High-Flux Ultrafiltration Membranes Combining Artificial Water Channels and Covalent Organic Frameworks
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of HC6H
2.3. Synthesis of Composite Matrix Membrane
2.4. Membrane Characterization
2.5. Membrane Separation Performance
3. Results and Discussion
3.1. Characterizations of HC6H
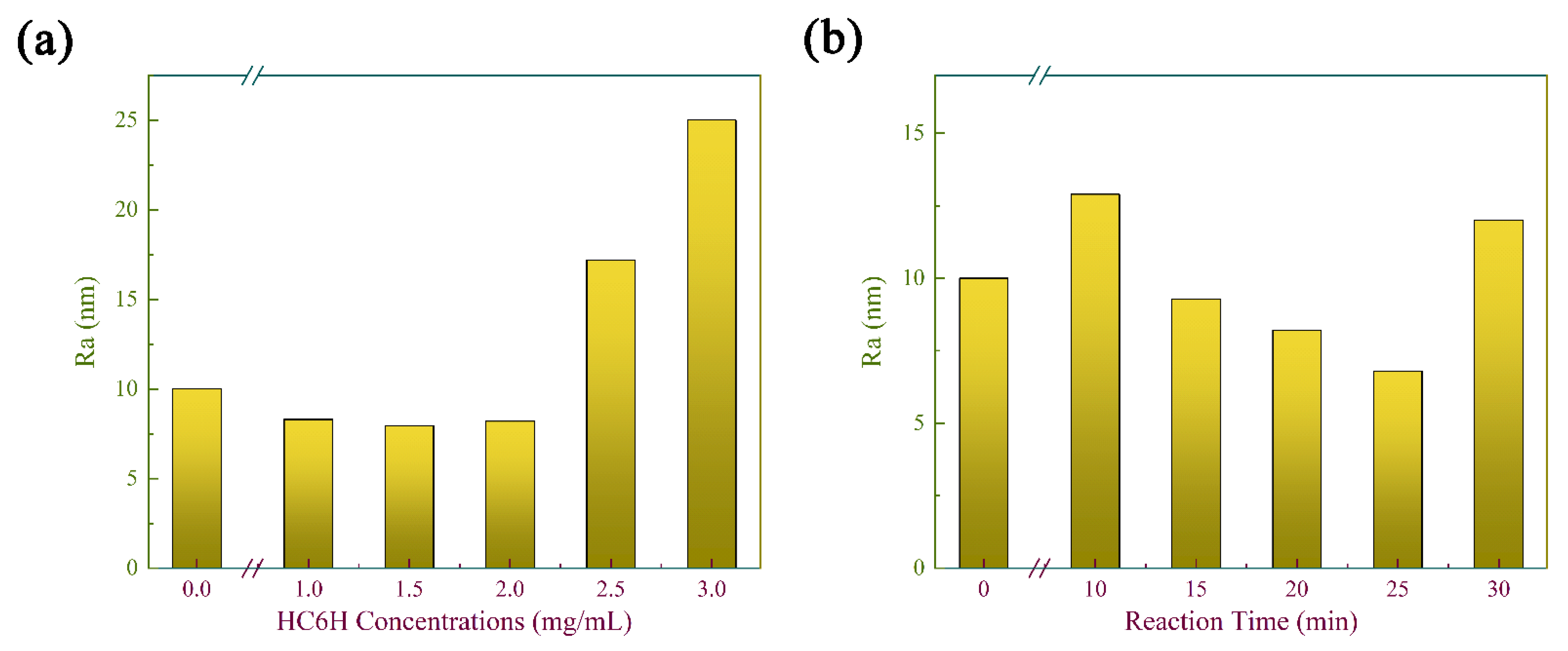
3.2. Morphologies of Composite Matrix Membranes
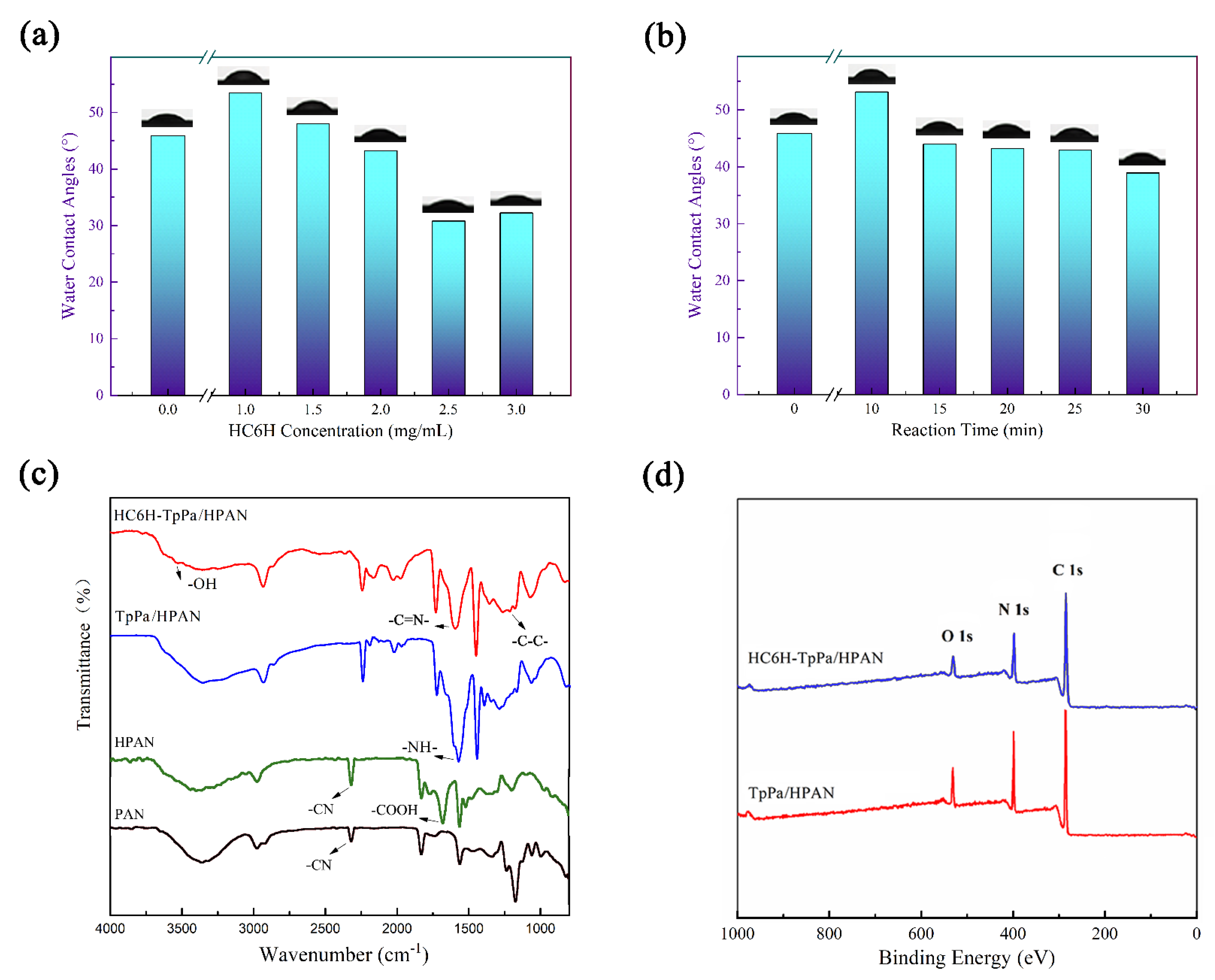
3.3. Surface Hydrophilicity of Composite Matrix Membranes
3.4. Chemical Composition of Composite Matrix Membranes
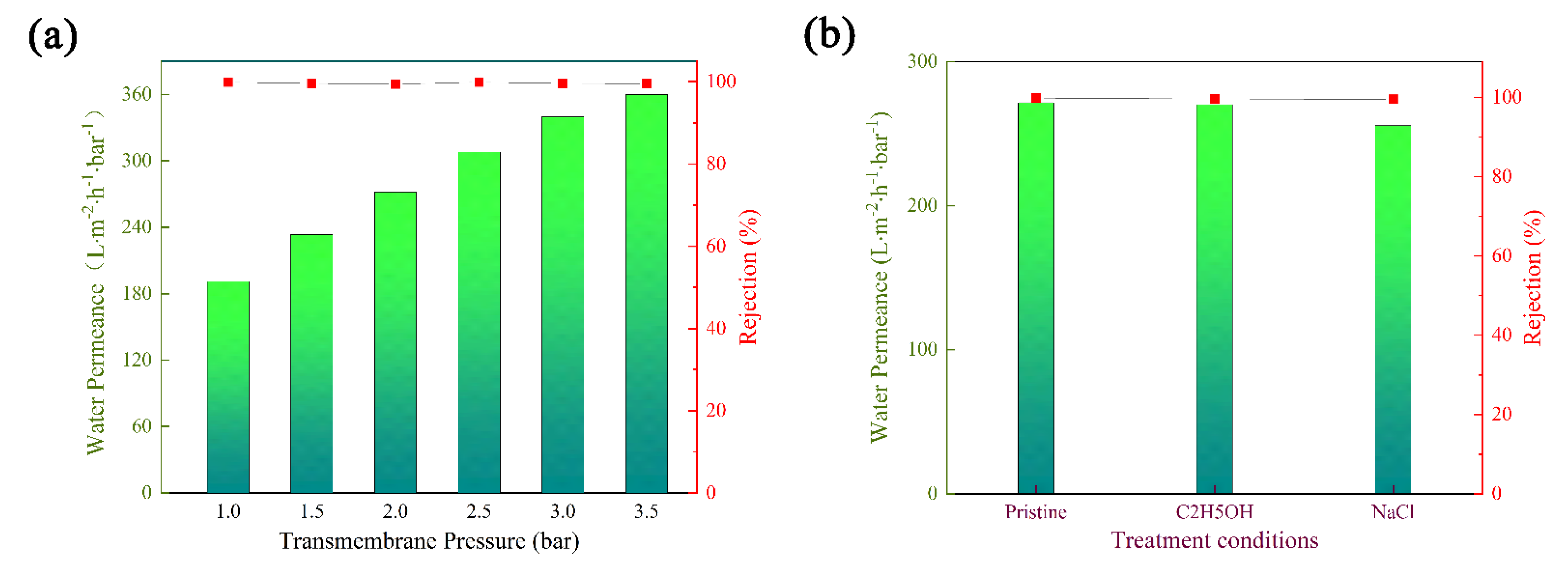
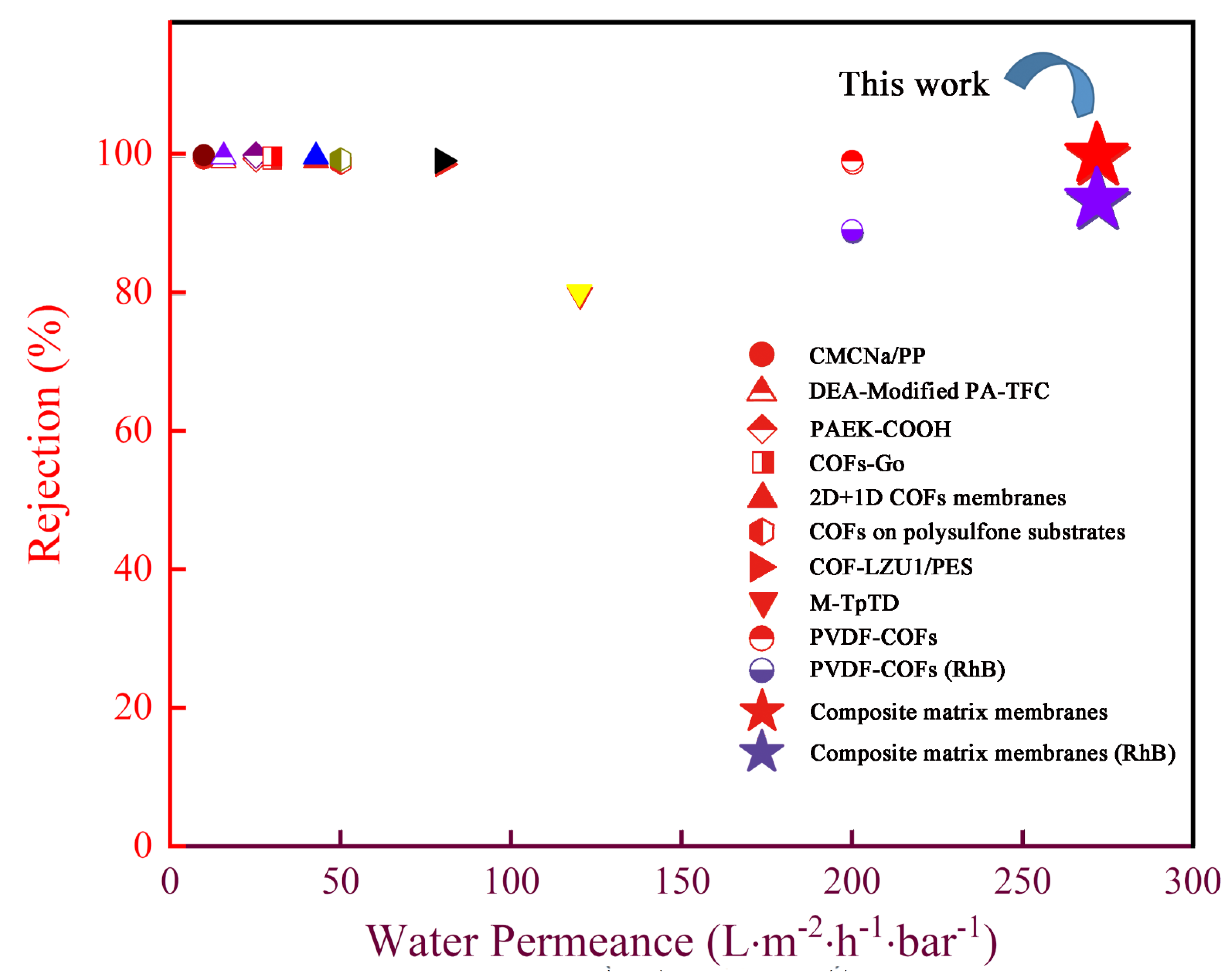
3.5. Seperation Performance of Composite Matrix Membranes
3.6. Stability of Membrane
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Loosdrecht, M.C.M.; Brdjanovic, D. Anticipating the next century of wastewater treatment. Science 2014, 344, 1452–1453. [Google Scholar] [CrossRef] [PubMed]
- An, A.K.; Guo, J.; Jeong, S.; Lee, E.-J.; Tabatabai, S.A.A.; Leiknes, T. High flux and antifouling properties of negatively charged membrane for dyeing wastewater treatment by membrane distillation. Water Res. 2016, 103, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, P.; Solomon, M.F.J.; Szekely, G.; Livingston, A.G. Molecular Separation with Organic Solvent Nanofiltration: A Critical Review. Chem. Rev. 2014, 114, 10735–10806. [Google Scholar] [CrossRef] [PubMed]
- Pohl, P.; Saparov, S.M.; Borgnia, M.J.; Agre, P. Highly selective water channel activity measured by voltage clamp: Analysis of planar lipid bilayers reconstituted with purified AqpZ. Proc. Natl. Acad. Sci. USA 2001, 98, 9624–9629. [Google Scholar] [CrossRef] [PubMed]
- Murata, K.; Mitsuoka, K.; Hirai, T.; Walz, T.; Agre, P.; Heymann, J.B.; Engel, A.; Fujiyoshi, Y. Structural determinants of water permeation through aquaporin-1. Nature 2000, 407, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Qiu, C.Q.; Li, X.S.; Vararattanavech, A.; Shen, W.M.; Torres, J.; Helix-Nielsen, C.; Wang, R.; Hu, X.; Fane, A.G.; et al. Synthesis of robust and high-performance aquaporin-based biomimetic membranes by interfacial polymerization-membrane preparation and RO performance characterization. J. Membr. Sci. 2012, 423, 422–428. [Google Scholar] [CrossRef]
- Huang, L.B.; Di Vincenzo, M.; Li, Y.; Barboiu, M. Artificial Water Channels: Towards Biomimetic Membranes for Desalination. Chemistry 2021, 27, 2224–2239. [Google Scholar] [CrossRef]
- Agre, P. Aquaporin water channels (Nobel lecture). Angew. Chem. Int. Ed. 2004, 43, 4278–4290. [Google Scholar] [CrossRef]
- Horner, A.; Siligan, C.; Cornean, A.; Pohl, P. Positively charged residues at the channel mouth boost single-file water flow. Faraday Discuss. 2018, 209, 55–65. [Google Scholar] [CrossRef]
- Eriksson, U.K.; Fischer, G.; Friemann, R.; Enkavi, G.; Tajkhorshid, E.; Neutze, R. Subangstrom Resolution X-ray Structure Details Aquaporin-Water Interactions. Science 2013, 340, 1346–1349. [Google Scholar] [CrossRef]
- Le Duc, Y.; Michau, M.; Gilles, A.; Gence, V.; Legrand, Y.M.; van der Lee, A.; Tingry, S.; Barboiu, M. Imidazole-quartet water and proton dipolar channels. Angew. Chem. Int. Ed. Engl. 2011, 50, 11366–11372. [Google Scholar] [CrossRef]
- Tunuguntla, R.H.; Allen, F.I.; Kim, K.; Belliveau, A.; Noy, A. Ultrafast proton transport in sub-1-nm diameter carbon nanotube porins. Nat. Nanotechnol. 2016, 11, 639–644. [Google Scholar] [CrossRef]
- Zhou, X.B.; Liu, G.D.; Yamato, K.; Shen, Y.; Cheng, R.X.; Wei, X.X.; Bai, W.L.; Gao, Y.; Li, H.; Liu, Y.; et al. Self-assembling subnanometer pores with unusual mass-transport properties. Nat. Commun. 2012, 3, 8. [Google Scholar] [CrossRef]
- Ogoshi, T.; Kanai, S.; Fujinami, S.; Yamagishi, T.A.; Nakamoto, Y. Para-bridged symmetrical pillar 5 arenes: Their Lewis acid catalyzed synthesis and host-guest property. J. Am. Chem. Soc. 2008, 130, 5022–5023. [Google Scholar] [CrossRef]
- Chen, L.; Si, W.; Zhang, L.; Tang, G.F.; Li, Z.T.; Hou, J.L. Chiral Selective Transmembrane Transport of Amino Acids through Artificial Channels. J. Am. Chem. Soc. 2013, 135, 2152–2155. [Google Scholar] [CrossRef]
- Liang, R.R.; Jiang, S.Y.; A, R.H.; Zhao, X. Two-dimensional covalent organic frameworks with hierarchical porosity. Chem Soc Rev. 2015, 7, 905–912. [Google Scholar] [CrossRef]
- Xu, H.; Gao, J.; Jiang, D. Stable, crystalline, porous, covalent organic frameworks as a platform for chiral organocatalysts. Nat. Chem. 2015, 7, 905–912. [Google Scholar] [CrossRef]
- Xu, F.; Xu, H.; Chen, X.; Wu, D.; Wu, Y.; Liu, H.; Gu, C.; Fu, R.; Jiang, D. Radical Covalent Organic Frameworks: A General Strategy to Immobilize Open-Accessible Polyradicals for High-Performance Capacitive Energy Storage. Angew. Chem. Int. Ed. 2015, 54, 6814–6818. [Google Scholar] [CrossRef]
- Su, Y.-Y.; Yan, X.; Chen, Y.; Guo, X.-J.; Chen, X.-F.; Lang, W.-Z. Facile fabrication of COF-LZU1/PES composite membrane via interfacial polymerization on microfiltration substrate for dye/salt separation. J. Membr. Sci. 2021, 618, 118706. [Google Scholar] [CrossRef]
- Yang, H.; Yang, L.; Wang, H.; Xu, Z.; Zhao, Y.; Luo, Y.; Nasir, N.; Song, Y.; Wu, H.; Pan, F.; et al. Covalent organic framework membranes through a mixed-dimensional assembly for molecular separations. Nat. Commun. 2019, 10, 2101. [Google Scholar] [CrossRef]
- Muniz, F.T.L.; Miranda, M.A.R.; Morilla dos Santos, C.; Sasaki, J.M. The Scherrer equation and the dynamical theory of X-ray diffraction. Acta Crystallogr. Sect. A 2016, 72, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Zhang, M.; Cheng, L.; Yuan, J.; Liu, G.; Huang, L.; Barboiu, M.; Jin, W. Bola-amphiphile-imidazole embedded GO membrane with enhanced solvent dehydration properties. J. Membr. Sci. 2020, 595, 117545. [Google Scholar] [CrossRef]
- Liu, K.; Hu, X.; Wei, Z.; Li, Y.; Jiang, Y. Modified Gaussian Process Regression Models for Cyclic Capacity Prediction of Lithium-Ion Batteries. IEEE Trans. Transp. Electrif. 2019, 5, 1225–1236. [Google Scholar] [CrossRef]
- Wu, C.; Wang, X.; Zhu, T.; Li, P.; Xia, S. Covalent organic frameworks embedded membrane via acetic-acid-catalyzed interfacial polymerization for dyes separation: Enhanced permeability and selectivity. Chemosphere 2020, 261, 127580. [Google Scholar] [CrossRef]
- Yu, S.; Chen, Z.; Cheng, Q.; Lü, Z.; Liu, M.; Gao, C. Application of thin-film composite hollow fiber membrane to submerged nanofiltration of anionic dye aqueous solutions. Sep. Purif. Technol. 2012, 88, 121–129. [Google Scholar] [CrossRef]
- Liu, M.; Chen, Q.; Lu, K.; Huang, W.; Lü, Z.; Zhou, C.; Yu, S.; Gao, C. High efficient removal of dyes from aqueous solution through nanofiltration using diethanolamine-modified polyamide thin-film composite membrane. Sep. Purif. Technol. 2017, 173, 135–143. [Google Scholar] [CrossRef]
- Xing, L.; Guo, N.; Zhang, Y.; Zhang, H.; Liu, J. A negatively charged loose nanofiltration membrane by blending with poly (sodium 4-styrene sulfonate) grafted SiO2 via SI-ATRP for dye purification. Sep. Purif. Technol. 2015, 146, 50–59. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Wang, J.; Peng, D.; Liu, J.; Zhang, Y. In-situ grown covalent organic framework nanosheets on graphene for membrane-based dye/salt separation. J. Membr. Sci. 2019, 581, 321–330. [Google Scholar] [CrossRef]
- Wang, R.; Shi, X.; Xiao, A.; Zhou, W.; Wang, Y. Interfacial polymerization of covalent organic frameworks (COFs) on polymeric substrates for molecular separations. J. Membr. Sci. 2018, 566, 197–204. [Google Scholar] [CrossRef]
- Kandambeth, S.; Biswal, B.P.; Chaudhari, H.D.; Rout, K.C.; Kunjattu, H.S.; Mitra, S.; Karak, S.; Das, A.; Mukherjee, R.; Kharul, U.K.; et al. Selective Molecular Sieving in Self-Standing Porous Covalent-Organic-Framework Membranes. Adv. Mater. 2017, 29, 1603945. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, K.; Guo, J.; Li, Y.; Chen, J.; Li, P. High-Flux Ultrafiltration Membranes Combining Artificial Water Channels and Covalent Organic Frameworks. Membranes 2022, 12, 824. https://doi.org/10.3390/membranes12090824
Liu K, Guo J, Li Y, Chen J, Li P. High-Flux Ultrafiltration Membranes Combining Artificial Water Channels and Covalent Organic Frameworks. Membranes. 2022; 12(9):824. https://doi.org/10.3390/membranes12090824
Chicago/Turabian StyleLiu, Kai, Jinwen Guo, Yingdong Li, Jinguang Chen, and Pingli Li. 2022. "High-Flux Ultrafiltration Membranes Combining Artificial Water Channels and Covalent Organic Frameworks" Membranes 12, no. 9: 824. https://doi.org/10.3390/membranes12090824
APA StyleLiu, K., Guo, J., Li, Y., Chen, J., & Li, P. (2022). High-Flux Ultrafiltration Membranes Combining Artificial Water Channels and Covalent Organic Frameworks. Membranes, 12(9), 824. https://doi.org/10.3390/membranes12090824




