyaniline nanocomposites as an efficient cathode catalyst in microbial fuel cells. Mater. Sci. Eng. C 2020, 108, 110498. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.; Zhang, H.; Ma, C.; Liu, J.; Cao, S.; Zhang, X. Enhancement of proton conductivity of chitosan membrane enabled by sulfonated graphene oxide under both hydrated and anhydrous conditions. J. Power Sources 2014, 269, 898–911. [Google Scholar] [CrossRef]
- Chu, F.; Lin, B.; Feng, T.; Wang, C.; Zhang, S.; Yuan, N.; Liu, Z.; Ding, J. Zwitterion-coated graphene-oxide-doped composite membranes for proton exchange membrane applications. J. Membr. Sci. 2015, 496, 31–38. [Google Scholar] [CrossRef]
- Özdemir, Y.; Üregen, N.; Devrim, Y. Polybenzimidazole based nanocomposite membranes with enhanced proton conductivity for high temperature PEM fuel cells. Int. J. Hydrogen Energy 2017, 42, 2648–2657. [Google Scholar] [CrossRef]
- Ross, R.D.; Roeder, R.K. Binding affinity of surface functionalized gold nanoparticles to hydroxyapatite. J. Biomed. Mater. Res. Part A 2011, 99A, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Żelechowska, K.; Prześniak-Welenc, M.; Łapiński, M.; Kondratowicz, I.; Miruszewski, T. Fully scalable one-pot method for the production of phosphonic graphene derivatives. Beilstein J. Nanotechnol. 2017, 8, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Etesami, M.; Abouzari-Lotf, E.; Ripin, A.; Mahmoud Nasef, M.; Ting, T.M.; Saharkhiz, A.; Ahmad, A. Phosphonated graphene oxide with high electrocatalytic performance for vanadium redox flow battery. Int. J. Hydrogen Energy 2018, 43, 189–197. [Google Scholar] [CrossRef]
- Zakeri, M.; Abouzari-lotf, E.; Miyake, M.; Mehdipour-Ataei, S.; Shameli, K. Phosphoric acid functionalized graphene oxide: A highly dispersible carbon-based nanocatalyst for the green synthesis of bio-active pyrazoles. Arab. J. Chem. 2019, 12, 188–197. [Google Scholar] [CrossRef]
- Li, X.; Chen, X.; Benicewicz, B.C. Synthesis and properties of phenylindane-containing polybenzimidazole (PBI) for high-temperature polymer electrolyte membrane fuel cells (PEMFCs). J. Power Sources 2013, 243, 796–804. [Google Scholar] [CrossRef]
- Tian, X.; Wang, S.; Li, J.; Liu, F.; Wang, X.; Chen, H.; Wang, D.; Ni, H.; Wang, Z. Benzimidazole grafted polybenzimidazole cross-linked membranes with excellent PA stability for high-temperature proton exchange membrane applications. Appl. Surf. Sci. 2019, 465, 332–339. [Google Scholar] [CrossRef]
- Sun, P.; Li, Z.; Jin, L.; Wang, S.; Yin, X. Construction of proton channels and reinforcement of physicochemical properties of oPBI/FeSPP/GF high temperature PEM via building hydrogen bonding network. Int. J. Hydrogen Energy 2017, 42, 14572–14582. [Google Scholar] [CrossRef]
- Kang, Y.; Zou, J.; Sun, Z.; Wang, F.; Zhu, H.; Han, K.; Yang, W.; Song, H.; Meng, Q. Polybenzimidazole containing ether units as electrolyte for high temperature proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2013, 38, 6494–6502. [Google Scholar] [CrossRef]
- Akhtar, F.H.; Kumar, M.; Villalobos, L.F.; Vovusha, H.; Shevate, R.; Schwingenschlögl, U.; Peinemann, K.V. Polybenzimidazole-based mixed membranes with exceptionally high water vapor permeability and selectivity. J. Mater. Chem. A 2017, 5, 21807–21819. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Z.; Fang, J.; Xu, H.; Yin, J. Graphene oxide/polybenzimidazole composites fabricated by a solvent-exchange method. Carbon 2011, 49, 1199–1207. [Google Scholar] [CrossRef]
- Mamlouk, M.; Ocon, P.; Scott, K. Preparation and characterization of polybenzimidzaole/diethylamine hydrogen sulphate for medium temperature proton exchange membrane fuel cells. J. Power Sources 2014, 245, 915–926. [Google Scholar] [CrossRef][Green Version]
- Kuo, Y.-J.; Lin, H.-L. Effects of mesoporous fillers on properties of polybenzimidazole composite membranes for high-temperature polymer fuel cells. Int. J. Hydrogen Energy 2018, 43, 4448–4457. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S.K. Graphene in electrocatalyst and proton conductiong membrane in fuel cell applications: An overview. Renew. Sustain. Energy Rev. 2017, 69, 862–870. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S.K. Recent advances in additive-enhanced polymer electrolyte membrane properties in fuel cell applications: An overview. Int. J. Energy Res. 2019, 43, 2756–2794. [Google Scholar] [CrossRef]
- Heo, Y.; Im, H.; Kim, J. The effect of sulfonated graphene oxide on Sulfonated Poly (Ether Ether Ketone) membrane for direct methanol fuel cells. J. Membr. Sci. 2013, 425–426, 11–22. [Google Scholar] [CrossRef]
- Chen, R.; Xu, F.; Fu, K.; Zhou, J.; Shi, Q.; Xue, C.; Lyu, Y.; Guo, B.; Li, G. Enhanced proton conductivity and dimensional stability of proton exchange membrane based on sulfonated poly(arylene ether sulfone) and graphene oxide. Mater. Res. Bull. 2018, 103, 142–149. [Google Scholar] [CrossRef]
- Özdemir, Y.; Özkan, N.; Devrim, Y. Fabrication and Characterization of Cross-linked Polybenzimidazole Based Membranes for High Temperature PEM Fuel Cells. Electrochim. Acta 2017, 245, 1–13. [Google Scholar] [CrossRef]
- Chen, H.; Wang, S.; Li, J.; Liu, F.; Tian, X.; Wang, X.; Mao, T.; Xu, J.; Wang, Z. Novel cross-linked membranes based on polybenzimidazole and polymeric ionic liquid with improved proton conductivity for HT-PEMFC applications. J. Taiwan Inst. Chem. Eng. 2019, 95, 185–194. [Google Scholar] [CrossRef]
- Mirza-Aghayan, M.; Molaee Tavana, M.; Boukherroub, R. Sulfonated reduced graphene oxide as a highly efficient catalyst for direct amidation of carboxylic acids with amines using ultrasonic irradiation. Ultrason. Sonochem. 2016, 29, 371–379. [Google Scholar] [CrossRef]
- Zhou, L.; Lin, X.; Huang, T.; Yu, A. Binder-free phenyl sulfonated graphene/sulfur electrodes with excellent cyclability for lithium sulfur batteries. J. Mater. Chem. A 2014, 2, 5117–5123. [Google Scholar] [CrossRef]
- De Silva, K.K.H.; Huang, H.-H.; Yoshimura, M. Progress of reduction of graphene oxide by ascorbic acid. Appl. Surf. Sci. 2018, 447, 338–346. [Google Scholar] [CrossRef]
- Firouz Tadavani, K.; Abdolmaleki, A.; Molavian, M.R.; Borandeh, S.; Sorvand, E.; Zhiani, M. Synergistic Behavior of Phosphonated and Sulfonated Groups on Proton Conductivity and Their Performance for High-Temperature Proton Exchange Membrane Fuel Cells (PEMFCs). Energy Fuels 2017, 31, 11460–11470. [Google Scholar] [CrossRef]
- Abouzari-Lotf, E.; Ghassemi, H.; Mehdipour-Ataei, S.; Shockravi, A. Phosphonated polyimides: Enhancement of proton conductivity at high temperatures and low humidity. J. Membr. Sci. 2016, 516, 74–82. [Google Scholar] [CrossRef]
- Keinath, S.E.; Morgan, R.J. Moisture content of aramid and polybenzimidazole fibers. Thermochim. Acta 1990, 166, 17–26. [Google Scholar] [CrossRef]
- Farrokhi, M.; Abdollahi, M. Enhancing medium/high temperature proton conductivity of poly(benzimidazole)-based proton exchange membrane via blending with poly(vinyl imidazole-co-vinyl phosphonic acid) copolymer: Proton conductivity-copolymer microstructure relationship. Eur. Polym. J. 2020, 131, 109691. [Google Scholar] [CrossRef]
- Atanasov, V.; Gudat, D.; Ruffmann, B.; Kerres, J. Highly phosphonated polypentafluorostyrene: Characterization and blends with polybenzimidazole. Eur. Polym. J. 2013, 49, 3977–3985. [Google Scholar] [CrossRef]
- Xue, C.; Zou, J.; Sun, Z.; Wang, F.; Han, K.; Zhu, H. Graphite oxide/functionalized graphene oxide and polybenzimidazole composite membranes for high temperature proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2014, 39, 7931–7939. [Google Scholar] [CrossRef]
- Krishnan, N.N.; Lee, S.; Ghorpade, R.V.; Konovalova, A.; Jang, J.H.; Kim, H.-J.; Han, J.; Henkensmeier, D.; Han, H. Polybenzimidazole (PBI-OO) based composite membranes using sulfophenylated TiO2 as both filler and crosslinker, and their use in the HT-PEM fuel cell. J. Membr. Sci. 2018, 560, 11–20. [Google Scholar] [CrossRef]
- Schaffer, M.; Licha, T. A guideline for the identification of environmentally relevant, ionizable organic molecule species. Chemosphere 2014, 103, 12–25. [Google Scholar] [CrossRef]
- Krishnan, N.N.; Joseph, D.; Duong, N.M.H.; Konovalova, A.; Jang, J.H.; Kim, H.-J.; Nam, S.W.; Henkensmeier, D. Phosphoric acid doped crosslinked polybenzimidazole (PBI-OO) blend membranes for high temperature polymer electrolyte fuel cells. J. Membr. Sci. 2017, 544, 416–424. [Google Scholar] [CrossRef]
- Lobato, J.; Cañizares, P.; Rodrigo, M.A.; Linares, J.J.; Manjavacas, G. Synthesis and characterisation of poly[2,2-(m-phenylene)-5,5-bibenzimidazole] as polymer electrolyte membrane for high temperature PEMFCs. J. Membr. Sci. 2006, 280, 351–362. [Google Scholar] [CrossRef]
- He, Y.; Tong, C.; Geng, L.; Liu, L.; Lü, C. Enhanced performance of the sulfonated polyimide proton exchange membranes by graphene oxide: Size effect of graphene oxide. J. Membr. Sci. 2014, 458, 36–46. [Google Scholar] [CrossRef]
- Xu, C.; Cao, Y.; Kumar, R.; Wu, X.; Wang, X.; Scott, K. A polybenzimidazole/sulfonated graphite oxide composite membrane for high temperature polymer electrolyte membrane fuel cells. J. Mater. Chem. 2011, 21, 11359–11364. [Google Scholar] [CrossRef]
- Xu, C.; Liu, X.; Cheng, J.; Scott, K. A polybenzimidazole/ionic-liquid-graphite-oxide composite membrane for high temperature polymer electrolyte membrane fuel cells. J. Power Sources 2015, 274, 922–927. [Google Scholar] [CrossRef]
- Singha, S.; Jana, T. Structure and Properties of Polybenzimidazole/Silica Nanocomposite Electrolyte Membrane: Influence of Organic/Inorganic Interface. ACS Appl. Mater. Interfaces 2014, 6, 21286–21296. [Google Scholar] [CrossRef]
- Hooshyari, K.; Javanbakht, M.; Shabanikia, A.; Enhessari, M. Fabrication BaZrO3/PBI-based nanocomposite as a new proton conducting membrane for high temperature proton exchange membrane fuel cells. J. Power Sources 2015, 276, 62–72. [Google Scholar] [CrossRef]
- Dippel, T.; Kreuer, K.D.; Lassègues, J.C.; Rodriguez, D. Proton conductivity in fused phosphoric acid; A 1H/31P PFG-NMR and QNS study. Solid State Ion. 1993, 61, 41–46. [Google Scholar] [CrossRef]
- Nayak, R.; Sundarraman, M.; Ghosh, P.C.; Bhattacharyya, A.R. Doped poly (2, 5-benzimidazole) membranes for high temperature polymer electrolyte fuel cell: Influence of various solvents during membrane casting on the fuel cell performance. Eur. Polym. J. 2018, 100, 111–120. [Google Scholar] [CrossRef]
- Lin, B.; Chu, F.; Yuan, N.; Shang, H.; Ren, Y.; Gu, Z.; Ding, J.; Wei, Y.; Yu, X. Phosphoric acid doped polybenzimidazole/imidazolium-modified silsesquioxane hybrid proton conducting membranes for anhydrous proton exchange membrane application. J. Power Sources 2014, 252, 270–276. [Google Scholar] [CrossRef]
- Elakkiya, S.; Arthanareeswaran, G.; Venkatesh, K.; Kweon, J. Enhancement of fuel cell properties in polyethersulfone and sulfonated poly (ether ether ketone) membranes using metal oxide nanoparticles for proton exchange membrane fuel cell. Int. J. Hydrogen Energy 2018, 43, 21750–21759. [Google Scholar] [CrossRef]
- He, S.; Dai, W.; Yang, W.; Liu, S.; Bian, X.; Zhang, C.; Lin, J. Nanocomposite proton exchange membranes based on phosphotungstic acid immobilized by polydopamine-coated halloysite nanotubes. Polym. Test. 2019, 73, 242–249. [Google Scholar] [CrossRef]
- Steffy, N.J.; Parthiban, V.; Sahu, A.K. Uncovering Nafion-multiwalled carbon nanotube hybrid membrane for prospective polymer electrolyte membrane fuel cell under low humidity. J. Membr. Sci. 2018, 563, 65–74. [Google Scholar] [CrossRef]
- Kim, Y.; Ketpang, K.; Jaritphun, S.; Park, J.S.; Shanmugam, S. A polyoxometalate coupled graphene oxide–Nafion composite membrane for fuel cells operating at low relative humidity. J. Mater. Chem. A 2015, 3, 8148–8155. [Google Scholar] [CrossRef]
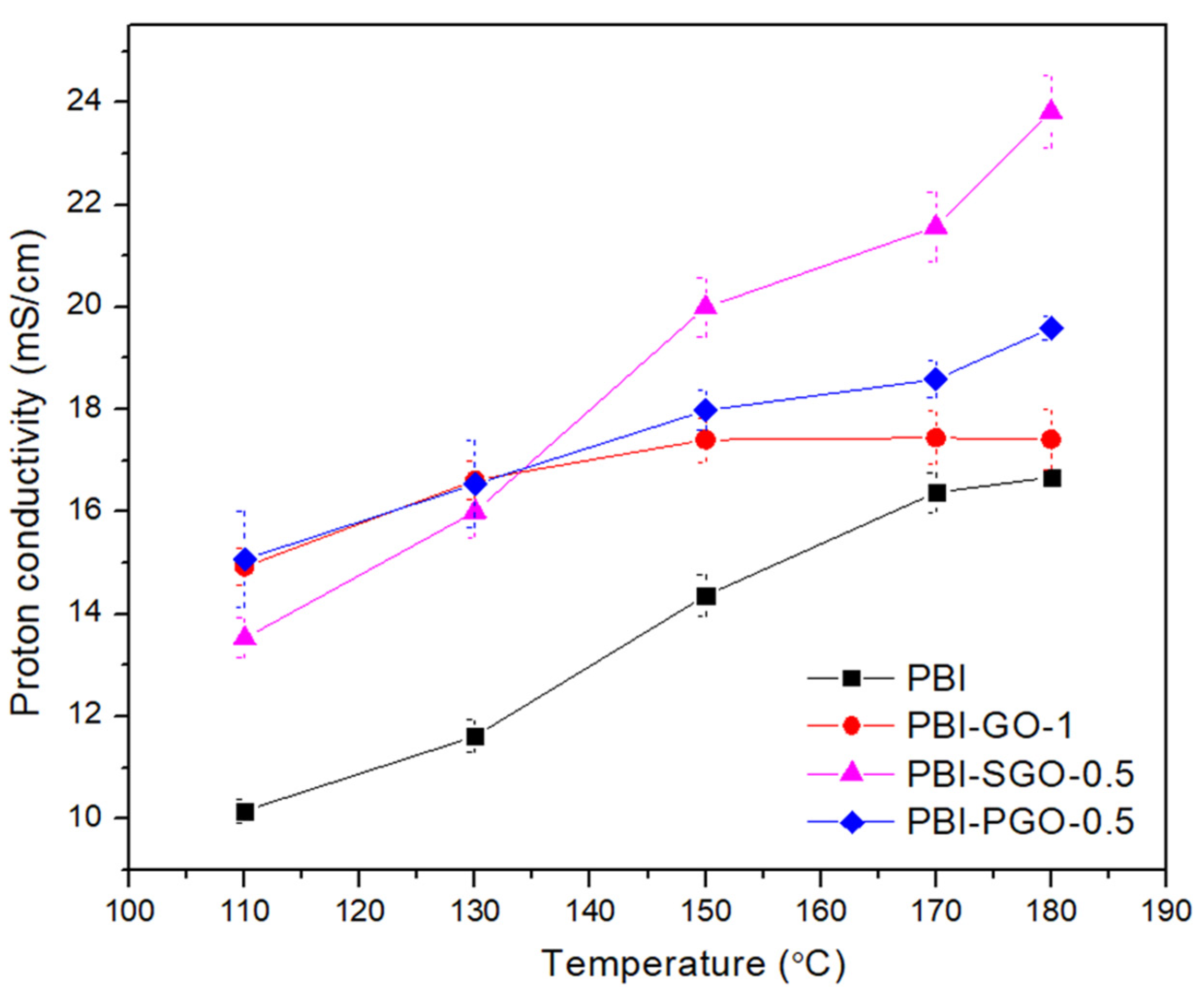
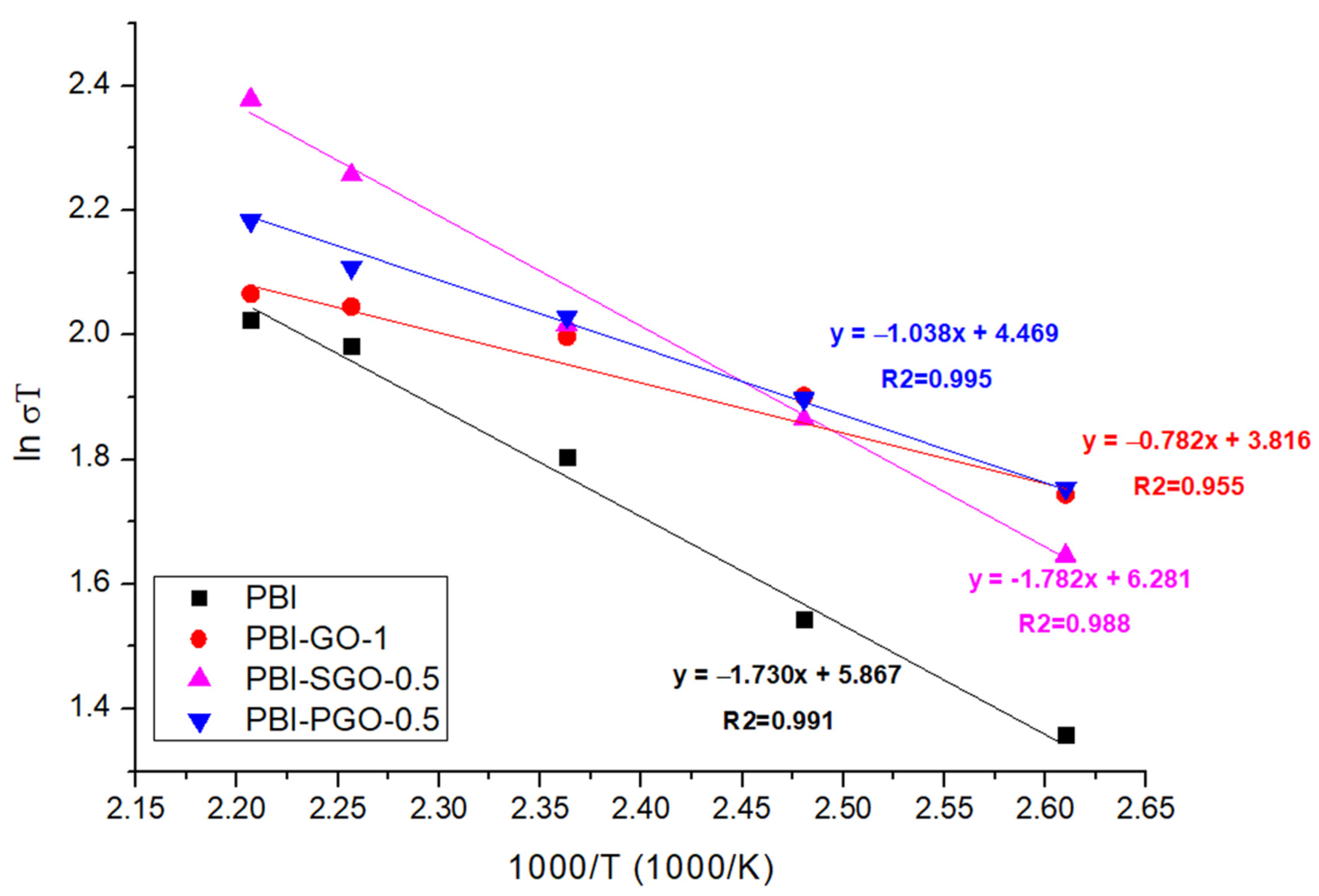
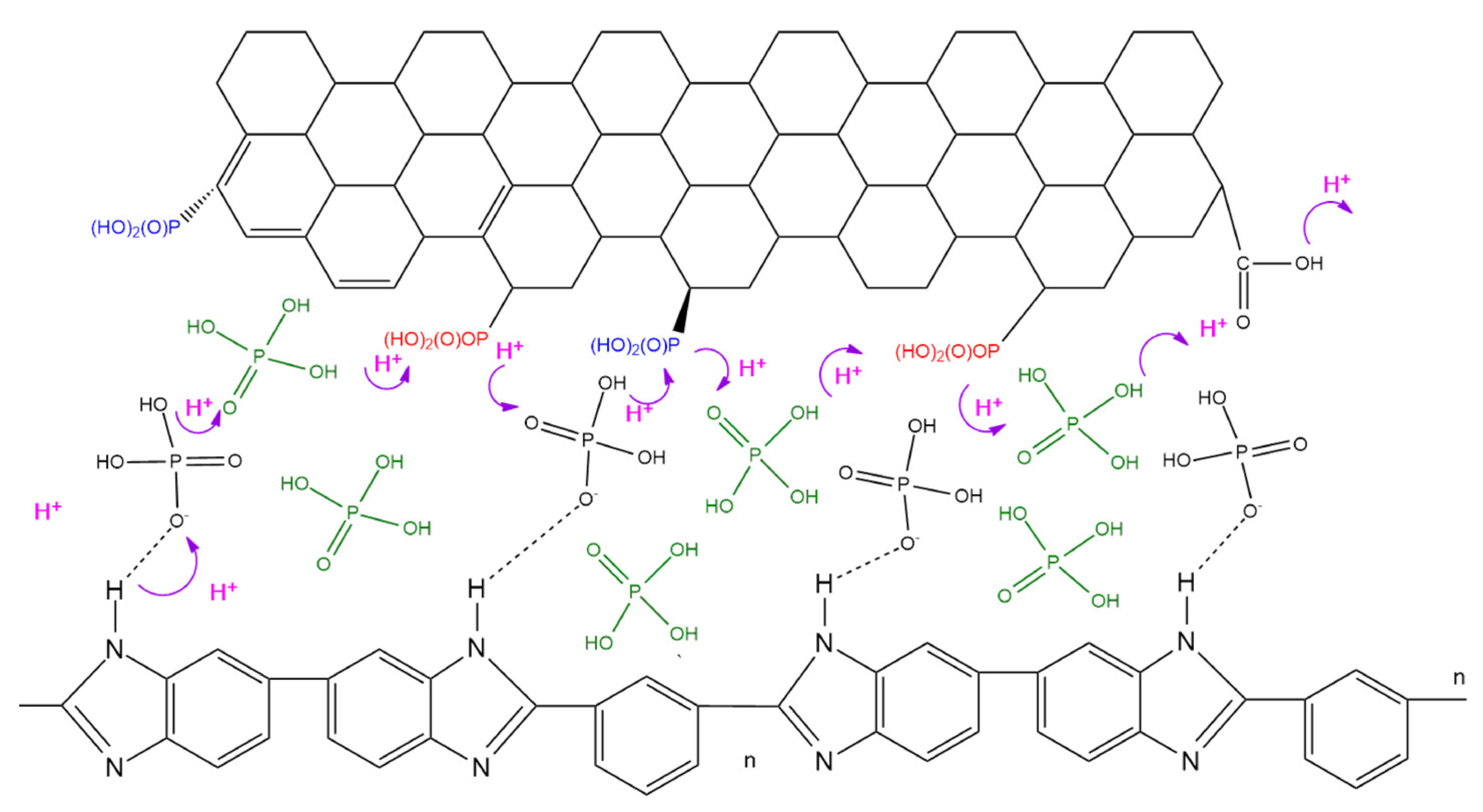
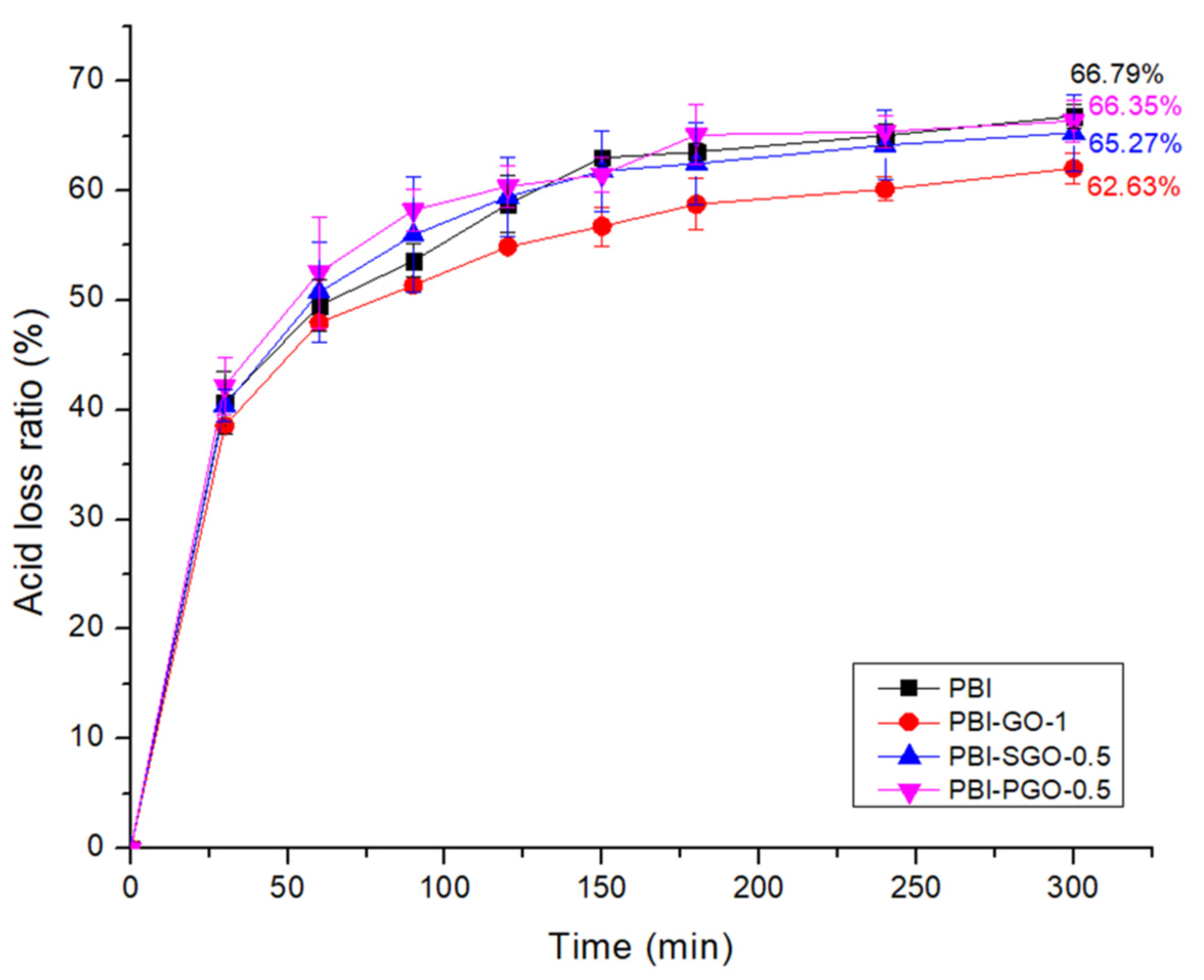
| Membranes | Ea (kJ/mol) |
|---|---|
| PBI | 14.38 |
| PBI-GO-1 | 6.50 |
| PBI-SGO-0.5 | 14.82 |
| PBI-PGO-0.5 | 8.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sulaiman, R.R.R.; Walvekar, R.; Wong, W.Y.; Khalid, M.; Pang, M.M. Proton Conductivity Enhancement at High Temperature on Polybenzimidazole Membrane Electrolyte with Acid-Functionalized Graphene Oxide Fillers. Membranes 2022, 12, 344. https://doi.org/10.3390/membranes12030344
Sulaiman RRR, Walvekar R, Wong WY, Khalid M, Pang MM. Proton Conductivity Enhancement at High Temperature on Polybenzimidazole Membrane Electrolyte with Acid-Functionalized Graphene Oxide Fillers. Membranes. 2022; 12(3):344. https://doi.org/10.3390/membranes12030344
Chicago/Turabian StyleSulaiman, Raja Rafidah Raja, Rashmi Walvekar, Wai Yin Wong, Mohammad Khalid, and Ming Meng Pang. 2022. "Proton Conductivity Enhancement at High Temperature on Polybenzimidazole Membrane Electrolyte with Acid-Functionalized Graphene Oxide Fillers" Membranes 12, no. 3: 344. https://doi.org/10.3390/membranes12030344
APA StyleSulaiman, R. R. R., Walvekar, R., Wong, W. Y., Khalid, M., & Pang, M. M. (2022). Proton Conductivity Enhancement at High Temperature on Polybenzimidazole Membrane Electrolyte with Acid-Functionalized Graphene Oxide Fillers. Membranes, 12(3), 344. https://doi.org/10.3390/membranes12030344