The Properties of Intermediate-Temperature Solid Oxide Fuel Cells with Thin Film Gadolinium-Doped Ceria Electrolyte
Abstract
:1. Introduction
2. Materials and Methods
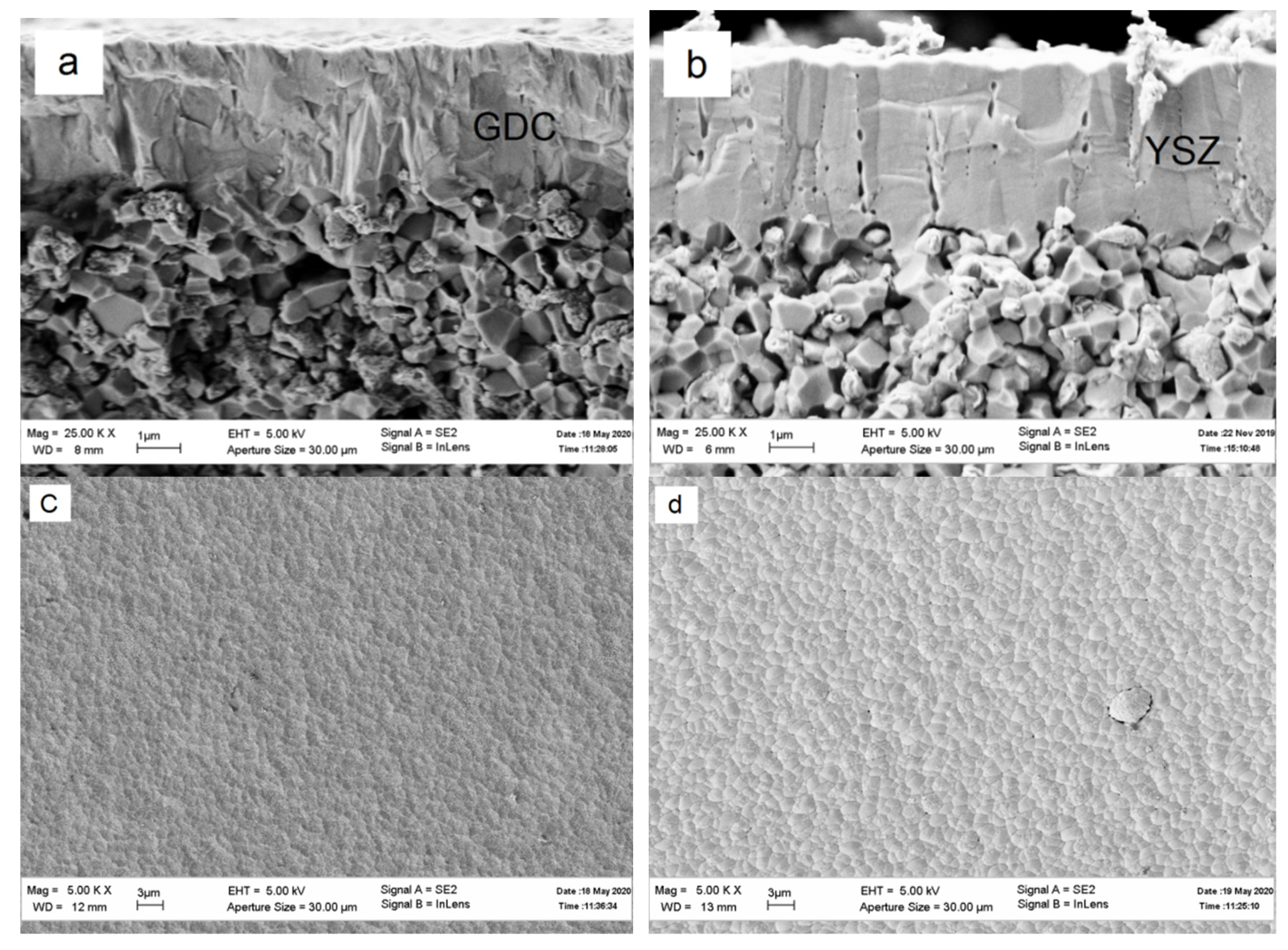
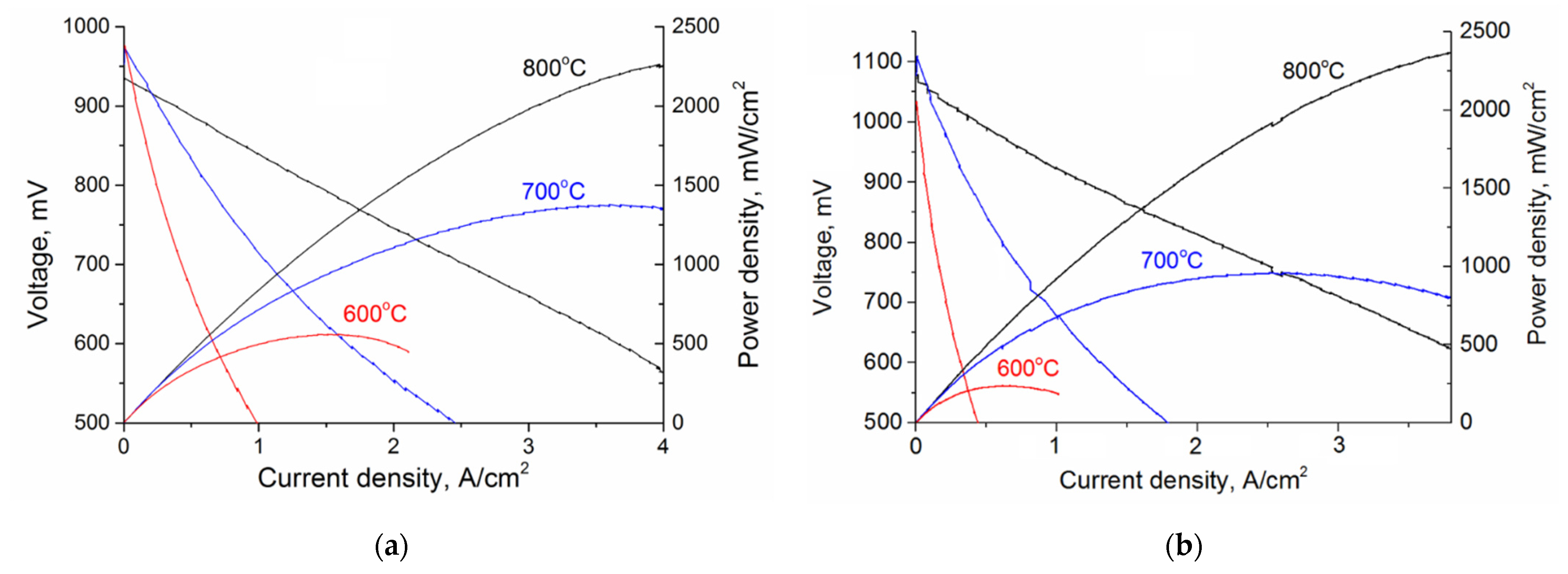
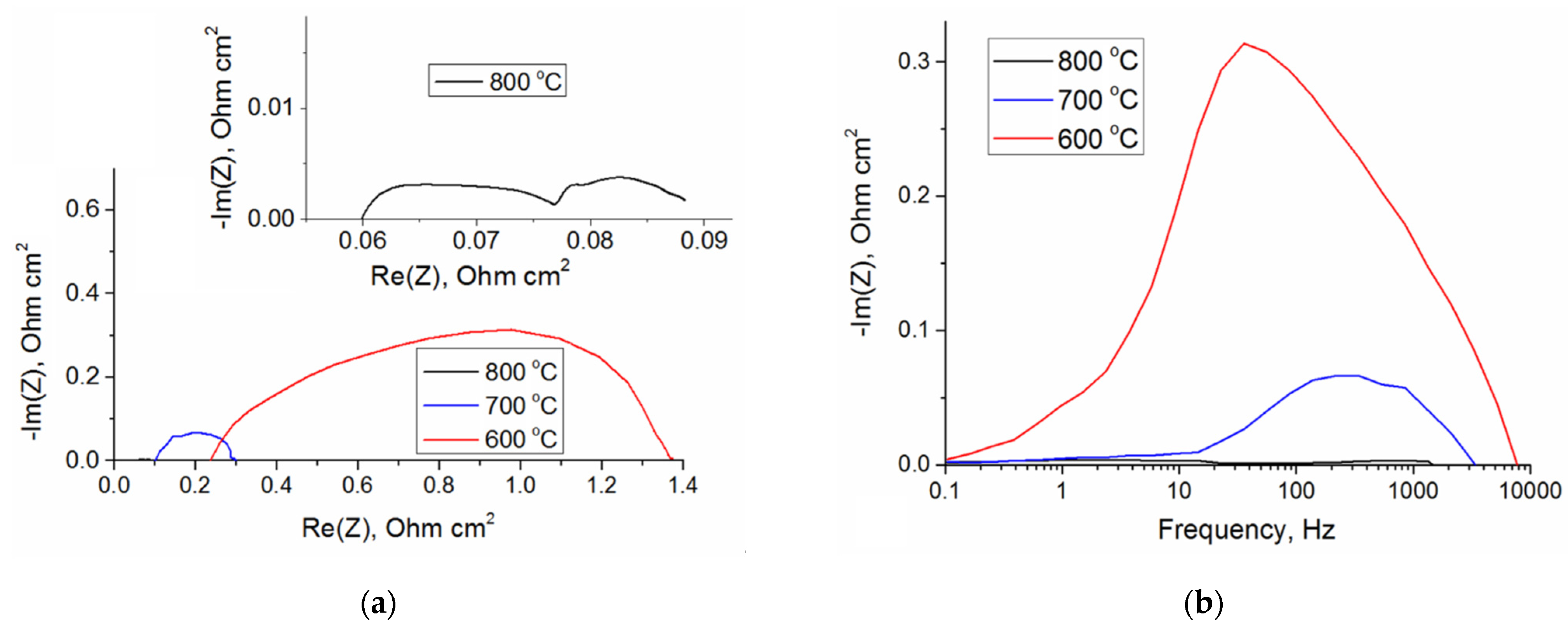
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Christen, H.M.; Eres, G. Recent advances in pulsed-laser deposition of complex oxides. J. Phys. Condens. Matter. 2008, 20, 264005. [Google Scholar] [CrossRef] [PubMed]
- Musil, J.; Vlcek, J.; Baroch, P. Magnetron discharges for thin films plasma processing. In Materials Surface Processing by Directed Energy Techniques; Pauleau, Y., Ed.; Elsevier Science Publisher B.V.: Oxford, UK, 2006; pp. 67–106. [Google Scholar] [CrossRef]
- Jiang, N.; Wachsman, E.D.; Jung, S.H. A higher conductivity Bi2O3-based electrolyte. Solid State Ion. 2002, 150, 347–353. [Google Scholar] [CrossRef]
- Liu, Y.X.; Wang, S.F.; Hsu, Y.F.; Yeh, W.Y. Solid oxide fuel cells incorporating doped lanthanum gallate films deposited by radio-frequency magnetron sputtering at various Ar/O2 ratios and annealing conditions. Surf. Coat. Technol. 2018, 344, 507–513. [Google Scholar] [CrossRef]
- Pikalova, E.; Osinkin, D.; Kalinina, E. Direct electrophoretic deposition and characterization of thin-film membranes based on doped BaCeO3 and CeO2 for anode-supported solid oxide fuel cells. Membranes 2022, 12, 682. [Google Scholar] [CrossRef] [PubMed]
- Kalyk, F.; Žalga, A.; Vasiliauskas, A.; Tamulevičius, T.; Tamulevičius, S.; Abakevičiene, B. Synthesis and electron-beam evaporation of gadolinium-doped ceria thin films. Coatings 2022, 12, 747. [Google Scholar] [CrossRef]
- Mogensen, M.; Sammes, N.M.; Tompsett, G.A. Physical, chemical and electrochemical properties of pure and doped ceria. Solid State Ion. 2000, 129, 63–94. [Google Scholar] [CrossRef]
- Jaiswal, N.; Tanwar, K.; Suman, R.; Kumar, D.; Upadhyay, S.; Parkash, O. A brief review on ceria based solid electrolytes for solid oxide fuel cells. J. Alloys Compd. 2019, 781, 984–1005. [Google Scholar] [CrossRef]
- Wilde, V.; Störmer, H.; Szász, J.; Wankmüller, F.; Ivers-Tiffée, E.; Gerthsen, D. Gd0.2Ce0.8O2 diffusion barrier layer between (La0.58Sr0.4)(Co0.2Fe0.8)O3-δ cathode and Y0.16Zr0.84O2 electrolyte for solid oxide fuel cells: Effect of barrier layer sintering temperature on microstructure. ACS Appl. Energy Mater. 2018, 1, 6790–6800. [Google Scholar] [CrossRef]
- Toor, S.Y.; Croiset, E. Reducing sintering temperature while maintaining high conductivity for SOFC electrolyte: Copper as sintering aid for Samarium Doped Ceria. Ceram. Int. 2020, 46, 1148–1157. [Google Scholar] [CrossRef]
- Kim, J.; Im, S.; Oh, S.H.; Lee, J.Y.; Yoon, K.J.; Son, J.W.; Yang, S.; Kim, B.K.; Lee, J.H.; Lee, H.W.; et al. Naturally diffused sintering aid for highly conductive bilayer electrolytes in solid oxide cells. Sci. Adv. 2021, 7, eabj8590. [Google Scholar] [CrossRef]
- Pikalova, E.Y.; Kalinina, E.G. Solid oxide fuel cells based on ceramic membranes with mixed conductivity: Improving efficiency. Russ. Chem. Rev. 2021, 90, 703–749. [Google Scholar] [CrossRef]
- Park, H.C.; Virkar, A.V. Bimetallic (Ni–Fe) anode-supported solid oxide fuel cells with gadolinia-doped ceria electrolyte. J. Power Source 2009, 186, 133–137. [Google Scholar] [CrossRef]
- Ding, C.; Lin, H.; Sato, K.; Hashida, T. A simple, rapid spray method for preparing anode-supported solid oxide fuel cells with GDC electrolyte thin films. J. Membrane Sci. 2010, 350, 1–4. [Google Scholar] [CrossRef]
- Reolon, R.P.; Halmenschlager, C.M.; Neagu, R.; Malfatti, C.F.; Bergmann, C.P. Electrochemical performance of gadolinia-doped ceria (CGO) electrolyte thin films for ITSOFC deposited by spray pyrolysis. J. Power Source 2014, 261, 348–355. [Google Scholar] [CrossRef]
- Shao, X.; Rickard, W.D.A.; Dong, D.; Dang, H.; Saunders, M.; Dodd, A.; Parkinson, G.; Li, C. High performance anode with dendritic porous structure for low temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2018, 43, 17849–17856. [Google Scholar] [CrossRef]
- Sivasankaran, V.; Combemale, L.; Pera, M.C.; Caboche, G. Initial preparation and characterization of single step fabricated intermediate temperature solid oxide fuel cells (IT-SOFC). Fuel Cells 2014, 14, 533–536. [Google Scholar] [CrossRef]
- Fu, C.J.; Chan, S.H.; Ge, X.M.; Liu, Q.L.; Pasciak, G. A promising Ni-Fe bimetallic anode for intermediate-temperature SOFC based on Gd-doped ceria electrolyte. Int. J. Hydrogen Energy 2011, 36, 13727–13734. [Google Scholar] [CrossRef]
- Yamaji, K.; Xiong, Y.; Kishimoto, H.; Horita, T.; Brito, M.E.; Yokokawa, H. Electronic conductivity and efficiency of SOFC electrolytes. ECS Trans. 2008, 12, 317–322. [Google Scholar] [CrossRef]
- Pérez-Coll, D.; Ruiz-Morales, J.C.; Marrero-López, D.; Núñez, P.; Frade, J.R. Effect of sintering additive and low temperature on the electrode polarization of CGO. J. Alloys Compd. 2009, 467, 533–538. [Google Scholar] [CrossRef]
- Solovyev, A.A.; Rabotkin, S.V.; Shipilova, A.V.; Ionov, I.V. Magnetron sputtering of gadolinium-doped ceria electrolyte for intermediate temperature solid oxide fuel cells. Int. J. Electrochem. Sci. 2019, 14, 575–584. [Google Scholar] [CrossRef]
- Solovyev, A.A.; Rabotkin, S.V.; Kuterbekov, K.A.; Koketay, T.A.; Nurkenov, S.A.; Opakhai, S.; Shipilova, A.V.; Ionov, I.V.; Eliseeva, G.M. Comparison of sputter-deposited single and multilayer electrolytes based on gadolinia-doped ceria and yttria-stabilized zirconia for solid oxide fuel cells. Int. J. Electrochem. Sci. 2020, 15, 231–240. [Google Scholar] [CrossRef]
- Matsui, T.; Kosaka, T.; Inaba, M.; Mineshige, A.; Ogumi, Z. Effects of mixed conduction on the open-circuit voltage of intermediate-temperature SOFCs based on Sm-doped ceria electrolytes. Solid State Ion 2005, 176, 663–668. [Google Scholar] [CrossRef]
- Duncan, K.L.; Lee, K.T.; Wachsman, E.D. Dependence of open-circuit potential and power density on electrolyte thickness in solid oxide fuel cells with mixed conducting electrolytes. J. Power Source 2011, 196, 2445–2451. [Google Scholar] [CrossRef]
- Fu, C.; Chan, S.H.; Liu, Q.; Ge, X.; Pasciak, G. Fabrication and evaluation of Ni-GDC composite anode prepared by aqueous-based tape casting method for low-temperature solid oxide fuel cell. Int. J. Hydrogen Energy 2010, 35, 301–307. [Google Scholar] [CrossRef]
- Riegraf, M.; Yurkiv, V.; Costa, R.; Schiller, G.; Friedrich, K.A. Evaluation of the effect of sulfur on the performance of Ni/CGO-based solid oxide fuel cell (SOFC) anodes. ChemSusChem 2017, 10, 587–599. [Google Scholar] [CrossRef]
- Kharton, V.V.; Viskup, A.P.; Figueiredo, F.M.; Naumovich, E.N.; Shaula, A.L.; Marques, F.M.B. Electrochemical properties of Pr-doped Ce(Gd)O2-δ. Mater. Lett. 2002, 53, 160–164. [Google Scholar] [CrossRef]
- Kurumchin, E.K. Isotope exchange studies of electrochemical systems with solid oxide electrolytes. Ionics 1998, 4, 390–394. [Google Scholar] [CrossRef]
- Uchida, H.; Yoshida, M.; Watanabe, M. Effects of ionic conductivities of zirconia electrolytes on polarization properties of platinum anodes in solid oxide fuel cells. J. Phys. Chem. 1995, 99, 3282–3287. [Google Scholar] [CrossRef]
- Huang, H.; Shim, J.H.; Chao, C.C.; Pornprasertsuk, R.; Sugawara, M.; Gur, T.M.; Prinz, F.B. Characteristics of oxygen reduction on nanocrystalline YSZ. J. Electrochem. Soc. 2009, 156, B392–B396. [Google Scholar] [CrossRef]
- Bai, J.; Han, Z.; Lv, B.; Chen, X.; Zhu, X.; Zhou, D. Preparation of 3D Structure High Performance Ba0.5Sr0.5Fe0.8Cu0.2O3-δ Nanofiber SOFC Cathode Material by Low-Temperature Calcination Method. Int. J. Hydrogen Energy 2021, 46, 8132–8142. [Google Scholar] [CrossRef]
- Meng, Y.; Zhang, Q.; Chen, Z.; Chen, X.; Zhou, J.; Zhu, X.; Wang, N.; Zhou, D. Novel Cobalt and Strontium-free Perovskite Pr0.5Ba0.5Fe1-xNixO3-δ (X = 0 and 0.2) as Cathode for Intermediate-Temperature Solid Oxide Fuel Cells. Ionics 2021, 27, 3951–3965. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Liang, Q.; Sun, X.; Zhu, X.; Zhou, D.; Meng, J. Pr2NiO4-Pr0.2Ce0.8O1.9 Composite Cathode as a Potential Cathode Material for Intermediate Temperature Solid Oxide Fuel Cells. Solid State Sci. 2020, 100, 106108–106116. [Google Scholar] [CrossRef]
- Meng, Y.; Zhu, X.; Meng, J.; Bai, J.; Chen, R.; Zhou, D.; Wang, N.; Tian, D. Heterointerface Effect in Accelerating the Cathodic Oxygen Reduction for Intermediate-Temperature Solid Oxide Fuel Cells. Front. Chem. 2022, 10, 959863. [Google Scholar] [CrossRef] [PubMed]
| Cell Configuration | Power Density, mW/cm−2 | Temperature, °C | Reference |
|---|---|---|---|
| NiO-GDC|GDC 180 μm|BSFC | 510 | 700 | [31] |
| NiO-GDC|GDC 300 μm|PBFN | 520 | 700 | [32] |
| NiO-GDC|GDC 320 μm|PNO-PCO | 570 | 800 | [33] |
| NiO-GDC|GDC 250 μm|PSFN | 699 | 800 | [34] |
| NiO/10ScCeSZ |GDC 3 μm|LSC | 1375 | 700 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solovyev, A.; Shipilova, A.; Smolyanskiy, E.; Rabotkin, S.; Semenov, V. The Properties of Intermediate-Temperature Solid Oxide Fuel Cells with Thin Film Gadolinium-Doped Ceria Electrolyte. Membranes 2022, 12, 896. https://doi.org/10.3390/membranes12090896
Solovyev A, Shipilova A, Smolyanskiy E, Rabotkin S, Semenov V. The Properties of Intermediate-Temperature Solid Oxide Fuel Cells with Thin Film Gadolinium-Doped Ceria Electrolyte. Membranes. 2022; 12(9):896. https://doi.org/10.3390/membranes12090896
Chicago/Turabian StyleSolovyev, Andrey, Anna Shipilova, Egor Smolyanskiy, Sergey Rabotkin, and Vyacheslav Semenov. 2022. "The Properties of Intermediate-Temperature Solid Oxide Fuel Cells with Thin Film Gadolinium-Doped Ceria Electrolyte" Membranes 12, no. 9: 896. https://doi.org/10.3390/membranes12090896
APA StyleSolovyev, A., Shipilova, A., Smolyanskiy, E., Rabotkin, S., & Semenov, V. (2022). The Properties of Intermediate-Temperature Solid Oxide Fuel Cells with Thin Film Gadolinium-Doped Ceria Electrolyte. Membranes, 12(9), 896. https://doi.org/10.3390/membranes12090896







