A Review of the Application of Modified Separators in Inhibiting the “shuttle effect” of Lithium–Sulfur Batteries
Abstract
:1. Introduction
2. Adsorption Effect Modification
2.1. Physical/Chemical Adsorption
2.2. Catalytic Adsorption
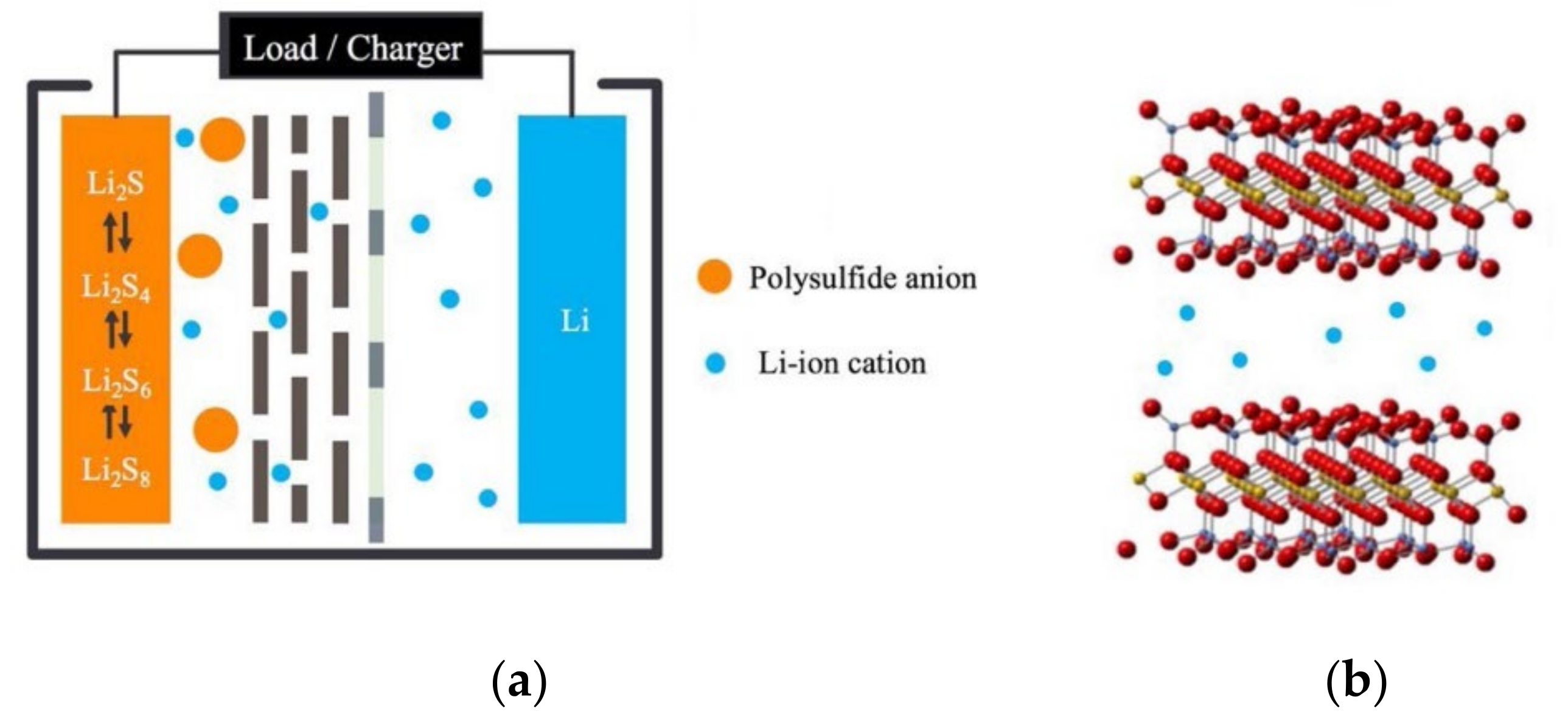
3. Electrostatic Effect
3.1. Negatively Charged Group
3.2. Polar Particles
4. Steric Hindrance Effect
4.1. Particle Buildup
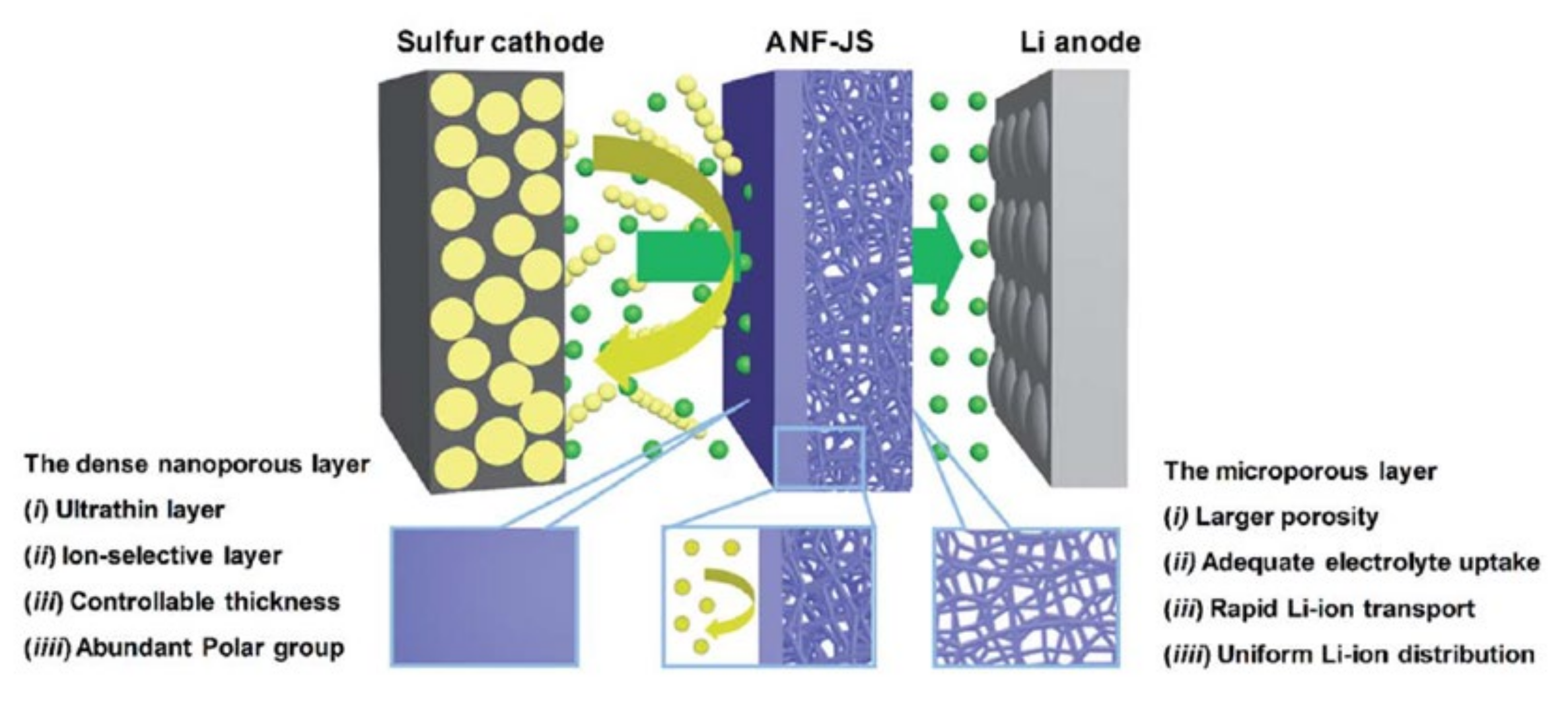
4.2. New Microporous Separator
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Islam, M.S.; Nazar, L.F. Advanced materials for lithium batteries. J. Mater. Chem. 2011, 21, 9810. [Google Scholar] [CrossRef]
- Ma, Y.; Xie, X.B.; Yang, W.Y.; Yu, Z.P.; Sun, X.Q.; Zhang, Y.P.; Yang, X.Y.; Kimura, H.; Hou, C.X.; Guo, Z.H.; et al. Recent advances in transition metal oxides with different dimensions as electrodes for high-performance supercapacitors. Adv. Compos. Hybrid Mater. 2021, 4, 906–924. [Google Scholar] [CrossRef]
- Zhai, I.Y.; Yang, W.; Xie, X.; Yu, Z.P.; Sun, X.Q.; Zhang, Y.P.; Yang, X.Y.; Kimura, H.; Hou, C.X.; Guo, Z.H.; et al. Co3O4 nanoparticle-dotted hierarchical-assembled carbon nanosheet framework catalysts with the formation/decomposition mechanisms of Li2O2 for smart lithium–oxygen batteries. Inorg. Chem. Front. 2022, 9, 1115–1124. [Google Scholar] [CrossRef]
- Ould, E.T.; Kamzabek, D.; Chakraborty, D.; Doherty, M.F. Lithium–sulfur batteries: State of the art and future directions. ACS Appl. Energy Mater. 2018, 1, 1783–1814. [Google Scholar] [CrossRef]
- Chen, H.; Wang, C.; Dong, W.; Lu, W.; Du, Z.L.; Chen, L.W. Monodispersed sulfur nanoparticles for lithium–sulfur batteries with theoretical performance. Nano Lett. 2015, 15, 798–802. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Li, B.Q.; Zhang, X.Q.; Huang, J.Q.; Zhang, Q. A perspective toward practical lithium–sulfur batteries. ACS Cent. Sci. 2020, 6, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, S.; Zhang, Y.; Li, M.; Chen, Z.W.; Lu, J. Revisiting the role of polysulfides in lithium–sulfur batteries. Adv. Mater. 2018, 30, 1705590. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.A.; Ding, J.; Wu, S.H.; Fang, J.; Boopathi, K.M.; Mohapatra, A.; Lee, L.W.; Wang, P.C.; Chang, C.C.; Chu, C.W. Modified separator performing dual physical/chemical roles to inhibit polysulfide shuttle resulting in ultrastable Li–S batteries. ACS Nano 2017, 11, 12436–12445. [Google Scholar] [CrossRef]
- He, Y.; Qiao, Y.; Chang, Z.; Cao, X.; Jia, M.; He, P.; Zhou, H. Developing A “Polysulfide-Phobic” Strategy to Restrain Shuttle Effect in Lithium-Sulfur Batteries. Angew. Chem. 2019, 58, 11774–11778. [Google Scholar] [CrossRef]
- Yan, J.; Liu, X.; Li, B. Capacity Fade Analysis of Sulfur Cathodes in Lithium-Sulfur Batteries. Adv. Sci. 2016, 3, 1600101–1600111. [Google Scholar] [CrossRef]
- Li, T.; Bai, X.; Gulzar, U.; Bai, Y.J.; Capiglia, C.; Deng, W.; Zhou, X.F.; Liu, Z.P.; Feng, Z.F.; Zaccaria, R.P. A Comprehensive Understanding of Lithium–Sulfur Battery Technology. Adv. Funct. Mater. 2019, 29, 1901730. [Google Scholar] [CrossRef]
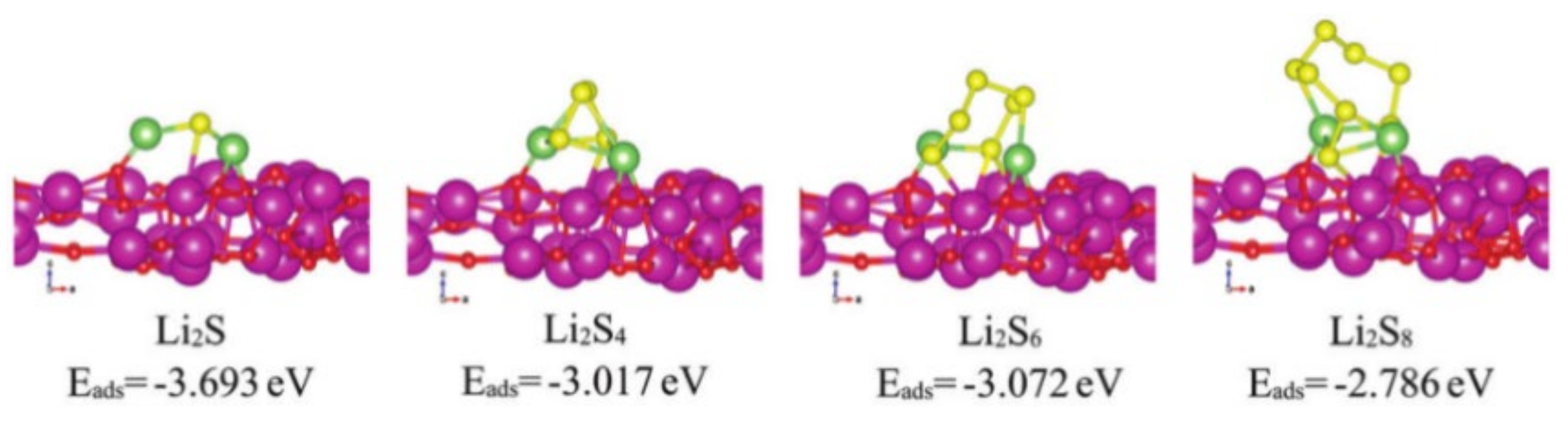
- Tao, X.; Wang, J.; Liu, C.; Wang, H.; Yao, H.; Zheng, G.Y.; Seh, Z.W.; Cai, Q.X.; Li, W.Y.; Zhou, G.; et al. Balancing surface adsorption and diffusion of lithium-polysulfides on nonconductive oxides for lithium-sulfur battery design. Nat. Commun. 2016, 7, 11203. [Google Scholar] [CrossRef]
- Manthiram, A.; Fu, Y.; Su, Y.S. Challenges and prospects of lithium–sulfur batteries. Acc. Chem. Res. 2013, 46, 1125–1134. [Google Scholar] [CrossRef]
- Manthiram, A.; Chung, S.H.; Zu, C. Lithium–sulfur batteries: Progress and prospects. Adv. Mater. 2015, 27, 1980–2006. [Google Scholar] [CrossRef]
- Dent, M.; Jakubczyk, E.; Zhang, T.; Lekakou, C. Kinetics of sulphur dissolution in lithium–sulphur batteries. J. Phys. Energy 2022, 4, 024001. [Google Scholar] [CrossRef]
- Deng, N.; Wang, Y.; Yan, J.; Ju, J.; Li, Z.J.; Fan, L.L.; Zhao, H.J.; Kang, W.M.; Cheng, B.W. A F-doped tree-like nanofiber structural poly-m-phenyleneisophthalamide separator for high-performance lithium-sulfur batteries. Power Sources 2017, 362, 243–249. [Google Scholar] [CrossRef]
- Dong, Q.; Shen, R.; Li, C.; Gan, R.Y.; Ma, X.T.; Wang, J.C.; Li, J.; Wei, Z.D. Construction of Soft Base Tongs on Separator to Grasp Polysulfides from Shuttling in Lithium-Sulfur Batteries. Small 2018, 14, 1804277. [Google Scholar] [CrossRef]
- Liu, Y.T.; Liu, S.; Li, G.R.; Guo, X.P. Strategy of enhancing the volumetric energy density for lithium–sulfur batteries. Adv. Mater. 2021, 33, 2003955. [Google Scholar] [CrossRef]
- Wang, P.; Xi, B.; Huang, M.; Chen, W.H.; Feng, J.K.; Xiong, S.L. Emerging catalysts to promote kinetics of lithium–sulfur batteries. Adv. Energy Mater. 2021, 11, 2002893. [Google Scholar] [CrossRef]
- Fang, R.; Zhao, S.; Sun, Z.; Wang, D.W.; Chen, H.M.; Li, F. More reliable lithium-sulfur batteries: Status, solutions and prospects. Adv. Mater. 2017, 29, 1606823. [Google Scholar] [CrossRef]
- Chung, S.H.; Chang, C.H.; Manthiram, A. Hierarchical sulfur electrodes as a testing platform for understanding the high-loading capability of Li-S batteries. J. Power Sources 2016, 334, 179–190. [Google Scholar] [CrossRef]
- Dang, C.; Mu, Q.; Xie, X.; Sun, X.; Yang, X.; Zhang, Y.P.; Maganti, S.; Huang, M.; Jiang, Q.L.; Seok, I.; et al. Recent progress in cathode catalyst for nonaqueous lithium oxygen batteries: A review. Adv. Compos. Hybrid Mater. 2022, 5, 606–626. [Google Scholar] [CrossRef]
- Zhang, K.; Chen, Z.X.; Ning, R.Q.; Xi, S.B.; Du, Y.H.; Liu, C.B.; Ren, Z.Y.; Chi, X.; Bai, M.H.; Shen, C.; et al. Single-Atom Coated Separator for Robust Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2019, 11, 25147–25154. [Google Scholar] [CrossRef]
- Song, C.W.; Peng, C.X.; Bian, Z.H.; Dong, F.; Xu, H.Y.; Zheng, S.Y. Stable and Fast Lithium–Sulfur Battery Achieved by Rational Design of Multifunctional Separator. Energy Environ. Mater. 2019, 2, 216–224. [Google Scholar] [CrossRef]
- Liao, H.Y.; Zhang, H.Y.; Hong, H.Q.; Li, Z.H.; Lin, Y.X. Novel flower-like hierarchical carbon sphere with multi-scale pores coated on PP separator for high-performance lithium-sulfur batteries. Electrochim. Acta 2017, 257, 210–216. [Google Scholar] [CrossRef]
- Chung, S.H.; Manthiram, A. Bifunctional Separator with a Light-Weight Carbon-Coating for Dynamically and Statically Stable Lithium-Sulfur Batteries. Adv. Funct. Mater. 2014, 24, 5299–5306. [Google Scholar] [CrossRef]
- Chung, S.H.; Manthiram, A. High-Performance Li-S Batteries with an Ultra-lightweight MWCNT-Coated Separator. J. Phys. Chem. Lett. 2014, 5, 1978–1983. [Google Scholar] [CrossRef]
- Zhai, P.Y.; Peng, H.J.; Cheng, X.B.; Zhu, L.; Huang, J.Q.; Zhu, W.C.; Zhang, Q. Scaled-up fabrication of porous-graphene-modified separators for high-capacity lithium–sulfur batteries. Energy Storage Mater. 2017, 7, 56–63. [Google Scholar] [CrossRef]
- Tan, L.; Li, X.H.; Wang, Z.X.; Guo, H.J.; Wang, J.X.; An, L. Multifunctional Separator with Porous Carbon/Multi-Walled Carbon Nanotube Coating for Advanced Lithium−Sulfur Batteries. Chemelectrochem 2018, 5, 71–77. [Google Scholar] [CrossRef]
- Lee, D.K.; Ahn, C.W.; Jeon, H.J. Web-structured graphitic carbon fiber felt as an interlayer for rechargeable lithium—sulfur batteries with highly improved cycling performance. J. Power Sources 2017, 360, 559–568. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, L.; Zhao, Y. Ultralight carbon flakes modified separator as an effective polysulfide barrier for lithium-sulfur batteries. Electrochim. Acta 2019, 295, 910–917. [Google Scholar] [CrossRef]
- Zhang, K.M.; Dai, L.Q.; Xie, L.J.; Kong, Q.Q.; Su, F.Y.; Shi, J.; Liu, Y.Z. Graphene/Carbon Black Co-modified Separator as Polysulfides Trapper for Li-S Batteries. Chemistryselect 2019, 4, 6026–6034. [Google Scholar] [CrossRef]
- Guo, Y.F.; Xiao, J.R.; Hou, Y.X.; Wang, Z.Y.; Jiang, A.H. Carbon nanotube doped active carbon coated separator for enhanced electrochemical performance of lithium–sulfur batteries. J. Mater. Sci. Mater. Electron. 2017, 28, 17453–17460. [Google Scholar] [CrossRef]
- Zhang, Z.A.; Wang, G.C.; Lai, Y.Q.; Li, J. A freestanding hollow carbon nanofiber/reduced graphene oxide interlayer for high-performance lithium–sulfur batteries. J. Alloys Compd. 2016, 663, 501–506. [Google Scholar] [CrossRef]
- Balach, J.; Jaumann, T.; Klose, M.; Oswald, S.; Eckert, J.; Giebeler, L. Functional Mesoporous Carbon-Coated Separator for Long-Life, High-Energy Lithium-Sulfur Batteries. Adv. Funct. Mater. 2015, 25, 5285–5291. [Google Scholar] [CrossRef]
- Chiu, L.L.; Chung, S.H. A Poly(ethylene oxide)/Lithium bis(trifluoromethanesulfonyl)imide-Coated Polypropylene Membrane for a High-Loading Lithium-Sulfur Battery. Polymers 2021, 13, 535. [Google Scholar] [CrossRef]
- Fan, Y.; Niu, Z.; Zhang, F.; Zhang, R.; Zhao, Y.; Lu, G. Suppressing the Shuttle Effect in Lithium-Sulfur Batteries by a UiO-66-Modified Polypropylene Separator. ACS Omega 2019, 4, 10328–10335. [Google Scholar] [CrossRef]
- Li, N.; Xie, Y.; Peng, S.T.; Xiong, X.; Han, K. Ultra-lightweight Ti3C2T MXene modified separator for Li–S batteries: Thickness regulation enabled polysulfide inhibition and lithium ion transportation. J. Energy Chem. 2020, 42, 116–125. [Google Scholar] [CrossRef]
- Zuo, X.; Zhen, M.; Wang, C. Ni@N-doped graphene nanosheets and CNTs hybrids modified separator as efficient polysulfide barrier for high-performance lithium sulfur batteries. Nano Res. 2019, 12, 829–836. [Google Scholar] [CrossRef]
- Li, P.Y.; Lv, H.W.; Li, Z.L.; Meng, H.P.; Lin, Z.; Wang, R.H.; Li, X.J. The Electrostatic Attraction and Catalytic Effect Enabled by Ionic-Covalent Organic Nanosheets on MXene for Separator Modification of Lithium-Sulfur Batteries. Adv. Mater. 2021, 33, 2007803. [Google Scholar] [CrossRef]
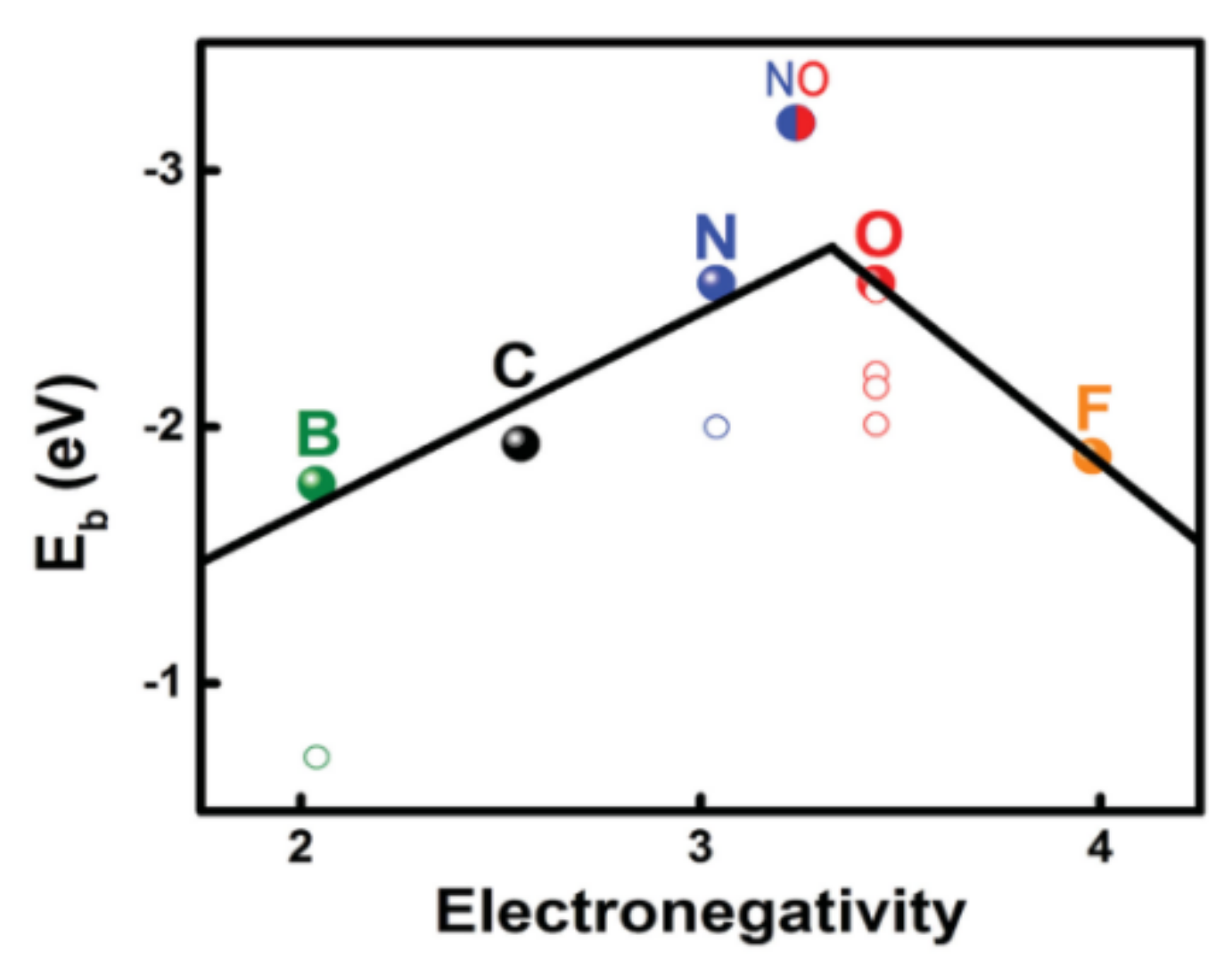
- Hou, T.Z.; Chen, X.; Peng, H.J.; Huang, J.Q.; Li, B.Q.; Zhang, Q.; Li, B. Design Principles for Heteroatom-Doped Nanocarbon to Achieve Strong Anchoring of Polysulfides for Lithium-Sulfur Batteries. Small 2016, 12, 3283–3291. [Google Scholar] [CrossRef]
- Zeng, P.; Huang, L.W.; Zhang, X.L.; Zhang, R.X.; Wu, L.; Chen, Y.G. Long-life and high-areal-capacity lithium-sulfur batteries realized by a honeycomb-like N, P dual-doped carbon modified separator. Chem. Eng. J. 2018, 349, 327–337. [Google Scholar] [CrossRef]
- Pei, C.B.; Li, J.D.; Lv, Z.Z.; Wang, H.M.; Dong, W.; Yao, Y.Y. Inhibiting polysulfides with PDA/PEI-functionalized separators for stable lithium-sulfur batteries. Int. J. Energy Res. 2021, 46, 10099–10110. [Google Scholar] [CrossRef]
- Zhang, H.; Lin, C.; Hu, X.H.; Zhu, B.K.; Yu, D.S. Effective Dual Polysulfide Rejection by a Tannic Acid/Fe(III) Complex-Coated Separator in Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2018, 10, 12708–12715. [Google Scholar] [CrossRef]
- Liu, N.; Wang, L.; Tan, T.Z.; Zhao, Y.; Zhang, Y.G. TiO2/GO-coated functional separator to suppress polysulfide migration in lithium-sulfur batteries. Beilstein J. Nanotechnol. 2019, 10, 1726–1736. [Google Scholar] [CrossRef]
- Xu, G.Y.; Yan, Q.B.; Wang, S.T.; Kushima, A.; Bai, P.; Liu, K.; Zhang, X.G.; Tang, Z.L.; Li, J. A thin multifunctional coating on a separator improves the cyclability and safety of lithium sulfur batteries. Chem. Sci. 2017, 8, 6619–6625. [Google Scholar] [CrossRef]
- Bhargav, A.; He, J.; Gupta, A.; Manthiram, A. Lithium-Sulfur Batteries: Attaining the Critical Metrics. Joule 2020, 4, 285–291. [Google Scholar] [CrossRef]
- Cheng, P.; Guo, P.Q.; Liu, D.Q.; Wang, Y.R.; Sun, K.; Zhao, Y.G.; He, D.Y. Fe3O4/RGO modified separators to suppress the shuttle effect for advanced lithium-sulfur batteries. J. Alloys Compd. 2019, 784, 149–156. [Google Scholar] [CrossRef]
- Lv, X.; Lei, T.; Wang, B.; Chen, W.; Jiao, Y.; Hu, Y.; Yan, Y.; Huang, J.; Chu, J.; Yan, C.; et al. An Efficient Separator with Low Li-Ion Diffusion Energy Barrier Resolving Feeble Conductivity for Practical Lithium–Sulfur Batteries. Adv. Energy Mater. 2019, 9, 1091800. [Google Scholar] [CrossRef]
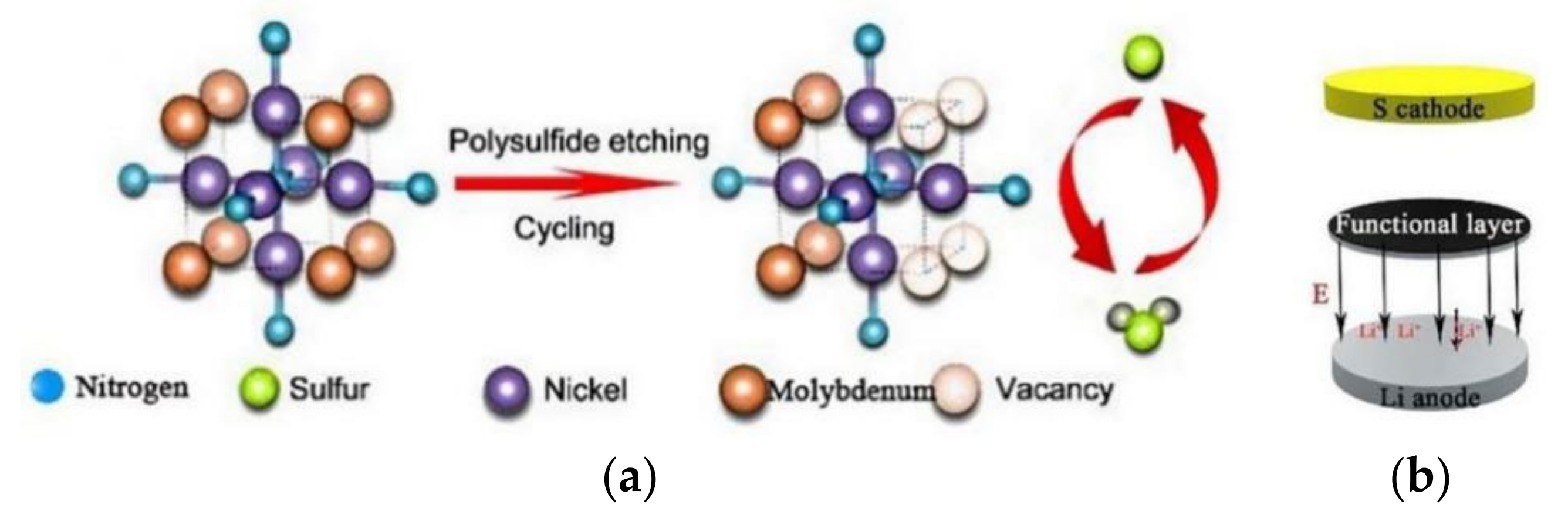
- Zhao, M.; Peng, H.J.; Zhang, Z.W.; Li, B.Q.; Chen, X.; Xie, J.; Chen, X.; Wei, J.Y.; Zhang, Q.; Huang, J.Q. Activating Inert Metallic Compounds for High-Rate Lithium-Sulfur Batteries through In Situ Etching of Extrinsic Metal. Angew. Chem. 2019, 58, 3779–3783. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Dai, R.Q.; Zhu, S.; Zhou, L.Z.; Xu, Q.J.; Min, Y.L. Bimetallic nitride modified separator constructs internal electric field for high-performance lithium-sulfur battery. Chem. Eng. J. 2022, 429, 132454. [Google Scholar] [CrossRef]
- Hong, X.J.; Song, C.L.; Yang, Y.; Tan, H.C.; Li, G.H.; Cai, Y.P.; Wang, H.X. Cerium Based Metal-Organic Frameworks as an Efficient Separator Coating Catalyzing the Conversion of Polysulfides for High Performance Lithium-Sulfur Batteries. ACS Nano 2019, 13, 1923–1931. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Yu, Z.J.; Zhang, M.K.; Zhen, S.Y.; Shen, J.H.; Chang, Y.; Wang, Y.; Deng, Y.F.; Li, A.J. A novel battery separator coated by a europium oxide/carbon nanocomposite enhances the performance of lithium sulfur batteries. Nanoscale 2021, 13, 16696–16704. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Feng, M.; Sun, H.; Li, G.R.; Fu, Q.; Li, H.B.; Liu, J.; Sun, L.Q.; Mauger, A.; Julien, A.M.; et al. Constructing metal-free and cost-effective multifunctional separator for high-performance lithium-sulfur batteries. Nano Energy 2019, 59, 390–398. [Google Scholar] [CrossRef]
- Lin, H.L.; Zhang, S.L.; Zhang, T.R.; Cao, S.; Ye, H.L.; Yao, Q.F.; Zheng, G.W.; Lee, J.Y. A Cathode-Integrated Sulfur-Deficient Co9S8 Catalytic Interlayer for the Reutilization of “Lost” Polysulfides in Lithium-Sulfur Batteries. ACS Nano 2019, 13, 7073–7082. [Google Scholar] [CrossRef]
- Song, S.L.; Shi, L.Y.; Lu, S.C.; Pang, Y.C.; Wang, Y.K.; Zhu, M.; Ding, D.W.; Ding, S.J. A new polysulfide blocker-poly(acrylic acid) modified separator for improved performance of lithium-sulfur battery. J. Membr. Sci. 2018, 563, 277–283. [Google Scholar] [CrossRef]
- Chiu, L.L.; Chung, S.H. Composite gel-polymer electrolyte for high-loading polysulfide cathodes. J. Mater. Chem. A 2022, 10, 13719–13726. [Google Scholar] [CrossRef]
- Li, D.D.; Yang, J.F.; Xu, X.; Wang, X.W.; Chen, J.T.; Xu, J.; Zhao, N. Synergistic inhibitory effect of ultralight CNTs-COOH@Fe3O4 modified separator on polysulfides shuttling for high-performance lithium–sulfur batteries. J. Membr. Sci. 2020, 611, 118300. [Google Scholar] [CrossRef]
- Wang, J.T.; Zhai, P.F.; Zhao, T.K.; Li, M.J.; Yang, Z.H.; Zhang, H.Q.; Huang, J.J. Laminar MXene-Nafion-modified separator with highly inhibited shuttle effect for long-life lithium–sulfur batteries. Electrochim. Acta 2019, 320, 134558. [Google Scholar] [CrossRef]
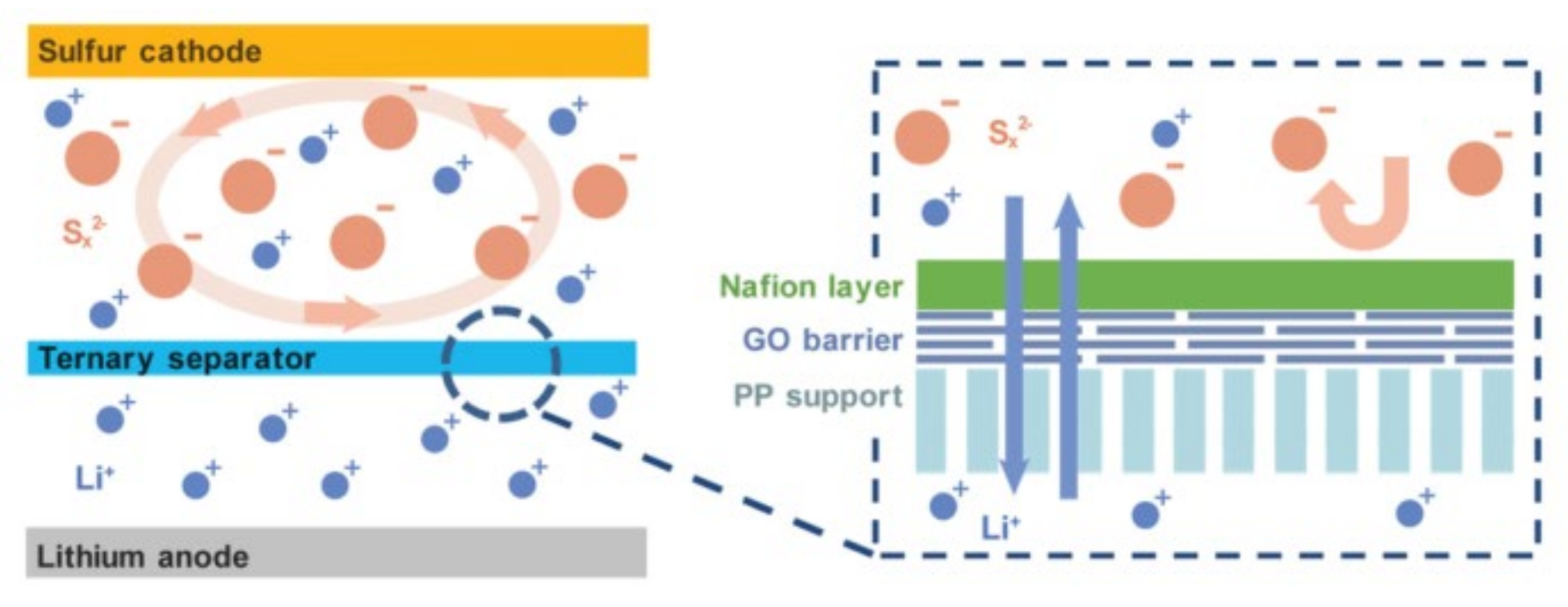
- Zhang, T.Z.; Huang, J.Q.; Peng, H.J.; He, L.Y.; Cheng, X.B.; Chen, C.M.; Zhang, Q. Rational Integration of Polypropylene/Graphene Oxide/Nafion as Ternary-Layered Separator to Retard the Shuttle of Polysulfides for Lithium-Sulfur Batteries. Small 2016, 12, 381–389. [Google Scholar] [CrossRef]
- Xu, R.; Sun, Y.Z.; Wang, Y.F.; Huang, J.Q.; Zhang, Q. Two-dimensional vermiculite separator for lithium sulfur batteries. Chin. Chem. Lett. 2017, 28, 2235–2238. [Google Scholar] [CrossRef]
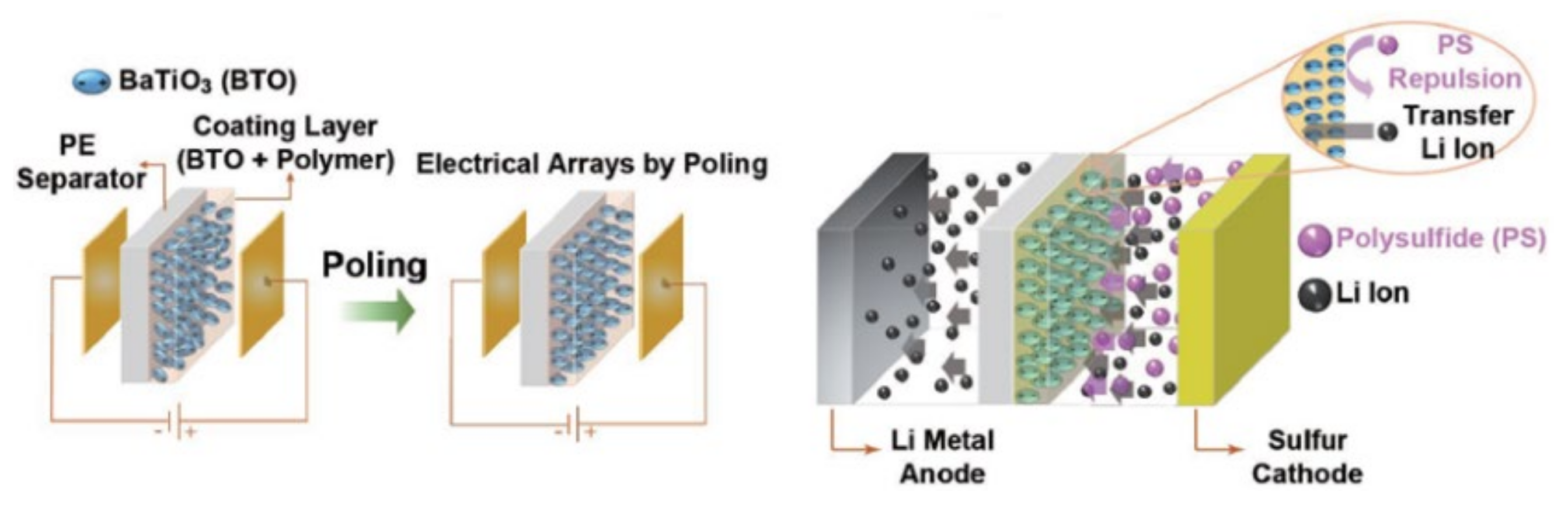
- Saroha, R.; Heo, J.; Li, X.Y.; Angulakshmi, N.; Lee, Y.; Ahn, H.J.; Ahn, J.H.; Kim, J.H. Asymmetric separator integrated with ferroelectric-BaTiO3 and mesoporous-CNT for the reutilization of soluble polysulfide in lithium-sulfur batteries. J. Alloys Compd. 2022, 893, 162272. [Google Scholar] [CrossRef]
- Yim, T.; Han, S.H.; Park, N.H.; Park, M.S.; Lee, J.H.; Shin, J.; Choi, J.W.; Jung, Y.J.; Jo, Y.N.; Yu, J.S.; et al. Effective Polysulfide Rejection by Dipole-Aligned BaTiO3Coated Separator in Lithium-Sulfur Batteries. Adv. Funct. Mater. 2016, 26, 7817–7823. [Google Scholar] [CrossRef]
- Babu, D.B.; Giribabu, K.; Ramesha, K. Permselective SPEEK/Nafion Composite-Coated Separator as a Potential Polysulfide Crossover Barrier Layer for Li-S Batteries. ACS Appl. Mater. Interfaces 2018, 10, 19721–19729. [Google Scholar] [CrossRef]
- Maletti, S.; Podetti, S.; Oswald, S.; Giebeler, L.; Barbero, C.A.; Balach, J. LiV3O8-Based Functional Separator Coating as Effective Polysulfide Mediator for Lithium–Sulfur Batteries. ACS Appl. Energy Mater. 2020, 3, 2893–2899. [Google Scholar] [CrossRef]
- Ghazi, Z.A.; He, X.; Khattak, A.M.; Khan, N.A.; Liang, L.B.; Iqbal, A.; Wang, J.X.; Sin, H.; Li, L.S.; Tang, Z.Y. MoS2 /Celgard Separator as Efficient Polysulfide Barrier for Long-Life Lithium-Sulfur Batteries. Adv. Mater. 2017, 29, 1606817. [Google Scholar] [CrossRef]
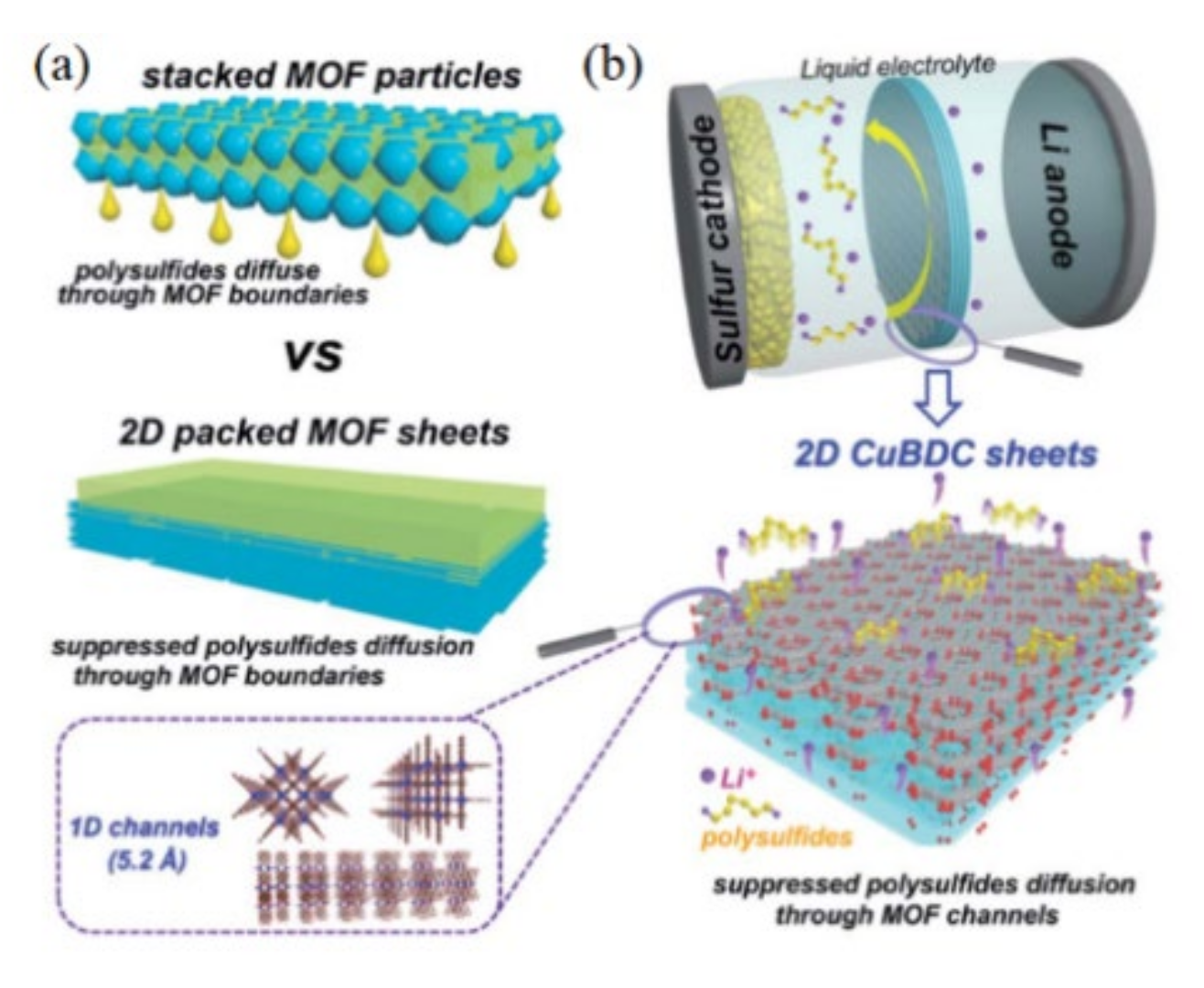
- Chang, Z.; Qiao, Y.; Wang, J.; Deng, H.; Zhou, H.S. Two-dimensional metal–organic framework with perpendicular one-dimensional nano-channel as precise polysulfide sieves for highly efficient lithium–sulfur batteries. J. Mater. Chem. A 2021, 9, 4870–4879. [Google Scholar] [CrossRef]
- Huang, Y.C.; Yen, Y.J.; Tseng, Y.H.; Chung, S.H. Module-Designed Carbon-Coated Separators for High-Loading, High-Sulfur-Utilization Cathodes in Lithium-Sulfur Batteries. Molecules 2021, 27, 228. [Google Scholar] [CrossRef]
- Wu, S.L.; Shi, J.Y.; Nie, X.L.; Yao, Y.M.; Jiang, F.; Wei, Q.F.; Huang, F.L. Microporous Cyclodextrin Film with Funnel-type Channel Polymerized on Electrospun Cellulose Acetate Membrane as Separators for Strong Trapping Polysulfides and Boosting Charging in Lithium–Sulfur Batteries. Energy Environ. Mater. 2022. [Google Scholar] [CrossRef]
- Pei, H.J.; Yang, C.Y.; Wu, Q.Y.; Zhou, X.P.; Xie, X.L.; Hwang, B.; Ye, Y.S. Ion-selective aramid nanofiber-based Janus separators fabricated by a dry-wet phase inversion approach for lithium–sulfur batteries. J. Mater. Chem. A 2022, 10, 5317–5327. [Google Scholar] [CrossRef]
- Sun, J.; Mu, Q.; Kimura, H.; Murugadoss, V.; He, M.; Du, W.; Hou, C.X. Oxidative degradation of phenols and substituted phenols in the water and atmosphere: A review. Adv. Compos. Hybrid Mater. 2022, 5, 627–640. [Google Scholar] [CrossRef]
- Guo, Y.; Li, J.; Pittcheri, R.; Zhu, J.H.; Wen, P.; Qiu, Y.J. Electrospun Ti4O7/C conductive nanofibers as interlayer for lithium-sulfur batteries with ultra long cycle life and high-rate capability. Chem. Eng. J. 2019, 355, 390–398. [Google Scholar] [CrossRef]
- Wang, X.B.; Luo, Y.H.; Wang, H.Y.; Wu, C.C.; Zhang, Z.S.; Lia, J.D. Tow-dimensional metal organic framework decorated porous carbon fiber as efficient interlayer for lithium-sulfur battery. J. Electroanal. Chem. 2021, 897, 115564. [Google Scholar] [CrossRef]

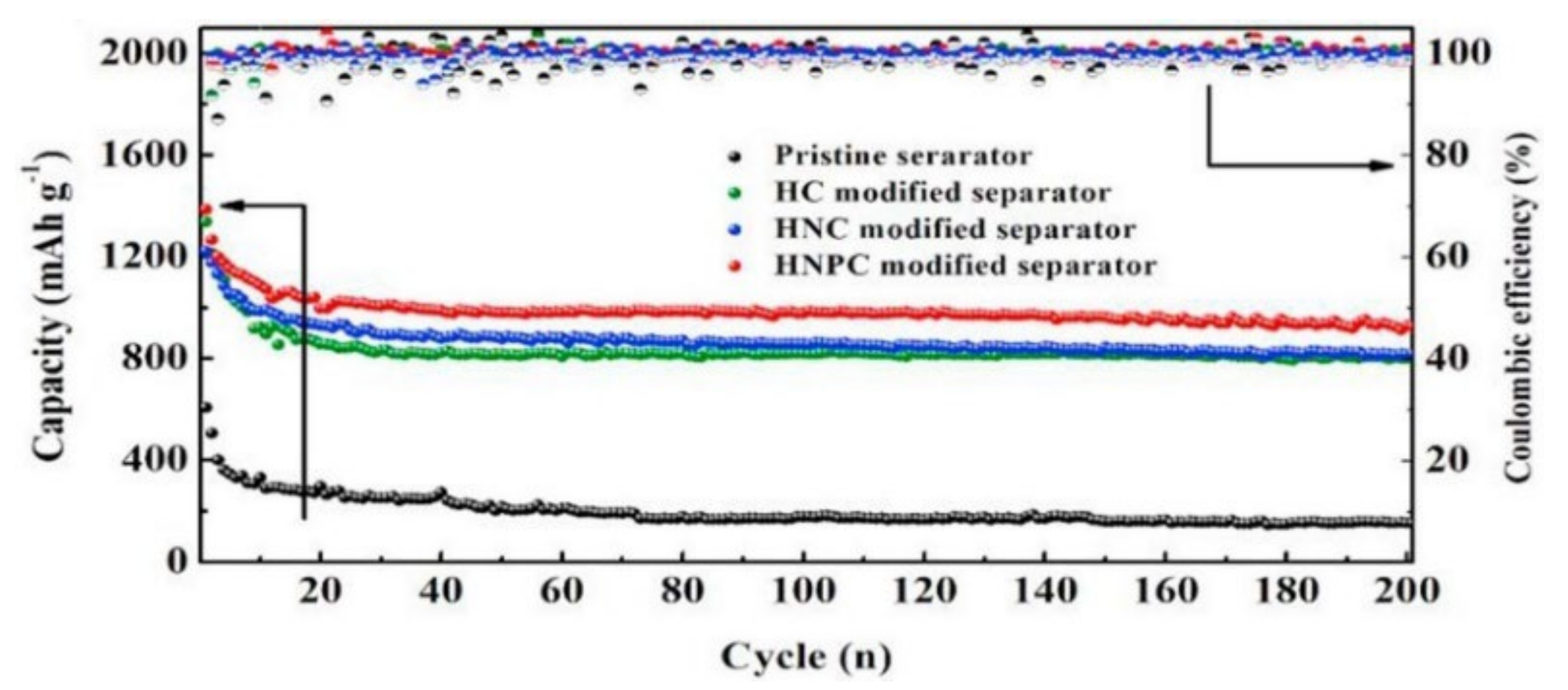





| Materials | Total Pore Volume/cm3 g−1 | Surface Area/m2 g−1 | Coating Binder Content | Area Density /mg cm−2 | Cathode Sulfur Content/wt% | S Loading/mg cm−2 | Capacity/(mAh g−1) (Rate) | Cycling Performance/(mAh g−1) (Cycles, Rate) | Capacity Decay/% | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Super P | - | - | None | 0.2 | 55 | 1.1–1.3 | 1289 (0.5 C) | 828 (200/0.2 C) | 0.19 | [26] |
| MWCNTs | 2.76 | 410.42 | None | 0.17 | 55 | - | 1107 (0.5 C) | 881 (150/0.2 C) | 0.14 | [27] |
| PG | 3.361 | 1443 | 20 wt% PVP | 0.54 | 63 | 1.8–2.0 | 1165 (0.5 C) | 877 (150/0.5 C) | - | [28] |
| PC/MWCNT | 0.17 | 83.4 | 10 wt% PVDF | 0.51 | 70 | 1.6–1.7 | 911 (0.5 C) | 659 (200/0.5 C) | 0.138 | [29] |
| GCFF | - | - | None | - | 60 | 0.7 | 1280.14 (0.2 C) | 1004.62 (100/0.2 C) | - | [30] |
| CFs | - | - | None | 0.16 | 60 | 1 | 1280.14 (0.2 C) | 683 (500/0.5 C) | 0.071 | [31] |
| rGO/CB | 2.334 | 861.12 | None | - | - | - | 1014.5 (0.2 C) | 850.9 (100/0.2 C) | 0.17 | [32] |
| CNT/AC | 0.19 | 1312 | 20 wt% PVDF | - | 70 | - | 1495.6 (0.2 C) | 742 (200/0.2 C) | 0.25 | [33] |
| HCNF/rGO | - | - | None | 1.2 | 60 | 1.4 | 1318.4 (0.2 C) | 779.1(100/1 C) | 0.13 | [34] |
| mesoC | 2.9 | 843 | 5 wt% super P and 10 wt% PVDF-HFP | 0.5 | 70 | 3.5 | 1378 (0.2 C) | 1021 (100/0.5 C) | 0.081 | [35] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.-W.; Sun, B.; Fu, P.; Liu, F.; Zhu, C.; Xu, B.-M.; Pan, Y.; Chen, C. A Review of the Application of Modified Separators in Inhibiting the “shuttle effect” of Lithium–Sulfur Batteries. Membranes 2022, 12, 790. https://doi.org/10.3390/membranes12080790
Zhang B-W, Sun B, Fu P, Liu F, Zhu C, Xu B-M, Pan Y, Chen C. A Review of the Application of Modified Separators in Inhibiting the “shuttle effect” of Lithium–Sulfur Batteries. Membranes. 2022; 12(8):790. https://doi.org/10.3390/membranes12080790
Chicago/Turabian StyleZhang, Bo-Wen, Bo Sun, Pei Fu, Feng Liu, Chen Zhu, Bao-Ming Xu, Yong Pan, and Chi Chen. 2022. "A Review of the Application of Modified Separators in Inhibiting the “shuttle effect” of Lithium–Sulfur Batteries" Membranes 12, no. 8: 790. https://doi.org/10.3390/membranes12080790
APA StyleZhang, B.-W., Sun, B., Fu, P., Liu, F., Zhu, C., Xu, B.-M., Pan, Y., & Chen, C. (2022). A Review of the Application of Modified Separators in Inhibiting the “shuttle effect” of Lithium–Sulfur Batteries. Membranes, 12(8), 790. https://doi.org/10.3390/membranes12080790





