Behavior of Mebendazole during NF/RO Adsorption and Photolysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Membranes
2.3. Adsorption
2.4. Photolysis
2.5. Toxicity
2.6. Chemical Analysis
2.7. Fourier Transformation Infrared Spectrometer—FTIR
3. Results and Discussion
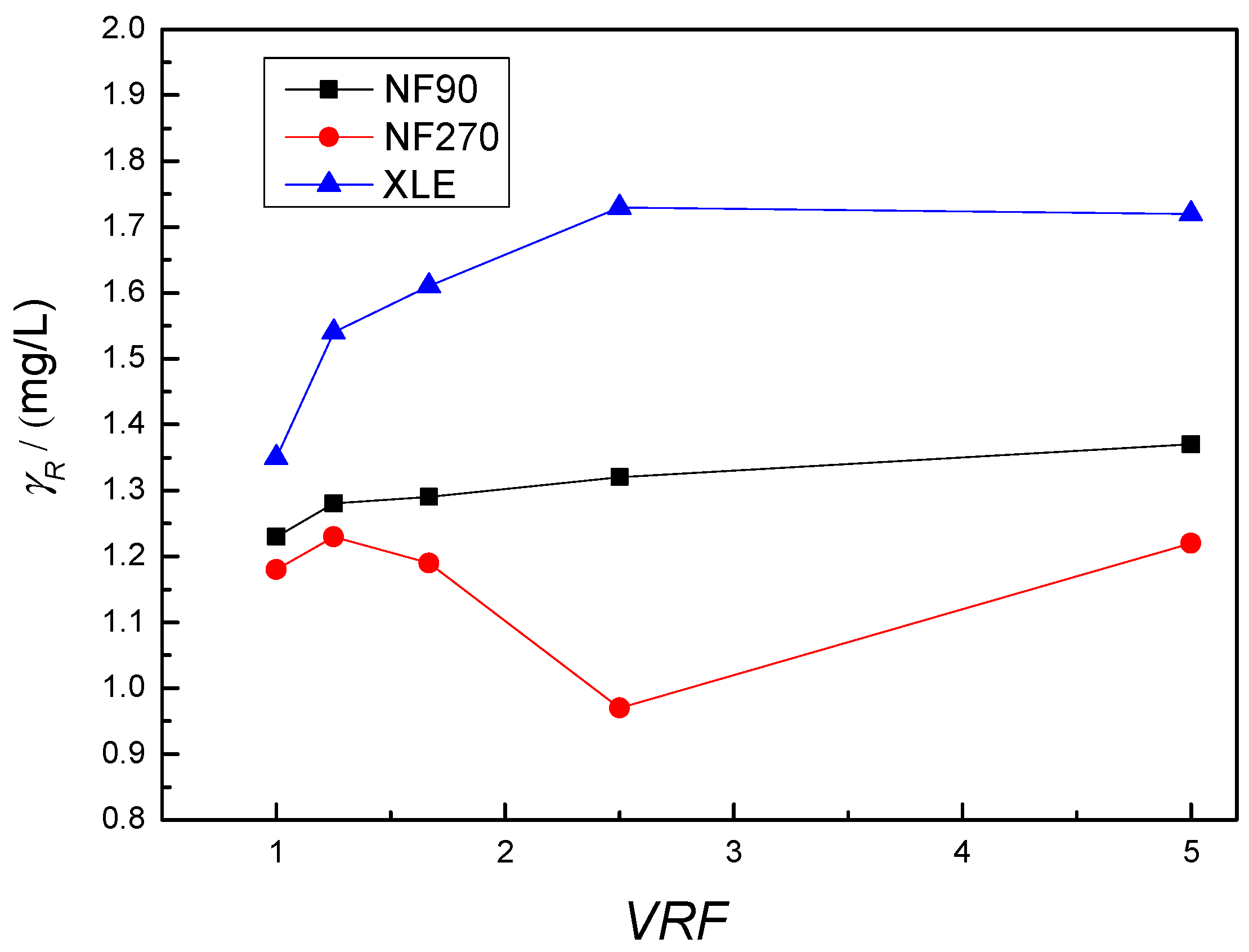
3.1. Removal of Mebendazole with RO/NF Membranes
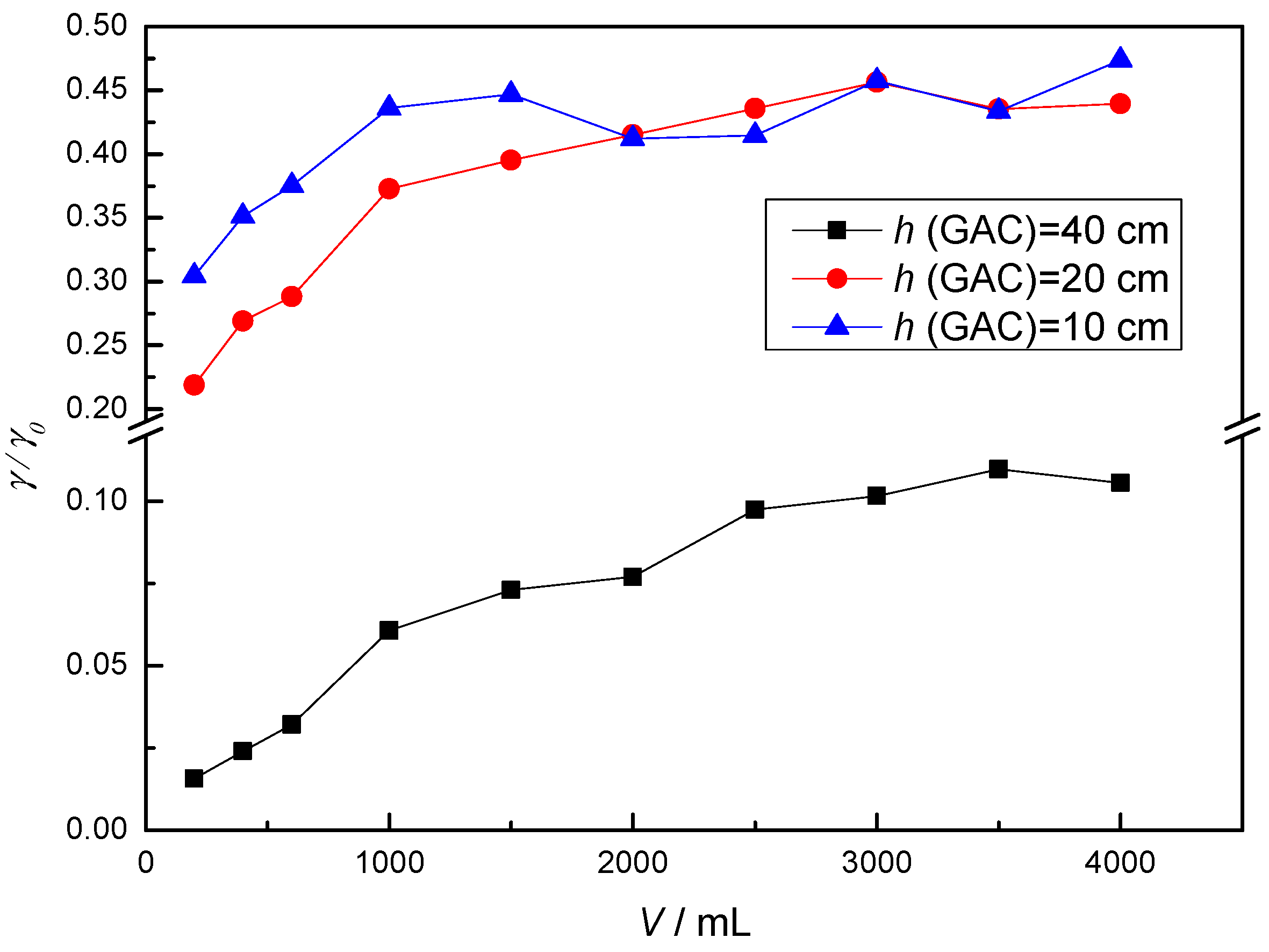
3.2. Adsorption
3.3. Photolysis and Toxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Charuaud, L.; Jarde, E.; Jaffrezic, A.; Thomas, M.F.; Le Bot, B. Veterinary pharmaceutical residues from natural water to tap water: Sales, occurrence and fate. J. Hazard. Mater. 2019, 361, 169–186. [Google Scholar] [CrossRef] [PubMed]
- Kwarciak-Kozlowska, A. Removal of pharmaceuticals and personal care products by ozonation, advance oxidation processes, and membrane separation. In Pharmaceuticals and Personal Care Products: Waste Management and Treatment Technology: Emerging Contaminants and Micro Pollutants, 1st ed.; Prasad, M.N.V., Vithanage, M., Kapley, A., Eds.; Butterworth-Heinemann Elseviere: Oxford, UK, 2019; Volume 3, pp. 151–171. [Google Scholar] [CrossRef]
- Elayapillai, S.; Ramraj, S.; Benbrook, D.M.; Bieniasz, M.; Wang, L.; Pathuri, G.; Isingizwe, Z.R.; Kennedy, A.L.; Zhao, Y.D.; Lightfoot, S.; et al. Potential and mechanism of mebendazole for treatment and maintenance of ovarian cancer. Gynecol. Oncol. 2021, 160, 302–311. [Google Scholar] [CrossRef]
- Hegazy, S.K.; El-Azab, G.A.; Zakaria, F.; Mostafa, M.F.; El-Ghoneimy, R.A. Mebendazole; from an anti-parasitic drug to a promising candidate for drug repurposing in colorectal cancer. Life Sci. 2022, 299, 120536. [Google Scholar] [CrossRef]
- Son, D.S.; Lee, E.S.; Adunyah, S.E. The antitumor potentials of benzimidazole anthelmintics as repurposing drugs. Immune Netw. 2020, 20, e29. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, F.; Black, M.; Vizeacoumar, F.; Pinto, N.; Ruicci, K.M.; Le, C.; Lowerison, M.R.; Leong, H.S.; Yoo, J.; Fung, K.; et al. Repurposing Albendazole: New potential as a chemotherapeutic agent with preferential activity against HPV-negative head and neck squamous cell cancer. Oncotarget 2017, 8, 71512–71519. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.J.; Luo, X.; Zhang, W.; Peng, F.; Cui, B.; Wu, S.J.; Zheng, F.M.; Xu, J.; Xu, L.Z.; Long, Z.J.; et al. Flubendazole, FDA-approved anthelmintic, targets breast cancer stem-like cells. Oncotarget 2015, 6, 6326–6340. [Google Scholar] [CrossRef]
- Li, Y.; Gan, Z.; Liu, Y.; Chen, S.; Su, S.; Ding, S.; Tran, N.H.; Chen, X.; Long, Z. Determination of 19 anthelmintics in environmental water and sediment using an optimized PLE and SPE method coupled with UHPLC-MS/MS. Sci. Total Environ. 2020, 719, 137516. [Google Scholar] [CrossRef]
- Sim, W.J.; Kim, H.Y.; Choi, S.D.; Kwon, J.H.; Oh, J.E. Evaluation of pharmaceuticals and personal care products with emphasis on anthelmintics in human sanitary waste, sewage, hospital wastewater, livestock wastewater and receiving water. J. Hazard. Mater. 2013, 248, 219–227. [Google Scholar] [CrossRef]
- Lian, Z.; Liang, Z.; Wang, J. Selective extraction and concentration of mebendazole in seawater samples using molecularly imprinted polymer as sorbent. Mar. Pollut. Bull. 2015, 91, 96–101. [Google Scholar] [CrossRef]
- Zajíčková, M.; Nguyen, L.T.; Skálová, L.; Raisová Stuchlíková, L.; Matoušková, P. Anthelmintics in the future: Current trends in the discovery and development of new drugs against gastrointestinal nematodes. Drug Discov. Today 2020, 25, 430–437. [Google Scholar] [CrossRef]
- Nixon, S.A.; Welz, C.; Woods, D.J.; Costa-Junior, L.; Zamanian, M.; Martin, R.J. Where are all the anthelmintics? Challenges and opportunities on the path to new anthelmintics. Int. J. Parasitol. Drugs Drug Resist. 2020, 14, 8–16. [Google Scholar] [CrossRef]
- Soil-Transmitted Helminth Infections. Available online: https://www.who.int/news-room/fact-sheets/detail/soil-transmitted-helminth-infections (accessed on 26 January 2021).
- Liang, C.; Wei, D.; Zhang, S.; Ren, Q.; Shi, J.; Liu, L. Removal of antibiotic resistance genes from swine wastewater by membrane filtration treatment. Ecotoxicol. Environ. Saf. 2021, 210, 111885. [Google Scholar] [CrossRef] [PubMed]
- Deegan, A.M.; Shaik, B.; Nolan, K.; Urell, K.; Oelgemöller, M.; Tobin, J.; Morrissey, A. Treatment options for wastewater effluents from pharmaceutical companies. Int. J. Environ. Sci. Technol. 2011, 8, 649–666. [Google Scholar] [CrossRef]
- Benner, J.; Salhi, E.; Ternes, T.; von Gunten, U. Ozonation of reverse osmosis concentrate: Kinetics and efficiency of beta blocker oxidation. Water Res. 2008, 42, 3003–3012. [Google Scholar] [CrossRef]
- Joo, S.H.; Tansel, B. Novel technologies for reverse osmosis concentrate treatment: A review. J. Environ. Manag. 2015, 150, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Saood Manzar, M.; Ahmad, T.; Ullah, N.; Velayudhaperumal Chellam, P.; John, J.; Zubair, M.; Brandão, R.J.; Meili, L.; Alagha, O.; Çevik, E. Comparative adsorption of Eriochrome Black T and Tetracycline by NaOH-modified steel dust: Kinetic and process modeling. Sep. Purif. Technol. 2022, 287, 120559. [Google Scholar] [CrossRef]
- Albatrni, H.; Qiblawey, H.; El-Naas, M.H. Comparative study between adsorption and membrane technologies for the removal of mercury. Sep. Purif. Technol. 2021, 257, 117833. [Google Scholar] [CrossRef]
- Saood Manzar, M.; Alshabib, M.; Alam, U.; Nawaz, M.; Zubair, M.; Ferreira Silva, A.; Dalhat Mu’azu, N.; Meili, L.; Çevik, E.; Alqahtani, H.A. Comparative adsorption of Eriochrome black T onto recyclable steel dust wastes: Isotherm, kinetics and thermodynamic studies. Colloids Surf. A Physicochem. Eng. Asp. 2022, 645, 128828. [Google Scholar] [CrossRef]
- Manzar, M.S.; Khan, G.; dos Santos Lins, P.V.; Zubair, M.; Khan, S.U.; Selvasembian, R.; Meili, L.; Blaisi, N.I.; Nawaz, M.; Abdul Aziz, H.; et al. RSM-CCD optimiza-tion approach for the adsorptive removal of Eriochrome Black T from aqueous system using steel slag-based adsorbent: Characterization, Isotherm, Kinetic modeling and thermodynamic analysis. J. Mol. Liq. 2021, 339, 116714. [Google Scholar] [CrossRef]
- Xu, L.; Campos, L.C.; Li, J.; Karu, K.; Ciric, L. Removal of antibiotics in sand, GAC, GAC sandwich and anthracite/sand biofiltration systems. Chemosphere 2021, 275, 130004. [Google Scholar] [CrossRef]
- Mompelat, S.; Le Bot, B.; Thomas, O. Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water. Environ. Int. 2009, 35, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran, D.; Sukul, P.; Lamshöft, M.; Maheswari, M.A.; Zühlke, S.; Spiteller, M. Photolysis of difloxacin and sarafloxacin in aqueous systems. Chemosphere 2009, 77, 739–746. [Google Scholar] [CrossRef]
- Dolar, D.; Pelko, S.; Košutić, K.; Horvat, A.J.M. Removal of anthelmintic drugs and their photodegradation products from water with RO/NF membranes. Process Saf. Environ. Prot. 2012, 90, 147–152. [Google Scholar] [CrossRef]
- Monteiro, N.K.V.; Firme, C.L. Teaching Thermodynamic, Geometric and Electronic Aspects of Diels-Alder Cycloadditions by Using Computational Chemistry-An Undergraduate Experiment. World J. Chem. Educ. 2015, 3, 141–149. [Google Scholar] [CrossRef]
- Racar, M.; Dolar, D.; Špehar, A.; Košutić, K. Application of UF/NF/RO membranes for treatment and reuse of rendering plant wastewater. Process Saf. Environ. Prot. 2017, 105, 386–392. [Google Scholar] [CrossRef]
- Dolar, D.; Vuković, A.; Ašperger, D.; Košutić, K. Effect of water matrices on removal of veterinary pharmaceuticals by nanofiltration and reverse osmosis membranes. J. Environ. Sci. 2011, 23, 1299–1307. [Google Scholar] [CrossRef]
- Racar, M.; Obajdin, K.; Dolar, D.; Košutić, K. Pretreatment for the reclamation of rendering plant secondary effluent with NF/RO: UF flat sheet versus UF hollow fiber membranes. Clean Technol. Environ. Policy 2020, 22, 399–408. [Google Scholar] [CrossRef]
- Dabić, D.; Babić, S.; Škorić, I. The role of photodegradation in the environmental fate of hydroxychloroquine. Chemosphere 2019, 230, 268–277. [Google Scholar] [CrossRef]
- Li, H.; Duan, L.; Wang, H.; Chen, Y.; Wang, F.; Zhang, S. Photolysis of sulfadiazine under UV radiation: Effects of the initial sulfadiazine concentration, pH, NO3− and Cd2+. Chem. Phys. Lett. 2020, 739, 136949. [Google Scholar] [CrossRef]
- Dolar, D.; Vukovic, A.; Asperger, D.; Kosutic, K. Efficiency of RO/NF membranes at the removai of veterinary antibiotics. Water Sci. Technol. 2012, 65, 317–323. [Google Scholar] [CrossRef]
- Dolar, D.; Drašinac, N.; Košutić, K.; Škorić, I.; Ašperger, D. Adsorption of hydrophilic and hydrophobic pharmaceuticals on RO/NF membranes: Identification of interactions using FTIR. J. Appl. Polym. Sci. 2017, 134, 17–21. [Google Scholar] [CrossRef]
- Košutić, K.; Dolar, D.; Kunst, B. On experimental parameters characterizing the reverse osmosis and nanofiltration membranes’ active layer. J. Membr. Sci. 2006, 282, 109–114. [Google Scholar] [CrossRef]
- Kimura, K.; Amy, G.; Drewes, J.E.; Heberer, T.; Kim, T.U.; Watanabe, Y. Rejection of organic micropollutants (disinfection by-products, endocrine disrupting compounds, and pharmaceutically active compounds) by NF/RO membranes. J. Membr. Sci. 2003, 227, 113–121. [Google Scholar] [CrossRef]
- Comerton, A.M.; Andrews, R.C.; Bagley, D.M.; Hao, C. The rejection of endocrine disrupting and pharmaceutically active compounds by NF and RO membranes as a function of compound and water matrix properties. J. Membr. Sci. 2008, 313, 323–335. [Google Scholar] [CrossRef]
- Nandiyanto, A.B.D.; Oktiani, R.; Ragadhita, R. How to read and interpret ftir spectroscope of organic material. Indones. J. Sci. Technol. 2019, 4, 97–118. [Google Scholar] [CrossRef]
- Jarusutthirak, C.; Amy, G.; Croué, J.P. Fouling characteristics of wastewater effluent organic matter (EfOM) isolates on NF and UF membranes. Desalination 2002, 145, 247–255. [Google Scholar] [CrossRef]
- Kimura, K.; Iwase, T.; Kita, S.; Watanabe, Y. Influence of residual organic macromolecules produced in biological wastewater treatment processes on removal of pharmaceuticals by NF/RO membranes. Water Res. 2009, 43, 3751–3758. [Google Scholar] [CrossRef]
- Li, C.; Yang, Y.; Liu, Y.; Hou, L. Removal of PhACs and their impacts on membrane fouling in NF/RO membrane filtration of various matrices. J. Membr. Sci. 2018, 548, 439–448. [Google Scholar] [CrossRef]
- ELAbadsa, M.; Varga, M.; Mihucz, V.G. Removal of selected pharmaceuticals from aqueous matrices with activated carbon under flow conditions. Microchem. J. 2019, 150, 104079. [Google Scholar] [CrossRef]
- Rizzo, L.; Malato, S.; Antakyali, D.; Beretsou, V.G.; Đolić, M.B.; Gernjak, W.; Heath, E.; Ivancev-Tumbas, I.; Karaolia, P.; Ribeiro, A.R.L.; et al. Consolidated vs new advanced treatment methods for the removal of contaminants of emerging concern from urban wastewater. Sci. Total Environ. 2019, 655, 986–1008. [Google Scholar] [CrossRef]
- Lin, L.; Jiang, W.; Xu, P. Comparative study on pharmaceuticals adsorption in reclaimed water desalination concentrate using biochar: Impact of salts and organic matter. Sci. Total Environ. 2017, 601, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Karim, E.F.I.A.; Ahmed, M.H.; Salama, R.B. Studies on the thermal and photochemical decomposition of mebendazole. Int. J. Pharm. 1996, 142, 251–255. [Google Scholar] [CrossRef]
- Kawabata, K.; Sugihara, K.; Sanoh, S.; Kitamura, S.; Ohta, S. Photodegradation of pharmaceuticals in the aquatic environment by sunlight and UV-A, -B and -C irradiation. J. Toxicol. Sci. 2013, 38, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Dayan, A.D. Albendazole, mebendazole and praziquantel. Review of non-clinical toxicity and pharmacokinetics. Acta Trop. 2003, 86, 141–159. [Google Scholar] [CrossRef]
- Marsboom, R. Toxicologic studies on mebendazole. Toxicol. Appl. Pharmacol. 1973, 24, 371–377. [Google Scholar] [CrossRef]


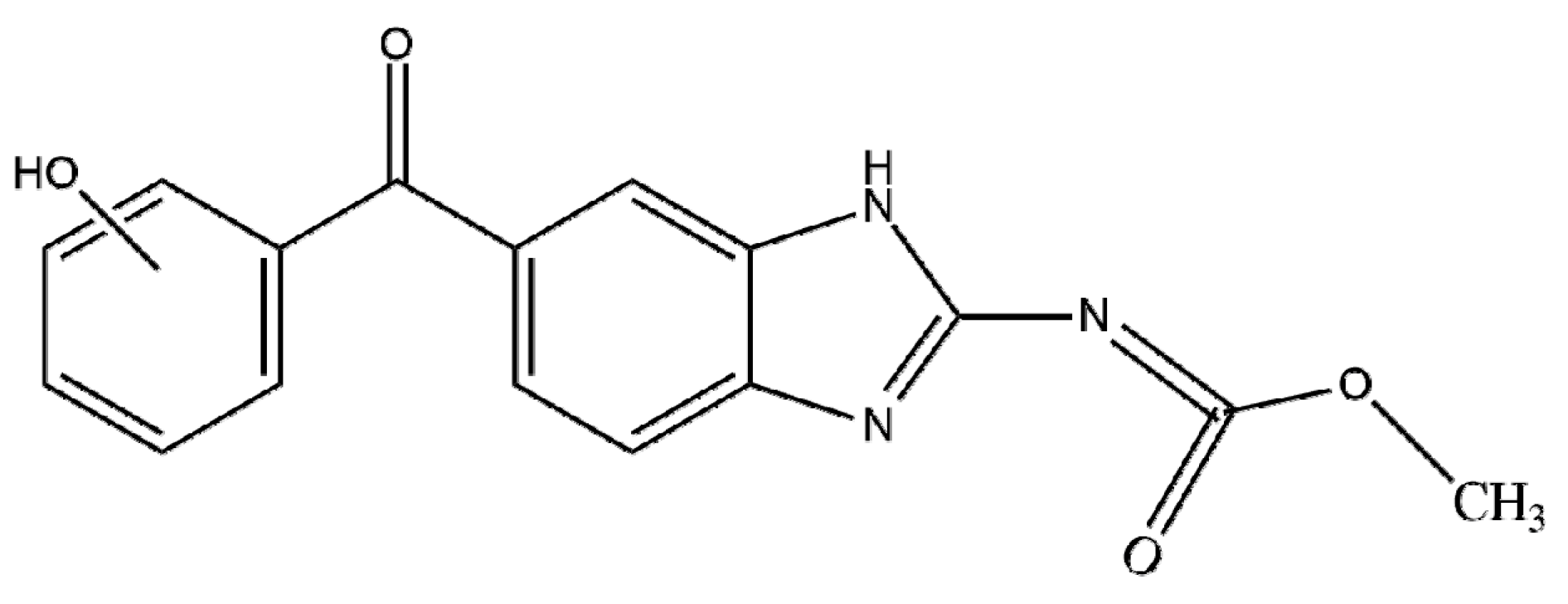
| IUPAC Name | Methyl N-(6-benzoyl-1H-benzimidazol-2-yl)carbamate |
|---|---|
| CAS number | 31431-39-7 |
| Mw (g/mol) | 295.293 |
| Molecular structures | 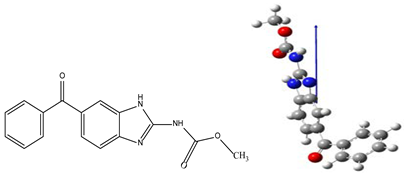 |
| width (nm) b | 0.511 b |
| height (nm) b | 0.418 b |
| length (nm) b | 1.572 b |
| logKow | 2.83 a |
| tt (°C) | 223.41 a |
| tv (°C) | 523.14 a |
| S (mg/L) | 71.3 at 25 °C a |
| Dipole moment, µ (D) | 6.318 c |
| XLE | NF90 | NF270 | |
|---|---|---|---|
| 1 mg/L | |||
| γ/(mg/L) | <LOD | <LOD | 0.60 |
| R/% | >99.9 | >99.9 | 41.9 |
| 3 mg/L | |||
| γ/(mg/L) | <LOD | <LOD | 1.29 |
| R/% | >99.9 | >99.9 | 57.0 |
| 5 mg/L | |||
| γ/(mg/L) | 0.11 | 0.10 | 0.33 |
| R/% | 97.7 | 97.9 | 93.2 |
| 10 mg/L | |||
| γ/(mg/L) | 0.17 | 0.17 | 0.36 |
| R/% | 98.4 | 98.4 | 96.6 |
| 1 mg/L | |||
| t/h | 0 | 4 | 12 |
| INH/% | 4.01 | 10.25 | 0 |
| 3 mg/L | |||
| t/h | 0 | 4 | 12 |
| INH/% | 6.66 | 9.71 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babić, B.; Andrić, D.; Farkaš, A.; Vuk, D.; Ašperger, D.; Dolar, D. Behavior of Mebendazole during NF/RO Adsorption and Photolysis. Membranes 2022, 12, 888. https://doi.org/10.3390/membranes12090888
Babić B, Andrić D, Farkaš A, Vuk D, Ašperger D, Dolar D. Behavior of Mebendazole during NF/RO Adsorption and Photolysis. Membranes. 2022; 12(9):888. https://doi.org/10.3390/membranes12090888
Chicago/Turabian StyleBabić, Bruna, Darko Andrić, Anamarija Farkaš, Dragana Vuk, Danijela Ašperger, and Davor Dolar. 2022. "Behavior of Mebendazole during NF/RO Adsorption and Photolysis" Membranes 12, no. 9: 888. https://doi.org/10.3390/membranes12090888
APA StyleBabić, B., Andrić, D., Farkaš, A., Vuk, D., Ašperger, D., & Dolar, D. (2022). Behavior of Mebendazole during NF/RO Adsorption and Photolysis. Membranes, 12(9), 888. https://doi.org/10.3390/membranes12090888


_Dionysiou.jpg)





