Development of Ultrafiltration Kaolin Membranes over Sand and Zeolite Supports for the Treatment of Electroplating Wastewater
Abstract
1. Introduction
2. Experiment
2.1. Materials
2.2. Fabrication and Characterization of Kaolin Membranes
- 8% of kaolin powder (ϕ < 53 µm) was mixed with 62% of water and 30% of PVA (12 wt% aqueous solution) for kaolin/sand membrane.
- 2% of kaolin powder (ϕ < 53 µm) was mixed with 68% of water and 30% of PVA (12 wt% aqueous solution) for kaolin/zeolite membrane.
2.3. Ultrafiltration Experiment
- −
- Determination of λmax.
- −
- Measurement of absorbance A for each standard solution and for the dosed solution.
- −
- The calibration curve is plotted for the standard solutions: A = f(C).
- −
- The absorbance A of the dosed solution is plotted on the calibration curve to determine its concentration.
2.4. Modeling of Membrane Fouling
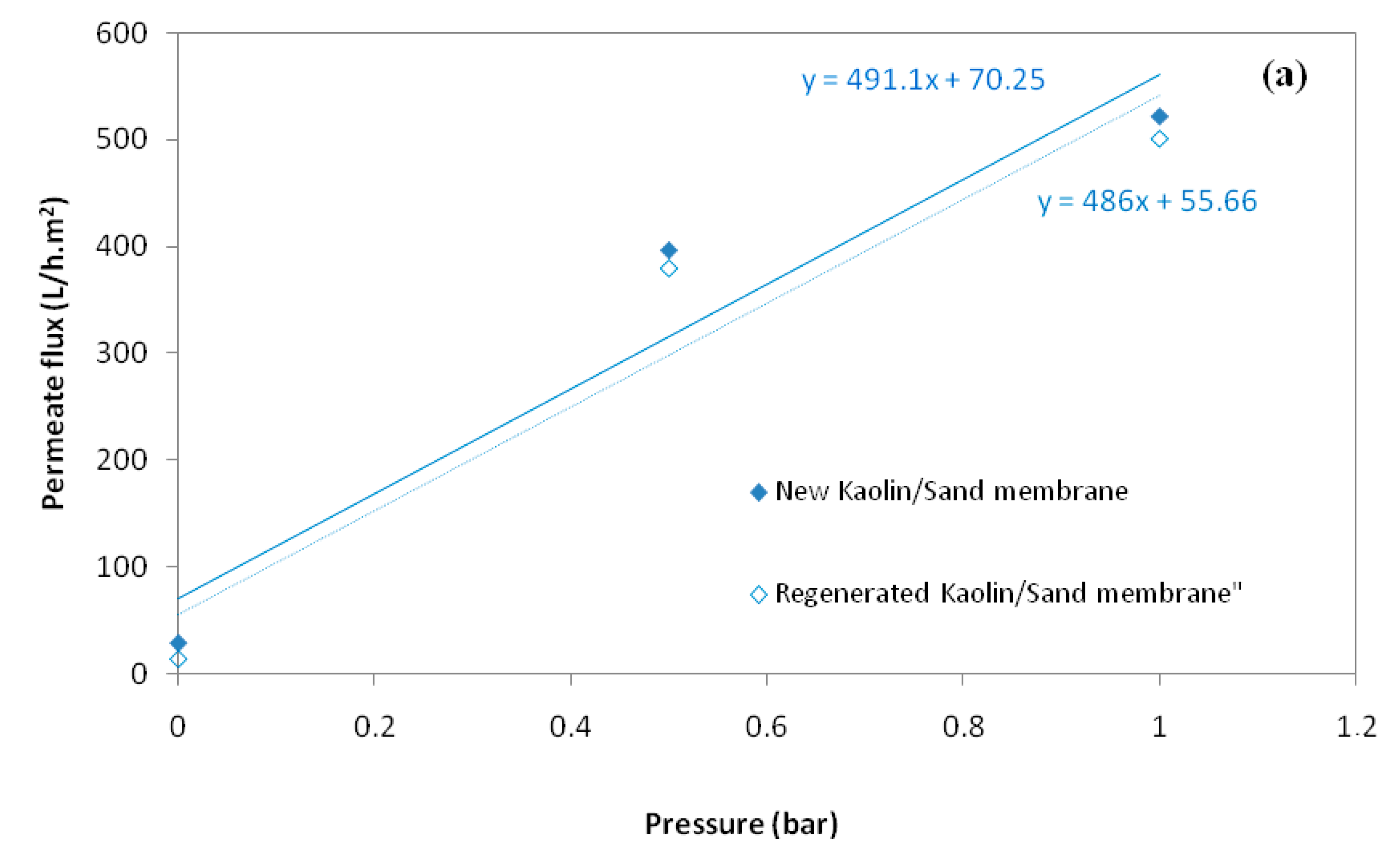
2.5. Determination of Fouling Resistances and Membrane Regeneration
3. Results and Discussion
3.1. Characterization of Kaolin Membranes
3.1.1. Membrane Morphology
3.1.2. Determination of Pore Diameters
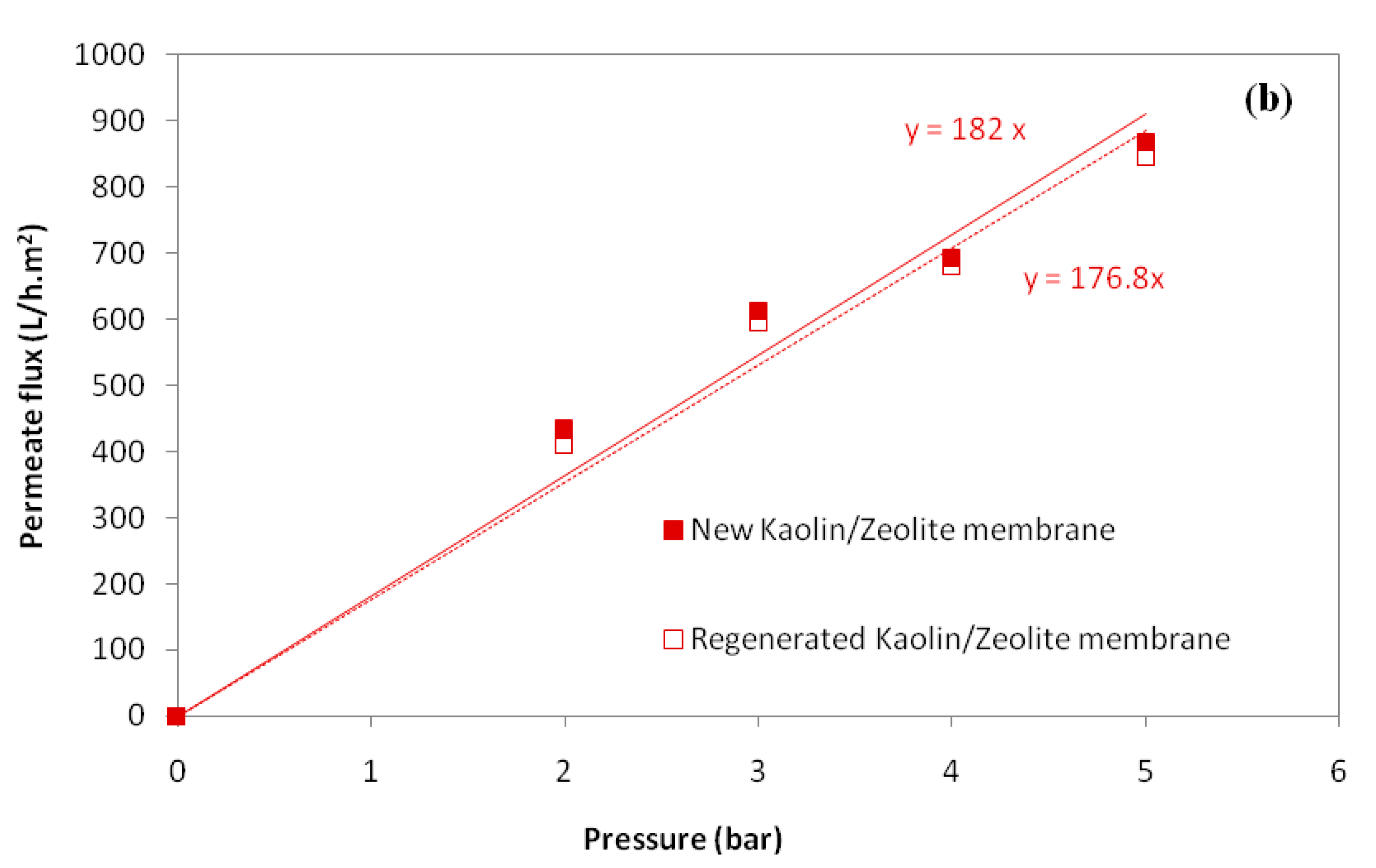
3.1.3. Determination of Water Permeability
3.2. Application to the Treatment of Electroplating Wastewater
3.2.1. Fluxes and Rejection Efficiencies
3.2.2. Fouling Mechanisms
3.2.3. Antifouling and Cleaning Study
3.3. Membranes’ Cost Estimation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aloulou, H.; Bouhamed, H.; Daramola, M.O.; Khemakhem, S.; Ben Amar, R. Fabrication of asymmetric ultrafiltration membranes from natural zeolite and their application in industrial wastewater treatment. Euro-Mediterr. J. Environ. Integr. 2020, 5, 36. [Google Scholar] [CrossRef]
- Aloulou, H.; Aloulou, W.; Daramola, M.O.; Ben Amar, R. Silane-grafted sand membrane for the treatment of oily wastewater via air gap membrane distillation: Study of the efficiency in comparison with microfiltration and ultrafiltration ceramic membranes. Mater. Chem. Phys. 2021, 261, 124186. [Google Scholar] [CrossRef]
- Beqqour, D.; Achiou, B.; Bouazizi, A.; Ouaddari, H.; Elomari, H.; Ouammou, M.; Bennazha, J.; Alami Younssi, S. Enhancement of microfiltration performances of pozzolan membrane by incorporation of micronized phosphate and its application for industrial wastewater treatment. J. Environ. Chem. Eng. 2019, 7, 102981. [Google Scholar] [CrossRef]
- Bousbih, S.; Errais, E.; Darragi, F.; Duplay, J.; Trabelsi-Ayadi, M.; Daramola, M.O.; Ben Amar, R. Treatment of Textile Wastewater using monolayered UltrafiltationCeramic Membrane Fabricated from Natural Kaolin Clay. Environ. Technol. 2021, 42, 3348–3359. [Google Scholar] [CrossRef] [PubMed]
- Manni, A.; Achiou, B.; Karim, A.; Harrati, A.; Sadik, C.; Ouammou, M.; Alamo Younssi, S.; El Bouari, A. New low-cost ceramic microfiltration membrane made from natural magnesite for industrial wastewater treatment. J. Environ. Chem. Eng. 2020, 8, 103906. [Google Scholar] [CrossRef]
- Belibi Belibi, P.; Nguemtchouin, M.M.G.; Rivallin, M.; Ndi Nsami, J.; Sieliechi, J.; Cerneaux, S.; Ngassoum, M.; Cretin, M. Microfiltration ceramic membranes from local Cameroonian clay applicable to water treatment. Ceram. Int. 2015, 41, 2752–2759. [Google Scholar] [CrossRef]
- Wei, Z.; Hou, J.; Zhu, Z. High-aluminum fly ash recycling for fabrication of cost-effective ceramic membrane supports. J. Alloys Compd. 2016, 683, 474–480. [Google Scholar] [CrossRef]
- Ben Ali, M.; Hamdi, N.; Rodriguez, M.A.; Srasra, E. Macroporous ceramic supports from natural clays. Improvement by the use of activated clays. Ceram. Int. 2017, 43, 1248. [Google Scholar] [CrossRef]
- Emani, S.; Uppaluri, R.; Purkait, M.K. Preparation and characterization of low-cost ceramic membranes for mosambi juice clarification. Desalination 2013, 317, 32–40. [Google Scholar] [CrossRef]
- Yoshino, Y.; Suzuki, T.; Nair, B.N.; Taguchi, H.; Itoh, N. Development of tubular substrates, silica based membranes and membrane modules for hydrogen separation at high temperature. J. Membr. Sci. 2005, 267, 8–17. [Google Scholar] [CrossRef]
- DeFriend, K.A.; Wiesner, M.R.; Barron, A.R. Alumina and aluminate ultrafiltration membranes derived from alumina nanoparticles. J. Membr. Sci. 2003, 224, 11–28. [Google Scholar] [CrossRef]
- Wang, Y.H.; Tian, T.F.; Liu, X.Q.; Meng, G.Y. Titania membrane preparation with chemical stability for very hash environments applications. J. Membr. Sci. 2006, 280, 261–269. [Google Scholar] [CrossRef]
- Das, B.; Chakrabarty, B.; Barkakati, P. Preparation and characterization of novel ceramic membranes for micro-filtration application. Ceram. Int. 2016, 42, 14326–14333. [Google Scholar] [CrossRef]
- Aloulou, H.; Bouhamed, H.; Ghorbel, A.; Ben Amar, R.; Khemakhem, S. Elaboration and characterization of ceramic microfiltration membranes from natural zeolite: Application to the treatment of cuttlefish effluents. Desalin. Water Treat. 2017, 95, 9–17. [Google Scholar] [CrossRef]
- Aloulou, H.; Bouhamed, H.; Ben Amar, R.; Khemakhem, S. New ceramic microfiltration membrane from Tunisian natural sand: Application for tangential wastewater treatment. Desal. Wat. Treat. 2017, 78, 41–48. [Google Scholar] [CrossRef]
- Weir, M.R.; Rutinduka, E.; Detellier, C.; Feng, C.Y.; Wang, Q.; Matsuura, T.; Le VanMao, R. Fabrication, characterization and preliminary testing of all-inorganic ultrafiltration membranes composed entirely of a naturally occurring sepiolite clay mineral. J. Membr. Sci. 2001, 182, 41. [Google Scholar] [CrossRef]
- Bouzerara, F.; Harabi, A.; Achour, S.; Larbot, A. Porous ceramic supports for membranes prepared from kaolin and doloma mixtures. J. Eur. Ceram. Soc. 2006, 26, 1663–1671. [Google Scholar] [CrossRef]
- Saja, S.; Bouazizi, A.; Achiou, B.; Ouammou, M.; Albizane, A.; Bennazha, J.; Younssi, S.A. Elaboration and characterization of low-cost ceramic membrane made from natural Moroccan perlite for treatment of industrial wastewater. J. Environ. Chem. Eng. 2018, 6, 451–458. [Google Scholar] [CrossRef]
- Sheikhi, M.; Arzani, M.; Mahdavi, H.R.; Mohammadi, T. Kaolinitic clay-based ceramic microfiltration membrane for oily wastewater treatment: Assessment of coagulant addition. Ceram. Int. 2019, 45, 17826–17836. [Google Scholar] [CrossRef]
- Barrouk, I.; Younssi, S.A.; Kabbabi, A.; Persin, M.; Albizane, A.; Tahiri, S. New ceramic membranes from natural Moroccan phosphate for microfiltration application. Desalin. Water Treat. 2015, 55, 53–60. [Google Scholar] [CrossRef]
- Malik, N.; Bulasara, V.K.; Basu, S. Preparation of novel porous ceramic microfiltration membranes from fly ash, kaolin and dolomite mixtures. Ceram. Int. 2019, 55, 53–60. [Google Scholar] [CrossRef]
- Mgbemena, C.O.; Ibekwe, N.O.; Sukumar, R.; Menon, A.R.R. Characterization of kaolin intercalates of oleochemicals derived from rubber seed (Hevea brasiliensis) and tea seed (Camelia sinensis) oils. J. King Saud. Univ. Sci. 2013, 25, 149–155. [Google Scholar] [CrossRef]
- Hedfi, I.; Hamdi, N.; Srasra, E.; Rodríguez, M.A. The preparation of micro-porous membranefrom Tunisian kaolin. Appl. Clay Sci. 2014, 101, 574–578. [Google Scholar] [CrossRef]
- Guesmi, Y.; Ridha, L.; Hassen, A.; Mahjoub, J.; Oun, A.; Swachchha, M.; Amor, H. Synthesis and characterization of alpha alumina-natural apatite based porous ceramic support for filtration application. Mater. Chem. Phys. 2020, 239, 122067. [Google Scholar] [CrossRef]
- Mestre, S.; Gozalbo, A.; Lorente-Ayza, M.M.; S’anchez, E. Low-cost ceramic membranes: A research opportunity for industrial application. J. Eur. Ceram. Soc. 2019, 39, 3392–3407. [Google Scholar] [CrossRef]
- Monash, P.; Pugazhenthi, G. Development of ceramic supports derived from low-cost raw materials for membrane applications and its optimization based on sintering temperature. Int. J. Appl. Ceram. Technol. 2011, 8, 227–238. [Google Scholar] [CrossRef]
- Harabi, A.; Zenikheri, F.; Boudaira, B.; Bouzerara, F.; Guechi, A.; Foughali, L. A new and economic approach to fabricate resistant porous membrane supports using kaolin and CaCO3. J. Eur. Ceram. Soc. 2014, 34, 1329–1340. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, R.; Wu, P.; He, J.; Liu, C.; Jiang, W. Three-step treatment of real complex, variable high-COD rolling wastewater by rational adjustment of acidification, adsorption, and photocatalysis using big data analysis. Sep. Purif. Technol. 2021, 270, 118865. [Google Scholar] [CrossRef]
- Yu, L.; Han, M.; He, F. A review of treating oily wastewater. Arab. J. Chem. 2017, 10, S1913–S1922. [Google Scholar] [CrossRef]
- Zsirai, T.; Qiblawey, H.; Buzatu, P.; Al-Marri, M.; Judd, S.J. Cleaning of ceramic membranes for produced water filtration. J. Pet. Sci. Eng. 2018, 166, 283–289. [Google Scholar] [CrossRef]
- Chen, R.; Liao, X.; Ge, Q. A novel multinuclear zinc complex Zn-Bet-Tf2N for electroplating wastewater treatment using forward osmosis technique. Chem. Eng. J. 2021, 404, 126569. [Google Scholar] [CrossRef]
- Yan, F.L.; Wang, Y.; Wang, W.H.; Zhao, J.X.; Feng, L.L.; Li, J.J.; Zhao, J.C. Application of biochars obtained through the pyrolysis of Lemna minor in the treatment of Ni-electroplating wastewater. J. Water Process Eng. 2020, 37, 101464. [Google Scholar] [CrossRef]
- Veréb, G.; Kovács, I.; Zakar, M.; Kertész, S.; Hodúr, C.; László, Z. Matrix effect in case of purification of oily waters by membrane separation combined with pre-ozonation. Environ. Sci. Pollut. Res. 2018, 25, 34976–34984. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Ren, H.; Huang, X.; Li, M.; Tang, Y.; Guo, F. Low-cost red mud modified graphitic carbon nitride for the removal of organic pollutants in wastewater by the synergistic effect of adsorption and photocatalysis. Sep. Purif. Technol. 2020, 237, 116477. [Google Scholar] [CrossRef]
- Priastomo, Y.; Setiawan, H.R.; Kurniawan, Y.S.; Ohto, K. Simultaneous removal of lead(II), chromium(III), and copper(II) heavy metal ions through an adsorption process using C-phenylcalix[4]pyrogallolarene material. J. Environ. Chem. Eng. 2020, 8, 103971. [Google Scholar]
- Sadeghi, M.H.; Tofighy, M.A.; Mohammadi, T. One-dimensional graphene for efficient aqueous heavy metal adsorption: Rapid removal of arsenic and mercury ions by graphene oxide nanoribbons (GONRs). Chemosphere 2020, 253, 126647. [Google Scholar] [CrossRef]
- Cambiella, A.; Benito, J.M.; Pazos, C.; Coca, J. Centrifugal separation efficiency in the treatment of waste emulsified oils. Chem. Eng. Res. Des. 2006, 84, 69–76. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, S.; Pan, S.Y.; Zhu, S.; Yu, Y.; Zheng, H. Performance evaluation and optimization of flocculation process for removing heavy metal. Chem. Eng. J. 2020, 385, 123911. [Google Scholar] [CrossRef]
- Chakrabarty, B.; Ghoshal, A.K.; Purkait, M.K. Ultrafiltration of stable oil-in-water emulsion by polysulfone membrane. J. Membr. Sci. 2008, 325, 427–437. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Harun, Z.; Ismail, A.F.; Rahman, M.A.; Jaafar, J. A novel green ceramic hollow fiber membrane (CHFM) derived from rice husk ash as combined adsorbent-separator for efficient heavy metals removal. Ceram. Int. 2017, 43, 4716–4720. [Google Scholar] [CrossRef]
- Aloulou, W.; Hamza, W.; Aloulou, H.; Oun, A.; Khemakhem, S.; Jada, A.; Chakraborty, S.; Curcio, S.; Ben Amar, R. Developing of titania-smectite nanocomposites UF membrane over zeolite based ceramic support. Appl. Clay. Sci. 2018, 155, 20–29. [Google Scholar] [CrossRef]
- Scherdel, C.; Reichenauer, G.; Wiener, M. Relationship between pore volumes and surface areas derived from the evaluation of N2- sorption data by Dr-, BET- and T-plot. Microporous Mesoporous Mater. 2010, 132, 572–575. [Google Scholar] [CrossRef]
- Aloulou, W.; Aloulou, H.; Ben Amar, R. Low-cost composite ultrafiltration membrane made from TiO2 and nanocomposite clay materials over zeolite support for oily wastewater purification and heavy metals removal. Desal. Wat. Treat. 2022, 246, 166–173. [Google Scholar] [CrossRef]
- Ahmad, T.; Guria, C.; Mandal, A. Optimal synthesis and operation of low-cost polyvinyl chloride/bentonite ultrafiltration membranes for the purification of oilfield produced water. J. Membr. Sci. 2018, 564, 859–877. [Google Scholar] [CrossRef]
- Achiou, B.; Elomari, H.; Ouammou, M.; Albizane, A.; Bennazha, J.; Younssi, S.A.; Amrani, I.E.E.; Aaddane, A. Elaboration and characterization of flat ceramic microfiltration membrane made from natural Moroccan pozzolan (Central Middle Atlas). J. Mater. Env. Sci. 2016, 7, 196–204. [Google Scholar]
- El Batouti, M.; Al-Harby, N.F.; Elewa, M.M. A Review on Promising Membrane Technology Approaches for Heavy Metal Removal from Water and Wastewater to Solve Water Crisis. Water 2021, 13, 3241. [Google Scholar] [CrossRef]
- Yao, Z.; Du, S.; Zhang, Y.; Zhu, B.; Zhu, L.; John, A.E. Positively charged membrane for removing low concentration Cr(VI) in ultrafiltration process. J. Water Process Eng. 2015, 8, 99–107. [Google Scholar] [CrossRef]
- Abidi, N.; Duplay, J.; Elmchaouri, A.; Jada, A.; Trabelsi-Ayadi, M. Textile Dye Adsorption Onto Raw Clay: Influence of Clay Surface Properties and Dyeing Additives. J. Colloid. Sci. Biotechnol. 2014, 3, 1. [Google Scholar]
- Mouiya, M.; Abourriche, A.; Bouazizi, A.; Benhammou, A.; El Hafiane, Y.; Abouliatim, Y.; Nibou, L.; Oumam, M.; Ouammou, M.; Smith, A.; et al. Flat ceramic microfiltration membrane based on natural clay and Moroccan phosphate for desalination and industrial wastewater treatment. Desalination 2018, 427, 42–50. [Google Scholar] [CrossRef]
- Kumar, R.V.; Ghoshal, A.K.; Pugazhenthi, G. Elaboration of novel tubular ceramic membrane from in expensive raw materials by extrusion method and its performance in microfiltration of synthetic oily wastewater treatment. J. Membr. Sci. 2015, 490, 92–102. [Google Scholar] [CrossRef]
- Veréb, G.; Kassai, P.; Santos, E.N.; Arthanareeswaran, G.; Hodúr, C.; László, Z. Intensification of the ultrafiltration of real oil-contaminated (produced) water with pre ozonation and/or with TiO2, TiO2/CNT nanomaterial-coated membrane surfaces. Environ. Sci. Pollut. Res. 2020, 27, 22195–22205. [Google Scholar] [CrossRef] [PubMed]
- Tahri, N.; Masmoudi, G.; Ellouze, E.; Jrad, A.; Drogui, P.; Ben Amar, R. Coupling microfiltration and nanofiltration processes for the treatment at source of dyeing-containing effluent. J. Clean. Prod. 2012, 33, 226. [Google Scholar] [CrossRef]
- Vasanth, D.; Pugazhenthi, G.; Uppaluri, R. Performance of Low-Cost Ceramic Microfiltration Membranes for the Treatment of Oil-in-water Emulsions. Sep. Sci. Technol. 2013, 48, 849–858. [Google Scholar] [CrossRef]
- Souza, M.Y.M.; Lira, H.L.; Santana, L.N.L. Rodríguez, M.A. Preparation and Application in Crude Oil-Water Separation of Clay-Based Membranes. Mater. Res. 2021, 24, e20200508. [Google Scholar] [CrossRef]
- Suresh, K.; Pugazhenthi, G. Development of ceramic membranes from low-cost clays for the separation of oil–water emulsion. Desal. Wat. Treat. 2016, 57, 1927–1939. [Google Scholar] [CrossRef]
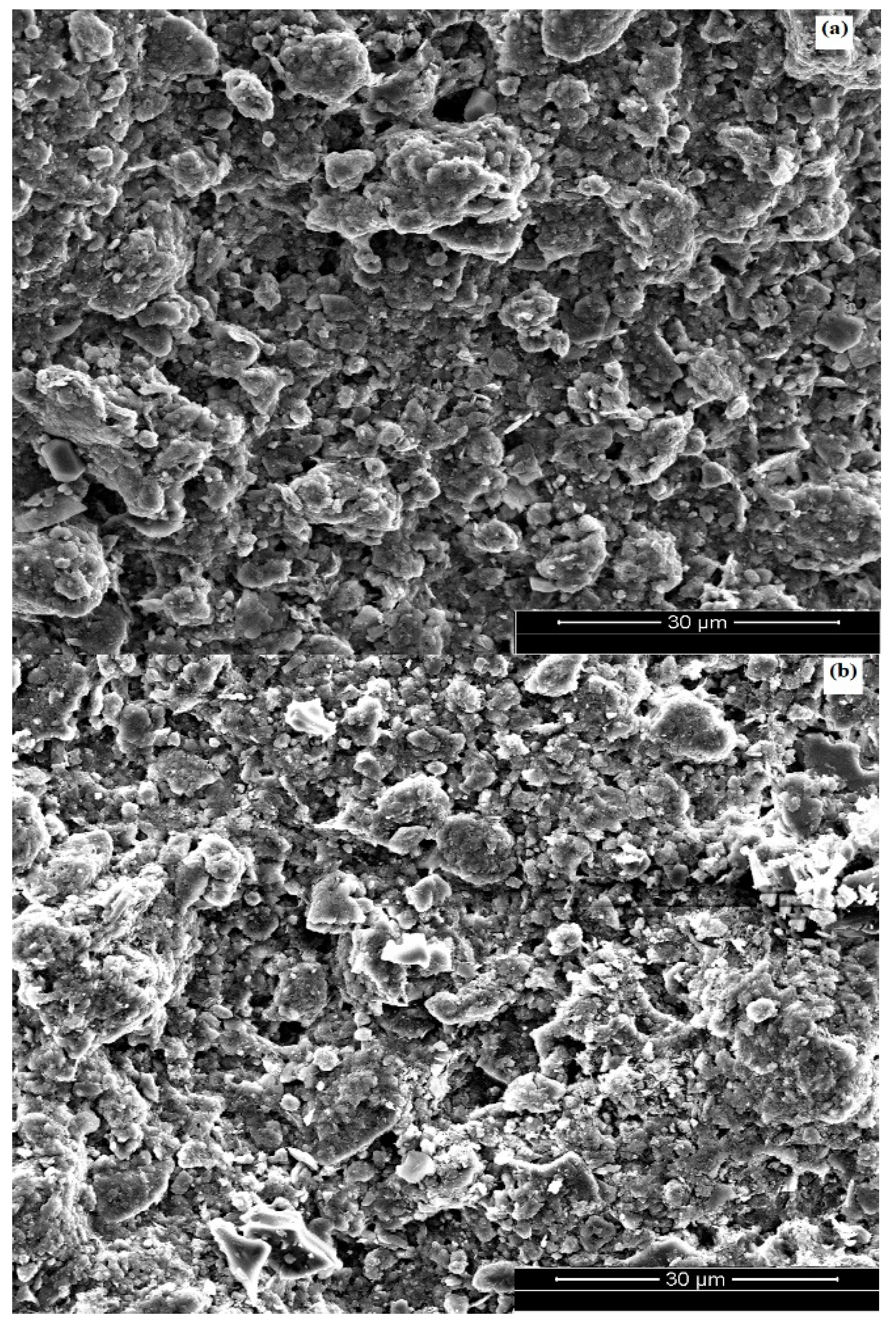

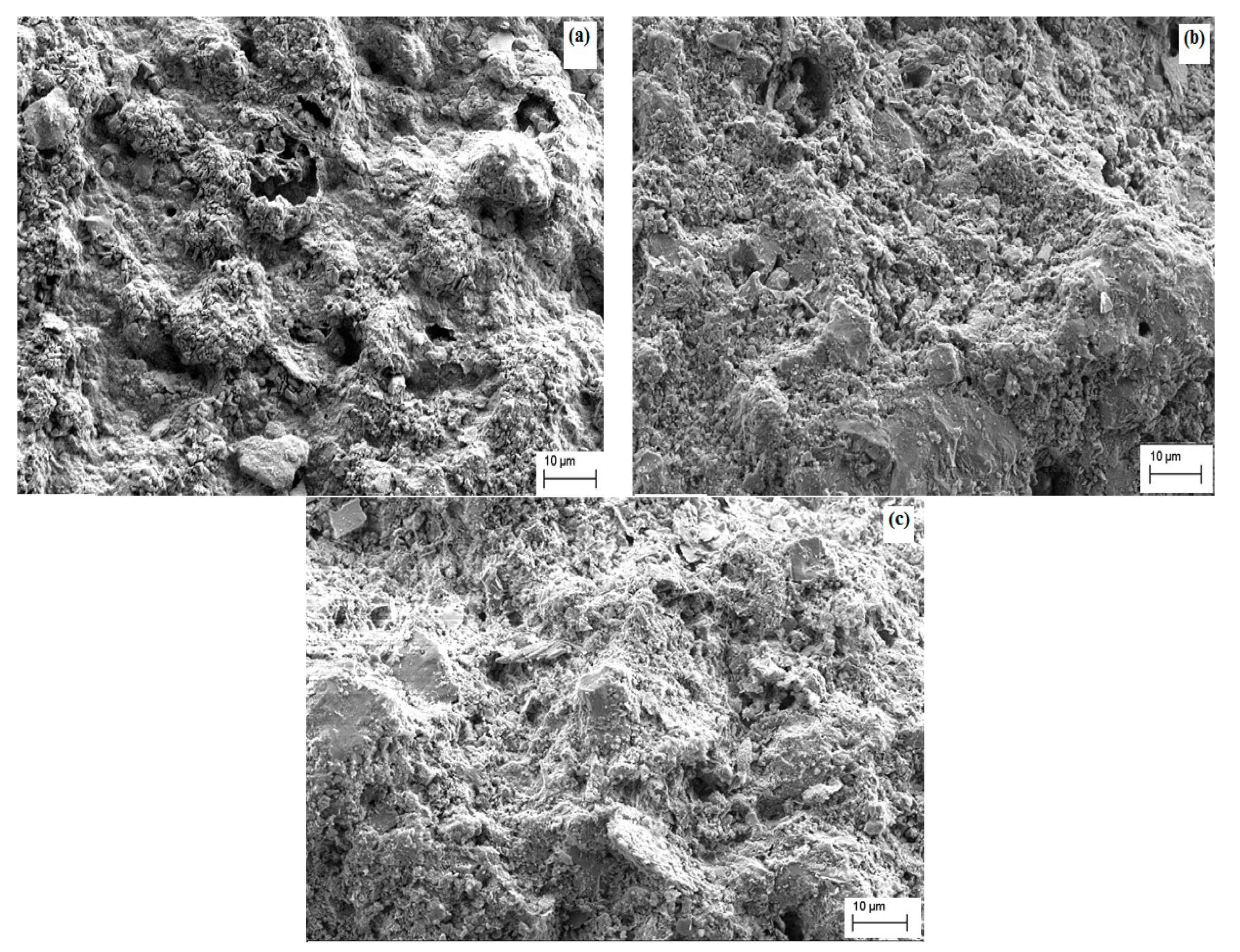


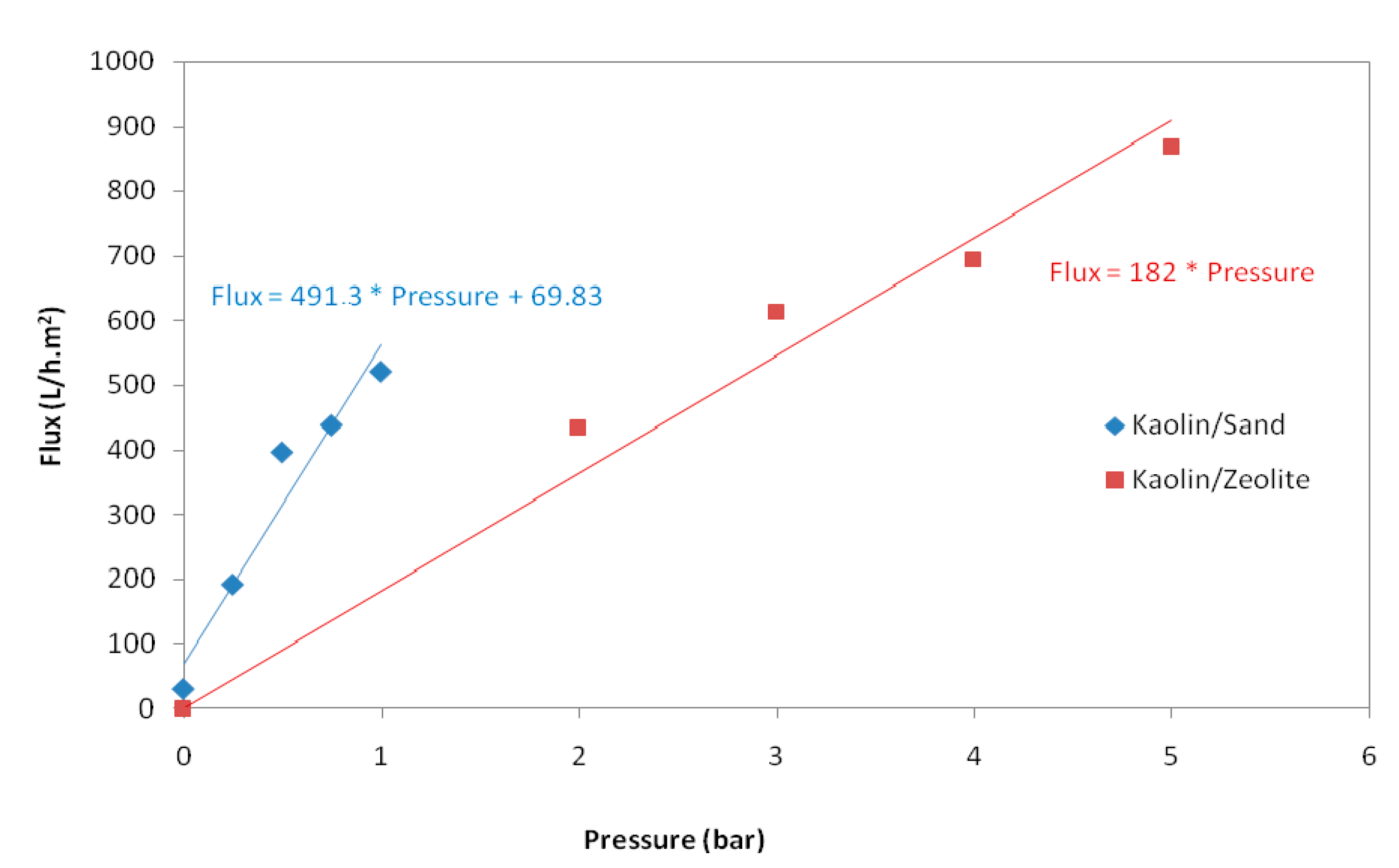

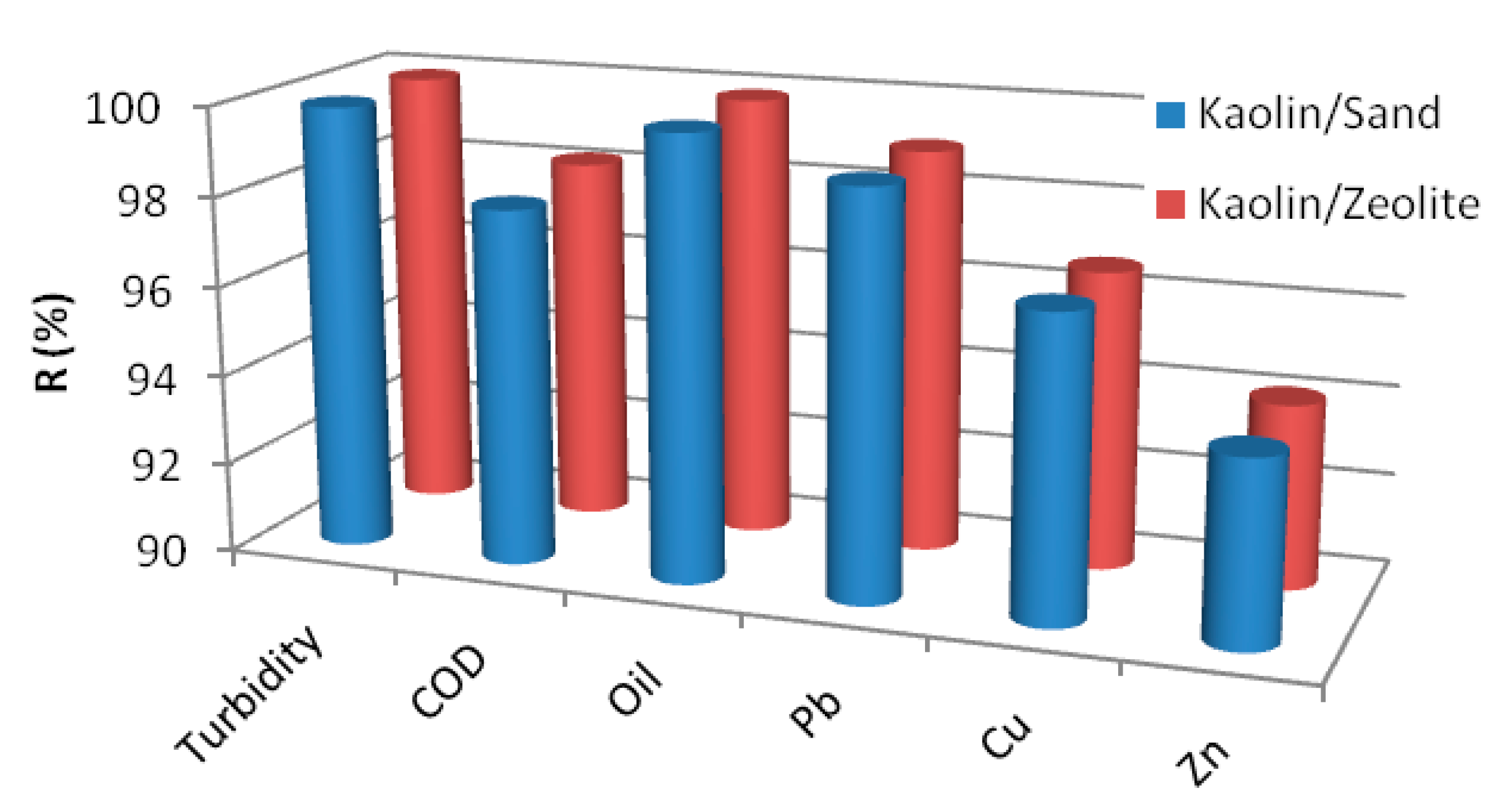
| Parameter | Sand Support | Zeolite Support |
|---|---|---|
| Sintering temperature (°C) | 1250 | 900 |
| Pore size (µm) | 10.36 | 0.55 |
| Mechanical strength (MPa) | 15.14 | 12.56 |
| Water permeability (L/h·m2·bar) | 3611 | 1218 |
| pH | Conductivity (ms/cm) | Oil Content (mg/L) | COD (mg/L) | Turbidity (NTU) | Pb (mg/L) | Zn (mg/L) | Cu (mg/L) | |
|---|---|---|---|---|---|---|---|---|
| Raw wastewater | 6.94 ± 0.2 | 3.33 ± 0.4 | 65,500 ± 500 | 4950 ± 200 | >6000 | 21.5 ± 0.02 | 10.4± 0.02 | 1.12 ± 0.02 |
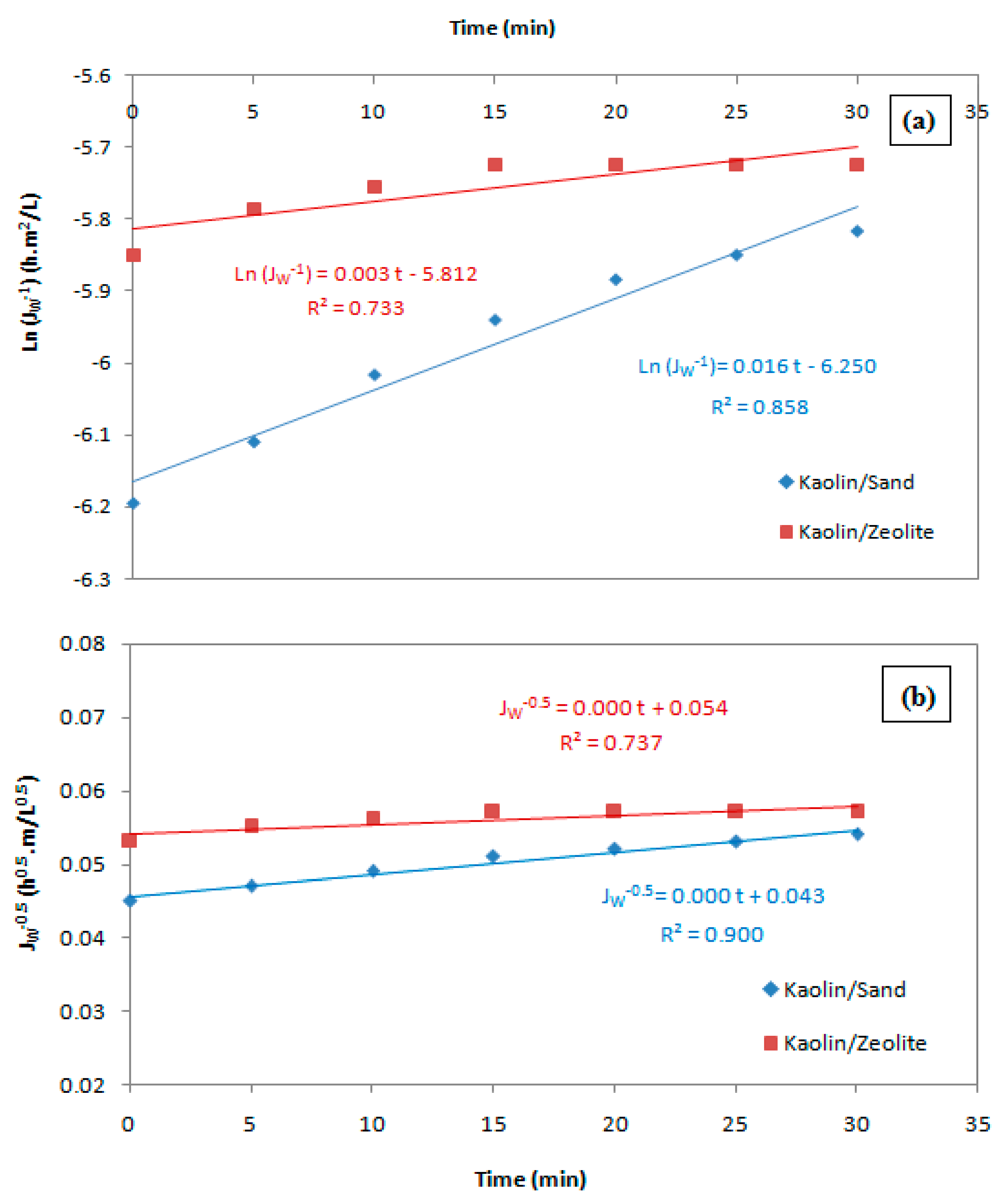
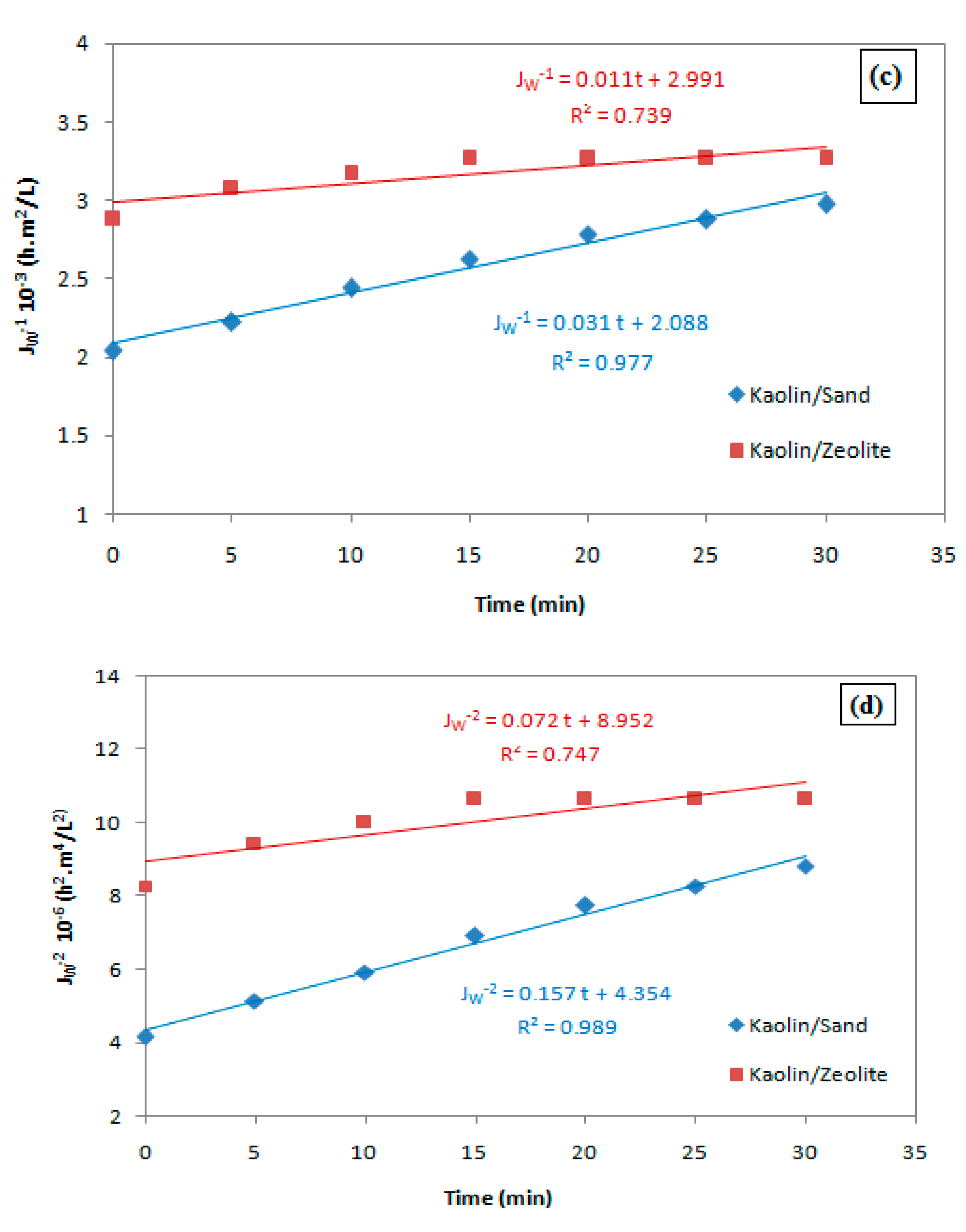
| Blocking Model | Kaolin/Sand | Kaolin/Zeolite | ||||
|---|---|---|---|---|---|---|
| K | J0 | R2 | K | J0 | R2 | |
| Complete pore blocking | 0.016 | −6.25 | 0.858 | 0.003 | −5.812 | 0.73 |
| Standard pore blocking | 0 | 0.043 | 0.9 | 0 | 0.054 | 0.737 |
| Intermediate pore blocking | 0.031 | 2.088 × 10−3 | 0.977 | 0.011 | 2.991 × 10−3 | 0.739 |
| Cake filtration | 0.157 | 4.354 × 10−6 | 0.989 | 0.072 | 8.952 × 10−6 | 0.747 |
| Cost of Raw Materials | |||||
|---|---|---|---|---|---|
| Raw Materials | Unit per Kg ($) | Amount of Raw Materials (g) | Cost ($) | ||
| Kaolin/Sand | Kaolin/Zeolite | Kaolin/Sand | Kaolin/Zeolite | ||
| Sand powder | 0.029 | 336 | - | 0.0097 | - |
| Zeolite powder | 0.174 | - | 336 | - | 0.058 |
| Kaolin powder | 0.16 | 8 | 2 | 0.0012 | 0.0003 |
| Distilled water | 0.28 | 172 | 278 | 0.048 | 0.077 |
| Porosity agents | 1.29 | 64 | 64 | 0.082 | 0.082 |
| Organic binder (PVA) | 0.8 | 30 | 30 | 0.024 | 0.024 |
| Total raw materials cost for fabrication of 15 membranes | 0.165 | 0.241 | |||
| Energy cost (Based on the power consumption) | |||||
| Kaolin/Sand | Kaolin/zeolite | ||||
| Mixer | 0.031 | 0.031 | |||
| Dry oven | 0.027 | 0.027 | |||
| Extruder | 0.138 | 0.138 | |||
| Furnace | 0.086 | 0.086 | |||
| Total production cost for fabrication of 15 membranes ($) (Surface of membrane = 1.7 × 10−3 m2) | 0.447 | 0.523 | |||
| Total production cost of membrane ($/m2) | 17.88 | 20.92 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aloulou, H.; Aloulou, W.; Duplay, J.; Baklouti, L.; Dammak, L.; Ben Amar, R. Development of Ultrafiltration Kaolin Membranes over Sand and Zeolite Supports for the Treatment of Electroplating Wastewater. Membranes 2022, 12, 1066. https://doi.org/10.3390/membranes12111066
Aloulou H, Aloulou W, Duplay J, Baklouti L, Dammak L, Ben Amar R. Development of Ultrafiltration Kaolin Membranes over Sand and Zeolite Supports for the Treatment of Electroplating Wastewater. Membranes. 2022; 12(11):1066. https://doi.org/10.3390/membranes12111066
Chicago/Turabian StyleAloulou, Hajer, Wala Aloulou, Joelle Duplay, Lassaad Baklouti, Lasâad Dammak, and Raja Ben Amar. 2022. "Development of Ultrafiltration Kaolin Membranes over Sand and Zeolite Supports for the Treatment of Electroplating Wastewater" Membranes 12, no. 11: 1066. https://doi.org/10.3390/membranes12111066
APA StyleAloulou, H., Aloulou, W., Duplay, J., Baklouti, L., Dammak, L., & Ben Amar, R. (2022). Development of Ultrafiltration Kaolin Membranes over Sand and Zeolite Supports for the Treatment of Electroplating Wastewater. Membranes, 12(11), 1066. https://doi.org/10.3390/membranes12111066







